Abstract
Acute lung injury (ALI) is a common, frequently hospital acquired condition with a high morbidity and mortality. The stress associated with invasive mechanical ventilation represents a potentially harmful exposure, and attempts to minimize deforming stress through low tidal ventilation have proven efficacious. Lung cells are both sensors and transducers of deforming stress, and are frequently wounded in the setting of mechanical ventilation. Cell wounding may be one of the drivers of the innate immunologic and systemic inflammatory response associated with mechanical ventilation. These downstream effects of mechano-transduction have been referred to collectively as “Biotrauma”. Our review will focus on cellular stress failure, that is cell wounding, and the mechanisms mediating subsequent plasma membrane repair, We hold that a better mechanistic understanding of cell plasticity, deformation associated remodeling and repair will reveal candidate approaches for lung protective interventions in mechanically ventilated patients. We will detail one such intervention, lung conditioning with hypertonic solutions as an example of ongoing research in this arena.
Keywords: Acute Lung Injury, Mechanical Ventilation, Plasma Membrane Repair, Deformation induced lipid trafficking, osmotic stress
1. Introduction
Acute lung injury (ALI) affects over 190,000 people and is responsible for approximately 74,000 deaths each year in the United States (Rubenfeld et al. 2007). Worldwide estimates range from 1.5 to 75 cases per 100,000 persons (Wheeler et al. 2007), translating to more than one million reported cases worldwide each year. ALI is rarely present at the time of hospital admission but develops over a period of hours to days in patients with predisposing conditions such as sepsis, trauma and shock, or who undergo certain medical and surgical interventions (Unpublished data from O. Gajic). Furthermore, certain supportive therapies including mechanical ventilation may lead to progression of ALI and the development of ventilator associated lung injury (VALI). As such patients may be victims of an avoidable, hospital-aquired complication whose consequent morbidity and mortality extract not only a dramatic emotional price, but also an increasingly burdensome monetary cost to the individual, the healthcare system, and society as a whole.
Central to the pathophysiology of VALI is the lungs’ response to deformation. As both sensors and transducers of deforming stress, the cells of the lung are well adapted to the periodic deformations of normal breathing. Cells possess well developed mechanisms to buffer stress concentrations associated with cellular shape changes induced under these conditions. However, in the injured, mechanically ventilated lung, the forces acting on cells may be excessive compared to those experienced by the healthy lung. Increased levels of alveolar ventilation often impose cellular shape changes associated with lytic tensions in stress bearing structures such as the cytoskeleton (CSK) and/or the plasma membrane (PM). The resulting cell wounds trigger or amplify a robust innate immune response often referred to as “biotrauma.” It is important to note that not all manifestations of biotrauma are associated with changes in cell and tissue architecture. (Caruso et al. 2003). However, the implication is that deforming stress, and therefore ventilator settings are critical in shaping lung injury and repair responses. In fact, to date low tidal volume ventilation has been the only therapy to attenuate VALI manifestations and improve overall patient outcomes (Acute Respiratory Distress Syndrome Network, 2000)
Since the lung cannot be casted and immobilized like a broken bone, even protective low tidal volume ventilation settings may not spare the injured lung from further damage. A great deal of research has focused on manipulating the innate immune response to deforming stress. A diverse set of bioactive molecules and pathways including immune response elements (Santos et al. 2005), coagulation proteins(Schultz et al. 2006), reactive oxygen species (Reddy et al. 2007), and fibro-proliferative and apoptotic pathways (Abraham 2003) have been targeted in experimental VALI models. Specific interventions include corticosteroid and immunosuppressive therapies, extracorporeal membrane oxygenation (ECMO) and the selective hemofiltration of key immunomodulatory molecules such as interleukins and TNF-alpha. Disappointingly, none have yielded significant gains, perhaps because most interventions target one or a few of the many complex downstream response elements to deforming stress, while low tidal ventilation targets the stimulus, deforming stress, itself.
Cell wounding amplifies the injured lungs’ pro-inflammatory response to deforming stress. Hence, making lung cells less susceptible to deformation injury ought to mitigate biotrauma. In the following review we will focus on the cellular injury and repair mechanisms relevant to the mechanically ventilated lung. We will share preliminary observations that suggest efficacy of hypertonic lung conditioning on alveolar epithelial and endothelial cell integrity in reduced experimental VALI models. Finally, restricting our comments largely to epithelial cells, we will detail the effects of osmotic stress on CSK structure, cell plasticity and deformation associated PM remodeling and associated membrane trafficking responses.
2. Topographical Distribution of Lung Parenchymal Stress
The lung may be described as a prestressed network of viscoelastic tissue elements that is deformed by surface tension. On a macroscopic scale the determinants of the topographical distribution of lung parenchymal stress and strain are generally understood. Accordingly, the gravitationally deformed lungs are contained within a gravitationally deformed but much stiffer thorax. Regional transpulmonary pressure and alveolar volume (stress and strain) distributions reflect the shape matching constraints of these two structures (Wilson 1986). In contrast, there remains a great deal of uncertainty about how an alveolus, let alone a lung resident cell, deforms during a breath. This is because of the limited spatial and temporal resolution of available in vivo imaging techniques and artifacts inherent in tissue preservation and labeling for histologic and morphometric analyses. In general, most data suggest that alveolar walls and the cells that decorate them unfold during breathing and that they undergo elastic deformations (stretch) only at high lung volumes (Tschumperlin et al. 2000; Bachofen et al. 2001; Weibel et al. 2007).
Near Functional Residual Capacity lung parenchyma and surface tension contribute approximately 50% each to lung recoil. Most of the parenchymal load is carried by a collagen and elastin rich connective tissue matrix, which provides the scaffolding to which lung cells adhere. While cells exert a deforming force on their surrounding tissue matrix, in aggregate the contribution of this force to overall lung recoil and parenchymal stiffness is small (<10%). Nevertheless, the cells’ load bearing elements, namely, CSK and/or PM risk failure, if large matrix deformations impose a large or sudden change in cell shape and dimensions. In the following sections we will briefly review four distinct ventilator induced lung injury mechanisms (Table 1) and explain why alveolar overdistension and so-called opening and collapse are associated with cell injury.
Table 1.
Mechanisms of cellular injury due to injurious ventilation
| Mechanism | Result | Similar toiler to… | Goal-directed Intervention |
|---|---|---|---|
| Overinflation | PM, BM wounding Permeability changes | Balloon | Low VT |
| Surfactant loss | Increased surface tension | Premature infant lung | Potential Replacement therapies |
| Low volume / repetitive stress | Cellular and BM Disruption | Repeatedly bending a paper clip | HFO, PEEP |
| Interdependence | Architectural & stress alteration, transudation | Card house | All of the above |
3. Physical determinants of cellular injury
Although often described in cartoon form as an air-filled sac, alveolar walls form hexagonal-like tissue networks that are partially unloaded by surface tension (Mead et al. 1970; Wilson et al. 1982; Stamenovic 1990). Electron microscopy-derived measurements of rat alveolar basement membrane area demonstrated a 35% increase during inflation to total lung capacity (Tschumperlin et al. 1999). The primary load-bearing tissue components, collagen and elastin, have differing characteristics that complement each other with regard to alveolar stability and protect the alveolus and its constituent cells from overdistension injury. Due to its flexible cross-linked polypeptides, elastin has a linear stress-strain relation up to 200% strain (Fung 1993), while the more organized structure of collagen has a highly nonlinear stress-strain curve (Stromberg et al. 1969). Thus elastin maintains tension at lower strains (Setnikar 1955), while collagen dominates under conditions of higher strain (Suki et al. 2005). Recent evidence suggests that the electrostatically charged proteoglycan matrix in which these fibers are embedded resists the tendency of collagen and elastin to fold at low to medium lung volumes, thereby further contributing to lung elasticity and the stabilization of the alveolar structure (Cavalcante et al. 2005). The interaction between collagen, elastin, and the matrix may therefore have two important functions with regard to ventilator-induced cellular injury that might enhance its attractiveness for therapeutic manipulation. First, the inherent stress-strain relationships of these molecules determine the level of overdistension necessary for stress failure. Second, the influence of the proteoglycan matrix stabilizes the alveolar structure as it deflates during end-expiration, and therefore affords protection against low volume injury.
Another mechanism for protection against overdistension has been observed under electron microscopy (EM) in rat alveoli (Bachofen et al. 2001). Observation of alveoli fixed at various stages of the respiratory cycle suggest that these structures do not inflate like a balloon, but instead unfold in a nonuniform manner. Moreover, EM images of plasma membrane folds, although dismissed by some as artifact of processing and fixation, have led to the following hypothesis (Figure 1): As the alveolus expands, the underlying structural elements and attached basement membrane are stretched, imposing a deformation on the adherent epithelial and endothelial cells. As the cells deform, PM pleats unfold, without significant increases in lateral plasma membrane tension. Moreover, any externally imposed cell shape is met with a lipid trafficking response in the form of non-secretory exocytosis, supplying the PM with excess substrate. PM unfolding, deformation induced lipid trafficking, along with cytoskeletal remodeling represent mechanisms, which allow cells to sustain large deformations without structural failure
Figure 1.
Schematic of alveolar septae contributing to acinar surface area compensation in response to volume-induced deformations. Ventilation at normal tidal volumes result in little change in acinar volume, whereas once volume increases such that septal unfolding is complete, wall stress will increase and acinar volume will change, potentially resulting in overdistension injury.
Injured lungs are particularly susceptible to overdistension because the number of aerated and recruitable alveoli is decreased (“the babylung concept”) (Gattinoni et al. 1987). Within the baby lung, both fully aerated and nonaerated but recruitable respiratory units exist in close proximity. This has been confirmed by computerized tomography (CT) in patients with acute respiratory distress syndrome (ARDS) (Maunder et al. 1986). A preferential distribution of ventilation to the less injured units places these units at a higher risk for hyperinflation injury (Figure 2). Supporting this hypothesis is recent histologic and CT imaging data in a rat VALI model that demonstrates a regional redistribution of ventilation from atelectatic to nonatelectatic areas resulting in overinflation injury (Tsuchida et al. 2006). In this study, a combination of high tidal volume and lack of recruitment of atelectatic regions consistently demonstrated histologic evidence of alveolar injury as measured by hyaline membrane formation, inflammatory cell infiltration, and the presence of alveolar epithelial cell lesions. Markers of biotrauma such as cytokines IL-6, IL-1β, and myeloperoxidase were elevated as well. Clearly the delivery of what would be a “normal” tidal volume to a healthy non-atelectatic lung, may indeed have the potential to be quite injurious to the injured, mechanically ventilated baby lung.
Figure 2.
The baby lung concept and regional overexpansion. White circles represent functional aerated or recruitable units. Black circles represent non-recruitable units. In the healthy lung (top), distribution of tidal volume across lung units results in normal, non-injurious ventilation whereas in the heterogeneously injured lung (bottom), nonuniform distribution of tidal volume occurs to preferentially recruitable units, causing overexpansion and potential injury to basement and plasma membranes.
The flip-side of overinflation and overdistension, the potential for low-volume injury was first supported by experimental evidence in 1993 by the Slutsky group (Muscedere et al. 1994). Their work in isolated rat lungs demonstrated that repetitive opening and collapse lead to a decrease in lung compliance and injury to the epithelial cells that line small airways and alveolar ducts. This hypothesis was validated by a series of elegant studies by Gaver et al. (Gaver et al. 1990; Naureckas et al. 1994; Bilek et al. 2003). In these experiments (Figure 3), an air bubble was passed through a narrow channel lined with pulmonary epithelial cells in an attempt to simulate the opening of a collapsed airway in a region of atelectatisis. Significant injury to the apical membranes of epithelial cells was demonstrated using a dual fluorescent (ethidium homodimer/calcein) labeling technique 30–150 seconds after the bubble passed over the cell surfaces and through the channel. Effective calcein staining requires the presence of an intact cellular plasma membrane, and conversely ethidium homodimer is impermeant to the plasma membrane and therefore its fluorescence within the cell indicates that membrane disruption has occurred. A number of cells stained positive for both indicators, suggesting that a plasma membrane disruption occurred and was resealed within 2–3 minutes after injury. This timeframe is consistent with previously measured plasma membrane resealing rates (McNeil et al. 1997).
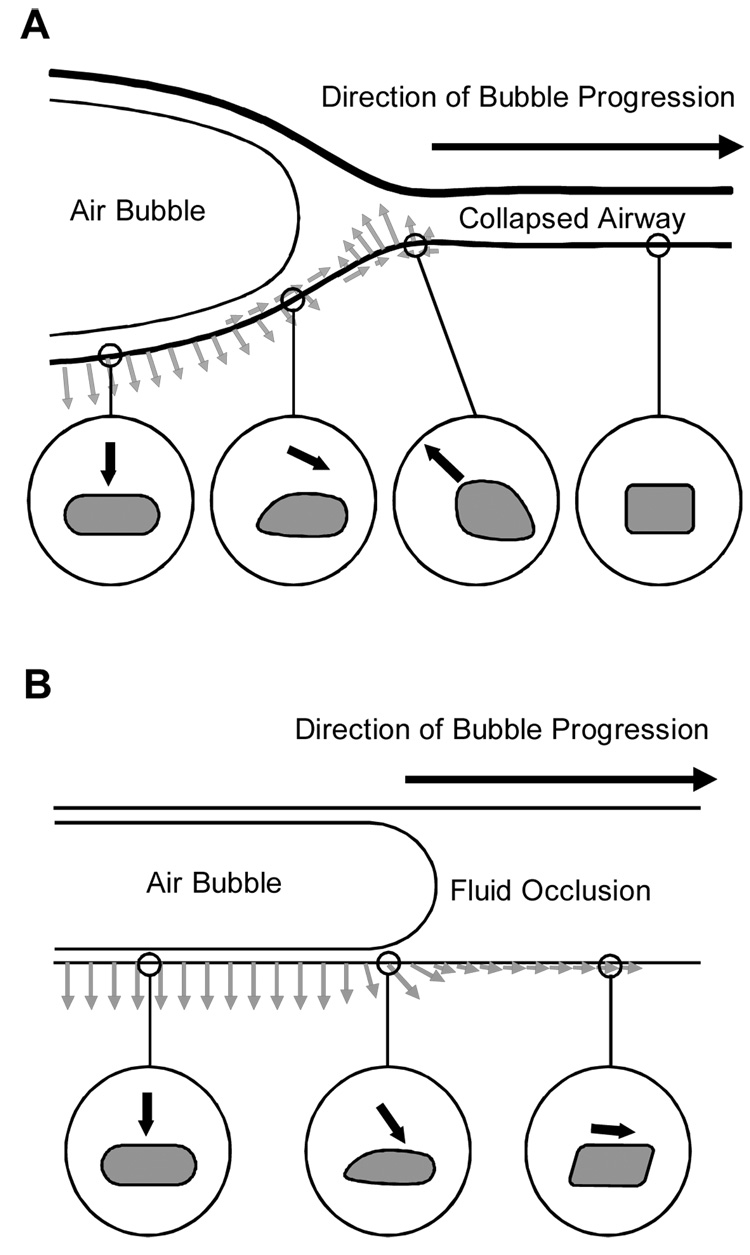
Figure 3.
Hypothetical stresses imparted on the epithelial cells of an airway during reopening (Reprinted with permission from Bilek et al(Bilek et al. 2003)). (A) A collapsed compliant airway shown to the right is forced open by a finger of air moving from left to right. A dynamic wave of stresses is imparted on the airway tissues as the bubble progresses. The circles show the cycle of stresses an airway epithelial cell might experience during reopening. The cell far downstream is nominally stressed. As the bubble approaches, the cell is a pulled up and toward the bubble. As the bubble passes, the cell is pushed away from the bubble. After the bubble has passed, the cell is pushed outward.
(B) A fluid occlusion in a rigid narrow channel is cleared by the progression of a finger of air moving from left to right. A dynamic wave of stresses is imparted on the pulmonary epithelial cells lining the channel wall. The circles show the cycle of stresses that the cells might experience during reopening. Far downstream the cell is pushed forward and slightly out. As the bubble approaches, a sudden rise in pressure and a peak in shear stress occurs, pushing the cell forward and outward with much greater force. After the bubble has passed, the cell is pushed outward.
Mathematical models supported by experimental data suggested that a steep pressure gradient at the leading edge of the bubble front (Bilek et al. 2003) is the most likely cause of epithelial cell damage during reopening. It is determined by dimensions of the collapse segment, the surface tension at the air/liquid interface, the viscosity of the airway fluid and the rate of bubble propagation. Importantly, as the experiments of the Gaver group demonstrate tube collapse is not a prerequisite for this injury mechanism.
Pulmonary surfactant is a complex lipid-protein mixture that stabilizes the lung by reducing the surface tension at the air-liquid interface (Goerke 1998). As early as 1966 Faridy had suggested that mechanical ventilation could impair the surfactant system and lead to lung injury (Faridy et al. 1966). More recent investigation in the isolated rat lung has elucidated two important effects of mechanical ventilation on surfactant. The first is a reduction in total surfactant content and active surfactant protein subsets(Shu et al. 2007) in animals with ALI. The second, is the observation that plasma proteins, which invariably gain access to airspaces of injured lungs alter surfactant kinetics and its biophysical properties (Hartog et al. 2000). Thirdly, large oscillations in alveolar surface area that accompany regional and/or global hyperventilation promote large to small aggregate conversions and loss of functionally competent surfactant (Veldhuizen et al. 2002). Low surface tension is critical for the maintenance of alveolar volume, and aeration surfactant dysfunction and/or inactivation greatly increases the risk of VALI associated cell injury. Surfactant replacement therapy while established as treatment for immature lungs has yet to find a place in the management of adults with ALI. Several synthetic surfactants are currently under investigation. A newer, highly active synthetic surfactant modified with a phospholipase-resistant diether phosphonolipid moiety (DEPN-8) has demonstrated resistance to deactivation by phospholipases in excised rat lungs (Wang et al. 2007), and recent in vitro and in vivo animal studies (Lewis et al. 2006; McCormack 2006) have begun to focus on the emerging anti-inflammatory and antibacterial functions of various surfactant components.
4. Evidence for deformation-induced injury
As early as 1974 Webb and Tierney had observed that rats ventilated with large tidal volumes developed hemorrhagic pulmonary edema (Webb et al. 1974), establishing a link between ventilator-induced injury to the blood-gas barrier and alteration in lung function. Later, Dreyfuss and colleagues (Dreyfuss et al. 1985) demonstrated that tidal volume was a more appropriate determinant of lung injury than peak airway pressure (Dreyfuss et al. 1998) and shortly thereafter the landmark Acute Respiratory Distress Syndrome Network (ARDSNet) trial confirmed the protective benefits of a low tidal volume ventilation strategy on clinical outcomes (Acute Respiratory Distress Syndrome Network, 2000).
The detailed morphological changes to the blood-gas barrier documented by Dreyfuss and colleagues (Dreyfuss et al. 1985) provide convincing electron micrographic evidence for dose- and time-dependent deformation-induced wounding at the cellular level as a result of mechanical ventilation. For example, the development of interstitial edema, plasma membrane blebbing, and loss of cellular contact with the basement membrane occurs after as little as a five minute period of injurious ventilation (Dreyfuss et al. 1998). Longer exposure at the same settings result in increasingly severe markers of cellular injury including inter- and intracellular gap formations, denuded basement membranes, and finally complete cellular destruction.
Electron microscopy provided the initial snapshots of cellular and plasma membrane wounding after injurious ventilation, but did not provide evidence of causality. To further explore this cause-effect relationship, Gajic and colleagues (Gajic et al. 2003) perfused isolated, mechanically ventilated rat lungs with propidium iodide (PI). PI is a plasma membrane impermeant fluorophore utilized in studies of membrane integrity; Its detection within the cell necessarily confirms a plasma membrane disruption has occurred (Figure 4). This series of experiments yielded two important results. First, the number of subpleural cells with membrane defects increased with length of exposure and increasing tidal volume, confirming the dose-dependent qualitative observations under EM. Second, by infusing PI at varying points in the timecourse and then comparing the number of PI positive cells in each, it was inferred that the majority (60%) of injured cells were able to repair the defect in their plasma membranes. These cells remained wholly viable after wounding.
Figure 4.
Propidium iodide imaging of a lung section. Propidium iodide (red color) is impermeable to the plasma membrane (PM). It is able to enter the cell after a PM disruption such as seen under conditions of cellular injury. Once in the cell, it intercalates with nucleic acid, fluorescing red after excitiation at the proper wavelength. The detection of PI necessarily means a PM break has occurred. See text for details.
5. Consequence of deformation-induced injury
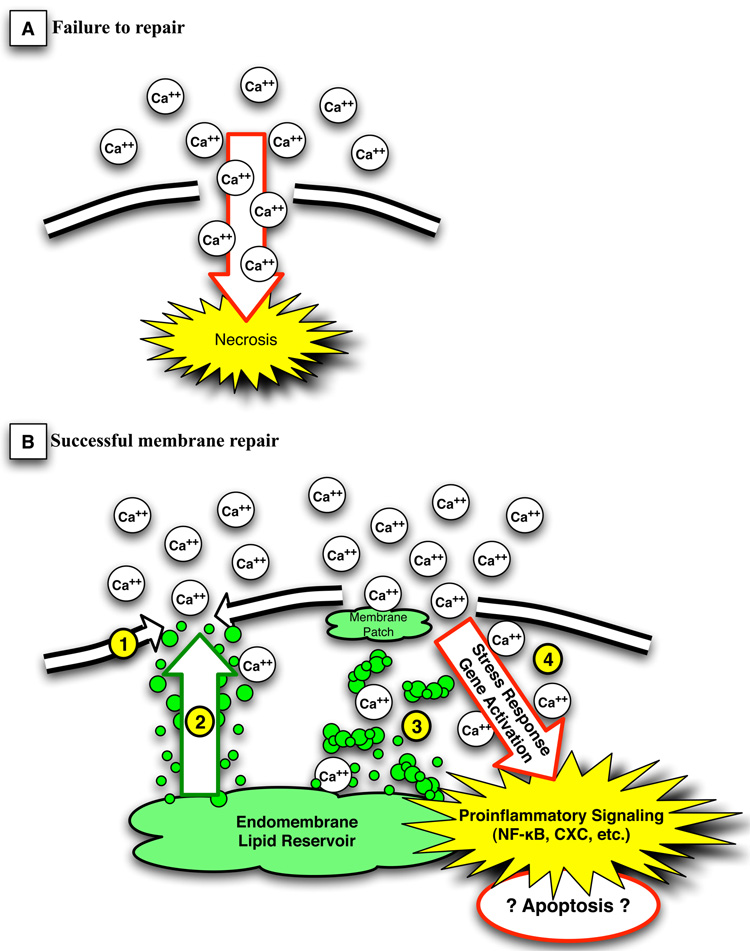
It has been demonstrated that ventilator settings correlate with inflammatory signaling responses (Tremblay et al. 1997), and that the ensuing robust immune response, or “biotrauma” (Tremblay et al. 1998), accounts for the systemic manifestations of ALI. It has been well established that mechanical stress positively regulates the expression of a number of genes, and cellular deformation has been directly implicated in the activation of immunomodulatory transcription factors including nuclear factor kappaB (NF-kappaB), activator protein 1 (AP-1) and cAMP-responsive element binding protein (Kirchner et al. 2005). The mechanism by which physical deformation is transduced has been an area of some debate, and likely is the result of inadequate temporal and spatial resolution of our current analysis tools. For instance, cellular injury appears to play a pivotal role in the activation of these responses, and deformation-induced plasma membrane disruptions have been directly linked to the activation of pro-inflammatory signaling cascades including early stress response genes, chemokine receptors, and adhesion molecules (Grembowicz et al. 1999). In this setting, membrane disruption acts as a mechanotransducer or “damage sensor,” whereby the influx of calcium after membrane injury leads to the upregulation of such mediators as fos and NF-kappaB (Figure 5).
Figure 5.
The two possible fates of a damaged cell.
In panel A, the plasma membrane is not resealed, calcium freely enters the cell, and cell death ensues by cellular necrosis. In panel B, the 3 mechanisms of plasma membrane repair are illustrated. Lateral lipid flow (#1) can fill the smallest defects, and is the primary mechanism in the human red blood cell. Lipid trafficking to the membrane (#2) accounts for the resealing and repair in most smaller membrane disruptions, while the formation of membrane plugs by coalescence of lipid vesicles in the presence of calcium influx fills larger membrane gaps (#3). Membrane disruption and ion flux (#4) can activate important stress response genes and proinflammatory signaling cascades such as NF-kappaB and CXC chemokines. This in turn may lead to prolonged response to the membrane defect or even apoptotic changes, which may result in damage in excess of that caused by necrotic cell death alone (see text for discussion).
Yet similar immune responses have been seen in cells after exposure to mechanical stress but without gross wounding of the plasma membrane or overt signs of injury. Is this really the case, or is it that our detection methods for cellular injury are inadequate? Because rapid plasma membrane resealing is imperative for the viability of the cell, and most survivable plasma membrane disruptions remain open less than 5 seconds (Grembowicz et al. 1999), commonly used methods to detect injury such as labeling studies and dye exclusion tests are inefficient and/or poor indicators in these situations. Moreover, the brevity of disruption suggests a priori that the usefulness of measuring surrogate markers of cellular injury such as lactate dehydrogenase will be quite low. As such, the sensitivity and specificity of our current methods to exclude plasma membrane injury as a driver of inflammation is unclear.
Whether the immune response of a cell that has experienced a sublethal plasma membrane wound with successful repair is different in scale or duration from that of a mortally wounded cell undergoing necrotic cell death is unknown. The comparison is of interest, however, in that the a priori supposition that cellular repair is beneficial may be not hold true in all cases. For instance, the previously described upregulation of immune response genes after plasma membrane wounding and repair may set up a prolonged inflammatory response by the viable cell. This would potentially result in more damage than would have necrotic death, where the loss of the cell would attenuate the inflammatory response. Of course the determination of an intervention’s effect on outcome will be based on the surrogate endpoints measured. One such example surrounds the effects of hypercapnia on cell wounding. Hypercapnia has been demonstrated to protect against, and hypocapnia exacerbate, vascular barrier dysfunction in a number of injury models (Laffey et al. 2000a; Laffey et al. 2000b; Kavanagh 2005). Our group has confirmed the protective effect of hypercapnia as measured by reduced injury-related edema formation in alveolar epithelial cells (Doerr et al. 2005). However, the use of a dual-labeling technique in cells injured by scratch under conditions of hypercapnia revealed a reduced resealing and repair rate when compared to cells injured under normo- or hypocapneic conditions. From this arises a paradox; Hypercapnia appears to have antinflammatory effects, as demonstrated by reduced edema formation, while at the same time leading to more cellular injury by impairing the ability for cell repair.
We have demonstrated previously that deformation-induced lipid trafficking is an important cytoprotective mechanism employed by the cell in the face of externally imposed shape change(Vlahakis et al. 2002). It is integral to the repair of all but the smallest plasma membrane wounds. Elements of this mechanism have been demonstrated to be sensitive to pH and/or PCO2 in other models, which in turn could explain the the inhibitory effects of hypercapnia on wound repair. For instance, low pH has been shown to inhibit endocytic membrane trafficking in secretory cells(Lippincott-Schwartz et al. 1997; Freedman et al. 1998). More recently, resealing of both mechanically wounded cells as well as pores created in several non-lung cell types by bacterial streptolysin O permeabilization was shown to be dependent on endocytosis (Idone et al. 2008). In cells in which endocytosis was inhibited by sterol depletion, repair was inhibited, while the opposite occurred when endocytosis was facilitated by cytoskeleton disrupting agents such as cytochalasin and latrunculin. Whether or not the same occurs in alveolar epithelial cells is yet to be determined, however it appears that the likelihood of plasma membrane repair can be summarized as follows: Plasma membrane regulation is a dynamic process in which both endo- and exocytic mechanisms are continually occurring. The balance of these pathways may be influenced by certain conditions that shift the equilibrium towards the favoring of either a pro-injury or pro-repair state. Certainly the ability to manipulate the tendency of one state over the other provides an attractive therapeutic target, and has driven much of our interest in this area.
6. Cell surface regulation and deformation induced lipid trafficking
Remodeling of the cellular stress-bearing elements and plasma membrane in response to deformation may provide a mechanism by which to minimize the risk of stress-induced structural failure in the short term (Vlahakis et al. 2002) and allow for adaptation to repetitive or chronic stress exposure in the long term. Remodeling is an active, energy requiring process guided by mechanosensing and mechanotransduction elements that include integrins and focal adhesion complexes uniquely positioned to act as gauges of local force and strain for the cell and its environment. Their central role as mechanotransducers is emphasized by the intracellular signal transduction mechanisms to which they are coupled: Tyrosine kinases, phospholipases, GTPases, and matrix metalloproteinases (Vlahakis et al. 2005). Signaling through these systems allows the cell to transduce a sensed, localized physical deformation into a robust, biochemical response.
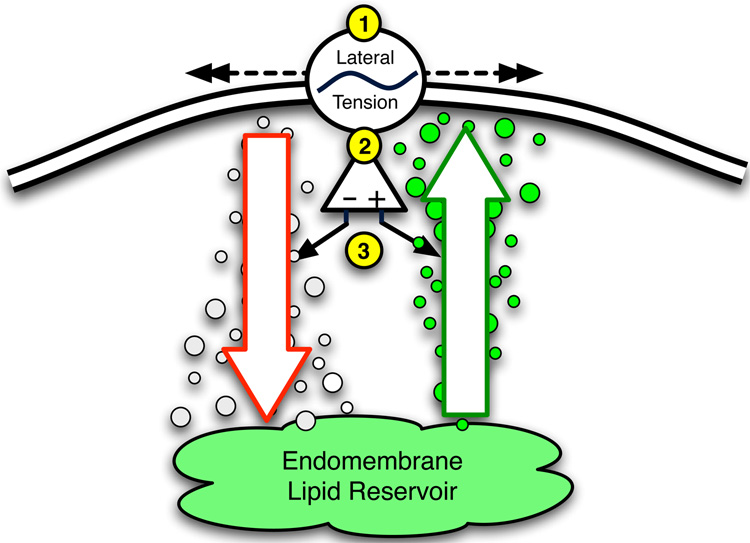
Such an example of a mechanosensing feedback system is the active regulation of plasma membrane surface area to cellular volume in response to strain, reducing the risk of plasma membrane stress failure. This process, illustrated in Figure 6, provides a mechanism for cellular integration of real-time lateral plasma membrane tension readout with responses that modulate the flux between two discreet but related pools: the plasma membrane, and the endomembrane, or intracellular plasma membrane reservoir (Vlahakis et al. 2001; Vlahakis et al. 2002; Fisher et al. 2004). Plasma membrane lateral tension affects the rate of shuttling between these pools, as has been suggested by the exposure of molluscan neurons to hyper- and hypotonic media (Sheetz 2001). In an elegant set of experiments utilizing an optical trap, coated beads were used to “grab” the plasma membrane and pull a pure lipid tether of plasma membrane away from the cell (Figure 7). Force and displacement measurements revealed that after an initial increase in force to pull a tether off the cell, force remained relatively unchanged over a subsequent range of displacement corresponding to a “buffer” of excess plasma membrane. Once this was exhausted, the force per displacement increased rather rapidly until exceeding the capacity of the tweezers. At this point the tether pulled out of the trap and it retracted to the plasma membrane surface. Exposure to hypotonic media resulted in cellular swelling, a subsequent rise in lateral plasma membrane tension, and consequent increase in net total plasma membrane fraction. The latter is interpreted from increasingly larger plateau phases (“buffers”) with each subsequent tether pull from the same cell under hypotonic conditions, whereas under hypertonic conditions the opposite occurred (Raucher et al. 1999).
Figure 6.
Schematic representation of deformation-induced lipid trafficking (DILT). Deformations of the plasma membrane (PM) result in changes in lateral PM tension. Increases in lateral tension lead to an increase in total PM content through a net increase in exocytic lipid trafficking from the endomembrane to PM, while endocytosis and a decrease in total PM predominates under conditions of decreased lateral tension.
Figure 7.
Measuring plasma membrane tension via optically trapped lipid tethers.
The top panel represents a schematic diagram of a plasma membrane (PM) lipid tether being pulled from a cell, while the bottom panel is a representative tracing of the force vs. displacement curve measured in realtime. A 0.5–1.0 micron, coated bead is optically trapped and inserted into the PM (#1). The bead acts as a handle, and as it is moved away from the cell, a lipid “tether.” Note the initial force increase to pull this tether from the PM (#1, lower panel). As the bead is pulled further toward #2, force plateaus over a finite displacement confirming the presence of a “lipid reservoir.” As the reservoir is used up, no further lipid can be trafficked to the membrane to buffer the tether and force begins to increase (#3) until the bead (and tether) are no longer able to be held by the optical trap. The recoil of the tether pulls the bead back to the PM surface (#1). See text for full discussion.
7. Plasma membrane resealing and repair
If wounding of the plasma membrane does occur, successful resealing and repair is imperative for the long-term viability of the cell. There are 3 mechanisms by which the cell may repair plasma membrane defects: Lateral lipid flow, lipid trafficking, and endomembrane patch formation and insertion (Figure 5). Lateral lipid flow is a passive, spontaneous, and rapid (milliseconds to seconds) closure of the lipid bilayer as a result of the thermodynamic drive to maintain the membrane’s phospholipid constituents in their lowest energy configuration. The human red blood cell utilizes this mechanism exclusively. From a historical perspective, however, lateral lipid flow could not explain the repair of larger breaks. The initial hypothesis that lipoprotein “plugs” pulled the injured membranes back together (McNeil 1993) in a calcium dependent manner(Xie et al. 1991) was challenged by a series of experiments in the mid 1990s (reviewed in (McNeil et al. 2003)) that led to the development of our modern concept of plasma membrane resealing via non-exocytic lipid trafficking. As an exhaustive review is beyond the scope of this article, the reader is directed to the excellent discussion of the key historical experiments leading to the current working hypothesis of exocytic cell membrane repair recently published by Steinhardt (Steinhardt 2005).
The concept of exocytosis as a repair mechanism evolved from earlier hypotheses surrounding membrane “plugging” as well as advances in neuronal models of synaptic vesicular targeting that lead to the identification of key mediators of neurotransmitter exocytosis. This body of literature laid the groundwork for understanding the machinery involved in the trafficking of reparative lipid constructs to the damaged plasma membrane. Of these, soluble NSF attachment receptor (SNARE) proteins are an important class of small, mostly plasma membrane-bound proteins that have been shown to mediate and direct vesicle fusion with the membrane in a number of cell types including neuronal cells and fibroblasts (Shen et al. 2005).
The three most studied SNARE proteins are the vesicular-bound syntaxin and synaptobrevin, and the target membrane-bound synaptosome-associated protein of 25,000 daltons (SNAP-25), which become associated, undergo a zipper-like transformation, and cinch the vesicle and membrane together in tight apposition. This primes the complex for release, which occurs by a triggering mechanism involving calcium binding to synaptotagmin resulting in membrane fusion. Once fusion completes, exocytosis occurs by such potential processes as hydration reactions and phase transitions.
The generation and trafficking of vesicles requires non-muscle myosin and is sensitive to disruption of the actin cytoskeleton. When a plasma membrane disruption occurs, calcium entry into the cell will activate this exocytic process. Larger influxes of calcium will also promote the coalescence of vesicular endomembrane, forming a patch that may be targeted en bloc to larger defects. Here it it can fuse with and reseal the plasma membrane (Figure 5).
The response to deformation induced plasma membrane wounding is biphasic. There is an immediate exocytic pathway and delayed response through a non-calcium dependent mechanism. In theory this allows for an initial, rapid response to reverse the injury, while the sustained trafficking may potentiate repair by restoring lipid to the membrane. This, in turn, may be used for lateral lipid flow repair and perhaps to prepare the cell for further deformations. By increasing the number of SNARE bound (but not fused) lipid vesicles to the membrane, the cell is effectively priming the mechanism such that it may respond more quickly to second and subsequent deformations. Moreover, the trafficking of additional lipid vesicles from the intracellular pool to the membrane after deformation may allow for a larger potentiated response upon repeat stimulation. This hypothesis was confirmed in a fibroblast model of repetitive plasma membrane wounding (Shen et al. 2005). Cells were infused with a fluorescent dye, wounded by micropipette, and the leakage of dye as a function of cell fluorescence tracked over time. After the cell was wounded, fluorescence decreased in a negative log fashion. If cells resealed, fluorescence levels stabilized, whereas in mortally wounded cells unable to repair their defect fluorescence decreased to background levels. The time to stabilization of fluorescence then corresponded to the time required for the cell to reseal. Supporting a adaptive, priming mechanism to plasma membrane disruption repeated wounding resulted in a doubling in the resealing rates with subsequent wounds and potentiation of calcium-mediated exocytosis as measured by a calcium sensitive dye. This adaptation mechanism may provide an advantage under conditions of repetitive or increased strain.
8. Manipulation of cellular response to deformation
In the unstressed state, cellular plasma membrane tension is low, and only approaches lytic tension if an externally imposed shape change forces a critical increase in cellular surface area. This increase is usually buffered by unfolding of pleats and recruitment of additional lipid to the plasma membrane as discussed earlier. Because changes in the cellular surface to volume ratio are such a critical determinant of lateral plasma membrane tension, our laboratory has become increasingly interested in the potential protective effects of an osmotic challenge. Exposure of the cell to hyperosmolar conditions leads to a sustained decrease in volume and relative excess in plasma membrane that should allow the cell to tolerate larger deformations with little change in lateral tension and lowered risk of plasma membrane wounding. Indeed experimental evidence from our laboratory in both A549 and rat AT1-like epithelial cells (AT2 cells transdifferentiated in culture for 5–7 days) demonstrate a lower susceptibility to deformation injury and a higher rate of repair under hypertonic conditions, and the opposite when exposed to a hypotonic milieu (Lee et al. 2007). Also, wounds formed under hypertonic conditions are likely smaller and contain a more tightly woven actin network at their base, making them faster and easier to reseal and repair.
While this data clearly demonstrates that hypertonic conditioning protects cells from physical stress failure, the effects of osmotic challenge are more complex than suggested by shape and volume change alone. Both cytoskeletal-plasma membrane interaction as well as cytoskeletal remodeling responses have been documented (Fuller et al. 1999; Parsegian et al. 2000; Pedersen et al. 2001; Despa 2005; Di Ciano-Oliveira et al. 2006).. The cytoskeleton is a complex network of biopolymers that behave like a hydrated gel, and the constituent polymers attract and order water molecules in a charge and surface topology dependent manner. In so doing, the cytoskleton competes for bulk water with other solutes inside the cell, effectively regulating water activity. In turn cytosolic water activity determines the relative hydration of membrane channels, enzymes, and DNA. The exposure of a cell to a hypertonic stress reduces water activity, and favors a state of cytoskeletal polymerization.
We have started to investigate some of these complex interactions by utilizing an optical trapping system similar to that of Sheetz to pull lipid tethers from A549, AT-1 and AT-2 cells exposed to hyper- and hypotonic conditions. During the course of the experiment tether retractive force is continually recorded in real-time over the range of displacement (Figure 7). Raucher and Sheetz demonstrated that molluscan neuron and 3T3 fibroblasts plasma membrane tension is primarily a result of lateral tension in the membrane, as indicated by a drop in tether retractive force to hypertonic cell shrinkage and increase in hypotonic cell swelling. Although we have confirmed Raucher and Sheetz results in 3T3 fibroblasts, our preliminary findings suggest that airway epithelial cells have a more complicated response that includes a significant contribution from interactions between the plasma membrane and underlying cytoskeleton. In these cells we see a complex response to hypertonic stress, with an immediate drop in force indicative of a decrease in lateral plasma membrane tension, that quickly progresses to a sustained increase over the next few minutes. This latter part of this biphasic response is inhibitable by cytoskeletal disruptive agents such as cytochalasin D or latrunculin, implicating a major role for cytoskeletal-plasma membrane interactions in AT-1,2, and A549 cells.
Evidence from the literature further suggests the protective effects of an osmotic challenge. For example, the immune-modulatory effect of hypertonic saline on polymorphonuclear neutrophils through decreased expression of adhesion molecules has motivated its use in the resuscitation of trauma victims with shock. Moreover, perfusion of the pulmonary circulation with sucrose promotes endothelial cytoskeletal polymerization, enhances vascular barrier properties and blocks pro-inflammatory secretory processes.
Osmotic challenge also results in the alteration of the biomechanical properties of cellular stress bearing elements. For example, cell shrinkage and swelling increase and decrease F actin content, respectively, and we have confirmed an increase in F-actin assembly in A549 and AT-1 like cells exposed to hypertonic stress in our laboratory (Hubmayr et al, unpublished results). These changes are associated with an increased stress tolerance of the subcortical cytoskeleton and therefore a decreased likelihood that the constituents reach lytic tensions.
9. Summary and conclusion
We have examined the mechanisms of cellular injury and repair relevant to mechanical ventilation and ALI. Central to the sequelae of VALI is cellular deformation, which can cause a robust innate immune response even at sublytic tensions in the absence of injury. The cell is able to respond to deformation-induced changes in lateral plasma membrane tension through a rapid, active trafficking of lipid constructs between the plasma membrane and its intracellular reservoir in order to maintain sublytic plasma membrane tension. However, if deformation exceeds the buffering capacity of this system, lytic tensions are reached and plasma membrane wounding will occur. Whether by overdistension or low-volume injury, resealing and repair of the defect must occur to assure the continued viability of the cell. This process is an active, orchestrated mechanism of non-exocytic vesicular trafficking that appears to be potentiated by secretagogues.
Numerous studies have attempted to parse potential therapeutic targets from the complex immune response mediators that are activated by deforming stress. In our laboratory we have taken a different approach, attempting to target the cell itself, instead of its downstream response elements that harken to the proverbial horse-already-out-of-the-barn. Our method has been to utilize an osmotic intervention with the goal of altering the sensitivity of cells to deformation, thereby preventing the deleterious responses normally seen. Current data suggests that hyperosmolar exposure may decrease the risk of deformation-induced wounding by altering the hydration state and therefore biomechanical properties of the cytoskeleton such that inherent lytic tensions are increased.
Finallly, missing from the evidence presented were confirmatory in vivo measurements and observations. For instance, questions surrounding small-scale intra-acinar stress and strain distributions remain unanswered by our current methodologies, yet technological advances with regard to temporal and spatial resolution are slowly coming on line and should begin to fill these important gaps. These measurements will become increasingly important as targeted genomic and physiomic interventions attempt to alter more and more esoteric experimental variables. Perhaps the bench may be able to supply feedback on this sort of treatment progress before such overt and tangible pathologic indicators of lung injury such as infiltrates, inflammation, or edema are obvious to the clinician at the bedside.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- Bachofen H, Schurch S. Alveolar surface forces and lung architecture. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:183–193. doi: 10.1016/s1095-6433(01)00315-4. [DOI] [PubMed] [Google Scholar]
- Bilek AM, Dee KC, Gaver DP., 3rd Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol. 2003;94:770–783. doi: 10.1152/japplphysiol.00764.2002. [DOI] [PubMed] [Google Scholar]
- Caruso P, Meireles SI, Reis LF, Mauad T, Martins MA, Deheinzelin D. Low tidal volume ventilation induces proinflammatory and profibrogenic response in lungs of rats. Intensive Care Med. 2003;29:1808–1811. doi: 10.1007/s00134-003-1908-7. [DOI] [PubMed] [Google Scholar]
- Cavalcante FS, Ito S, Brewer K, Sakai H, Alencar AM, Almeida MP, Andrade JS, Jr, Majumdar A, Ingenito EP, Suki B. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol. 2005;98:672–679. doi: 10.1152/japplphysiol.00619.2004. [DOI] [PubMed] [Google Scholar]
- Despa F. Biological water: Its vital role in macromolecular structure and function. Ann N Y Acad Sci. 2005;1066:1–11. doi: 10.1196/annals.1363.023. [DOI] [PubMed] [Google Scholar]
- Di Ciano-Oliveira C, Thirone AC, Szaszi K, Kapus A. Osmotic stress and the cytoskeleton: the R(h)ole of Rho GTPases. Acta Physiol (Oxf) 2006;187:257–272. doi: 10.1111/j.1748-1716.2006.01535.x. [DOI] [PubMed] [Google Scholar]
- Doerr CH, Gajic O, Berrios JC, Caples S, Abdel M, Lymp JF, Hubmayr RD. Hypercapnic acidosis impairs plasma membrane wound resealing in ventilator-injured lungs. Am J Respir Crit Care Med. 2005;171:1371–1377. doi: 10.1164/rccm.200309-1223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss D, Basset G, Soler P, Saumon G. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis. 1985;132:880–884. doi: 10.1164/arrd.1985.132.4.880. [DOI] [PubMed] [Google Scholar]
- Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- Faridy EE, Permutt S, Riley RL. Effect of ventilation on surface forces in excised dogs' lungs. J Appl Physiol. 1966;21:1453–1462. doi: 10.1152/jappl.1966.21.5.1453. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Levitan I, Margulies SS. Plasma membrane surface increases with tonic stretch of alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31:200–208. doi: 10.1165/rcmb.2003-0224OC. [DOI] [PubMed] [Google Scholar]
- Freedman SD, Kern HF, Scheele GA. Acinar lumen pH regulates endocytosis, but not exocytosis, at the apical plasma membrane of pancreatic acinar cells. Eur J Cell Biol. 1998;75:153–162. doi: 10.1016/S0171-9335(98)80057-5. [DOI] [PubMed] [Google Scholar]
- Fuller N, Rand RP. Water in actin polymerization. Biophys J. 1999;76:3261–3266. doi: 10.1016/S0006-3495(99)77478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. Biomechanics: Mechanical Properties of Living Tissues. New York: Springer-Verlag; 1993. [Google Scholar]
- Gajic O, Lee J, Doerr CH, Berrios JC, Myers JL, Hubmayr RD. Ventilator-induced cell wounding and repair in the intact lung. Am J Respir Crit Care Med. 2003;167:1057–1063. doi: 10.1164/rccm.200208-889OC. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M. Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis. 1987;136:730–736. doi: 10.1164/ajrccm/136.3.730. [DOI] [PubMed] [Google Scholar]
- Gaver DP, 3rd, Samsel RW, Solway J. Effects of surface tension and viscosity on airway reopening. J Appl Physiol. 1990;69:74–85. doi: 10.1152/jappl.1990.69.1.74. [DOI] [PubMed] [Google Scholar]
- Goerke J. Pulmonary surfactant: functions and molecular composition. Biochim Biophys Acta. 1998;1408:79–89. doi: 10.1016/s0925-4439(98)00060-x. [DOI] [PubMed] [Google Scholar]
- Grembowicz KP, Sprague D, McNeil PL. Temporary disruption of the plasma membrane is required for c-fos expression in response to mechanical stress. Mol Biol Cell. 1999;10:1247–1257. doi: 10.1091/mbc.10.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartog A, Gommers D, Haitsma JJ, Lachmann B. Improvement of lung mechanics by exogenous surfactant: effect of prior application of high positive end-expiratory pressure. Br J Anaesth. 2000;85:752–756. doi: 10.1093/bja/85.5.752. [DOI] [PubMed] [Google Scholar]
- Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180:905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh BP. Therapeutic hypercapnia: careful science, better trials. Am J Respir Crit Care Med. 2005;171:96–97. doi: 10.1164/rccm.2410003. [DOI] [PubMed] [Google Scholar]
- Kirchner EA, Mols G, Hermle G, Muehlschlegel JD, Geiger KK, Guttmann J, Pahl HL. Reduced activation of immunomodulatory transcription factors during positive end-expiratory pressure adjustment based on volume-dependent compliance in isolated perfused rabbit lungs. Br J Anaesth. 2005;94:530–535. doi: 10.1093/bja/aei078. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Kavanagh BP. Biological effects of hypercapnia. Intensive Care Med. 2000a;26:133–138. doi: 10.1007/s001340050027. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Tanaka M, Engelberts D, Luo X, Yuan S, Tanswell AK, Post M, Lindsay T, Kavanagh BP. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med. 2000b;162:2287–2294. doi: 10.1164/ajrccm.162.6.2003066. [DOI] [PubMed] [Google Scholar]
- Lee HB, Rasmussen DL, Wang SH, Lee WY, Mullon JJ, Walters BJ, Stroetz RW, Hubmayr RD. Osmotic Effects on Mechanical Alveolar Epithelial Cell Deformation Injury and Repair. ATS. 2007 [Google Scholar]
- Lewis JF, Veldhuizen RA. The future of surfactant therapy during ALI/ARDS. Semin Respir Crit Care Med. 2006;27:377–388. doi: 10.1055/s-2006-948291. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Smith CL. Insights into secretory and endocytic membrane traffic using green fluorescent protein chimeras. Curr Opin Neurobiol. 1997;7:631–639. doi: 10.1016/s0959-4388(97)80082-7. [DOI] [PubMed] [Google Scholar]
- Maunder RJ, Shuman WP, McHugh JW, Marglin SI, Butler J. Preservation of normal lung regions in the adult respiratory distress syndrome. Analysis by computed tomography. JAMA. 1986;255:2463–2465. [PubMed] [Google Scholar]
- McCormack FX. New concepts in collectin-mediated host defense at the air-liquid interface of the lung. Respirology. 2006;11 Suppl:S7–S10. doi: 10.1111/j.1440-1843.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- McNeil PL. Cellular and molecular adaptations to injurious mechanical stress. Trends Cell Biol. 1993;3:302–307. doi: 10.1016/0962-8924(93)90012-p. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Loss, restoration, and maintenance of plasma membrane integrity. J Cell Biol. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149:1327–1334. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- Naureckas ET, Dawson CA, Gerber BS, Gaver DP, 3rd, Gerber HL, Linehan JH, Solway J, Samsel RW. Airway reopening pressure in isolated rat lungs. J Appl Physiol. 1994;76:1372–1377. doi: 10.1152/jappl.1994.76.3.1372. [DOI] [PubMed] [Google Scholar]
- Parsegian VA, Rand RP, Rau DC. Osmotic stress, crowding, preferential hydration, and binding: A comparison of perspectives. Proc Natl Acad Sci U S A. 2000;97:3987–3992. doi: 10.1073/pnas.97.8.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Hoffmann EK, Mills JW. The cytoskeleton and cell volume regulation. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:385–399. doi: 10.1016/s1095-6433(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Raucher D, Sheetz MP. Characteristics of a membrane reservoir buffering membrane tension. Biophys J. 1999;77:1992–2002. doi: 10.1016/S0006-3495(99)77040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SP, Hassoun PM, Brower R. Redox imbalance and ventilator-induced lung injury. Antioxid Redox Signal. 2007;9:2003–2012. doi: 10.1089/ars.2007.1770. [DOI] [PubMed] [Google Scholar]
- Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest. 2007;131:554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- Santos CC, Zhang H, Liu M, Slutsky AS. Bench-to-bedside review: Biotrauma and modulation of the innate immune response. Crit Care. 2005;9:280–286. doi: 10.1186/cc3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MJ, Haitsma JJ, Zhang H, Slutsky AS. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia--a review. Crit Care Med. 2006;34:871–877. [PubMed] [Google Scholar]
- Setnikar I. [Origin and significance of the mechanical property of the lung.] Arch Fisiol. 1955;55:349–374. [PubMed] [Google Scholar]
- Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2:392–396. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- Shen SS, Tucker WC, Chapman ER, Steinhardt RA. Molecular regulation of membrane resealing in 3T3 fibroblasts. J Biol Chem. 2005;280:1652–1660. doi: 10.1074/jbc.M410136200. [DOI] [PubMed] [Google Scholar]
- Shu LH, Xue XD, Liu CF, Han XH, Wu HM, Shang YX, Cai XX, Wei KL. Effect of dexamethasone on the content of pulmonary surfactant protein D in young rats with acute lung injury induced by lipopolysaccharide. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:155–158. [PubMed] [Google Scholar]
- Stamenovic D. Micromechanical foundations of pulmonary elasticity. Physiol Rev. 1990;70:1117–1134. doi: 10.1152/physrev.1990.70.4.1117. [DOI] [PubMed] [Google Scholar]
- Steinhardt RA. The mechanisms of cell membrane repair: A tutorial guide to key experiments. Ann N Y Acad Sci. 2005;1066:152–165. doi: 10.1196/annals.1363.017. [DOI] [PubMed] [Google Scholar]
- Stromberg DD, Wiederhielm CA. Viscoelastic description of a collagenous tissue in simple elongation. J Appl Physiol. 1969;26:857–862. doi: 10.1152/jappl.1969.26.6.857. [DOI] [PubMed] [Google Scholar]
- Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol. 2005;98:1892–1899. doi: 10.1152/japplphysiol.01087.2004. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay LN, Slutsky AS. Ventilator-induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians. 1998;110:482–488. [PubMed] [Google Scholar]
- Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol. 1999;86:2026–2033. doi: 10.1152/jappl.1999.86.6.2026. [DOI] [PubMed] [Google Scholar]
- Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med. 2000;162:357–362. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- Tsuchida S, Engelberts D, Peltekova V, Hopkins N, Frndova H, Babyn P, McKerlie C, Post M, McLoughlin P, Kavanagh BP. Atelectasis causes alveolar injury in nonatelectatic lung regions. Am J Respir Crit Care Med. 2006;174:279–289. doi: 10.1164/rccm.200506-1006OC. [DOI] [PubMed] [Google Scholar]
- Veldhuizen RA, Welk B, Harbottle R, Hearn S, Nag K, Petersen N, Possmayer F. Mechanical ventilation of isolated rat lungs changes the structure and biophysical properties of surfactant. J Appl Physiol. 2002;92:1169–1175. doi: 10.1152/japplphysiol.00697.2001. [DOI] [PubMed] [Google Scholar]
- Vlahakis NE, Hubmayr RD. Cellular stress failure in ventilator-injured lungs. Am J Respir Crit Care Med. 2005;171:1328–1342. doi: 10.1164/rccm.200408-1036SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Deformation-induced lipid trafficking in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L938–L946. doi: 10.1152/ajplung.2001.280.5.L938. [DOI] [PubMed] [Google Scholar]
- Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Role of deformation-induced lipid trafficking in the prevention of plasma membrane stress failure. Am J Respir Crit Care Med. 2002;166:1282–1289. doi: 10.1164/rccm.200203-207OC. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chang Y, Schwan AL, Notter RH. Activity and inhibition resistance of a phospholipase-resistant synthetic surfactant in rat lungs. Am J Respir Cell Mol Biol. 2007;37:387–394. doi: 10.1165/rcmb.2006-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- Weibel DB, Siegel AC, Lee A, George AH, Whitesides GM. Pumping fluids in microfluidic systems using the elastic deformation of poly(dimethylsiloxane) Lab Chip. 2007;7:1832–1836. doi: 10.1039/b714664g. [DOI] [PubMed] [Google Scholar]
- Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- Wilson T. Solid Mechanics. In: Fishman A, editor. Handbook of Physiology. Baltimore, MD: The Williams and Wilkins Co; 1986. pp. 35–40. [Google Scholar]
- Wilson TA, Bachofen H. A model for mechanical structure of the alveolar duct. J Appl Physiol. 1982;52:1064–1070. doi: 10.1152/jappl.1982.52.4.1064. [DOI] [PubMed] [Google Scholar]
- Xie XY, Barrett JN. Membrane resealing in cultured rat septal neurons after neurite transection: evidence for enhancement by Ca(2+)-triggered protease activity and cytoskeletal disassembly. J Neurosci. 1991;11:3257–3267. doi: 10.1523/JNEUROSCI.11-10-03257.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]