Abstract
Superoxide radical anion is a biologically important oxidant that has been linked to tissue injury and inflammation in several diseases. Here we carried out a structure-activity study on 6 different carboxyfullerene superoxide dismutase (SOD) mimetics with distinct electronic and biophysical characteristics. Neurotoxicity via NMDA receptors, which involves intracellular superoxide, was used as a model to evaluate structure-activity relationships between reactivity towards superoxide and neuronal rescue by these drugs. A significant correlation between neuroprotection by carboxyfullerenes and their ki towards superoxide radical was observed. Computer-assistant molecular modeling demonstrated that the reactivity towards superoxide is sensitive to changes in dipole moment which are dictated not only by the number of carboxyl groups, but also by their distribution on the fullerene ball. These results indicate that the SOD activity of these cell-permeable compounds predicts neuroprotection, and establishes a structure-activity relationship to aid in future studies on the biology of superoxide across disciplines.
Keywords: Superoxide, neuroprotection, carboxyfullerenes, SOD mimetics
Background
Superoxide radical is a biologically important reactive oxygen species (ROS) which is proposed to contribute to inflammation, altered cellular metabolism, and tissue injury in diabetes, stroke, cardiovascular disease and cancer. Specific intracellular targets of superoxide include ras, aconitase, numerous ion channels and transporters, polyunsaturated lipids, and many redox-sensitive transcription factors [1–3]. However, a full understanding of how superoxide modulates intracellular signaling and gene expression has been hampered by the lack of potent cell-permeable small-molecule SOD mimetics. The development of such compounds would not only provide potential therapeutic approaches, but also valuable tools to understand the basic biology of ROS and their contribution to pathological states. In the present work, we describe the rational design of a new class of SOD mimetics based on malonic acid derivatized fullerene C60 compounds which are cell and mitochondria permeable. We employed N-methyl-D-aspartate (NMDA) mediated neuronal injury as a model paradigm reported to involve intracellular superoxide to carry out these structure-activity studies.
NMDA receptors are members of the superfamily of ligand-gated ion channels that are involved in several important functions in the central nervous system (CNS). It is now known that calcium entry through the NMDA receptor channel plays important roles in development and in forms of synaptic plasticity that may underlie higher order processes such as learning and memory [4–7]. Excitotoxicity associated with overactivation of glutamate receptors, however, has been linked to a number of pathological conditions, ranging from acute insults such as stroke and trauma, to chronic neurodegenerative diseases including as Huntington’s disease, Parkinson’s disease (PD), Alzheimer’s disease (AD), and amyotrophic lateral sclerosis (ALS) [8–11]. It has been reported that exposure of neurons to NMDA produces higher levels of ROS, likely through the mitochondrial electron transport chain [12–15] and previous studies have linked ROS production to NMDA-induced excitotoxic neuronal death [16–19].
Under physiological conditions, ROS homeostasis is maintained in vivo by the interplay between oxidant production and the antioxidant defense capacity. However, during episodes of oxidative stress, increased free radical production or reduced antioxidant reserves may shift this balance towards a more pathologic state. Since superoxide radical is much less reactive than other ROS, it is sufficiently long-lived to cross cell membranes and reach more vulnerable cellular domains. Consequently, effective targeting of superoxide radical intracellularly is a potentially viable strategy to alleviate pathologic conditions attributed to increased oxidative stress. Attempts to develop Mn-based SOD mimetics, which can be extremely effective in vitro, were however hampered by the poor membrane penetration of these compounds [20], while nitroxide-based SOD mimetics suffer from very low reactivity towards superoxide [21] and very fast cell clearance [22].
Fullerenes are 3D carbon networks in which the spherical ring strain forces atoms to undergo partial pyramidalization, therefore imparting them with an increased s-orbital character [23–25]. This has been shown to imbue them with metal-like electronic properties [25], and to result in enhanced reactivity of the outer surface of the fullerene derivative towards electron-rich reagents including oxygen free radicals[26]. In a previous report, we introduced evidence that supported a catalytic metalloenzyme-like antioxidant activity of the C3 malonic acid C60 derivative versus superoxide radical [27]. Our data also suggested that the metal-like properties of fullerene compounds are amenable to targeted modifications to achieve other desirable enzyme-like activities. Indeed, C3 was recently shown by our group to decrease brain oxidative stress and therefore to extend lifespan of mice when chronically administered in their drinking water[28] while improving their cognitive performance[29]. We have also reported that acute C3 treatment rescues a subset of crucial population of inhibitory interneurons that are lost due to induced NADPH oxidase activity; i.e. increased superoxide levels in the brain of a schizophrenic model mouse.[30] The present work was carried out to provide insight on the rational design of carboxyfullerenes possessing variable chemical and biophysical properties as tools to study the biology of superoxide and as potential therapeutic candidates to ameliorate disease states resulting from oxidative stress. We therefore establish correlations between the specific antioxidant reactivity of carboxyfullerenes, structural features of the compounds; i.e., number and distribution of malonic acid groups over the C60 surface, and their neuroprotective efficacy against NMDA-induced excitotoxicity in cortical neuronal cultures. The structural requirements for neuronal protection by antioxidant strategies are also discussed in terms of in vitro reactivity towards superoxide radical, drug hydrophilicity, and dipole moment, using other benchmark antioxidants for comparison.
Methods
Chemicals
3-morpholino-sydnonimine hydrochloride (SIN-1), cytochrome c from bovine heart (cyt cIII), superoxide dismutase from bovine erythrocytes (SOD), xanthine (Xn), hypoxanthine (HX), xanthine oxidase (XO) and toluene, were purchased from Sigma Chemical Co. (Saint Louis, MO). 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (CPTIO) was obtained from Calbiochem (CA, U.S.A.).
Instrumentation
Spectrophotometric measurements were carried out using an Agilent 8453 UV-visible Spectroscopy System (Agilent Technologies, Germany) controlled by UV-vis ChemStation Rev. A.09.01 software or a Spectramax 190 plate reader, Molecular Devices, Sunnyvale, CA, supported by SOFTmax Pro version 3.1.2. All of the HPLC methods used a Hewlett Packard/Agilent 1100 series HPLC with a quaternary pump and diode array detector. Electron paramagnetic resonance (EPR) experiments were carried out on Bruker e-scan benchtop spectrometer (Bruker Biospin, USA).
Preparation of malonic acid and acetic acid C60 derivatives
C60 was dissolved in toluene and stirred overnight. Dimethyl bromomalonate was then added, followed by 1,8-diazabicyclo (5.4.0) undec-7-ene (DBU). The reaction mixture was stirred for 2 hours, filtered through a pad of silica gel and solvent was removed in vacuo. The product was then chromatographed on a silica gel column (Merck, 280–400 mesh) using toluene-hexane (1:1 by volume) as eluent. Unreacted C60 is eluted first. Various malonic acid C60 esters are then eluted sequentially by gradually stepping up the ratio of toluene to hexane (4:1, then 9:1). Each isomer is observable as a colored band on the column, allowing collection of each fraction separately. Finally, toluene with 5% ethylacetate (EtOAc) is then used to elute the band corresponding to C3, followed by three additional derivatives. Purity of the C60 malonic ester fractions was monitored by thin layer chromatography (TLC) and high performance liquid chromatography (HPLC). Fractions were evaporated and chromatographed a second time if necessary.
Free acids of the collected esters were then produced by base-catalyzed hydrolysis as described [29]. Esters are dissolved in toluene and purged with nitrogen. Addition of sodium methoxide resulted in precipitation of the corresponding malonic acid products within minutes. The mixture was stirred continuously at room temperature under nitrogen for one hour, and then water was added and the mixture was stirred overnight. The malonic acids partition into the aqueous phase. The layers were separated, and the aqueous layer chilled and acidified with 20% sulfuric acid. The solution was then extracted with ethyl acetate three times, resulting in all products malonic acid C60 derivatives going into the organic layer, which was washed several times with water to extract any remaining contaminants. The ethyl acetate was then evaporated, and the resulting powder lyophilized to remove residual solvent and water.
To produce P2 or C3lite, C3 is dissolved in acetonitrile:water (1:1), and heated to 60°C to produce sequential mono-decarboxylation of the malonic acids to produce acetic acid groups. After ~ 1.5 hours of heating, the P2 concentration is near maximal, and the reaction is stopped. To obtain C3 lite, heating is continued for 6 hours. Solvents are removed in vacuo and the resulting solid products are redissolved in water. To obtain pure P2 or C3lite, the products of thermal decomposition of C3 are separated by preparative HPLC and collected be automated fraction collector. The HPLC was equipped with a Zorbax SB-C18 4.6×250 mm column maintained at room temperature. Solvents were 0.1% TFA in water (solvent A) and 0.1% TFA in 95% acetonitrile and 5% water (solvent B). Samples were eluted from the column using a gradient from 40:60; A:B, to 10:90; A:B, over 15 min., with an additional 15 minutes at 10:90; A:B. Compounds were monitored and identified by their UV-vis absorbance using an in-line diode array detector. Solvents were then removed in vacuo and the resulting dry solid product stored at 4 °C.
NMR and mass spectroscopy were performed at the Washington University Resource for Biomedical and Bioorganic Mass Spectrometry to confirm identity and purity of the derivatives. Both conventional mass spectrometry with negative ion analysis (increased sensitivity for negatively charged groups such as carboxylic acids), and electrospray mass spectrometry were used.
Neuronal cell cultures
Neocortical cell cultures were prepared from fetal (E15) Swiss-Webster mice (Simonson) as described previously [13]. Cortical hemispheres are dissected away from the rest of the brain and placed in trypsin (0.25%, Gibco) for 15 min. The cortices are briefly centrifuged, the trypsin removed and the hemispheres resuspended in plating medium, which consists of media stock (MS: Eagle’s Minimal Essential Media minus L-glutamine, Gibco 11430-022) with 20 mM glucose, 26.2 mM NaHCO3), 5% fetal calf serum, and 5% horse serum (Hyclone) for pure neuronal cultures (<0.5% astrocytes). For neuron-astrocyte co-cultures, the same media, supplemented with L-glutamine (2 mM) is used. After trituration, cell suspensions are diluted and plated onto a pre-existing bed of mouse cortical astrocytes in 24-well Primaria culture plates. Cultures are fed biweekly with growth medium (media stock with 10% horse serum, 2 mM L-glutamine), until the final feeding at day 12, when cultures are fed with media stock supplemented with 2 mM L-glutamine. Cells are used for experiments at 14–15 days in culture. All animal studies were approved by the Animal Welfare Committee of the University of California, San Diego and were in accordance with the PHS Guide for the Care and Use of Laboratory Animals, USDA Regulations, and the AVMA Panel on Euthanasia guidelines.
Injury Paradigms
Brief exposure to N-methyl-D-aspartate (NMDA) was carried out as described [13]. The culture media is exchanged twice with HEPES, bicarbonate-buffered balanced salt solution (HBBSS), and then NMDA (150 µM) is added alone, or with a C60 derivative for 10 min. Exposure is terminated by exchanging the medium four times with MS. The cells are returned to the 37°C (5% CO2) incubator for 24 h, when injury is assessed by determination of LDH release [31], and by visual examination of cultures by bright-field microscopy for degeneration of cell bodies, fragmentation of processes, and development of pyknotic nuclei.
EPR Experiments
A Bruker e-scan benchtop EPR spectrometer was used to determine the concentration of superoxide generated through hypoxanthine/xanthine oxidase metabolism and the scavenging by vitamin E. Mixtures containing variable vitamin E concentration (0–100 µM final) were incubated with xanthine oxidase (0.01 U/mL), hypoxanthine (85 µM), and 35 mM DEPMPO for 10 minutes before injected into the EPR cavity of Bruker e-scan benchtop spectrometer via a Teflon tube with inner diameter of ~ 0.4 mm. The EPR spectra were recorded using the following setting: Receiver gain 1 × 103, scan width 200 G centered at 3484.9 G, modulation amplitude 2 G, time constant 5.16 ms, modulation frequency 86 kHz, microwave power 5.04 mW, 5.24-s sweep time, and the spectrometer’s operating frequency 9.784 GHz. Each spectrum was the average of 10 scans.
Calculations and Modeling
Molecular models, geometry optimization, and energy minimization for all molecules were carried out using MM2 force field provided by CS Chem3D Ultra® Version 7.0 (CambridgeSoft, Cambridge, MA, U.S.A.) and then verified and refined using CAChe WorkSystem Pro Version 5.04 (Fujitsu America Inc., OR, U.S.A). Energy minimization of molecules, molecular properties, and electrostatic superdelocalizability potential maps were generated under the Project Manager environment using the following procedures: Firstly, a thorough conformational search had been carried out within Mechanics using CAChe Augmented MM2 or MM3 parameters through the conjugate gradient method until the gradient was below 0.001 Kcal/mol within 3000 steps. Then the unique minimum was fully optimized with MOPAC (PM3 force field). The PRECISE keyword was also used in order to increase the geometric and electronic convergence criteria. In some cases, the GEO-OK keyword was used to prevent calculation routines from unexpected termination. Finally, electron density isosurface map colored by electrostatic potential was accomplished by tabulating at an isosurface value of 0.01 e/Å3. Calculations for nucleophilic superdelocalizability included solvation effects of water (dielectric constant 78.4 and effective radius of 1.3 Å) simulated by COSMO (Conductor-like Screening Model). Superdelocalizability calculations by CAChe® create isosurfaces that display areas in space where the frontier electrons are most likely to be found, depending on the attacking reagent energy. A good value for the reagent energy is one which is lower than the LUMO energy of the substrate and which approximates the energy of the reagent's frontier orbital. A value of −5 eV was estimated for the superoxide anion through energy level calculations using CAChe®. Same procedure was applied to obtain the nucleophilic superdelocalizability of α-tocopherol.
Molecular docking studies of the native ligands and carboxyfullerenes into the binding pockets in NR1 and NR2A subunits of the NMDA receptor were carried out using the BioMedCAChe suite of algorithms (Fujitsu America Inc., OR, U.S.A). The X-ray structure of the ligand binding core of the NR1-NR2A heterodimer subunit at 2.0 Å resolution in complex with glycine and glutamate was downloaded from the Protein Data Bank website (PDB) (http://www.rcsb.org/, entry 2A5T, [32, 33]). After correcting the crystal structure of NR1/NR2A protein complex for valency and H-bonding, glycine or glutamate were re-docked into their identified pocket (defined to include all atoms and groups within 7 Å radii of the co-crystallized ligands) by holding the residues of the protein fixed and allowing the ligand to be flexible. The orientation and hydrogen bonding of the re-docked complex were close to that in the crystal structure and the flexibility of the protein residues of the active site was consequently allowed. The docking scores were then calculated using a genetic algorithm (1500 generations) with a fast, simplified potential of mean force (PMF, grid spacing 0.3 Å). Native ligands were subsequently replaced with each of the studied carboxyfullerenes or known competitive inhibitors in the respective biding sites and the docking scores were evaluated using the routine described above.
Results
Structures of carboxyfullerenes used in this study
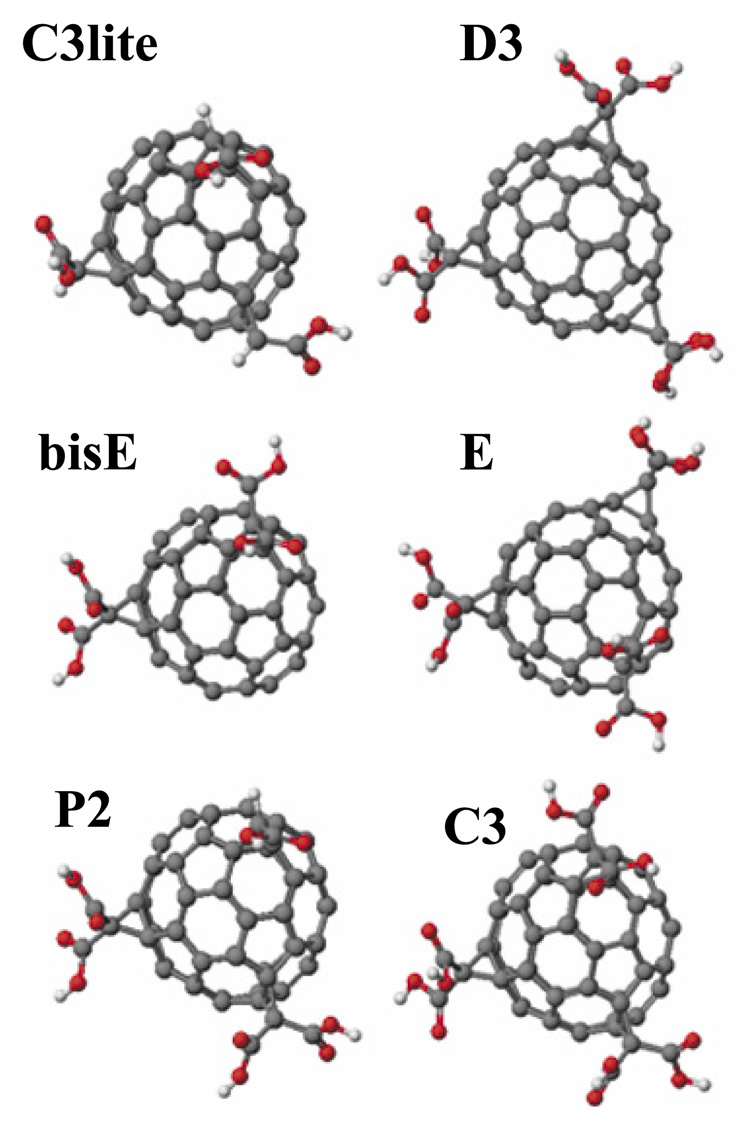
In Figure 1 we provide the structural models of all the six compounds employed in the present study. These models were generated as described in the Modeling and Calculation section above. The selection of these compounds was based on structural variability that illustrates the functional dependence on two molecular structural aspects: First, the number of carboxylic acid groups attached to the fullerene moiety was increased from 3 to 6 in the order C3lite < bisE < P2 < C3; and secondly, the distribution of a fixed number of carboxylic groups over the fullerene was varied in the hexacarboxylic acid isomers (C3, E, and D3).
Figure 1.
Structural models of the carboxyfullerenes employed in the present study. Molecular models, geometry optimization, and energy minimization for all molecules were carried out to arrive at the indicated structural models. The selection of these compounds was based on structural variability that illustrates the functional dependence on two molecular structural aspects: First, the number of carboxylic acid groups attached to the fullerene moiety was increased from 3 to 6 in the order C3lite < bisE < P2 < C3; and secondly, the distribution of a fixed number of carboxylic groups over the fullerene surface was varied in the hexa isomers (C3, E, and D3).
The IUPAC nomenclatures of these compounds are: bisE, 3’H,3’’H-dicyclopropa[1,9:21,40]-(C60 Ih)[5,6]fullerene-3’,3’,3’’,3’’-tetracarboxylic acid; C3lite, 3’H,3’’H,3’’’H tricyclopropa[1,9:21,40:44,45]-(C60-Ih)[5,6]fullerene-3’,3’’,3’’’-tricarboxylic acid; P2, 3’H,3’’H,3’’’H-tricyclopropa[1,9:21,40:44,45]-( C60-Ih)[5,6]fullerene-3’,3’,3’’,3’’,3’’’- pentacarboxylic acid; E, 3’H,3’’H,3’’’H-tricyclopropa[1,9:21,40:34,35]-( C60-Ih)[5,6]fullerene-3’,3’,3’’,3’’,3’’’,3’’’-hexacarboxylic acid; C3, 3’H,3’’H,3’’’H-tricyclopropa[1,9:21,40:44,45]-(C60-Ih)[5,6]fullerene-3’,3’,3’’,3’’,3’’’,3’’’-hexacarboxylic acid; D3, 3’H,3’’H,3’’’H-tricyclopropa[1,9:34,35:43,57]-(C60-Ih)[5,6]fullerene-3’,3’,3’’,3’’,3’’’,3’’’-hexacarboxylic acid.
Neuroprotective efficacy of carboxyfullerenes against NMDA toxicity in cortical neurons
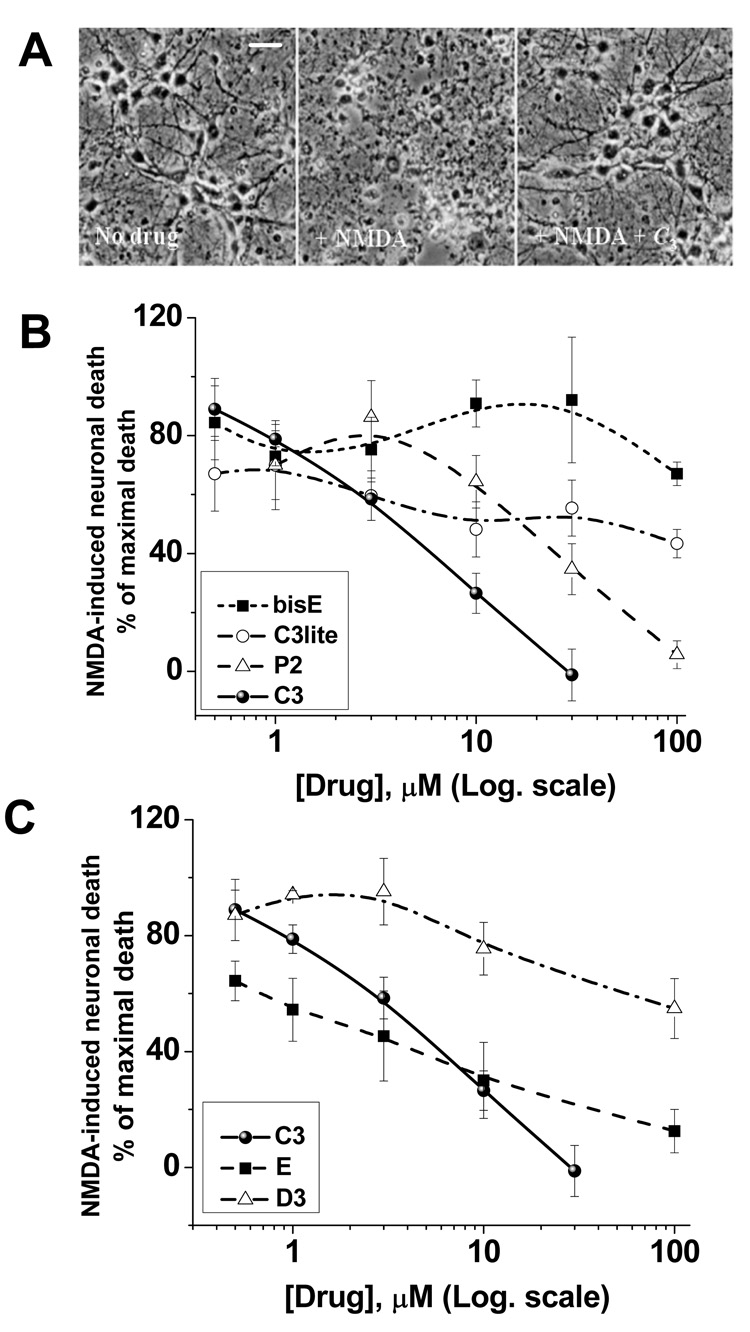
To evaluate the effect of different carboxyfullerenes on NMDA receptor-mediated excitotoxicity, mouse cortical neuronal cultures were exposed to 150 µM NMDA for 10 minutes, with cell death assayed 24 h later. Carboxyfullerenes were included only during the period of NMDA exposure. This is a commonly used paradigm to study excitotoxic neuronal injury through NMDA receptors, and to probe downstream events which contribute to the excitotoxic cascade. Figure 2 shows neuroprotection by C3 at 30 µM, demonstrated by bright-field photomicrographs 24 h after NMDA (Fig. 2A), and by cell death as assayed by LDH release from degenerating neurons (Figures 2B, C). By constructing dose-protection curves for all compounds, it was concluded from Figure 2B that increasing the number of carboxylic groups enhances neuroprotective efficacy. That is, while 30 µM C3 (6 carboxylic groups) was sufficient to completely block neuronal death, concentrations of up to 100 µM of C3lite (3 carboxylic groups), bisE (4 carboxylic groups), and P2 (5 carboxylic groups) only partially blocked NMDA-mediated neuronal death. Neuroprotection was also dependant on the symmetry of distribution of the carboxylic groups over the C60 core (Figure 2C), with more clustered malonic acid groups conferring better neuroprotection, in general. Values for IC50, and the concentrations at which each carboxyfullerene compound provided maximal neuroprotection are given in Table 1. Values for α-tocopherol, the metalloporphyrin, MnTMPyp, and the spin-trap phenylbutyl nitrone, PBN, are included for comparison. Data in Figure 2 and Table 1 indicate that C3, E, and P2 are much more potent neuroprotective compounds than bisE, C3lite, and D3, and that all are more potent and effective than three commonly-used antioxidants, α-tocopherol, MnTMPyp, and PBN.
Figure 2.
Neuroprotection by carboxyfullerenes in NMDA receptor-mediated excitotoxic injury. Neurotoxicity produced by exposure to NMDA is reduced by co-application of the carboxyfullerenes. Data are presented as the percentage of cell death (LDH release) produced by NMDA alone, mean ± SEM, n > 12. In (A), phase contrast images of cultured cortical neuronal 24 hr after drug exposure are shown: control (left), NMDA (middle), NMDA plus 30 µM C3 (right). (B) Increasing the number of carboxylic group on the C60 surface increases neuronal survival, with the exception of the bisE compound (reviewed in more detail in the Discussion). (C) Shows neuronal protection provided by carboxyfullerenes that posses the same number of carboxylic groups, but differ in their distribution over the C60 sphere, see the structures in Figure 1. The white bar is 25 µm.
Table 1. Comparison of the efficacy of carboxyfullerene isomers with other commonly studied antioxidants.
Mixed cortical cultures were exposed to NMDA for 10 min in the presence of antioxidant compounds, and cell death assessed at 24 h by LDH release. A range of concentrations for each antioxidant were tested; the maximal protection and the concentration at which it was achieved are listed. Values are expressed as % of NMDA-induced death in the absence of test compound.
| Drug | IC50, µM | %Maximum achievable protection [µM] |
|---|---|---|
| C3lite | 10 | 66 [10] |
| BisE | >100 | 43 [100] |
| P2 | 17 | 93 [100] |
| C3 | 4 | 100 [30] |
| E | 9 | 87 [100] |
| D3 | >100 | 46 [100] |
| α-tocopherol | >100 | 22 [100] |
| MnTMPyp | >100 | 16 [50] |
| Phenylbutyl nitrone | >100 | 15 [300] |
Efficacy of the different carboxyfullerenes against superoxide radical in vitro
A direct implication of the neuroprotection pattern observed with different carboxyfullerenes is that it appears to require not only an increasing number of carboxylic acid groups, but also specific positioning of these groups on the fullerene surface. In a previous paper we reported that C3 is a potent antioxidant that eliminates superoxide radical catalytically [27]. Therein we suggested a mechanism in which C3 facilitates the superoxide dismutation through electrostatic stabilization of the negatively charged superoxide on electron-deficient regions of C3 in coordination with the carboxylic acid groups. Here we attempt to evaluate and compare the efficacy of the different malonic acid derivatives as superoxide-eliminating antioxidant. In doing this, our goal was to explore the structural criteria that may dictate the potency of a particular carboxyfullerene as an antioxidant in vitro and to eventually establish a correlation with its efficacy as neuroprotective compound in neuronal cell culture.
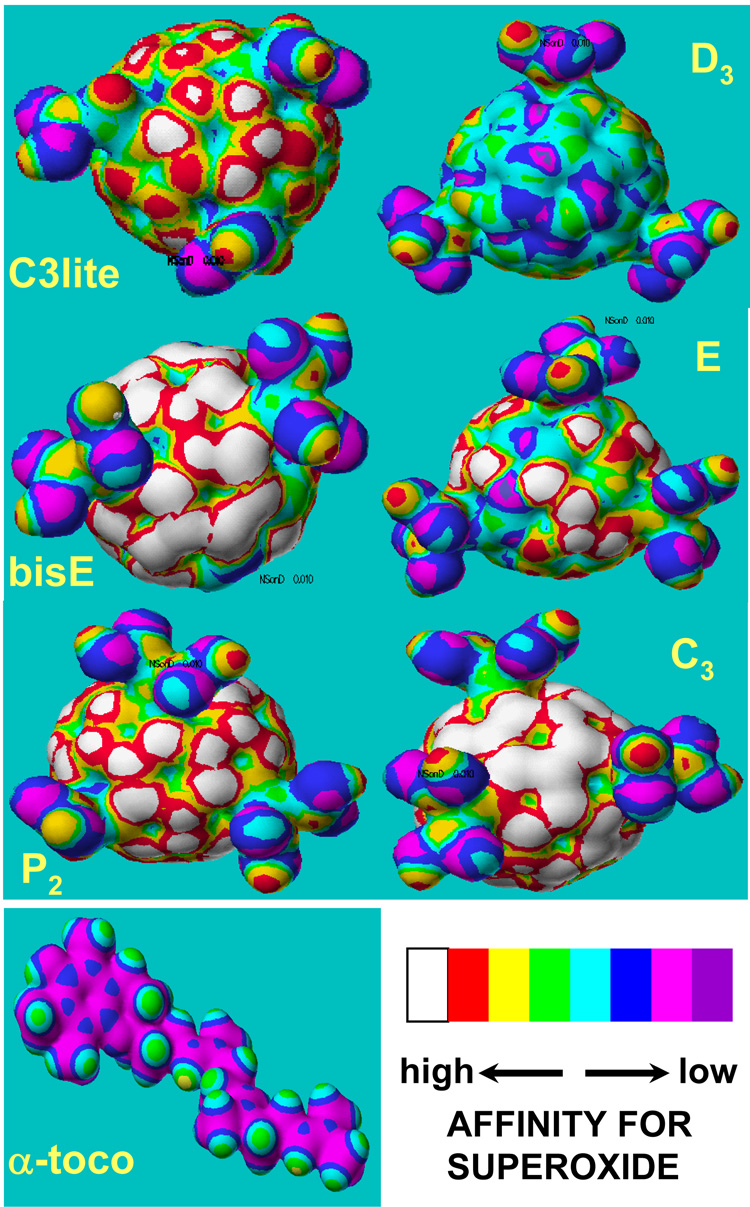
Based on the observation that dismutation of superoxide by C3 is catalytic and is not due to direct scavenging of O2-˙, we attempted to model the interaction between superoxide and each compound employed in this study. Semi-empirical quantum-mechanical calculations were carried out to predict the electron distribution on the surface of each compound and for α-tocopherol as a control antioxidant. The mapping depicted in Figure 3 indicates the nucleophilic superdelocalizability contour, which is a measure of the susceptibility of each fullerene derivative to be attacked by a nucleophile (superoxide anion in this case). Each contour in Figure 3 can be seen as a qualitative indicator of the reactivity of the molecule towards O2-˙ which is electrostatically driven toward electron-deficient areas on the C60 surface (white/red regions, Figure 3). One should therefore expect that the larger the white/red regions on the C60 surface, the more reactive the molecule towards superoxide radical will be. The carboxyl groups are important because they can stabilize O2-˙ by hydrogen bonding until a second O2-˙ arrives to combine with the original O2-˙, allowing dismutation of O2-˙ with the help of protons from the carboxyl groups and/or local water molecules. In Figure 3, and for the sake of comparison, we also provide the superdelocalizability map for α-tocopherol. It appears in terms of the electrostatic drive that α-tocopherol is much less reactive towards superoxide radical relative to any of the studied carboxyfullerenes. This finding is important because, although α-tocopherol is clearly a highly effective chain-breaking lipid peroxidation inhibitor [34] (106 M−1 s−1), its low reactivity towards superoxide and lack of significant neuroprotective efficacy against NMDA supports the concept that superoxide elimination is key to neuroprotection.
Figure 3.
Nucleophilic superdelocalizability maps for carboxyfullerenes and vitamin E. Electron density mapping reflects nucleophilic superdelocalizability indicating on the resulting isosurface locations that are susceptible to attack by superoxide, and designated by a color scale showing decreasing susceptibility in the order white through cyan (legend).
The maps in Figure 3 confirm that regions around the malonic acid groups are the most electron-deficient areas and are therefore electrostatically attractive for the approaching superoxide anion. Charge distribution is however sensitive to the symmetry of each molecule as expected. As a result, the highest symmetry of the D3 hexa-isomer is associated with the least electronic detraction due to opposing charge withdrawal by equivalent malonic groups. In the case of C3 it is reasonable to conclude that the maximum number of malonic acid groups that are clustered in the vicinity of each other on the C60 surface optimizes electronic, steric, as well as topological conditions to eliminate O2-˙ according to the proposed mechanism. It is important to note that the neuroprotective efficacies of the studied drugs (Figure 2 and Table 1) qualitatively correlate with their superoxide-affinity trend except in the case of bisE which exhibits weak neuroprotection while seem to posses widely spread electron deficient regions. This anomaly can however be understood if we consider the factor of solubility of bisE as discussed below.
Kinetics and pH-dependence of superoxide elimination by carboxyfullerenes
Next, we assayed the in vitro reactivity of each carboxyfullerene and of α-tocopherol towards O2-˙. To determine the kinetics of the reactions of different carboxyfullerenes with superoxide anion in vitro, O2-˙ was generated enzymatically through xanthine oxidase (XO) metabolism of hypoxanthine (HX). The concentration of O2-˙ was determined using a microplate assay [35], based on the SOD-inhibitable reduction of ferricytochrome c [36], and followed as absorbance at 550 nm [27, 35]. To confirm that carboxyfullerenes do not inhibit xanthine oxidase activity, we measured both purine metabolites and O2 consumption during the reaction, and observed that even at 300 µM, C3 inhibited xanthine oxidase by less than 10%.
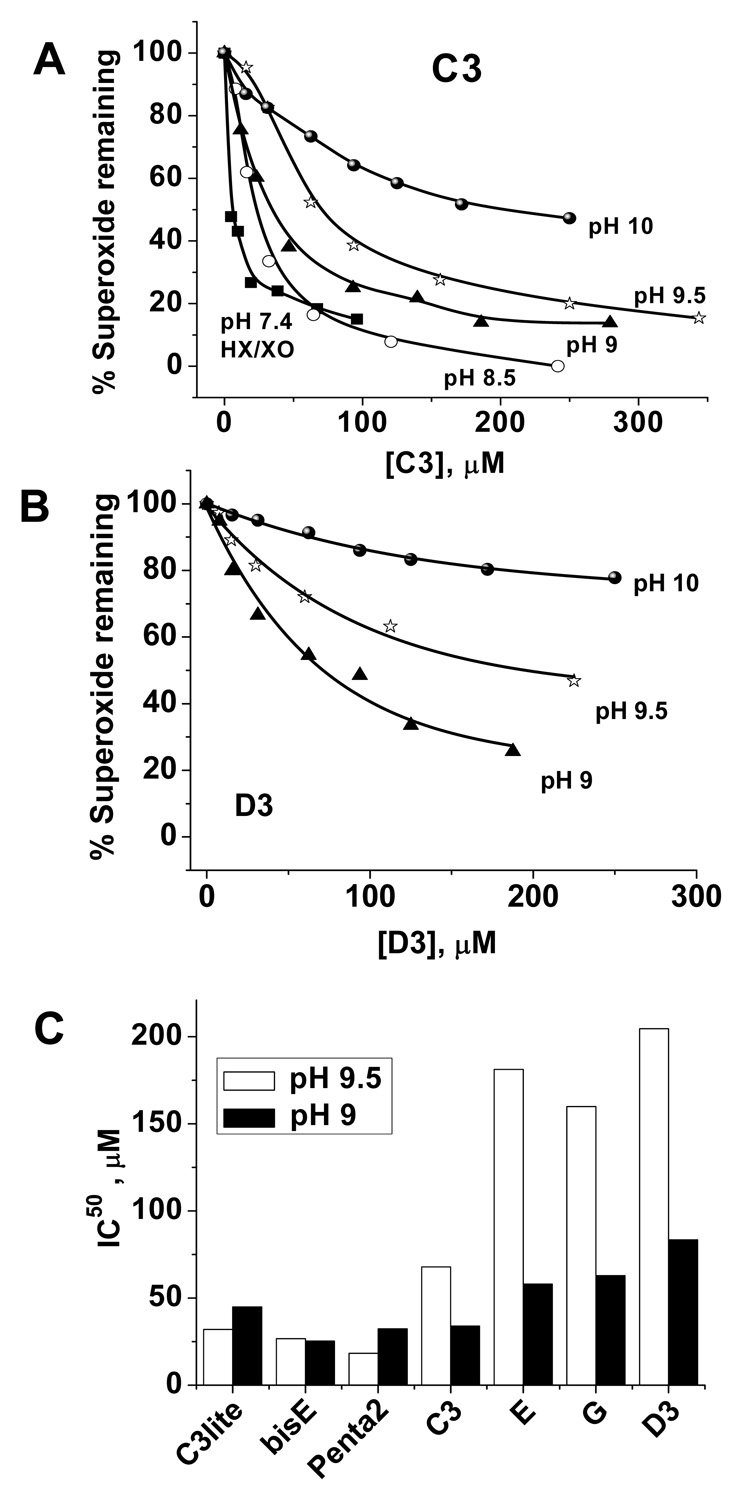
The solubility of fullerenes is strongly dependent on the number of carboxylic groups, and hence on the pH of the working solutions. Consequently, and in order to enhance the solubility of less hydrophilic compounds we employed thermal decomposition of SIN-1 as a second method to generate superoxide at higher pH values (8.5 to 10.5) [27]; the results are shown in Figure 4 (A–C). The SIN-1 system provides another advantage since the decomposition of SIN-1 is controllable by both pH and temperature. This allowed control of the rate of superoxide generation and hence enabled us to perform kinetic and mechanistic studies at variable temperature and pH. Kinetic analyses and determination of ki and IC50 values were carried out as described earlier[26].
Figure 4.
Carboxyfullerenes eliminate superoxide faster at lower pH. C3 exhibits an order of magnitude faster rate of superoxide elimination as compared to D3, compare (A) and (B). Both drugs showed an inverse relationship between the efficiency of superoxide removal and pH; a trend that was more pronounced in the hexa isomers as shown in (C). Superoxide was generated by HX/XO metabolism at pH 7.4, or through thermal decomposition of SIN-1 at pH values of 8.5, 9.0, 9.5, and 10, see experimental section for details.
Representative dose-response curve at pH 7.4 (PBS buffer, HX/XO system) in the case of C3 is given in Figure 4A along with curves obtained using the SIN-1 system in glycine/NaOH buffer adjusted to pH values of 8.5, 9, 9.5, and 10. Similar curves obtained for D3 at pH values of 9, 9.5, and 10 are provided in Figure 4B to confirm that these compounds react with superoxide through the same mechanism. It is clear from Figure 4 (A, B) that carrying out the reaction at lower pHs was associated with systematic increases in the efficacy of the drug in removing superoxide as marked by decreased IC50 values. In fact we observed an order of magnitude increase in the rate constant of the carboxyfullerene/superoxide reactions when the pH of the reaction medium was reduced by one unit. This relation was independent on the source of superoxide employed and was more pronounced in the case of the hexa-isomers (Figure 4C) indicating the importance of shifting the carboxylic groups towards their neutral acid form as a prerequisite to augment the reactivity of the molecule against superoxide anion. Taken together, these observations support the suggested catalytic dismutation of superoxide by carboxyfullerenes which appear to follow similar mechanism albeit with different reactivity and hence variable kinetics.
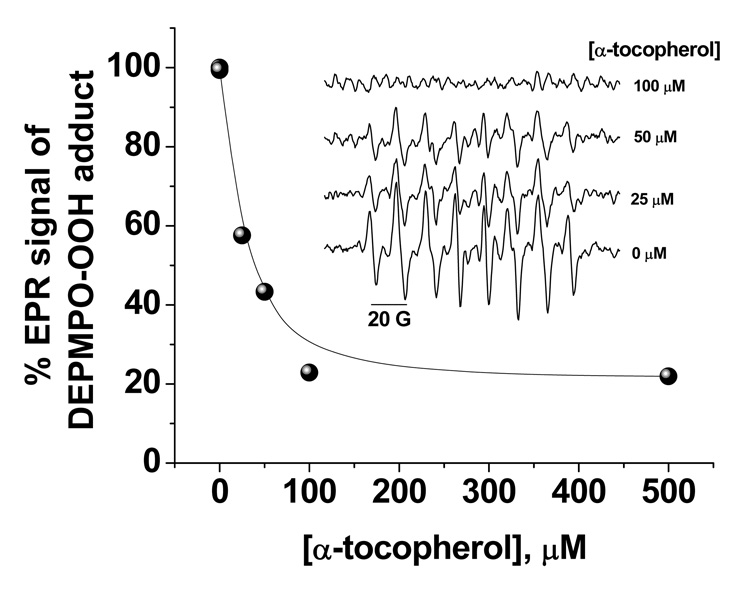
We also carried out a spin-trapping competitive EPR experiment to determine the rate constant of superoxide removal by α-tocopherol and the results are given in Figure 5. Other assays for superoxide determination such as those dependent on optical methods including cyt c assay and fluorescence read-outs are not accurate in this case because of turbidity artifacts caused by the lipophilic α-tocopherol when mixed with the aqueous assay media. In this experiment, superoxide radical was generated by HX/XO system in PBS buffer at pH 7.4 in the presence of 35 mM DEPMPO and the reaction mixture was incubated for 20 minutes at 25 °C with different concentrations of α-tocopherol (0 – 100 µM). When competitive kinetics were applied on the results obtained through Figure 5 and using the reported kDEPMPO range of values of 4 – 58 M−1 s−1 [37], kα-toco was calculated to be in the range 0.35 – 5.8 × 104 M−1 s−1 versus superoxide. This is comparable with the value, for example, of kα-toco = 4.9 × 103 M−1 s−1 obtained for α-tocopherol incorporated into soybean and dimyristoyl phosphatidylcholine liposomal membranes [38]. We will use the upper limit of the estimated kα--toco ~ 6 × 104 M−1 s−1 against superoxide to compare with carboxyfullerenes in the discussion section below.
Figure 5.
Kinetics of superoxide elimination by α-tocopherol. The effect of increasing the concentration of α-tocopherol from 0 – 100 µM on the EPR signal from the DEPMPO/superoxide spin adduct is shown. Superoxide was generated by HX/XO metabolism, and measured by EPR spectroscopy using the spin-trap, DEPMPO. EPR settings were: receiver gain 1 × 103, scan width 150 G centered at 3484.9 G, modulation amplitude 2 G, time constant 5.16 ms, modulation frequency 86 kHz, microwave power 5.04 mW, 5.24-s sweep time, and the spectrometer’s operating frequency 9.784 GHz. Each spectrum was the average of 10 scans. The rate constant for the reaction between superoxide and α-tocopherol is kα-toco = 6.0 × 104 M−1 s−1.
Carboxyfullerenes do not bind to the glutamate or glycine binding sites on the NMDA receptor: molecular docking calculations
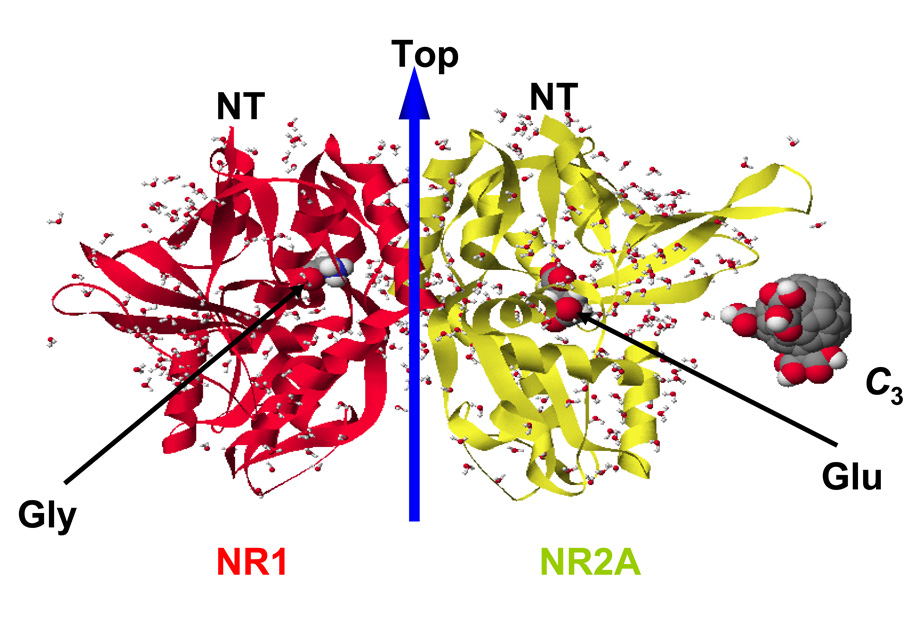
We have previously reported that co-application of C3 or D3 with NMDA does not decrease NMDA-stimulated 45Ca2+ influx into cultured neurons, and does not reduce the immediate neuronal swelling induced by NMDA [19, 39], as would be expected by blockade of the NMDA receptor directly. However, to further eliminate the possibility of direct ion-channel regulation by carboxyfullerenes, we examined the binding of all compounds in the glutamate and glycine active sites of the NR1 and NR2A subunits of the NMDA receptor and found that this binding is energetically unfeasible due to highly destabilizing steric blocking between the protein and carboxyfullerenes (Figure 6). To validate our findings, we calculated the docking scores for the native ligands; i.e. glycine and glutamate, in their respective binding sites and those for their reported competitors NMDA, D-AP5, D-serine, and ACPC [38]. All of these compounds exhibited stabilizing interactions in their defined binding pockets as revealed by their exothermic binding scores which indicate that our calculations can accurately predict the binding affinity of carboxyfullerenes. The calculated docking scores in kcal/mole were: Glutamate, −69.3; NMDA, −76.8; D-AP5, −76.2; Glycine, −27.6; D-Serine, −46.0; ACPC, −16.6; Carboxyfullerenes (all compounds) > +500. Taken together with our previous results, the current molecular docking calculations support the conclusion that the rescue from NMDA-induced neuronal death by carboxyfullerenes is not due to NMDA receptor blockade.
Figure 6.
Carboxyfullerenes do not compete with the native ligands of the NMDA receptor. Side view of the NR1-NR2A S1S2 heterodimer in complex with glycine (left) and glutamate (right). As a representative carboxyfullerene, C3 was placed by the protein to illustrate the size of carboxyfullerenes in comparison with that of the native ligands. Although the calculated binding scores for native ligands and known competitive agonists/antagonists were exothermic and therefore favorable, those for all carboxyfullerenes were highly unfavorable; see text for additional details.
Reactivity towards superoxide and bioavailability determine neuroprotection
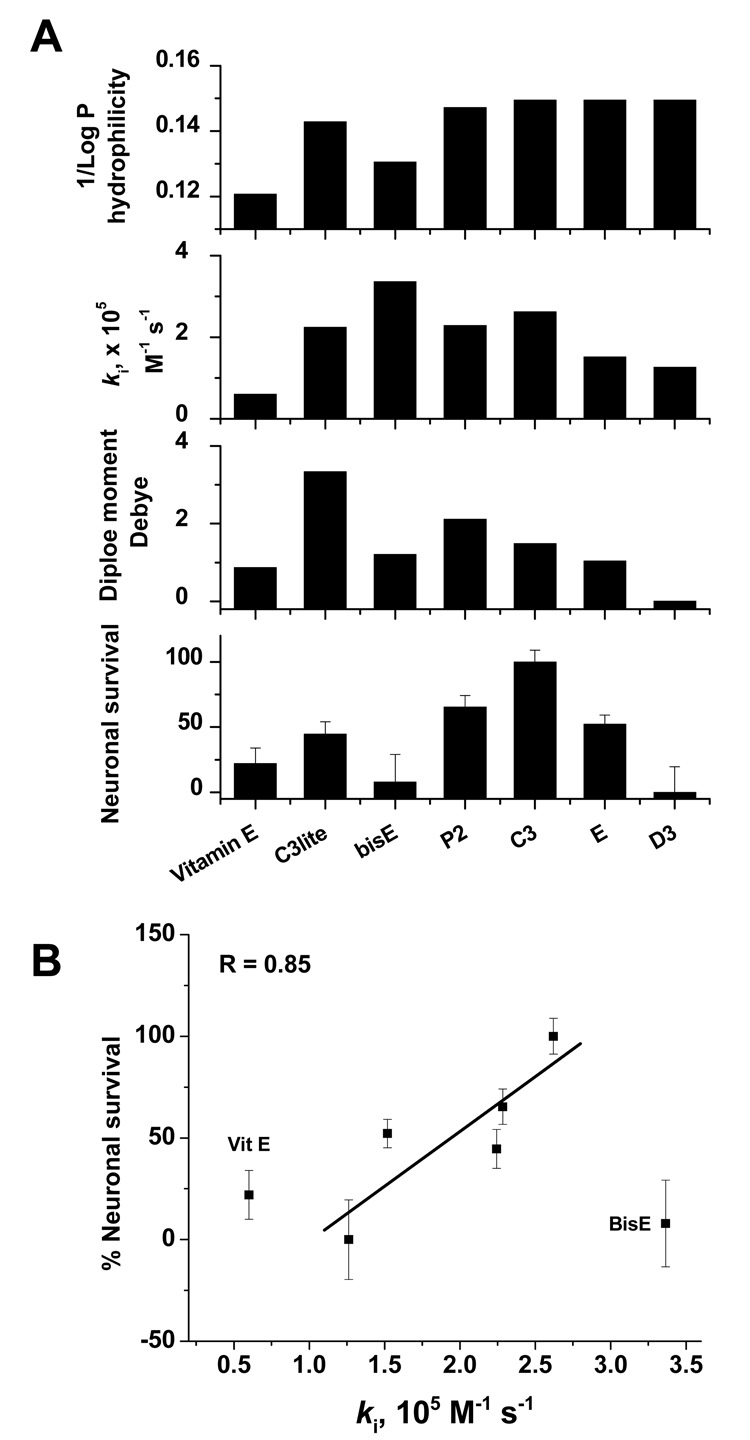
The results in Figure 2–Figure 4 and Table 1 indicate that both the number of carboxylic groups and their distribution are important factors in determining the efficacy of the drug. In Figure 7, we compare neuroprotective efficacy, the calculated dipole moments as indicators for reactivity trend towards superoxide, the in vitro rates of elimination of superoxide, and expected rate of cellular drug uptake based on their partition coefficients. It can be seen from Figure 7 that, with the exception of bisE, the neuroprotective efficacy increases with the number of carboxylic groups, moving from C3lite to C3. Although somewhat similar trends for both dipole moment and ki values were observed, we believe that more significant correlations between these parameters may be modified by other factors, such as rate of cellular uptake. Although the overall performance of each drug, including α-tocopherol, can be qualitatively explained in terms of these parameters, bisE represented an exception, since it showed the maximum ki for superoxide, but less neuroprotection than expected. Its low hydrophilicity is expected to slow its ability to enter cells, and therefore, this anomaly may reflect lack of uptake into cells during the very brief (10 minute) exposure time.
Figure 7.
Correlation between rescue from NMDA-induced neuronal death and calculated dipole moment, experimentally determined rate of superoxide elimination, and calculated hydrophilicity for each drug at 30 µM (100 µM in the case of α-tocopherol) (A). Maximum protection exhibited by the C3 isomer is consistent with it having optimal hydrophilicity, symmetry, and a high rate of superoxide elimination. Although the overall performance of each drug, including α-tocopherol, can be qualitatively explained in terms of the parameters, bisE represented an exception, since it showed the maximum ki for superoxide, but less neuroprotection than expected. Its low hydrophobicity is expected to slow its ability to enter cells, and therefore, this anomaly may reflect lack of uptake into cells during the brief (10 minute) exposure time. (B) Regression analysis indicates a good correlation (R = 0.85) between neuroprotective efficacy and ki for superoxide across isomers, with the exception of BisE, whose low aqueous solubility counterbalances it high ki. α-tocopherol is also included for comparison.
When the number of carboxylic groups is kept constant in the hexa isomer series, the hydrophilicity (inverse partition coefficient) was also constant as seen in the upper panel of Figure 7. Under this structural constraint, the correlation between neuroprotection and the efficacy towards superoxide followed a clear decreasing trend in the order C3>E>D3. In this particular order, the drug’s nucleophilic superdelocalizability maps clearly exhibit decreased reactivity towards superoxide (Figure 3), the dipole moment decreases, and the kinetics of superoxide removal get slower, while neuroprotection drops from 100% by C3 to 50% by E to virtually no protection by D3 at 30 µM doses (Figure 7).
Discussion
The goals of the current study were three-fold: 1) to determine the structural requirements for neuronal protection by antioxidant strategies vis-à-vis in vitro reactivity towards superoxide radical, drug hydrophilicity, and dipole moment, using several benchmark antioxidants as references, 2) to determine whether neuroprotection against NMDA toxicity correlated with and was predicted by the ki-superoxide of the compounds, and finally 3) to provide a versatile panel of water-soluble, cell-permeable SOD mimetics, supported by structure-activity data, as tools to investigate the biology of superoxide more broadly.
NMDA receptor-mediated neurotoxicity is implicated in many human disease states including trauma, ischemia, epilepsy, and chronic neurodegenerative disorders such as AD, PD, and ALS. Excitotoxicity due to NMDA receptor overactivation can result in excess elevation of intracellular calcium and activation of subsequent events which lead to neuronal death [40, 41]. Although NMDA-receptor antagonists prevent neuronal injury in cellular and animal models, in humans, their side effects including nausea, vomiting, and memory impairment have removed them as viable candidates for clinical applications [42].
Studies have shown that overactivation of NMDA receptors leads to oxidative stress, and compounds which possess antioxidant properties protect against NMDA neurotoxicity, although subsequent work on many of these compounds indicate that they may act through non-antioxidant mechanisms. One such study on the metalloporphyrin class of SOD mimetics indicated that neuroprotection by these potent antioxidant compounds does not correlate with their SOD activity [20], and that compounds which had no antioxidant properties were also protective. Neuroprotection was attributed to the ability of the metalloporphyrins to limit delayed calcium dysregulation after NMDA receptor overactivation. Because this study also indicated that these compounds did not enter cells during the exposure period (30 min.), the authors concluded that metalloporphyrins functioned at the plasma membrane to suppress intracellular Ca2+ increases following NMDA receptor activation, and not through their actions as SOD mimetics. However, the fact that these compounds, while potent SOD mimetics, were not cell permeable revives the question of whether increased intracellular superoxide levels are critical to NMDA receptor-mediated injury.
In the present paper we wanted to establish the criteria for an optimized carboxyfullerene antioxidant and to determine whether the observed neuroprotection is due to the elimination of superoxide radical. A set of six different carboxyfullerenes were synthesized and their reactivity against superoxide radical was studied theoretically as well as experimentally. In the selected compounds, we varied the functionalization to follow the dependence of reactivity on an increasing number of carboxylic acid groups attached to the fullerene moiety in the order C3lite < bisE < P2 < C3; and the distribution of a fixed number of carboxylic groups over the fullerene ball in the hexa-carboxylic isomers (C3, E, and D3).
We observed a significant correlation between the neuroprotective efficacy of different carboxyfullerenes and their SOD activities (ki). Neuroprotection also correlated with the dipole moment. A clear exception to both of these is the BisE compound, discussed further below. The dipole moment is a property we previously suggested to be important to superoxide elimination by carboxyfullerenes based on electrostatic interaction between superoxide and the compound [27]. The high degree of correlation between neuroprotection and these two parameters, both of which predict efficacy as SOD mimetics, provide strong support for the view that superoxide radical contributes to NMDA receptor-mediated neurotoxicity. In addition to SOD activity, biophysical properties that predict the ability of compounds to enter and distribute throughout cells were also found to be associated with improved neuroprotection. The compounds which best highlight this relationship are C3lite, BisE, and C3, which show neuroprotection in the rank order C3 > C3lite > BisE. Based on their ki’s against superoxide alone, however, one would expect that neuroprotection would be in the order BisE > C3 ≥ C3lite. BisE is the least hydrophilic compound (other than α-tocopherol), and is predicted to have lower water solubility than the other carboxyfullerenes (confirmed experimentally). We have previously reported that the C3 and D3 compounds, which are actually less lipophilic than both the BisE and C3lite compounds, can intercalate into lipid bilayers [19]. Studies using an anti- C60 antibody and Western blot analysis [43] or immunocytochmistry [27] to detect C3 have shown rapid uptake and intracellular distribution of C3 into neurons, and specifically into mitochondria [27, 43]. Based on this, we believe that the BisE compound likely becomes “trapped” in the plasma membrane, with limited transfer into the more aqueous cell interior. C3lite, which also has a lower 1/Log P than C3, may likewise have slower uptake into intracellular compartments. Thus, the rate and degree of cell permeability may be optimized at a 1/Log P of ~ 0.15, and likely modifies the predicted neuroprotective efficacy of the carboxyfullerenes based on their SOD mimetic activities alone. Of note, we interpreted the lack of significant neuroprotection provided by α-tocopherol to suggest that lipid peroxidation, while an observed consequence of NMDA receptor overactivation, may be downstream of the point at which cells can be effectively rescued.
The C3 and D3 compounds have been shown to be neuroprotective in a range of in vitro injury models, including many that do not involve the NMDA receptor [19, 44], and they did not block NMDA-stimulated 45Ca2+ influx [19]. Moreover, C3 compound, administered long-term to mice, not only extended lifespan [28, 29], but improved spatial learning and memory performance on the Morris water maze [29], a task that is quite sensitive to NMDA receptor inhibition. However, to further confirm that neuroprotection was not through direct antagonism at either the glutamate or glycine binding sites, we carried out molecular docking calculations for all carboxyfullerenes, and found that binding was sterically prohibited and thermodynamically highly unfavorable at both binding sites, as shown in Figure 6.
In summary, molecular modeling and in vitro studies have been employed to arrive at an optimum compromise between the SOD activity of carboxyfullerenes and their biophysical properties, and correlations between these parameters and neuroprotective efficacy were then established. Chemical features that enhanced the biological effectiveness of a carboxyfullerene included maximum number of carboxylic groups that are distributed as close to each other as possible, largest dipole moment, and significant solubility in water. Based on these factors, the C3 compound would be predicted to provide the greatest degree of neuroprotection, as was observed experimentally. Taken together, our results indicate that developing SOD mimetics with a high ki for superoxide, moderate dipole moment, and a balance between lipophilic and hydrophilic properties (i.e. 1/Log P of ~ 0.15–0.16) may be an attractive strategy for drug development for disease and injury conditions in which superoxide is involved. We believe these data also provide strong support for the idea that superoxide is an important downstream mediator of neurotoxicity triggered by NMDA receptors. Further studies should provide insights into how superoxide interacts with delayed calcium dysregulation and other pathways implicated in NMDA-R-mediated neuronal injury. Finally, our results indicate that the malleability of fullerenes to functionalization can confer selected biological properties and biophysical characteristics, giving this class of molecules a unique ability to be adapted or tailored for specific biological applications and molecular targets.
Acknowledgments
This work was funded by a Paul Beeson Physician Scholar Award, the Selma I. Hartke fund for aging research, and NIH NS47966 (LLD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15(2):247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97(10):967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 3.McCord JM, Edeas MA. SOD, oxidative stress and human pathologies: a brief history and a future vision. Biomed Pharmacother. 2005;59(4):139–142. doi: 10.1016/j.biopha.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Maren S, Baudry M. Properties and mechanisms of long-term synaptic plasticity in the mammalian brain: relationships to learning and memory. Neurobiol Learn Mem. 1995;63(1):1–18. doi: 10.1006/nlme.1995.1001. [DOI] [PubMed] [Google Scholar]
- 5.Asztely F, Gustafsson B. Ionotropic glutamate receptors. Their possible role in the expression of hippocampal synaptic plasticity. Mol Neurobiol. 1996;12(1):1–11. doi: 10.1007/BF02740744. [DOI] [PubMed] [Google Scholar]
- 6.Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi S, Nakajima Y, Masu M, Ueda Y, Nakahara K, Watanabe D, Yamaguchi S, Kawabata S, Okada M. Glutamate receptors: brain function and signal transduction. Brain Res Brain Res Rev. 1998;26(2–3):230–235. doi: 10.1016/s0165-0173(97)00033-7. [DOI] [PubMed] [Google Scholar]
- 8.Brauner-Osborne H, Egebjerg J, Nielsen EO, Madsen U, Krogsgaard-Larsen P. Ligands for glutamate receptors: design and therapeutic prospects. J Med Chem. 2000;43(14):2609–2645. doi: 10.1021/jm000007r. [DOI] [PubMed] [Google Scholar]
- 9.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- 10.Choi D. Antagonizing excitotoxicity: a therapeutic strategy for stroke? Mt Sinai J Med. 1998;65(2):133–138. [PubMed] [Google Scholar]
- 11.Beal MF, Howell N, Bodis-Wollner I. Mitochondria and Free Radicals in Neurodegenerative Diseases. New York: John Wiley & Sons; 1997. [Google Scholar]
- 12.Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15(5 Pt 1):3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-asparate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16(4):1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sengpiel B, Preis E, Krieglstein J, Prehn JH. NMDA-induced superoxide production and neurotoxicity in cultured rat hippocampal neurons: role of mitochondria. Eur J Neurosci. 1998;10(5):1903–1910. doi: 10.1046/j.1460-9568.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- 16.Dugan LL, Gabrielesen JK, Yu SP, Lin T-S, Choi DW. Buckminsterfullerenol free radical scavengers reduce ex-citotoxic and apoptotic death of cultured cortical neurons. Neurobiol Dis. 1996;3:129–135. 305–310. doi: 10.1006/nbdi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 17.Budd SL, Nicholls DG. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem. 1996;67(6):2282–2291. doi: 10.1046/j.1471-4159.1996.67062282.x. [DOI] [PubMed] [Google Scholar]
- 18.Monyer H, Hartley DM, Choi DW. 21-Aminosteroids attenuate excitotoxic neuronal injury in cortical cell cultures. Neuron. 1990;5(2):121–126. doi: 10.1016/0896-6273(90)90302-v. [DOI] [PubMed] [Google Scholar]
- 19.Dugan LL, Turetsky DM, Du C, Lobner D, Wheeler M, Almli CR, Shen CK, Luh TY, Choi DW, Lin TS. Carboxyfullerenes as neuroprotective agents. Proc Natl Acad Sci U S A. 1997;94(17):9434–9439. doi: 10.1073/pnas.94.17.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tauskela JS, Brunette E, O'Reilly N, Mealing G, Comas T, Gendron TF, Monette R, Morley P. An alternative Ca2+-dependent mechanism of neuroprotection by the metalloporphyrin class of superoxide dismutase mimetics. Faseb J. 2005;19(12):1734–1736. doi: 10.1096/fj.05-3795fje. [DOI] [PubMed] [Google Scholar]
- 21.Rosen GM, Britigan BE, Halpern HJ, Pou S. Free Radicals, Biology and Detection by Spin Trapping. New York: Oxford University Press; 1999. [Google Scholar]
- 22.Utsumi H, Yamada K, Ichikawa K, Sakai K, Kinoshita Y, Matsumoto S, Nagai M. Simultaneous molecular imaging of redox reactions monitored by Overhauser-enhanced MRI with 14N- and 15N-labeled nitroxyl radicals. Proc Natl Acad Sci U S A. 2006;103(5):1463–1468. doi: 10.1073/pnas.0510670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sola M, Mestres J, Duran M. Molecular Size and Pyramidalization: Two Keys for Understanding the Reactivity of Fullerenes. J Phys Chem. 1995;99:10752–10758. [Google Scholar]
- 24.Haddon RC, Palmer RE, Kroto HW, Sermon PA. The Fullerenes: Powerful Carbon-Based Electron Acceptors [and Discussion] Philosophical Transactions: Physical Sciences and Engineering. 1993;343(1667):53–62. [Google Scholar]
- 25.Diener MD, Alford JM. Isolation and properties of small-bandgap fullerenes. Nature. 1998;393:668–671. [Google Scholar]
- 26.Ali SS, Hardt JI, Quick KL, Kim-Han JS, Erlanger BF, Huang TT, Epstein CJ, Dugan LL. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free Radic Biol Med. 2004;37(8):1191–1202. doi: 10.1016/j.freeradbiomed.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Ali SS, Hardt JI, Quick KL, Sook Kim-Han J, Erlanger BF, Huang TT, Epstein CJ, Dugan LL. A biologically effective fullerene (C(60)) derivative with superoxide dismutase mimetic properties. Free Radic Biol Med. 2004;37(8):1191–1202. doi: 10.1016/j.freeradbiomed.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Ali SS, Xiong C, Lucero J, Behrens MM, Dugan LL, Quick KL. Gender differences in free radical homeostasis during aging: shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell. 2006;5(6):565–574. doi: 10.1111/j.1474-9726.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 29.Quick KL, Ali SS, Arch R, Xiong C, Wozniak D, Dugan LL. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol Aging. 2008;29(1):117–128. doi: 10.1016/j.neurobiolaging.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-Induced Loss of Phenotype of Fast-Spiking Interneurons Is Mediated by NADPH-Oxidase. Science. 2007;318(5856):1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 31.Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987;20(1):83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 32.Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438(7065):185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- 33.Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. Embo J. 2003;22(12):2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Quinn PJ. The location and function of vitamin E in membranes. Molecular Membrane Biology. 2000;17:143–156. doi: 10.1080/09687680010000311. [DOI] [PubMed] [Google Scholar]
- 35.Quick KL, Hardt JI, Dugan LL. Rapid microplate assay for superoxide scavenging efficiency. J Neurosci Methods. 2000;97(2):139–144. doi: 10.1016/s0165-0270(00)00179-5. [DOI] [PubMed] [Google Scholar]
- 36.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 37.Gotoh N, Niki E. Rates of interactions of superoxide with vitamin E, vitamin C and related compounds as measured by chemiluminescence. Biochimica et Biophysica Acta (BBA) - General Subjects. 1992;1115(3):201–207. doi: 10.1016/0304-4165(92)90054-x. [DOI] [PubMed] [Google Scholar]
- 38.Chen PE, Wyllie DJ. Pharmacological insights obtained from structure-function studies of ionotropic glutamate receptors. Br J Pharmacol. 2006;147(8):839–853. doi: 10.1038/sj.bjp.0706689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dugan LL, Lovett E, Cuddihy S, Almli CR, Lin T-S, Choi DW. Carboxyfullerenes as neuroprotective antioxidants. In: Kreiglstein J, editor. Pharmacology of Cerebral Ischemia. New York: Academic Press; 1998. [Google Scholar]
- 40.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 41.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 42.Gardoni F, Di Luca M. New targets for pharmacological intervention in the glutamatergic synapse. Eur J Pharmacol. 2006;545(1):2–10. doi: 10.1016/j.ejphar.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, Seta P, Larroque C. Cellular localization of a water-soluble fullerene derivative. Biochem Biophys Res Commun. 2002;294(1):116–119. doi: 10.1016/S0006-291X(02)00445-X. [DOI] [PubMed] [Google Scholar]
- 44.Lotharius J, Dugan LL, O'Malley KL. Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J Neurosci. 1999;19(4):1284–1293. doi: 10.1523/JNEUROSCI.19-04-01284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]