Abstract
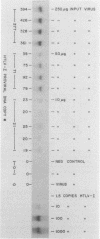
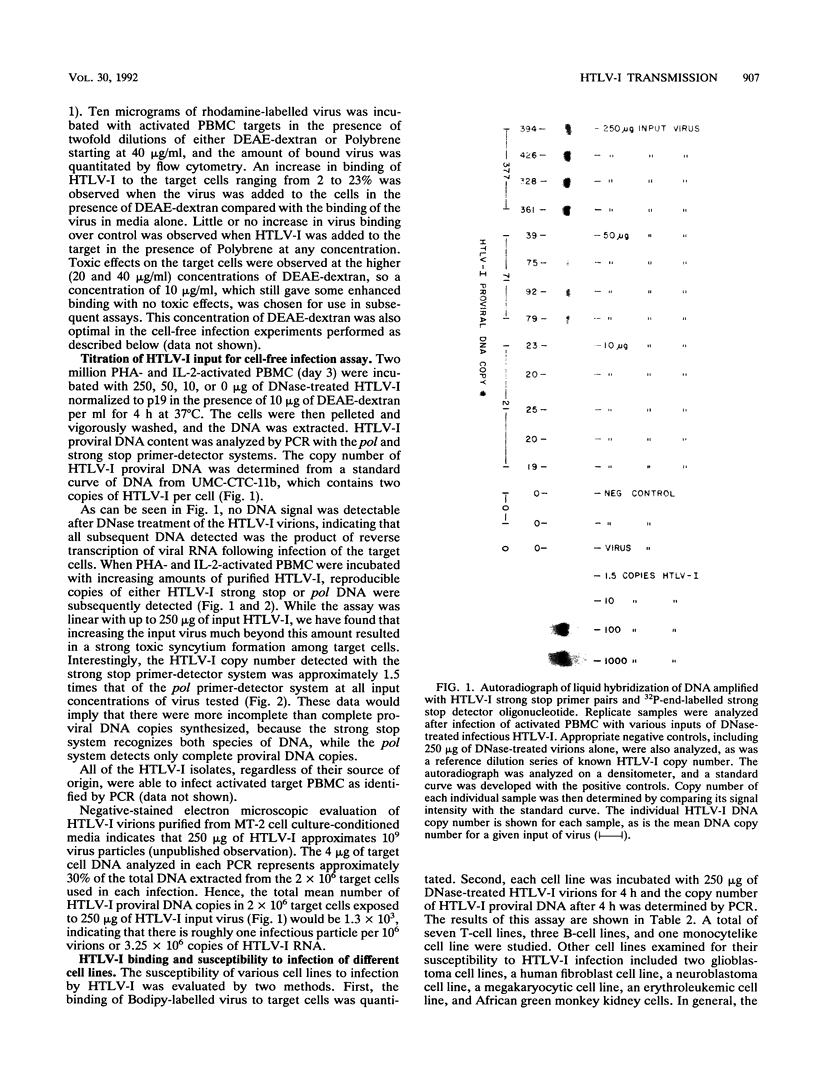
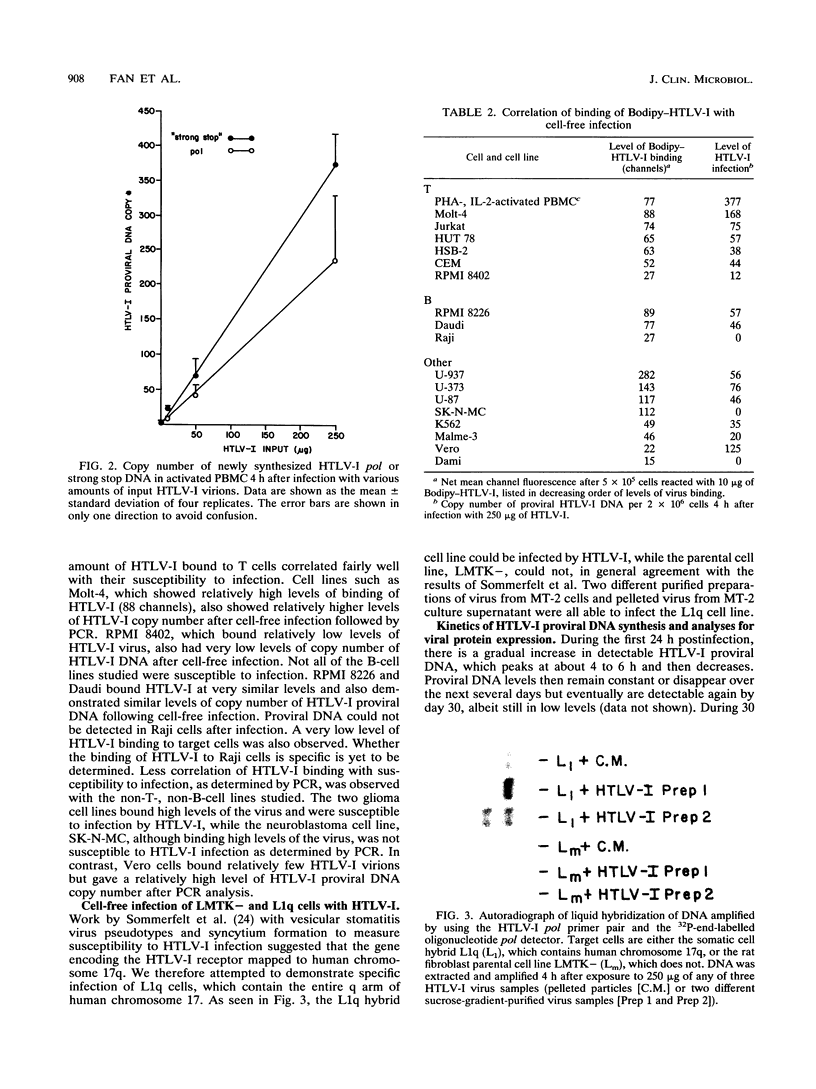
Previous studies of in vitro infection by human T-cell lymphoma/leukemia virus type I (HTLV-I) have required cocultivation of target cells with HTLV-I cell lines or vesicular stomatitis virus pseudotypes containing HTLV-I envelope proteins. We report here the development of a cell-free infection assay for HTLV-I. Target cells were incubated with purified, DNase-treated HTLV-I virions for 4 h at 37 degrees C. Target cell DNA was then analyzed for the presence of newly synthesized HTLV-I proviral DNA by the highly sensitive polymerase chain reaction. Using this assay system, we have been able to consistently detect in vitro infection of a variety of cellular targets by different HTLV-I isolates. Optimal infection required the presence of 10 micrograms of DEAE-dextran per ml. The assay was dose dependent with respect to virus input. In general, the amount of proviral DNA detected correlated with the level of HTLV-I receptors present on the surface of the target cells, as measured by fluorochrome-labelled HTLV-I binding. Finally, the specificity of the assay was confirmed by demonstrating that the cell line, L1q, a somatic cell hybrid containing human chromosome 17q, to which the gene for the HTLV-I receptor has been mapped, was susceptible to infection by HTLV-I, while the parental mouse cell line from which it was derived, LMTK-, which lacks human chromosome 17q, was not.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhagavati S., Ehrlich G., Kula R. W., Kwok S., Sninsky J., Udani V., Poiesz B. J. Detection of human T-cell lymphoma/leukemia virus type I DNA and antigen in spinal fluid and blood of patients with chronic progressive myelopathy. N Engl J Med. 1988 May 5;318(18):1141–1147. doi: 10.1056/NEJM198805053181801. [DOI] [PubMed] [Google Scholar]
- Byrne B. C., Li J. J., Sninsky J., Poiesz B. J. Detection of HIV-1 RNA sequences by in vitro DNA amplification. Nucleic Acids Res. 1988 May 11;16(9):4165–4165. doi: 10.1093/nar/16.9.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham P., Nagy K., Cheingsong-Popov R., Exley M., Weiss R. A. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983 Dec 9;222(4628):1125–1127. doi: 10.1126/science.6316502. [DOI] [PubMed] [Google Scholar]
- Ehrlich G. D., Davey F. R., Kirshner J. J., Sninsky J. J., Kwok S., Slamon D. J., Kalish R., Poiesz B. J. A polyclonal CD4+ and CD8+ lymphocytosis in a patient doubly infected with HTLV-I and HIV-1: a clinical and molecular analysis. Am J Hematol. 1989 Mar;30(3):128–139. doi: 10.1002/ajh.2830300304. [DOI] [PubMed] [Google Scholar]
- Ehrlich G. D., Glaser J. B., LaVigne K., Quan D., Mildvan D., Sninsky J. J., Kwok S., Papsidero L., Poiesz B. J. Prevalence of human T-cell leukemia/lymphoma virus (HTLV) type II infection among high-risk individuals: type-specific identification of HTLVs by polymerase chain reaction. Blood. 1989 Oct;74(5):1658–1664. [PubMed] [Google Scholar]
- Graziano S. L., Lehr B. M., Merl S. A., Ehrlich G. D., Moore J. L., Hallinan E. J., Hubbell C., Davey F. R., Vournakis J., Poiesz B. J. Quantitative assay of human T-cell leukemia/lymphoma virus transformation. Cancer Res. 1987 May 1;47(9):2468–2473. [PubMed] [Google Scholar]
- Greenberg S. M., Rosenthal D. S., Greeley T. A., Tantravahi R., Handin R. I. Characterization of a new megakaryocytic cell line: the Dami cell. Blood. 1988 Dec;72(6):1968–1977. [PubMed] [Google Scholar]
- Hansen J. E., Nielsen C., Mathiesen L. R., Nielsen J. O. Involvement of lymphocyte function-associated antigen-1 (LFA-1) in HIV infection: inhibition by monoclonal antibody. Scand J Infect Dis. 1991;23(1):31–36. doi: 10.3109/00365549109023371. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of human endothelial cells by human T-lymphotropic virus type I. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7588–7590. doi: 10.1073/pnas.81.23.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. C., Hou Y., Woods L. K., Moore G. E., Minowada J. Cytogenetic study of human lymphoid T-cell lines derived from lymphocytic leukemia. J Natl Cancer Inst. 1974 Sep;53(3):655–660. doi: 10.1093/jnci/53.3.655. [DOI] [PubMed] [Google Scholar]
- Krichbaum-Stenger K., Poiesz B. J., Keller P., Ehrlich G., Gavalchin J., Davis B. H., Moore J. L. Specific adsorption of HTLV-I to various target human and animal cells. Blood. 1987 Nov;70(5):1303–1311. [PubMed] [Google Scholar]
- Merl S., Kloster B., Moore J., Hubbell C., Tomar R., Davey F., Kalinowski D., Planas A., Ehrlich G., Clark D. Efficient transformation of previously activated and dividing T lymphocytes by human T cell leukemia-lymphoma virus. Blood. 1984 Nov;64(5):967–974. [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Papsidero L. D., Dittmer R. P., Vaickus L., Poiesz B. J. Monoclonal antibodies and chemiluminescence immunoassay for detection of the surface protein of human T-cell lymphotropic virus. J Clin Microbiol. 1992 Feb;30(2):351–358. doi: 10.1128/jcm.30.2.351-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papsidero L., Swartzwelder F., Sheu M., Montagna R., Ehrlich G., Bhagavati S., Dosik H., Sninsky J., Poiesz B. Immunodetection of human T-cell lymphotropic virus type I core protein in biological samples by using a monoclonal antibody immunoassay. J Clin Microbiol. 1990 May;28(5):949–955. doi: 10.1128/jcm.28.5.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Mier J. W., Woods A. M., Gallo R. C. T-cell lines established from human T-lymphocytic neoplasias by direct response to T-cell growth factor. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6815–6819. doi: 10.1073/pnas.77.11.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Reitz M. S., Kalyanaraman V. S., Gallo R. C. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature. 1981 Nov 19;294(5838):268–271. doi: 10.1038/294268a0. [DOI] [PubMed] [Google Scholar]
- Ratner L., Poiesz B. J. Leukemias associated with human T-cell lymphotropic virus type I in a non-endemic region. Medicine (Baltimore) 1988 Nov;67(6):401–422. doi: 10.1097/00005792-198811000-00004. [DOI] [PubMed] [Google Scholar]
- Saida T., Saida K., Funauchi M., Nishiguchi E., Nakajima M., Matsuda S., Ohta M., Ohta K., Nishitani H., Hatanaka M. HTLV-I myelitis: isolation of virus, genomic analysis, and infection in neural cell cultures. Ann N Y Acad Sci. 1988;540:636–638. doi: 10.1111/j.1749-6632.1988.tb27196.x. [DOI] [PubMed] [Google Scholar]
- Sanford J. A., Stubblefield E. General protocol for microcell-mediated chromosome transfer. Somat Cell Mol Genet. 1987 May;13(3):279–284. doi: 10.1007/BF01535210. [DOI] [PubMed] [Google Scholar]
- Seto A., Isono T., Ogawa K. Infection of inbred rabbits with cell-free HTLV-I. Leuk Res. 1991;15(2-3):105–110. doi: 10.1016/0145-2126(91)90090-g. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Williams B. P., Clapham P. R., Solomon E., Goodfellow P. N., Weiss R. A. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science. 1988 Dec 16;242(4885):1557–1559. doi: 10.1126/science.3201246. [DOI] [PubMed] [Google Scholar]
- Wells K. H., Byrne B. C., Poiesz B. J. Detection, prevention, and treatment of retroviral infections. Semin Oncol. 1990 Jun;17(3):295–320. [PubMed] [Google Scholar]
- Yamamoto N., Matsumoto T., Koyanagi Y., Tanaka Y., Hinuma Y. Unique cell lines harbouring both Epstein-Barr virus and adult T-cell leukaemia virus, established from leukaemia patients. Nature. 1982 Sep 23;299(5881):367–369. doi: 10.1038/299367a0. [DOI] [PubMed] [Google Scholar]
- Yanagihara R., Nerurkar V. R., Garruto R. M., Miller M. A., Leon-Monzon M. E., Jenkins C. L., Sanders R. C., Liberski P. P., Alpers M. P., Gajdusek D. C. Characterization of a variant of human T-lymphotropic virus type I isolated from a healthy member of a remote, recently contacted group in Papua New Guinea. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1446–1450. doi: 10.1073/pnas.88.4.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- de Rossi A., Aldovini A., Franchini G., Mann D., Gallo R. C., Wong-Staal F. Clonal selection of T lymphocytes infected by cell-free human T-cell leukemia/lymphoma virus type I: parameters of virus integration and expression. Virology. 1985 Jun;143(2):640–645. doi: 10.1016/0042-6822(85)90405-2. [DOI] [PubMed] [Google Scholar]