Abstract
Cisplatin, carboplatin, and oxaliplatin anticancer drugs are commonly used to treat lung, colorectal, ovarian, breast, head/neck, and genitourinary cancers. However, the efficacy of platinum-based drugs is often compromised because of the substantial risk for severe toxicities, including neurotoxicity. Neurotoxicity can result in both acute and chronic debilitation. Moreover, colorectal cancer patients treated with oxaliplatin more often discontinue therapy due to peripheral neuropathy than for tumor progression, potentially compromising patient benefit. Numerous methods to prevent neurotoxicity have so far proven unsuccessful. In order to circumvent this life-altering side effect, while taking advantage of the antitumor activities of the platinum agents, efforts to identify mechanism-based biomarkers are underway. In this review, we detail findings from the current literature for genetic markers associated with neurotoxicity induced by single agent and combination platinum chemotherapy. These data have the potential for broad clinical implications if mechanistic associations lead to the development of toxicity modulators to minimize the noxious sequelae of platinum chemotherapy.
The chemotherapy drugs cisplatin, carboplatin, and oxaliplatin are commonly used for the treatment of lung, colorectal, ovarian, breast, head/neck, bladder and testicular cancers (Fig. 1). Cisplatin was approved for the treatment of both ovarian and testicular cancer in 1978 (1) and is also administered for many other types of solid tumors. Subsequently, carboplatin was approved in March, 1989 for treatment of ovarian cancer. In 2002, a third-generation platinum drug, oxaliplatin, was approved for treatment of metastatic colorectal cancer (2). Cisplatin is the most commonly used chemotherapy drug in the USA (3). Unfortunately, the benefit of these frequently prescribed drugs is compromised by severe side effects including neurotoxicity.
Figure 1.
Chemical structures of platinum compounds
Recent meta-analyses have compared platinum and non-platinum treatments for various cancer types. A meta-analysis on individual patient data concluded that cisplatin was superior to carboplatin for non-small cell lung cancer (NSCLC) in terms of response rate and prolonged survival without an increase in severe side effects (4). By analyzing over 1700 ovarian cancer patients from six randomized trials, another meta-analysis determined that the inclusion of cisplatin in front-line therapy of stage III ovarian cancers improves survival (5). Platinum-based regimens had been shown to have a slightly higher 1-year survival rate than non-platinum-based regimens in metastatic non-small lung cancer patients (p=0.03). Analysis of 17 trials that included 4920 NSCLC patients indicated that the platinum-based treatments are associated with a higher risk of anemia, nausea, and neurotoxicity (p=0.02) (6). Clearly, the use of platinum drugs is essential in the fight against cancer, however the toxic side effects must be attenuated in order to reap the maximum benefits.
The second generation platinum drug carboplatin is a more stable analogue but has equivalent activity, in come cancer types, to cisplatin. Carboplatin is part of first-line therapy for ovarian cancer, typically in combination with a taxane. Lung cancer is also treated with carboplatin, in combination therapy with vinorelbine, bevacizumab, etopside, gemcitabine, or paclitaxel (among others) (7). The negative side effects of carboplatin combinations vary with the partnering agent. For example, vinorelbine/carboplatin treatment has less incidence of grade 3–4 toxicities, than gemcitabine/carboplatin (8). The efficacy of single agent therapy for NSCLC is generally improved by the addition of carboplatin (9).
Oxaliplatin, the third-generation platinum drug, is the standard of treatment in conjunction with 5-fluorouracil/ leucovorin (5-FU/LV) for locally advanced and metastatic cancer of the colon or rectum. It has shown improved survival in the adjuvant setting among Stage III patients compared to 5-FU/ LV treatment as well as in first-line treatment of metastatic disease, compared with 5-FU/irinotecan therapy (10). Importantly, the incidence of neurotoxicity is increased with the addition of oxaliplatin (11). The FDA noted that over 70% of the patients receiving oxaliplatin are affected by some degree of sensory neuropathy (12). Most importantly, neurotoxicity, and not tumor progression, is often the cause of treatment discontinuation. An additional recent study examining 383 patients treated with oxaliplatin and irinotecan showed that 52% of patients required dose reduction due to adverse events, including neurotoxicity, and that 26% required hospitalization because of these negative events (13).
Platinum Toxicity
The general toxicity profile differs between the three platinum drugs. Cisplatin may cause severe renal tubular damage and reduces glomerular filtration. Optimal administration requires concurrent saline hydration and mannitol diuresis to minimize the likelihood of potentially lethal damage to the kidneys (14). Cisplatin causes the most severe nausea and vomiting of the approved platinum compounds which can usually be prevented or managed with current antiemetic regimens. Peripheral neurotoxicity is the most common dose-limiting problem associated with modern cisplatin therapy. Cisplatin neurotoxicity is first characterized by painful paresthesias and numbness which typically occurs during the first few drug cycles. Loss of vibration sense, paraesthesia and ataxia can become apparent after several treatment cycles. Additionally, 75–100% of patients treated with cisplatin show some level of ototoxicity. Ototoxicity caused by cisplatin is cumulative and can be irreversible, therefore monitoring by audiograms should be considered. Cisplatin also causes mild hematological toxicity.
The dose-limiting toxicity of carboplatin is thrombocytopenia, which is dose-dependent and varies in severity based on the individual. Because the toxicities are dose-dependent, alternative methods of ensure appropriate dose are in practice. By measuring glomerular filtration rate (GFR), Barrett et al were able to take into account the body mass index of all patients individually (15). They determined that there was no association between body mass index and prognosis in ovarian cancer patients. However, Ekhart and colleagues established the importance of the Calvert formula (based on GFR) for dose determination with targeted carboplatin exposures (16). Alternatively, a patient with normal renal function should be given a flat dose based on the mean population carboplatin clearance(16). Patients treated with carboplatin are also at a higher risk of anemia than those treated with cisplatin (p=0.02) (6).
The neurotoxicity resulting from carboplatin administration is less frequent (4–6%) (17) than that observed with cisplatin or oxaliplatin (15–60%) (18), and is typically less severe. Risk of carboplatin peripheral neurotoxicity increased in patients older than 65 years and in patients previously treated with cisplatin. Additionally, carboplatin does not cause the loss of hearing that is seen in the majority of patients treated with cisplatin (19–21). Carboplatin-treated patients also experience a lower incidence of nausea and/or vomiting and renal toxicity, when compared to cisplatin based regimens (4, 6).
Oxaliplatin frequently causes neutropenia, but is dose-limited in many cases by a purely sensory neuropathy, which appears to be cumulative and, at least in a large part, reversible with drug cessation. There are two patterns of neuropathy; an acute cold aggravated but transient condition and a more chronic form which has onset after multiple exposures to the drug and which often improves but does not disappear with drug cessation. Acute oxaliplatin neurotoxicity can occur within hours of dosing as this may be precipitated or exacerbated by exposure to cold temperature or cold objects and typically resolves within hours to days (22). This acute neurotoxicity is dose-related and reversible. The more chronic pattern of sensory neuropathy was observed in about 50% of study patients who received oxaliplatin with infusional 5-FU/LV (23).
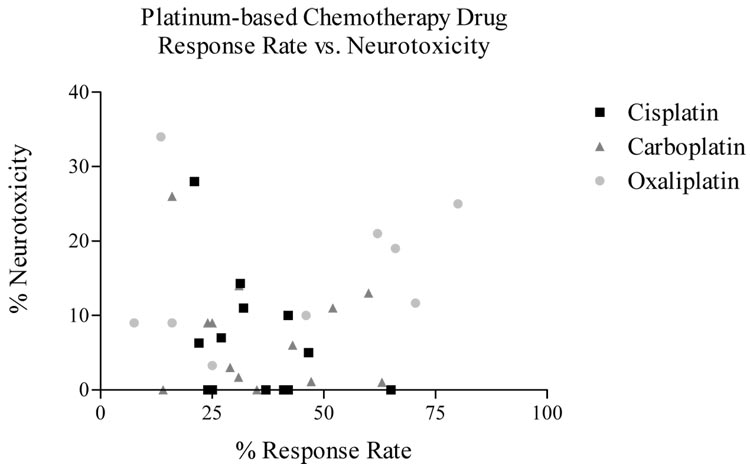
Platinum-induced peripheral neuropathy has elements common to all three agents with some distinctive patterns. The neurologic complications of these three drugs occur in most cases, in a cumulative manner. Cisplatin and carboplatin related neuropathies are often not completely reversible and are seen as paresthesias in a stocking-glove distribution, areflexia, and loss of proprioception and vibratory sensation. Loss of motor function has also been reported in patients treated with cisplatin. After discontinuation of cis- or oxaliplatin therapy, the neurotoxic symptoms may progress for up to 2 months. Patients may experience gradual improvement, however more severe cases may have incomplete recovery (24). The incidence of clinical neurotoxicity does not appear to be directly associated with anti-tumor response (Fig. 2). Therefore, neurotoxicity is not merely a ‘necessary evil’, but can be approached as an avoidable side effect of platinum agents.
Figure 2.
Percentage of neurotoxicity vs. response rate (RR) for each platinum drug treatment from platinum-containing trials (4, 11, 51, 62–74).
Proposed platinum-drug mechanism of neurotoxicity
Cisplatin, carboplatin, and oxaliplatin differ in their solubility, chemical reactivity, oxygenated leaving groups, pharmacokinetics, and toxicology (14). The leaving groups on each molecule lead to differences in each platinum drug’s reactivity with nucleophiles, which is likely contributing to the differences in toxicity (25). Platinum drugs undergo aquation, which is a key step in the drug forming a complex with the target DNA (26). The result of this hydrolysis is the formation of a positively charged molecule that then crosslinks to DNA, forming the DNA/platinum adducts (26). The amount of DNA cross-links in DRG neurons at a given cumulative dose was significantly correlated with the degree of neurotoxicity (27). Patient trials of platinum agents have shown that the severity of neurotoxicity is commonly cisplatin > oxaliplatin >> carboplatin. Based on the data from cisplatin, the differences in the degree of neurotoxicity could be associated with their different plasma concentrations of the intermediary products of the aquation to DNA adduct formation process (26). Cisplatin and oxaliplatin undergo hydrolysis to a greater extent than carboplatin, which may contribute to the difference in the associated neurotoxicity severity patterns.
Although the cellular damage is thought to be caused by the formation of these DNA adducts, an additional complex composed of platinum-DNA-protein crosslinks (DPCLs) has been proposed as a mechanism for the platinum antitumor activities, and studied specifically with cisplatin (28). By covalently linking DNA with protein complexes, these DPCLs are able to disrupt nuclear metabolism and spatial organization of chromatin, as well as inhibit DNA replication and repair.
The platinum drugs appear to effect the axons, myelin sheath, neuronal cell body and the glial structures of the neurons (29). At the cellular level, the chemotherapy interferes with DNA replication and metabolic function of the neurons (30). Platinum-based agents have the propensity to enter the dorsal root ganglia and peripheral nerves (31) as opposed to the brain, as these drugs have poor penetration through the blood-brain barrier (32). Levels of platinum have been shown to be significantly higher in the dorsal root ganglia than in the brain and spinal cord, which are protected by that barrier (32). It was previously thought that platinum drugs entered the dorsal root ganglia through passive diffusion (20), although current data indicates the presence of metal transporters that may be involved in their entrance into the cell (33).
Current research has shown insight into the mechanisms of nerve damage caused by the platinum agents. After entering the DRG, the platinum agent forms an adduct with DNA. Apoptosis has been observed in DRG neurons following cisplatin treatment both in vitro and in vivo (34), and is correlated with increased platinum-DNA binding in these DRG neurons (34). Oxaliplatin and cisplatin differ in their severity of neurotoxicity to the DRG. Cisplatin produced about three times more platinum- DNA adducts in the DRG (35) than equimolar doses of oxaliplatin, consistent with clinical observations that cisplatin is associated with greater neurotoxicity.
Although the mechanism of the transition from a platinum-DNA adduct to neuronal apoptosis is not fully understood, one proposal has suggested that the DNA repair machinery is unable to repair the damaged DNA. Polymorphisms in the DNA repair genes, including genes in base excision repair, nucleotide excision repair, mismatch repair, and double-strand break repair pathways, cause the individuals to be less proficient in repairing carcinogen-induced damage (36). It has also been proposed that the platinum-DNA adducts interfere with the normal function of cellular proteins such as binding or interactions with other proteins (37).
Neurotoxicity in the peripheral nervous system is therefore a significant factor affecting the efficacy of the platinum-based drugs, as patients may experience either more negative side-effects than benefits from this drug class or be forced to forego further therapy with an active agent. Recent studies have examined the effectiveness of a number of potential neuroprotectant agents, such as erythropoietin (38), amofistine (39), carbemazepine (40), and supplements such as Vitamin E or intravenous calcium or magnesium transfusions (29). The use of magnesium and calcium supplementation with oxaliplatin appeared to reduce the anti-tumor effect in advanced colorectal cancer in one study and is no longer a viable approach to avoid neurotoxicity (41). Additionally, there has been a clinical trial using nimodipine, a calcium-channel antagonist, in conjunction with cisplatin. This trial resulted in premature cessation of treatment plus nimodipine due to significantly increased gastrointestinal toxicity. Acetyl-L-carnitine is an additional intervention method used to treat chemotherapy-induced neurotoxicity (42). Xaliproden is a non-peptidic neurotrophic drug that has recently been used in oxaliplatin-treated patients experiencing neurotoxicity (43). A cytokine called LIF, or leukemia inhibiting factor, was shown to have a role in diminishing peripheral neurotoxicity in animal models, but was not confirmed in clinical samples. Because the platinum drugs target the dorsal root ganglion and accumulate there, glutathione (GSH) had also been evaluated as a neuroprotectant. Reduced GSH has a high affinity for heavy metals and could prevent the platinum accumulation in the DRG (44, 45). Org 2766 was shown to attenuate cisplatin-associated neuropathy in ovarian cancer patients without adversely affecting the efficacy (46). The efforts to establish a neuroprotective agent against cisplatin-induced neurotoxicity have been to date unsuccessful, therefore future randomized controlled clinical trials must be performed to continue this issue (47). For this reason, we must turn to genetics for efforts to prevent this debilitating side-effect.
In addition to efforts to identify a successful neuroprotective agent, there have been numerous studies attempting to establish the role of various phenotypic markers for chemotherapy-induced neurotoxicity. An accurate marker of neurotoxicity that would enable a quantitative monitoring of progress of neurotoxicity or provide a prediction of the ultimate severity would prove valuable in controlling this toxicity. Cavaletti et al. indicated a highly significant correlation between the decrease in circulating levels of nerve growth factor (NGF) and the severity of chemotherapy-induced neurotoxicity in patients treated with cisplatin and paclitaxel, however it did not predict final neurological outcome (48). In addition, nerve electrophysiological studies have been used to detect the progression of drug-induced neuropathy (49). However, further studies to evaluate the effectiveness of both blood markers and electrophysiology in detection of neurotoxicity progression must be performed to conclude that these provide any sufficient benefit to the patient.
Many recent efforts have been made to determine genetic linkages as a cause of platinum-based toxicity, in order to ultimately diminish these effects and augment the beneficial anti-tumor qualities. The first and all-encompassing step of identifying genes that may play a role in the variation of drug efficacy and individual response to the drug, a number of genome-wide studies have been pursued. After discussing these broad studies, we review the specific targeted genetic data and research efforts designed to circumvent this life-altering side-effect of platinum-based cancer treatment.
Genome-wide studies to establish loci for chemotherapy response and toxicity
Genome-wide studies are an effective tool to narrow the genome to a specific region that is associated with drug response or other important phenotypes related to that drug. This allows for the identification of putative candidate genes. Genome-wide expression analysis studies have been used to correlate particular genomic signatures with drug response and development of toxicity (50). Furthermore, genome-wide polymorphism studies describe variation between individuals and associate these with response.
Platinum drug disposition
The disposition of a chemotherapeutic agents is often the key to determining the mechanism by which they cause toxic effects in the human body. The disposition of a drug includes its metabolism, absorption, distribution, and excretion. There are plausible connections between genetic variability in candidate drug disposition genes variability in neurotoxicity rise (Table 1).
Table 1.
| Tumor Type | Gene | N | Therapy | Neurotoxicity Relationship |
|---|---|---|---|---|
| Ovarian | ABCB1, ABCC1, ABCC2, ABCG2, CDKN1A, CYP1B1, CYP2C8, CYP3A4, CYP3A5, ERCC1, ERCC2, GSTP1, MAPT, MPO, TP53, XRCC1 | 914 | Carboplatin plus either paclitaxel or docetaxel | Selected SNPs in each gene show no significant association |
| Marsh, et al (60) | ||||
| Advanced Colorectal | GSTP1 | 166 | FOLFOX | I105V homozygous Ile alleles show increase in neurotoxicity (p<0.01) |
| Ruzzo, et al (51) | ||||
| Colorectal | SCN1A | 152 | 5-FU/Oxaliplatin | T1067A T/T genotype have decreased neurotoxicity (p=0.002) |
| Nagashima, et al (61) | ||||
| GI Cancer | GSTP1 | 64 | Oxaliplatin | I105V homozygous Ile allele shows increased frequency of grade 3 neurotoxicity (p=0.002) |
| Lecomte, et al (52) |
Glutathione S- Transferases (GST) are a family of enzymes that catalyze the conjugation of glutathione to electrophilic toxins to inactivate them and aid in their excretion from the body. The GST genes encode metabolizing enzymes that decrease the reactivity of toxins with substrates in the body. They are divided into five classes. Genetic polymorphisms have been found in a number of these, including GSTM1, GSTT1, GSTP1. Two independent studies in advanced colorectal cancer patients treated with oxaliplatin looked at the GST genes for variants patients that experienced grade 3 cumulative neuropathy. Ruzzo et al described 166 patients in which there was evidence of an association between the GSTP1 105 Val G/G allele and the development of grade 3 neuropathy from oxaliplatin treatment (Table 1) (51). Additionally, Lecomte and colleagues indicated that in a cohort of 64 patients, there was a significant association between the GSTP1 105 Val G/G allele and risk of developing neurotoxicity(52). A phase II study of 42 patients treated with irinotecan and carboplatin in advanced non-small cell lung cancer also showed an association with this allele, where 5/9 (26%) of those with the GSTP1 105 G/A or G/G genotypes had partial response when compared to individuals with A/A who had no response (p = 0.057) (53).
Gamelin et al proposed that key components of the oxalate synthesis pathway could be associated with platinum-drug neurotoxicity. In a study of an initial 10 patients followed by an additional 135 patients treated with oxaliplatin, a minor haplotype in AGXT was able to predict both acute and chronic neurotoxicity(54). Although this is the first study to indicate the contribution of AGXT, it warrants further analysis in larger patient cohorts to determine the predictive power of this haplotype.
Cell-entry mechanisms differ between drugs. As a heavy metal, platinum drugs must have a particular method of entering their cell of interest. Metal transporters, such as the copper transporters CTR1, ATP7A and ATP7B, have been of particular interest. Deletion of the CTR1 gene in yeast leads to a significant accumulation of all three clinically available platinum agents (55). Forced overexpression of human CTR1 in ovarian cancer cells increased cisplatin uptake. CTR1 mediates cellular accumulation of platinum-containing drugs used in patients. In order for neurotoxicity to develop, the drug must be capable of entering its target cells to cause damage. Therefore any genes or proteins involved in the transport of platinum into or out of cells could play a role in neurotoxicity development. However, there are no clinical studies assessing the influence of genetic variation in platinum transporters on patient toxicity or outcome.
Repair/ Resistance
DNA repair is an important mechanism for resistance to platinum-based therapy and possibly the development of neurotoxicity. If the cell is able to repair the DNA that is attacked by the platinum agent, then that agent will be unsuccessful in inducing apoptosis. Nucleotide excision repair (NER) genes, such as excision repair cross-complementation group 1 (ERCC1) have been hypothesized to play a role in the efficacy of platinum-based drugs. Among the numerous studies performed assessing the association between NER genes and chemotherapy clinical outcome, many have provided concrete evidence of an association (56). Park et al described an association between the ERCC1 codon 118 polymorphism and clinical output in colorectal cancer patients treated with platinum-based chemotherapy. This genotype could be a useful predictor of clinical outcome not only for colorectal cancer patients, but also for patients with epithelial ovarian cancer (57, 58). To date, there have not been an association shown between ERCC1 and chemotherapy neurotoxicity (51), however because the primary target of platinum is DNA, the possibility remains. There are many genes whose effect on neurotoxicity have yet to be studied. In a recent study in a population of ovarian cancer samples, there were a number of genes examined that were treated with combination therapy, paclitaxel/carboplatin or docetaxel/carboplatin, of which no associations with neurotoxicity were found (59).
Conclusions
There are many avenues that will need to be pursued to avoid or minimize neurotoxicity caused by platinum drugs. Several are underway, such as studies to determine drug combinations to prevent or minimize the toxicities. Unfortunately, these have resulted in only minor contributions. An individual’s unique genetic code effects the disposition of each drug within their body. To date, there have been many studies done examining DNA repair and drug disposition genes in various patient populations treated with a number of different drugs, including platinum-based drugs. Despite these efforts, specific targets have yet to be elucidated that determine the precise association between platinum-agents and sensory neurotoxicity or any of the other toxic side-effects that these drugs are associated with. By further and more detailed examination of the drug disposition as well as the specific phenotype it is causing, researchers may identify concrete phenotype/genotype associations.
Data from clinical trials indicate a lack of correlation between the incidence of neurotoxicity and tumor response rate. This supports the hypothesis that by eliminating or decreasing neurotoxicity, the effectiveness of the drug will not be diminished. However, it will take a more concerted effort to discover meaningful predictors of this serious adverse drug effect. Until the concrete phenotype/genotype associations have been established to enable individualized therapy, a physician should be aware of the risk of the severe, life-altering side-effect of neurotoxicity. When the symptoms become extreme, it may be advisable to cease therapy or to offer an alternative treatment in order to salvage the patient’s quality of life.
Acknowledgments
The authors are supported in part by NIH grants U01 GM63340, P50 CA106991, P30 CA016086, and R21 CA102461.
Footnotes
The authors do not have any conflicts of interest.
References
- 1.Higby DJ, Wallace HJ, Jr, Albert DJ, Holland JF. Diaminodichloroplatinum: a phase I study showing responses in testicular and other tumors. Cancer. 1974;33:1219–1225. doi: 10.1002/1097-0142(197405)33:5<1219::aid-cncr2820330505>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.FDA. Oncology Tools. Listing of Approved Oncology Drugs with Approved Indications. 2007 [cited; Available from: http://www.accessdata.fda.gov/scripts/cder/onctools/druglistframe.htm.
- 3.Seiwert TY, Salama JK, Vokes EE. The chemoradiation paradigm in head and neck cancer. Nat Clin Pract Oncol. 2007;4:156–171. doi: 10.1038/ncponc0750. [DOI] [PubMed] [Google Scholar]
- 4.Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst. 2007;99:847–857. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 5.Hess LM, Benham-Hutchins M, Herzog TJ, et al. A meta-analysis of the efficacy of intraperitoneal cisplatin for the front-line treatment of ovarian cancer. Int J Gynecol Cancer. 2007;17:561–570. doi: 10.1111/j.1525-1438.2006.00846.x. [DOI] [PubMed] [Google Scholar]
- 6.Rajeswaran A, Trojan A, Burnand B, Giannelli M. Efficacy and side effects of cisplatin- and carboplatin-based doublet chemotherapeutic regimens versus non-platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non-small cell lung carcinoma: A systematic review of randomized controlled trials. Lung Cancer. 2007 doi: 10.1016/j.lungcan.2007.07.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.de Cos Escuin JS, Delgado IU, Rodriguez JC, Lopez MJ, Vicente CD, Miranda JA. Stage IIIA and IIIB non-small cell lung cancer: results of chemotherapy combined with radiation therapy and analysis of prognostic factors. Arch Bronconeumol. 2007;43:358–365. doi: 10.1016/s1579-2129(07)60086-x. [DOI] [PubMed] [Google Scholar]
- 8.Helbekkmo N, Sundstrom SH, Aasebo U, et al. Vinorelbine/carboplatin vs gemcitabine/carboplatin in advanced NSCLC shows similar efficacy, but different impact of toxicity. Br J Cancer. 2007;97:283–289. doi: 10.1038/sj.bjc.6603869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abratt RP, Hart GJ. 10-year update on chemotherapy for non-small cell lung cancer. Ann Oncol. 2006;17 Suppl 5:v33–v36. doi: 10.1093/annonc/mdj947. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 11.Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim A, Hirschfeld S, Cohen MH, Griebel DJ, Williams GA, Pazdur R. FDA drug approval summaries: oxaliplatin. Oncologist. 2004;9:8–12. doi: 10.1634/theoncologist.9-1-8. [DOI] [PubMed] [Google Scholar]
- 13.Ashley AC, Sargent DJ, Alberts SR, et al. Updated efficacy and toxicity analysis of irinotecan and oxaliplatin (IROX) : intergroup trial N9741 in first-line treatment of metastatic colorectal cancer. Cancer. 2007;110:670–677. doi: 10.1002/cncr.22831. [DOI] [PubMed] [Google Scholar]
- 14.McKeage MJ. Comparative adverse effect profiles of platinum drugs. Drug Saf. 1995;13:228–244. doi: 10.2165/00002018-199513040-00003. [DOI] [PubMed] [Google Scholar]
- 15.Barrett SV, Paul J, Hay A, Vasey PA, Kaye SB, Glasspool RM. Does body mass index affect progression-free or overall survival in patients with ovarian cancer? Results from SCOTROC I trial. Ann Oncol. 2008 doi: 10.1093/annonc/mdm606. [DOI] [PubMed] [Google Scholar]
- 16.Ekhart C, de Jonge ME, Huitema AD, Schellens JH, Rodenhuis S, Beijnen JH. Flat dosing of carboplatin is justified in adult patients with normal renal function. Clin Cancer Res. 2006;12:6502–6508. doi: 10.1158/1078-0432.CCR-05-1076. [DOI] [PubMed] [Google Scholar]
- 17.Pharmacists ASoH-S. Carboplatin. [cited; Available from: http://www.ashp.org/mngrphs/ahfs/a395017.htm.
- 18.FDA. FDA Oncology Tools Product Label Details in Conventional Order for Oxaliplatin. [cited; Available from: http://www.accessdata.fda.gov/scripts/cder/onctools/labels.cfm?GN=oxaliplatin.
- 19.Chaney SG, Campbell SL, Bassett E, Wu Y. Recognition and processing of cisplatin- and oxaliplatin-DNA adducts. Crit Rev Oncol Hematol. 2005;53:3–11. doi: 10.1016/j.critrevonc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 21.Kartalou M, Essigmann JM. Recognition of cisplatin adducts by cellular proteins. Mutat Res. 2001;478:1–21. doi: 10.1016/s0027-5107(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 22.Cavaletti G, Tredici G, Petruccioli MG, et al. Effects of different schedules of oxaliplatin treatment on the peripheral nervous system of the rat. Eur J Cancer. 2001;37:2457–2463. doi: 10.1016/s0959-8049(01)00300-8. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan AV, Goldstein D, Friedlander M, Kiernan MC. Oxaliplatin-induced neurotoxicity and the development of neuropathy. Muscle Nerve. 2005;32:51–60. doi: 10.1002/mus.20340. [DOI] [PubMed] [Google Scholar]
- 24.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 25.Hah SS, Stivers KM, de Vere White RW, Henderson PT. Kinetics of carboplatin-DNA binding in genomic DNA and bladder cancer cells as determined by accelerator mass spectrometry. Chemical research in toxicology. 2006;19:622–626. doi: 10.1021/tx060058c. [DOI] [PubMed] [Google Scholar]
- 26.Zhu C, Raber J, Eriksson LA. Hydrolysis process of the second generation platinum-based anticancer drug cis-amminedichlorocyclohexylamineplatinum(II) J Phys Chem B Condens Matter Mater Surf Interfaces Biophys. 2005;109:12195–12205. doi: 10.1021/jp0518916. [DOI] [PubMed] [Google Scholar]
- 27.Dzagnidze A, Katsarava Z, Makhalova J, et al. Repair capacity for platinum-DNA adducts determines the severity of cisplatin-induced peripheral neuropathy. J Neurosci. 2007;27:9451–9457. doi: 10.1523/JNEUROSCI.0523-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chvalova K, Brabec V, Kasparkova J. Mechanism of the formation of DNA-protein cross-links by antitumor cisplatin. Nucleic acids research. 2007;35:1812–1821. doi: 10.1093/nar/gkm032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stillman M, Cata JP. Management of chemotherapy-induced peripheral neuropathy. Curr Pain Headache Rep. 2006;10:279–287. doi: 10.1007/s11916-006-0033-z. [DOI] [PubMed] [Google Scholar]
- 30.Dunlap B, Paice JA. Chemotherapy-induced peripheral neuropathy: A need for standardization in measurement. J Support Oncol. 2006;4:398–399. [PubMed] [Google Scholar]
- 31.Sul JK, Deangelis LM. Neurologic complications of cancer chemotherapy. Semin Oncol. 2006;33:324–332. doi: 10.1053/j.seminoncol.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 32.McKeage MJ, Hsu T, Screnci D, Haddad G, Baguley BC. Nucleolar damage correlates with neurotoxicity induced by different platinum drugs. Br J Cancer. 2001;85:1219–1225. doi: 10.1054/bjoc.2001.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Safaei R. Role of copper transporters in the uptake and efflux of platinum containing drugs. Cancer Lett. 2006;234:34–39. doi: 10.1016/j.canlet.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 34.McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18:305–313. doi: 10.1016/j.nbd.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology. 2006;27:992–1002. doi: 10.1016/j.neuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Suk R, Gurubhagavatula S, Park S, et al. Polymorphisms in ERCC1 and grade 3 or 4 toxicity in non-small cell lung cancer patients. Clin Cancer Res. 2005;11:1534–1538. doi: 10.1158/1078-0432.CCR-04-1953. [DOI] [PubMed] [Google Scholar]
- 37.Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998;25:4–12. [PubMed] [Google Scholar]
- 38.Bianchi R, Brines M, Lauria G, et al. Protective effect of erythropoietin and its carbamylated derivative in experimental Cisplatin peripheral neurotoxicity. Clin Cancer Res. 2006;12:2607–2612. doi: 10.1158/1078-0432.CCR-05-2177. [DOI] [PubMed] [Google Scholar]
- 39.DiPaola RS, Schuchter L. Neurologic protection by amifostine. Semin Oncol. 1999;26:82–88. [PubMed] [Google Scholar]
- 40.Lersch C, Schmelz R, Eckel F, et al. Prevention of oxaliplatin-induced peripheral sensory neuropathy by carbamazepine in patients with advanced colorectal cancer. Clin Colorectal Cancer. 2002;2:54–58. doi: 10.3816/CCC.2002.n.011. [DOI] [PubMed] [Google Scholar]
- 41.Hochster HS, Grothey A, Childs BH. Use of Calcium and Magnesium Salts to Reduce Oxaliplatin-Related Neurotoxicity. J Clin Oncol. 2007 doi: 10.1200/JCO.2007.13.5251. [DOI] [PubMed] [Google Scholar]
- 42.De Grandis D. Acetyl-L-carnitine for the treatment of chemotherapy-induced peripheral neuropathy: a short review. CNS drugs. 2007;21 Suppl 1:39–43. doi: 10.2165/00023210-200721001-00006. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 43.Susman E. Xaliproden lessens oxaliplatin-mediated neuropathy. The lancet oncology. 2006;7:288. doi: 10.1016/s1470-2045(06)70639-8. [DOI] [PubMed] [Google Scholar]
- 44.Schmidinger M, Budinsky AC, Wenzel C, et al. Glutathione in the prevention of cisplatin induced toxicities. A prospectively randomized pilot trial in patients with head and neck cancer and non small cell lung cancer. Wiener klinische Wochenschrift. 2000;112:617–623. [PubMed] [Google Scholar]
- 45.Cascinu S, Catalano V, Cordella L, et al. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2002;20:3478–3483. doi: 10.1200/JCO.2002.07.061. [DOI] [PubMed] [Google Scholar]
- 46.van der Hoop RG, Vecht CJ, van der Burg ME, et al. Prevention of cisplatin neurotoxicity with an ACTH(4–9) analogue in patients with ovarian cancer. The New England journal of medicine. 1990;322:89–94. doi: 10.1056/NEJM199001113220204. [DOI] [PubMed] [Google Scholar]
- 47.Albers J, Chaudhry V, Cavaletti G, Donehower R. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane database of systematic reviews (Online) 2007 doi: 10.1002/14651858.CD005228.pub2. CD005228. [DOI] [PubMed] [Google Scholar]
- 48.Cavaletti G, Bogliun G, Marzorati L, et al. Early predictors of peripheral neurotoxicity in cisplatin and paclitaxel combination chemotherapy. Ann Oncol. 2004;15:1439–1442. doi: 10.1093/annonc/mdh348. [DOI] [PubMed] [Google Scholar]
- 49.Mileshkin L, Stark R, Day B, Seymour JF, Zeldis JB, Prince HM. Development of neuropathy in patients with myeloma treated with thalidomide: patterns of occurrence and the role of electrophysiologic monitoring. J Clin Oncol. 2006;24:4507–4514. doi: 10.1200/JCO.2006.05.6689. [DOI] [PubMed] [Google Scholar]
- 50.Potti A, Dressman HK, Bild A, et al. Genomic signatures to guide the use of chemotherapeutics. Nat Med. 2006;12:1294–1300. doi: 10.1038/nm1491. [DOI] [PubMed] [Google Scholar]
- 51.Ruzzo A, Graziano F, Loupakis F, et al. Pharmacogenetic profiling in patients with advanced colorectal cancer treated with first-line FOLFOX-4 chemotherapy. J Clin Oncol. 2007;25:1247–1254. doi: 10.1200/JCO.2006.08.1844. [DOI] [PubMed] [Google Scholar]
- 52.Lecomte T, Landi B, Beaune P, Laurent-Puig P, Loriot MA. Glutathione S-transferase P1 polymorphism (Ile105Val) predicts cumulative neuropathy in patients receiving oxaliplatin-based chemotherapy. Clin Cancer Res. 2006;12:3050–3056. doi: 10.1158/1078-0432.CCR-05-2076. [DOI] [PubMed] [Google Scholar]
- 53.Pillot GA, Read WL, Hennenfent KL, et al. A phase II study of irinotecan and carboplatin in advanced non-small cell lung cancer with pharmacogenomic analysis: final report. J Thorac Oncol. 2006;1:972–978. [PubMed] [Google Scholar]
- 54.Gamelin L, Capitain O, Morel A, et al. Predictive factors of oxaliplatin neurotoxicity: the involvement of the oxalate outcome pathway. Clin Cancer Res. 2007;13:6359–6368. doi: 10.1158/1078-0432.CCR-07-0660. [DOI] [PubMed] [Google Scholar]
- 55.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan P, Miao XP, Zhang XM, et al. Polymorphisms in nucleotide excision repair genes XPC and XPD and clinical responses to platinum-based chemotherapy in advanced non-small cell lung cancer. Zhonghua Yi Xue Za Zhi. 2005;85:972–975. [PubMed] [Google Scholar]
- 57.Park DJ, Zhang W, Stoehlmacher J, et al. ERCC1 gene polymorphism as a predictor for clinical outcome in advanced colorectal cancer patients treated with platinum-based chemotherapy. Clin Adv Hematol Oncol. 2003;1:162–166. [PubMed] [Google Scholar]
- 58.Kang S, Ju W, Kim JW. Association between excision repair cross-complementation group 1 polymorphism and clinical outcome of platinum-based chemotherapy in patients with epithelial ovarian cancer. Exp Mol Med. 2006;38:320–324. doi: 10.1038/emm.2006.38. [DOI] [PubMed] [Google Scholar]
- 59.Mori T, Hosokawa K, Kinoshita Y, et al. A pilot study of docetaxel-carboplatin versus paclitaxel-carboplatin in Japanese patients with epithelial ovarian cancer. International journal of clinical oncology / Japan Society of Clinical Oncology. 2007;12:205–211. doi: 10.1007/s10147-007-0656-z. [DOI] [PubMed] [Google Scholar]
- 60.Marsh S, Paul J, King CR, Gifford G, McLeod HL, Brown R. Pharmacogenetic assessment of toxicity and outcome after platinum plus taxane chemotherapy in ovarian cancer: the Scottish Randomised Trial in Ovarian Cancer. J Clin Oncol. 2007;25:4528–4535. doi: 10.1200/JCO.2006.10.4752. [DOI] [PubMed] [Google Scholar]
- 61.Nagashima WZ F, Yang D, Gordon M, Schultheis A, Fazzone W, Azuma M, El-Khoueiry A, Iqbal S, Lenz HJ. Polymorphism in sodium-channel alpha 1-subunit (SCN1A) predicts response, TTP, survival, and toxicity in patients with metastatic colorectal cancer treated with 5-FU/oxaliplatin. ASCO; 2006. Journal of Clinical Oncology. 2006 [Google Scholar]
- 62.Caruba T, Cottu PH, Madelaine-Chambrin I, Espie M, Misset JL, Gross-Goupil M. Gemcitabine-oxaliplatin combination in heavily pretreated metastatic breast cancer: a pilot study on 43 patients. Breast J. 2007;13:165–171. doi: 10.1111/j.1524-4741.2007.00391.x. [DOI] [PubMed] [Google Scholar]
- 63.Ferrero JM, Weber B, Geay JF, et al. Second-line chemotherapy with pegylated liposomal doxorubicin and carboplatin is highly effective in patients with advanced ovarian cancer in late relapse: a GINECO phase II trial. Ann Oncol. 2007;18:263–268. doi: 10.1093/annonc/mdl376. [DOI] [PubMed] [Google Scholar]
- 64.Choi CH, Kim TJ, Lee SJ, et al. Salvage chemotherapy with a combination of paclitaxel, ifosfamide, and cisplatin for the patients with recurrent carcinoma of the uterine cervix. Int J Gynecol Cancer. 2006;16:1157–1164. doi: 10.1111/j.1525-1438.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- 65.Belani CP, Fossella F. Elderly subgroup analysis of a randomized phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for first-line treatment of advanced nonsmall cell lung carcinoma (TAX 326) Cancer. 2005;104:2766–2774. doi: 10.1002/cncr.21495. [DOI] [PubMed] [Google Scholar]
- 66.Mardiak J, Salek T, Sycova-Mila Z, et al. Gemcitabine plus cisplatine and paclitaxel (GCP) in second-line treatment of germ cell tumors (GCT): a phase II study. Neoplasma. 2005;52:243–247. [PubMed] [Google Scholar]
- 67.Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 68.Pujol JL, Milleron B, Molinier O, et al. Weekly paclitaxel combined with monthly carboplatin in elderly patients with advanced non-small cell lung cancer: a multicenter phase II study. J Thorac Oncol. 2006;1:328–334. [PubMed] [Google Scholar]
- 69.Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24:2786–2792. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 70.Kakolyris S, Ziras N, Vamvakas L, et al. Gemcitabine plus oxaliplatin combination (GEMOX regimen) in pretreated patients with advanced non-small cell lung cancer (NSCLC): a multicenter phase II study. Lung Cancer. 2006;54:347–352. doi: 10.1016/j.lungcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Fracasso PM, Blessing JA, Molpus KL, Adler LM, Sorosky JI, Rose PG. Phase II study of oxaliplatin as second-line chemotherapy in endometrial carcinoma: a Gynecologic Oncology Group study. Gynecologic oncology. 2006;103:523–526. doi: 10.1016/j.ygyno.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 72.Cappuzzo F, Novello S, De Marinis F, et al. Phase II study of gemcitabine plus oxaliplatin as first-line chemotherapy for advanced non-small-cell lung cancer. Br J Cancer. 2005;93:29–34. doi: 10.1038/sj.bjc.6602667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steer CB, Chrystal K, Cheong KA, et al. Gemcitabine and oxaliplatin followed by paclitaxel and carboplatin as first line therapy for patients with suboptimally debulked, advanced epithelial ovarian cancer. A phase II trial of sequential doublets. The GO-First Study. Gynecologic oncology. 2006;103:439–445. doi: 10.1016/j.ygyno.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 74.Delaloge S, Laadem A, Taamma A, et al. Pilot study of the paclitaxel, oxaliplatin, and cisplatin combination in patients with advanced/recurrent ovarian cancer. American journal of clinical oncology. 2000;23:569–574. doi: 10.1097/00000421-200012000-00007. [DOI] [PubMed] [Google Scholar]