SUMMARY
The biochemical mechanisms that underlie hypoxia-induced NF-κB activity have remained largely undefined. We found that prolonged hypoxia-induced NF-κB activation is restricted to cancer cell lines infected with high risk human papilloma virus (HPV) serotypes. The HPV-encoded E6 protein is necessary and sufficient for prolonged hypoxia-induced NF-κB activation in these systems. The molecular target of E6 in the NF-κB pathway is the CYLD lysine 63 (K63) deubiquitinase, a negative regulator of the NF-κB pathway. Specifically, hypoxia stimulates E6-mediated ubiquitination and proteasomal degradation of CYLD. Given the established role of NF-κB in human carcinogenesis, these findings provide a potential molecular/viral link between hypoxia and the adverse clinical outcomes observed in HPV-associated malignancies.
SIGNIFICANCE
Intratumoral hypoxia is a critical factor in the poor clinical outcomes of human malignancies, most notably cervix and head and neck cancers. We found that prolonged hypoxia-induced NF-κB activation is not a generalized phenomenon amongst cancer cells but rather is restricted to human papilloma (HPV)-positive cancers, such as cervix and head and neck cancers. Under hypoxic conditions, the HPV-encoded E6 protein inactivates the CYLD tumor suppressor, a negative regulator of the NF-κB pathway and thereby allows for unrestricted activation of NF-κB. Because NF-κB-induced genes promote survival, proliferation, and angiogenesis, our findings illustrate how a common human virus adapts to hypoxia and helps account for the aggressive tumor biology associated with hypoxia.
INTRODUCTION
It has been over 50 years since the seminal observation by Thomlinson and Gray that intratumoral hypoxia is associated with resistance to radiation therapy (Thomlinson and Gray, 1955). In the ensuing decades, mounting clinical and experimental evidence has established the influence of hypoxia on tumor biology. For example, the identification of intratumoral hypoxia in patients with cervical and head and neck cancer is associated with an increased risk for local recurrence after radiation, the presence of lymphatic and hematogenous metastases, and reduced overall survival (Tatum et al., 2006). Moreover, hypoxia is associated with resistance to not only radiation therapy but also cytotoxic chemotherapy (Harris, 2002; Le et al., 2004; Subarsky and Hill, 2003). Additional investigations have further extended the importance of hypoxia in the malignant progression of other tumor models, such as sarcomas, breast cancer and prostate cancer (Tatum et al., 2006; Vaupel et al., 2001; Vaupel et al., 2002).
As a solid tumor grows, hypoxia invariably occurs as a consequence of aberrant neo-angiogenesis, cancer associated anemia that results in reduced oxygen carrying capacity of blood, increased oxygen demand of the growing tumor, and abnormal oxygen diffusion due to imbalances in directional microcirculation (Hockel and Vaupel, 2001). In addition to reduced oxygen tension, the hypoxic environment is also characterized by acidosis and diminished micronutrient availability. Thus, during carcinogenesis, pre-malignant and malignant cells must adapt to the harsh hypoxic microenvironment and does so through both genomic and non-genomic mechanisms (Hockel and Vaupel, 2001). Indeed, hypoxia results in genomic instability manifested by increased rates of gene amplification, point mutation, and chromosomal rearrangement that add to the genomic complexity of tumor cells (Hockel and Vaupel, 2001; Reynolds et al., 1996; Young et al., 1988). Through selection pressures, this genomic instability leads to outgrowth of clones which manifest survival and even proliferation advantages as well as resistance to anti-neoplastic therapy.
Non-genomic cellular adaptations to hypoxia have been more well studied and include upregulation of angiogenesis, oxygen transport, glycolysis, and glucose uptake (Harris, 2002). Many of these adaptations are mediated by the transcription factor hypoxia-inducible alpha (HIFα), which drives expression of hypoxia-response genes, such as vascular endothelial growth factor. In addition to HIFα, other transcription factors have been reported to be activated by hypoxia. For example, hypoxia-induced activation of the NF-κB family of transcription factors has been observed in some tumor models (Koong et al., 1994; Royds et al., 1998). NF-κB is a family of transcription factors that induces a transcriptional response that results in the expression of proteins that promote survival, proliferation, angiogenesis, invasion and metastasis (Baldwin, 2001), .Accordingly, hypoxia-induced NF-κB activation represents another potential mechanism whereby tumor cells could adapt to the inhospitable hypoxic milieu.
Whereas constitutive NF-κB activity has been implicated in the malignant progression of numerous hematologic and solid malignancies (Basseres and Baldwin, 2006), a comprehensive analysis of the timing and duration of hypoxia-induced NF-κB activation has not been performed to our knowledge. Moreover, the biochemical mechanisms that underlie hypoxia-induced NF-κB activity have remained largely undefined. In the current study, we observed that prolonged hypoxia-induced NF-κB activation was restricted to HPV-positive cancer cells and was mediated by an effect of the HPV-encoded E6 protein on polyubiquitination and subsequent degradation of the CYLD K63 deubiquitinase.
RESULTS
Prolonged hypoxia-induced NF-κB activation is restricted to HPV-infected cell types
Although hypoxia-induced NF-κB activation has been reported in various cell systems, a thorough analysis of the timing and extent of hypoxia-induced NF-κB activation across a wide range of malignant cell types has not been undertaken. We performed electrophoretic mobility shift assays (EMSAs) to screen 32 human cancer cell lines of epithelial or mesenchymal origin for hypoxia-induced (1% O2) NF-κB activation. Only 4/32 cell lines exhibited hypoxia-induced NF-κB activation at 24 or 48 hour time points, and all four of these cell lines represented squamous cell carcinomas of the cervix (HeLa, SiHa, and Me180; n =3) or head and neck (HEp2; n = 1), all of which are infected with high-risk HPV serotypes (Figure 1A and Table S1). Strikingly, in HPV-negative cervix (HT3 and C33A) and head and neck cancer cell lines (CAL27), hypoxia resulted either in no change or a decrease in NF-κB activity (Figure 1A, and Table S1). These results were confirmed by transient transfection of a NF-κB driven reporter (Figure 1b and Supplementary Table 1). Electrophoretic mobility supershift analyses revealed that the NF-κB complexes were composed of p65 and p50, components of the “classical” NF-κB pathway (Figure 1C). Of note, the p65 antibody employed for this assay did not retard the migration of the band representing p65-p50 heterodimers, but rather prevented p65-p50 heterodimers from binding to the radiolabeled NF-κB oligonucleotide probe and therefore resulted in a reduced signal attributable to p65-p50 DNA binding (Lee and Ziegler, 2007).
Figure 1. Effects of hypoxia and reoxygenation on NF-κB activation.
A. Representative EMSAs for measurement of NF-κB activity under normoxic (21% 02) and hypoxic (1% 02) conditions for the indicated times. WT and M = cold-competition with 100-fold molar excess of wild-type and mutant NF-κB DNA response elements, respectively. Red = HPV-positive squamous cell carcinoma; blue = HPV-negative squamous cell carcinoma; black = non-squamous cell carcinoma. B. NF-κB reporter assays performed 48 hrs after transfection. N = normoxia; H = hypoxia. Results are averages of three experiments ± standard deviation (s.d.). Firefly luciferase expression was normalized to that of Renilla luciferase (see Methods). C. Electrophoretic mobility supershift assays were performed with the indicated antibodies after cells were exposed to hypoxia for 24 hours. D. Top panels: EMSAs demonstrate detailed time course of hypoxia-induced NF-κB activity. Second row: Oct-1 EMSAs serves as a control. Third row: HIF1α Western blots confirm exposure to hypoxia. Bottom row: γ-tubulin Westerns as loading controls. E. Effects of reoxygenation on NF-κB activity (measured by EMSA). EMSAs for AP1 or Oct-1 served as controls. Cells were exposed to hypoxia for 24 hrs followed by return to normoxic conditions (reoxygenation) for the indicated times. F. Transient hypoxia-induced NF-κB activation in HPV-negative cancer cells. Top panels: Shorter course hypoxia exposure (1 hr) of HPV-negative cancer cells. Bottom panels: detailed time course of transient NF-κB activation.
We evaluated HPV-positive and –negative tumor xenografts for hypoxia-induced NF-κB activation. HeLa (HPV18-positive) and CAL27 (HPV-negative squamous cell carcinoma of the head and neck) subcutaneous xenografts were harvested from the flanks of nude mice one hour after intraperitoneal injection of the hypoxia marker pimonidazole. In HeLa xenografts, regions of hypoxia, as detected by pimonidazole staining, were associated with nuclear p65 staining, whereas relatively non-hypoxic regions manifested predominantly cytoplasmic p65 expression (Figure S1A). We did not observe any nonspecific staining by a control antibody of the same isotype (rabbit IgG) as the p65 antibody (data not shown). In contrast, p65 nuclear staining was actually reduced in hypoxic regions in CAL27 xenografts (Figure S1B), a result which is concordance with the diminished NF-κB activation observed in CAL27 cells in vitro (Figure 1A).
A detailed time course analysis of HPV-positive HeLa and SiHa (HPV serotype 16) cells revealed that hypoxia-induced NF-κB activation occurred rapidly (i.e. within 15 minutes) and was sustained for at least 48 hours (Figure 1D, top panels). Hypoxia did not affect the electrophoretic mobility of AP1 or Oct-1 complexes (Figures 1D and 1E), indicating that hypoxia-induced transcription factor activation is not a generalized phenomenon. When hypoxic HPV-positive cells were returned to normoxia (21% O2), NF-κB levels gradually declined (Figure 1E). Specifically, NF-κB activity was maintained for several hours prior to a return to the baseline level of NF-κB activity exhibited at normoxia prior to initial exposure to hypoxic conditions. Some HPV-negative cell lines exhibited a modest degree of transient hypoxia-induced NF-κB activation that abated after three hours (Figure 1F). For example, DLD-1 cells, an HPV-negative colon cancer cell line, exhibited activation of NF-κB after one and three hours of hypoxia exposure, but at later time points cells no longer demonstrated heightened NF-κB activity. Taken together, these data indicate that prolonged hypoxia-induced NF-κB activation is restricted to HPV-positive cancer cell models.
Activation of NF-κB is typically mediated by biochemical signaling events that converge on the I kappa B kinase (IKK) complex, which consists of the IKKγ (NEMO), IKKα and IKKβ isoforms (Karin, 2006; Chen, 2005). Whereas IKKγ is the essential regulatory unit of the IKK complex, IKKαand IKKβ are catalytic subunits that are operative in the “alternative” and “classical” NF-κB pathways, respectively, and mediate NF-κB activation by phosphorylating IκB inhibitory proteins, thereby targeting IκB for ubiquitination and subsequent proteasomal degradation. Because our electrophoretic mobility supershift experiments indicated that hypoxia-induced NF-κB activation involved members of the classical NF-κB pathway, we evaluated the effects of hypoxia on IKKβ activity. Prolonged hypoxia (24 hours) induced IKKβ kinase activity in HeLa and SiHa cells (Figure S2A). We also investigated the timing of IKKβ activation in HPV-positive and –negative cell models. In HPV-positive cells (HeLa and SiHa), hypoxia-induced IKKβ occurred rapidly and was sustained (Figure S2B, top panels). In contrast, in HPV-negative cells, hypoxia either failed to induce IKKβ altogether (C33A) or resulted in transient IKKβ activation at one hour that returned to baseline levels after four hours (HT3 and DLD-1 colon cancer cells; Figure S2B, bottom panels). The timing of IKKβ activation correlated with the timing of NF-κB activation observed in the gel shift assays (Figures 1D and F) and further establishes the model that the phenomenon of prolonged hypoxia-induced activation of the NF-κB pathway is restricted to HPV-positive tumor cells.
HPV E6 protein is necessary and sufficient for prolonged hypoxia-induced NF-κB activation
Next, we explored the hypothesis that an HPV-encoded protein mediates prolonged hypoxia-induced NF-κB activation. Only two HPV-encoded proteins, E6 and E7, are expressed in HPV-positive squamous cell carcinomas (Munger et al., 2004). The E6 and E7 proteins inactivate and inhibit the expression of the p53 and pRb tumor suppressor proteins, respectively, although p53- and pRB-independent oncogenic functions of these HPV-encoded proteins have been well-documented (Munger et al., 2004; Scheffner and Whitaker, 2003). To investigate the effects of E6 and E7 on hypoxia-induced NF-κB activation, we performed RNA interference (RNAi) with small interfering RNAs (siRNAs) to silence expression of the E6 and E7 proteins in HeLa cells. Because E6 and E7 are expressed as a bicistronic transcript, RNAi that is specific for either E6 or E7 have variably been reported to silence expression of one or both of the proteins (Butz et al., 2003; Favre-Bonvin et al., 2005; Jiang and Milner, 2002). E6 siRNA selectively induced p53 expression in HeLa cells (E6 cannot be detected by direct immunoblotting, so p53 expression serves as a surrogate for E6 expression; Figure 2A, middle set of panels), but did not alter E7 or pRB levels (Figure 2A, panels on right and second from left). Scrambled control siRNAs had no effect on E6 or E7 expression. Silencing of E6 was associated with inhibition of hypoxia-induced NF-κB activation, as well as reduction in the constitutive NF-κB activity observed at normoxia, whereas control siRNA did not affect NF-κB activity (Figures 2B and 2C). The finding of increased NF-κB activity in E6-silenced hypoxic versus normoxic cells suggests the possibility of E6-independent mechanisms that may mediate a component of the prolonged hypoxia-induced NF-κB activation observed in HPV-positive cells. Similar results were obtained for E6 silencing in SiHa cells (Figure S3). To further demonstrate specificity of the effects of E6 siRNA for HPV-positive cells, we demonstrated that E6 siRNA did not influence constitutive or transient hypoxia-induced NF-κB activation observed in HPV-negative DLD-1 cells (Figure S4). RNAi-based silencing of E7 expression in HeLa cells also resulted in a decrease in hypoxia-induced and constitutive NF-κB activation (Figures 2B and 2C). However, we cannot attribute this effect to loss of E7 expression, because E7 RNAi led not only to reduction in E7 levels with an associated increase in pRB expression, but also to silencing of E6 expression (Figure 2A).
Figure 2. HPV E6 is necessary and sufficient for prolonged hypoxia-induced NF-κB activation.
A. Western blots for p53, E7 and pRB (a surrogate for E7 expression). Cells were transfected with the indicated siRNAs and immunoblotting was performed with the indicated antibodies after 48 hours. C = scrambled control siRNA. B. EMSAs show the effects of E6 and E7 RNAi on NF-κB activity. C. Same as B, but NF-κB activity measured by reporter assay performed under hypoxic conditions. Results were normalized to that of the C siRNA. D. NF-κB reporter activity due to transfection of HPV16 E6 under hypoxic conditions. Cells were exposed to normoxia or hypoxia for 48 hours and results are represented as the ratio of NF-κB reporter activity in hypoxia:normoxia. E. NF-κB reporter activity resulting from transfection of E6 plus increasing concentrations of E7. Results calculated as in D. F. NF-κB reporter activity resulting from transfection of HPV16 E6 or E7. G. NF-κB reporter activity resulting from transfection of E6 plus increasing amounts of E7. Results for C through G are averages of three experiments ± s.d. In D through G, the results were normalized to that of the vector controls, and firefly luciferase expression was normalized to that of Renilla luciferase (see Methods). H. EMSAs in primary murine keratinocytes stably transduced with control, E6 or E7 retroviral vectors. I. IKKβ in vitro kinase assays (top two panels) were performed on extracts exposed to normoxia or hypoxia. Successful silencing of E6 is shown in the bottom two panels.
To further explore the role of E6 and E7 in mediating hypoxia-induced NF-κB activation, we expressed HPV16 E6 or E6 plus increasing amounts of E7 in C33A cells. Whereas ectopic expression of E6 resulted in a dose-dependent increase in hypoxia-induced NF-κB activation in C33A cells, E7 did not augment this response (Figure 2D) nor did it have any effect on its own (not shown). In addition, co-expression of E7 with E6 did not enhance the E6-mediated effects on NF-κB activation (Figure 2E). We obtained similar results in HEK293 cells (human embryonic kidney cells that are HPV-negative), although experiments in HEK293 cells were performed only under normoxia because prolonged hypoxia induced massive cell death (Figures 2F and 2G). Because squamous cell carcinomas arise from the malignant transformation of keratinocytes, we examined the effects of ectopic expression of E6 or E7 introduced by retroviral transduction into human keratinocytes. Similar to our results in HEK293 and C33A cells, E6 but not E7 expression in human keratinocytes resulted in activation of NF-κB under hypoxic conditions (Figure 2H). We next tested whether activation of IKKβ under hypoxic conditions was dependent upon E6 expression. Indeed, hypoxia-induced IKK activation in HPV-positive cells was negated by silencing E6 protein expression with E6-specific siRNA (Figure 2I), thereby establishing that E6-mediated regulation of hypoxia-induced NF-κB activation involves activation of IKKβ. Based on these gene transfer experiments combined with the results of the RNA interference studies, we conclude that the HPV E6 protein is necessary and sufficient for prolonged hypoxia-induced NF-κB activation in HPV-positive cells.
Given the importance of HIFα in cellular adaptations to hypoxia, we also sought to determine if HIFα plays a role in prolonged hypoxia-induced NF-κB activation. Toward this end, we silenced expression of HIFα in HeLa and SiHa cells (Figure S5A).Downregulation of HIFα protein levels by HIFα-specific siRNA had no effect on hypoxia-induced NF-κB activation (Figure S5B), indicating that the effects of hypoxia on NF-κB occur in a HIF-α independent fashion.
In HPV-positive cells, hypoxia induced NF-κB activation is TRAF6-dependent, and hypoxia-induced TRAF6 poly-ubiquitination is E6-dependent
In response to activation of cell-surface receptors, including members of the tumor necrosis factor receptor superfamily, toll-like receptors and the interleukin-1 receptor, the tumor necrosis factor receptor associated factors (TRAFs) activate the IKK complex (Chen, 2005). We focused on TRAF6 (one of seven TRAF family members), which in combination with a trimeric protein complex that consists of TGFβ-activated kinase (TAK1) and TAK1-binding proteins 1 and 2, activate the IKKβ/IKKγ complex (Chen, 2005). Transfection of a TRAF6-dominant negative expression plasmid into HeLa or SiHa cells inhibited hypoxia-induced NF-κB activation in a dose-dependent fashion (Figure 3A). When TRAF6 itself receives upstream signals from cell-surface receptors, TRAF6 oligomerizes and trans-auto-ubiquitinates, a process which results in lysine 63 (K63) linked polyubiquitination of TRAF6 (Chen, 2005; Wang et al., 2001). In contrast to lysine 48 (K48) polyubiquitination, which results in proteasome-mediated degradation of ubiquitinated proteins, K63 polyubiquitination is a proteasome-independent activating event in the NF-κB pathway (Krappmann and Scheidereit, 2005). In HeLa and SiHa cells exposed to hypoxia, TRAF6 polyubiquitination increased (Figure 3B). Hypoxia-induced TRAF6 polyubiquitination was not observed in HPV-negative DLD-1 cells (Figure 3C). Moreover, hypoxia-induced TRAF6 polyubiquitination was prevented by gene silencing of E6 expression with E6-specific siRNA (Figure 3D). Although these results indicate that E6 mediates hypoxia-induced NF-κB activation through a TRAF6-dependent mechanism, we rejected the possibility that TRAF6 was the direct molecular target of E6 within the NF-κB signaling pathway based on the following rationale.
Figure 3. Hypoxia induces TRAF6 ubiquitination in an E6-dependent fashion.
A. Cells were co-transfected with the TRAF6-DN or a control expression plasmid plus the NF-κB luciferase reporter. Results are means of three experiments ± s.d. and are represented as the ratio of NF-κB reporter activity in hypoxia:normoxia (48 hrs). Firefly luciferase expression was normalized to that of Renilla luciferase (see Methods). B. Hypoxia-induced TRAF6 polyubiquitination. TRAF6 immunoprecipitates were immunoblotted with ubiquitin (top) or TRAF6 (bottom) antibodies. C. TRAF6 polyubiquitination in normoxia and hypoxia (1 hr). D. TRAF6 polyubiquitination was assessed as in B but after transfection of control or E6 siRNA. All immunoprecipitations were performed under denaturing conditions.
E6-mediated activation of NF-κB under hypoxia is dependent upon CYLD
HPV-encoded E6 recruits p53 to a protein complex in which the cellular protein, E6-associated protein (E6-AP), polyubiquitinates p53 through K48 linkages and thereby targets p53 for proteasome-dependent degradation (Munger et al., 2004). Because E6 functions in the context of a K48 ubiquitin ligase complex and TRAF6 activation occurs as a consequence of K63 polyubiquitination, it did not seem plausible that E6 is involved in both K48 and a K63 ubiquitin ligase activity. Along these lines, there are two K63 deubiquitinases, A20 and CYLD, which function to cleave K63 ubiquitin linkages on signaling components (e.g. TRAF2, TRAF6, and IKKγ) in the NF-κB pathway and consequently inactivate these signaling proteins, thereby providing negative feedback signals to the NF-κB pathway (Chen, 2005). We concentrated on CYLD, because the CYLD deubiquitinase is operative in keratinocytes (Massoumi et al., 2006), the cells from which squamous cell carcinomas originate.
We determined whether CYLD expression was required for hypoxia inducible activation of NF-κB in various cell models with endogenous or ectopic expression of E6. When we silenced CYLD expression in HeLa and SiHa cells with CYLD-specific siRNA (Figure 4A), hypoxia-induced NF-κB activation as measured by reporter assay was markedly reduced compared to control siRNA (Figure 4B). Similar results were obtained in the C33A cells transiently transfected with E6 (Figures 4C and 4D). That is, inhibition of endogenous CYLD expression by CYLD siRNA in C33A cells (Figure 4C) abrogated the hypoxia-induced NF-κB activation associated with ectopic E6 expression (Figure 4D).
Figure 4. E6-mediated activation of NF-κB is dependent upon CYLD.
A. Western blots for CYLD in cells transfected with CYLD or C siRNA. B. Effects of CYLD RNAi on hypoxia-induced NF-κB reporter activity. Results are reported as the ratio of NF-κB activity in hypoxia to normoxia. C. Cells were transfected with an HPV16 E6 expression vector and control or E6-specific siRNA. Protein was harvested at 48 hrs. Western blots for CYLD and actin demonstrate effective silencing of CYLD. D. Effects of CYLD RNAi on hypoxia-induced NF-κB reporter activity. Results are reported as the ratio of NF-κB activity in hypoxia:normoxia. E. Effects of CYLD RNA interference on hypoxia-induced NF-κB activity as measured by EMSA. Top panel: NF-κB EMSA. Bottom panel: Oct-1 EMSA. F. Ratio of NF-κB reporter activity (hypoxia:normoxia) in primary wild-type (CYLD+/+) or CYLD “knock-out” (CYLD−/−) primary epidermal keratinocytes transfected with a HPV16E6 or vector control plasmid. All reporter results are averages of three experiments ± s.d. Duration of hypoxia was 24 hours. G. Effects of E6 expression on NF-κB activation in CYLD+/+ or CYLD−/− primary epidermal keratinocytes assayed by EMSA. C = lentiviral control transduction. E6 = HA-E6 lentiviral transduction. N = normoxia; H = hypoxia for 12 hours.
When we performed this experiment in HeLa cells with an EMSA as the read-out, we again found that cells treated with control siRNA manifested hypoxia-induced NF-κB activation (Figure 4E). Importantly, CYLD silencing resulted in heightened NF-κB activation under normoxic conditions to a degree that approximated that of the hypoxia-induced NF-κB activation in control siRNA treated cells (Figure 4E), suggesting that abrogation of CYLD expression in HPV-positive cells is sufficient to recapitulate prolonged hypoxia-induced NF-κB activation. A low level of residual hypoxia-induced NF-κB activation was observed in CYLD siRNA treated HeLa cells and may have been attributable to incomplete silencing of CYLD expression (Figure 4E, compare lane 2 to lane 4). Thus, induction of NF-κB activity by hypoxia occurred only in the setting of CYLD expression. NF-κB activation induced by tumor necrosis factor alpha (TNFα) was greater than that observed in normoxic conditions in CYLD siRNA treated cells (Figure 4E, compare lane 6 to lane 2). Thus, the absence of significant hypoxia-induced NF-κB activation in CYLD siRNA treated cells was not attributable to a hypothetical limit in the level of NF-κB activation in HeLa cells.
To more fully characterize the role of CYLD in mediating E6 effects on NF-κB, we studied hypoxia-induced NF-κB activation in CYLD-deficient (CYLD−/−) and wild-type (CYLD+/+) murine keratinocytes transiently transfected with E6 or a vector control. In the absence of E6 expression (vector control transfection), hypoxia-induced NF-κB reporter activation was not observed in either CYLD−/− or CYLD+/+ keratinocytes (Figure 4F). E6 transfection into CYLD+/+ keratinocytes resulted in hypoxia-induced NF-κB activation, whereas NF-κB inducibility under hypoxic conditions was lost in CYLD−/− keratinocytes despite ectopic expression of E6 (Figure 4F). To corroborate these reporter gene data, we analyzed NF-κB activation by EMSA in CYLD−/− and CYLD+/+ keratinocytes with or without ectopic expression of HPV16 E6. These studies required high transfection efficiency of E6. Accordingly, we cloned an HA-tagged version of HPV16 E6 into a lentiviral vector in which the green fluorescent protein (GFP) is co-expressed downstream of the EMCV internal ribosome entry site. One hundred percent of lentivirally transduced CYLD−/− and CYLD+/+ keratinocytes exhibited green fluorescence under UV microscopy (not shown), and functional expression of E6 was documented by downregulation of p53 expression (Figure 4G, top panels). In the setting of E6 expression, hypoxia induced marked activation of NF-κB in CYLD+/+ but not in CYLD−/− keratinocytes (Figure 4G, lanes 2, 4, 6, and 8). In the absence of E6, very little or no hypoxia-induced NF-κB activation was observed in either CYLD−/− or CYLD+/+ keratinocytes (Figure 4G, lanes 1, 3, 5, and 7). A slight increase in the intensity of the NF-κB gel shifted band in control transduced CYLD+/+ cells under hypoxia is likely an artifact of unequal protein loading, as the gel shifted band for Oct-1 was also increased under these conditions (Figure 4 G, compare lane 1 to 5). Interestingly, constitutive NF-κB activation under normoxic conditions was not significantly different in CYLD−/− and CYLD+/+ keratinocytes transduced with control lentivirus, a finding supported by a previous report (Massoumi et al., 2006). In summary, these data in murine keratinocytes in combination with the CYLD siRNA experiments in HeLa and SiHa cells demonstrate that E6-mediation of hypoxia-induced NF-κB activation is dependent upon CYLD expression.
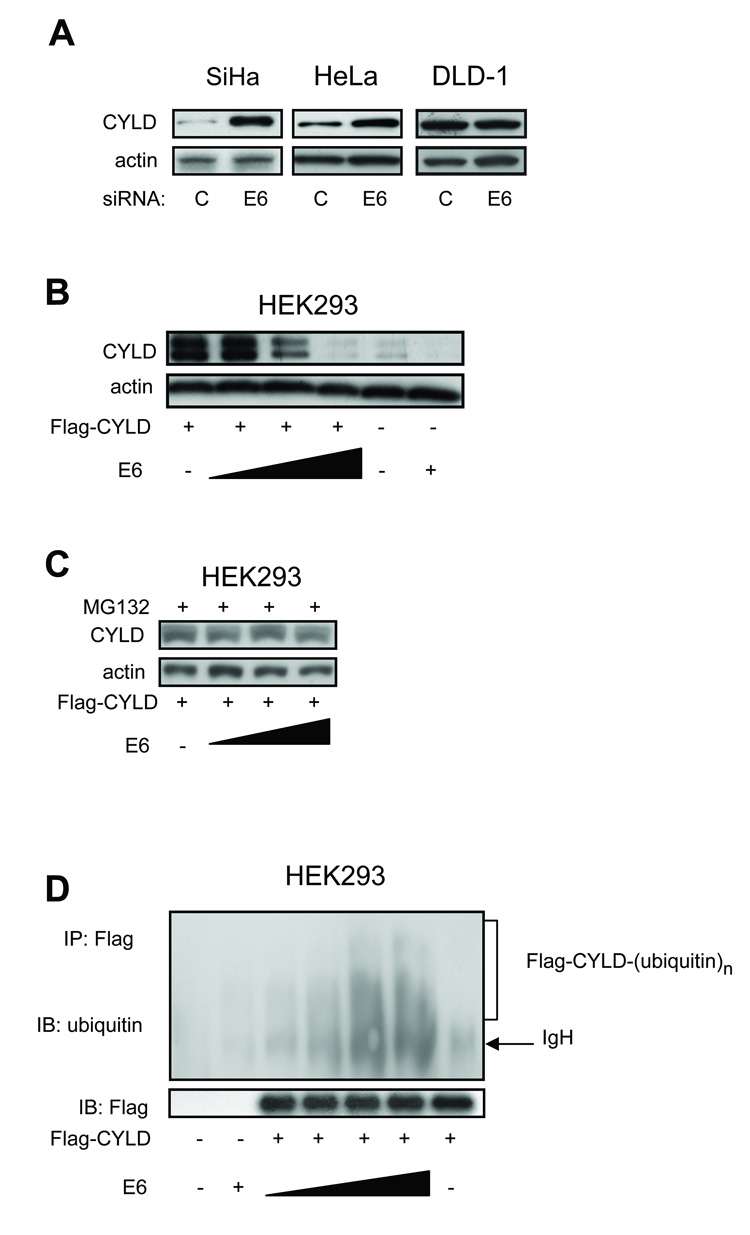
E6 reduces CYLD expression through a proteasome-dependent mechanism
Given the established role of E6 in degradation of cellular proteins through K48 polyubiquitination, we postulated that if CYLD were the molecular target of E6, then an inverse correlation between E6 and CYLD protein levels should exist. In HeLa and SiHa cells in which E6 protein levels were reduced by RNAi, the corresponding CYLD levels increased (Figure 5A). In contrast, E6 siRNA had no effect on CYLD levels in DLD-1 cells (Figure 5A). Transient transfection studies involving E6 and Flag-tagged CYLD constructs in HEK293 cells demonstrated that CYLD expression was reduced by E6 in a dose-dependent manner (Figure 5B). Ectopic expression of E6 also decreased endogenous CYLD levels in HEK293 cells (Figure 5B, rightmost two lanes). Importantly, degradation of CYLD by E6 was prevented by treatment of cells with the proteasome inhibitor, MG132 (Figure 5C). Based on these results, we concluded that E6 targets CYLD for proteasome-mediated degradation.
Figure 5. E6 reduces CYLD expression through a proteasome-dependent mechanism.
A. Western blots for CYLD expression in cells transfected with E6 or control siRNA. B. CYLD expression in cells transfected with combinations of HPV16 E6 or Flag-tagged CYLD. C. Same as B except cells were treated with the proteasome inhibitor, MG132 (10 µM), two hrs prior to harvesting protein. D. Ubiquitination of CYLD in the presence of E6. Cells were exposed to MG132 (10 µM) for two hrs prior to harvesting protein. IP = immunoprecipitation; IB = immunoblot. Ubiquitination assays were performed under denaturing conditions.
To further define the relationship between E6 and CYLD, we tested whether E6 expression is associated with CYLD polyubiquitination. Flag-CYLD was co-transfected with HA-tagged ubiquitin and increasing amounts of E6 into HEK293 cells treated with MG132 prior to harvesting protein to prevent CYLD degradation. Flag-CYLD was immunoprecipitated, and polyubiquitination of CYLD was analyzed by immunoblotting with an anti-ubiquitin antibody. As shown in Figure 5D, the degree of Flag-CYLD polyubiquitination correlated with the amount of transfected E6. These findings further establish CYLD as a molecular target of E6.
Hypoxia-induced degradation of CYLD is associated with E6-mediated CYLD ubiquitination
Because E6 effects on NF-κB are predominantly observed under hypoxic conditions, we reasoned that CYLD expression should be modulated by hypoxia in HPV-positive cells, whereby hypoxia would lead to decreased CYLD expression and consequent disinhibition of the NF-κB pathway. Indeed, hypoxia led to a rapid and prolonged decrease in CYLD protein levels in both HeLa and SiHa cells, which was abrogated by proteasome inhibition (Figure 6A). The suppression of CYLD protein levels during hypoxia-induced NF-κB activation is particularly noteworthy, because NF-κB has been shown to transcriptionally activate the CYLD gene (Jono et al., 2004). In contrast to HPV-positive cells, CYLD protein levels were unaffected by hypoxia in HPV-negative cell lines (Figure 6B).
Figure 6. Effects of hypoxia and hydroxylation inhibition on CYLD expression and ubiquitination.
A. Top and middle panels: Western blots for CYLD and actin after cells were exposed to hypoxia for the indicated times. Bottom panel: Same as top panels, but cells were treated with MG132 for 2 hours prior to protein extraction. B. CYLD and actin Western blots on protein harvested from the indicated HPV-negative cells exposed to hypoxia. C. CYLD ubiquitination status under normoxia, hypoxia, and upon inhibition of hydroxylase activity with DMOG. Cells were exposed to hypoxia or DMOG for six hrs in the presence of MG132 (10µM) to prevent CYLD degradation. Protein was immunoprecipitated with an anti-CYLD antibody (or IgG isotype control) and then subjected to immunoblotting with the indicated antibodies. The bottom panels demonstrate equivalent input of CYLD into all lanes. D. Effects of E6 gene silencing on hypoxia-induced CYLD ubiquitination. Cells were transfected with E6-specific or control siRNA and, after 24 hrs, exposed to normoxia or hypoxia for an additional 6 hrs. Co-immunoprecipitation to assess CYLD ubiquitination was performed as in C. The bottom panels demonstrated effective silencing of E6 protein expression. (Current antibodies cannot detect E6 by direct immunoblotting, so E6 immunoprecipitation followed by E6 immunoblotting with two different E6 antibodies was performed).
Next, we studied the ubiquitination status of CYLD under hypoxic conditions. Exposure of HPV-positive cells to hypoxia led to a marked increase in ubiquitinated CYLD (Figure 6C, compare lanes 2 to 4 and 6 to 8). In contrast, the ubiquitination status of CYLD was unaffected by hypoxia in C33A cells (Figure 6C, compare lanes 10 and 12). We then tested whether hypoxia-induced CYLD ubiquitination was dependent upon expression of E6. For these studies, we studied the state of CYLD ubiquitination in SiHa and HeLa cells that were treated with E6-specific siRNA or control siRNA. As expected, silencing of E6 expression (but not control siRNA) abrogated hypoxia-induced CYLD ubiquitination (Figure 6D). These results indicate that CYLD ubiquitination is enhanced under hypoxia in an E6-dependent fashion.
CYLD-mediated inactivation of the NF-κB pathway occurs through K63 deubiquitination of TRAFs (e.g. TRAF2 and TRAF6) as well as IKKγ, the latter of which is required for IKKβ kinase activity. As such, we reasoned that IKKβ kinase activity should be inversely related to CYLD protein levels. That is, as cellular levels of CYLD and its associated NF-κB inhibitory effects decline under hypoxic conditions, IKKβ activity would be expected to increase. Indeed, in both HeLa and SiHa cells, hypoxia-induced CYLD repression was temporally associated with activation of IKKβ and treatment of cells with a proteasome inhibitor not only prevented the decline in CYLD levels but also inhibited the IKKβ activation observed during hypoxia (Figure S6).
In addition to its deubiquitination effects on TRAFs and IKKγ, CYLD also deubiquitinates Bcl-3, and in so doing prevents its nuclear localization (Massoumi et al., 2006). Bcl-3 is a transcriptional coactivator that, when recruited to p50 homodimers, activates the transcriptional properties of DNA-bound p50 (Watanabe et al., 1997; Fujita et al., 1993). Because p50 lacks a transactivation domain, p50 homodimers will not induce gene transcription in the absence of bound Bcl-3. Given that p50 homodimers form in response to hypoxia (see Figure 1C), we hypothesized that as CYLD levels decrease under hypoxic conditions Bcl-3 should increasingly accumulate in the nucleus. In fact, nuclear Bcl-3 levels increased in a time-dependent fashion under hypoxic conditions (Figure S7), a finding which further implicates a central role for CYLD in the development of hypoxia-induced NF-κB activation.
Hypoxia leads to enhanced E6-CYLD protein-protein interactions
Our results, so far, suggest a model in which E6, in its capacity to function as part of an E3 ubiquitin ligase complex, ubiquitinates CYLD, and does so more efficiently under hypoxic conditions, thereby extinguishing the inhibitory effects of CYLD on the NF-κB pathway and leading to heightened NF-κB activation (see schematic, Figure 7A). To gain more insight into the differential effects of E6 on CYLD in normoxia versus hypoxia, we considered that the biochemical effects of hypoxia are frequently mediated by altered protein-protein interactions. Indeed, post-translational hydroxylation mediated by cellular hydroxylases can enhance or diminish protein-protein interactions. For example, asparagine hydroxylation of the transactivation domain of HIF1α by an asparaginyl hydroxylase known as factor inhibitor of HIF (FIH) prevents the recruitment of the transcriptional coactivator CBP/p300 (Lando et al., 2002), yet prolyl hydroxylation by prolyl hydroxylases enhances the HIF1α interaction with the von Hippel-Lindau ubiquitin ligase complex (Hirota and Semenza, 2005). Thus, we postulated that hypoxia would lead to an enhanced protein-protein interaction between E6 and CYLD, thereby allowing for more efficient E6-mediated ubiquitination.
Figure 7. The E6-CYLD protein-protein interaction is enhanced by hypoxia and hydroxylase inhibition.
A. Schematic diagram depicting the mechanism whereby HPVencoded E6 activates NF-κB through ubiquitination and subsequent proteasomal degradation of CYLD. B. Protein extracts (same extracts as Figure 6C) were exposed to normoxia, hypoxia or DMOG for six hrs in the presence of MG132 (10 µM) and then subjected to co-immunoprecipitation studies to evaluate E6-CYLD protein-protein interactions (top panels). E6-p53 co-immunoprecipitations (bottom panels). Immunoprecipitations with an isotype control antibody (lanes 1 and 5). C. CYLD does not immunoprecipitate with AKT. Experiments were performed as in B with indicated antibodies. For B and C, the protein extracts were the same as those employed for Figure 6. D. CYLD protein expression was assessed cells treated with DMOG or vehicle (DMSO).
We performed co-immunoprecipitation studies in HeLa and SiHa cells to compare the intensity of the putative E6-CYLD protein-protein interaction in normoxia versus hypoxia. HeLa and SiHa cells were exposed to MG132 (10 µM) for one hour prior to harvesting protein in order to prevent E6-mediated, proteasome-dependent degradation of CYLD. Indeed, these immunoprecipitation studies demonstrated that E6 and CYLD weakly interact in SiHa and HeLa cells under normoxia (Figure 7B, lanes 2 and 6). Importantly, the interaction was markedly augmented by hypoxia (Figure 7B, compare lanes 2 to 4 and lanes 6 to 8). As a positive control, we co-immunoprecipitated p53 with the E6 antibody (Figure 7B). The specificity of the interaction was established through pull-downs with an isotype control antibody (Figure 7B, lanes 1 and 5), as well as an anti-AKT antibody (Figure 7C, lanes 1, 2, and 4 and lanes 6, 7, and 9), neither of which revealed CYLD in the immunoprecipitate. These results support the notion that the E6-dependent effects on CYLD are mediated through protein-protein interactions that are enhanced during hypoxia.
HPV E6-mediated degradation of p53 is dependent upon E6-AP, which, in conjunction with E6, forms a ternary complex with p53 thereby leading to polyubiquitination and proteasome-dependent degradation of p53 (Scheffner et al., 1990; Huibregtse et al., 1991; Scheffner et al., 1993). Many other E6-AP dependent targets of E6, such as ErbB2, have been reported (Narisawa-Saito et al., 2007). Given the established role of E6-AP in mediating many E6 effects, we explored the role of E6-AP in E6-dependent, hypoxia-induced NF-κB activation. Toward this end, we introduced lentiviral constructs that expressed E6-AP-specific short hairpin RNA (shRNA) or scrambled control shRNA into HeLa and SiHa cells. Functional knockdown of E6-AP was demonstrated by restoration of p53 expression in E6-AP shRNA expressing cells (Figure S8A). Silencing of E6-AP did not influence hypoxia-induced effects on CYLD expression, IKKβ kinase activity, or NF-κB EMSAs (Figure S8, B-D). Similar findings were found with two other E6-AP shRNAs (not shown). These results indicate that E6-AP is not involved in E6-dependent, hypoxia-induced effects on the NF-κB pathway.
The hypoxia-induced, E6-mediated effects on CYLD are recapitulated by hydroxylase inhibition
To begin to explore the biochemical effects that mediate suppression of CYLD expression in hypoxia, we considered that hypoxia-induced post-translational effects are frequently mediated by oxygen-dependent prolyl or asparaginyl hydroxylases (Schofield and Ratcliffe, 2005). Because the effects of E6 on CYLD are more pronounced under hypoxic conditions, we postulated that reduced activity of oxygen-dependent hydroxylases that results when molecular oxygen is a limiting substrate enhances the E6-mediated effects on CYLD. Accordingly, we determined if treatment of HeLa and SiHa cells with the pan-hydroxylase inhibitor, dimethyloxaloylglycine (DMOG), would recapitulate the effects of hypoxia. DMOG treatment (but not vehicle control) led to a drop in CYLD protein levels (Figure 7D). Hydroxylase inhibition also resulted in heightened CYLD ubiquitination and enhanced the protein-protein interaction between E6 and CYLD (Figure 6C, compare lanes 2 to 3 and lanes 6 to 7; Figure 7B, compare lanes 2 to 3 and lanes 6 to 7; Figure 7C). The E6-dependence of this effect is highlighted by the fact that DMOG failed to alter CYLD ubiquitination in HPV-negative cells (Figure 6C, compare lanes 10 to 11). These findings implicate a role for hydroxylation as a post-translational modification that mediates the effects of E6 on CYLD.
NF-κB activation is required for anchorage-independent growth under hypoxic conditions in HPV-positive cells
To establish a biologic role for hypoxia-induced NF-κB activation in HPV-positive squamous cell carcinomas, we determined whether NF-κB activation was necessary for anchorage-independent growth. SiHa and HeLa cells were transduced with an adenoviral vector containing an IκB-“super repressor” (IκB-SR), in which serine phosphorylation sites are mutated to alanines rendering the IκB-SR resistant to proteasome-mediated degradation (Karin, 2006). Ectopic expression of the IκB-SR effectively inhibited hypoxia-induced NF-κB activation in SiHa and HeLa cells (Figure 8A). Both the size and number of SiHa and HeLa colonies transduced with a control adenovirus actually increased in hypoxic compared to normoxic conditions (Figure 8B). Importantly, this effect was completely abolished by expression of the IκB-SR (Figure 8B), a finding that confirms that heightened NF-κB activation due to hypoxia exposure is critical to anchorage-independent growth of HPV-positive squamous cell carcinomas. Next, we assessed whether CYLD suppresses hypoxia-induced anchorage-independent growth. HeLa cells were transfected with a plasmid encoding wild-type CYLD or a CYLD mutant (C/S-CYLD) in which its enzymatic activity is abolished by an amino acid substitution in the cysteine box of the deubiquitinase domain (Brummelkamp et al., 2003). Compared to transfection with an empty vector control, wild-type CYLD transfection reduced hypoxia-induced colony formation, whereas as the C/S-CYLD mutant did not (Figure 8C), findings which indicates that CYLD inhibits hypoxia-induced anchorage-independent growth.
Figure 8. Hypoxia-induced enhancement of anchorage-independent growth is NF-κB dependent.
A. EMSAs and reporter assays to define the multiplicity of infection (moi) of the Ad-IB-SR that blocks hypoxia-induced NF-κB activity. The EMSAs were performed after 24 hrs of hypoxia exposure, and reporter activity was assayed after 48 hrs of hypoxia. The reporter results are the means of three experiments ± s.d.. B. Results of anchorage-independent growth assays (at 14 days) were normalized to that of the normoxic group transduced with the control virus (Ad-CMV). Representative phase-contrast photomicrographs are shown (final magnification = 200X). C. Anchorage-independent growth of HeLa cells transfected with the indicated plasmids (CYLD, C/S-CYLD or vector control) under G418 selection. D. Model of cellular NF-κB responses to hypoxia (see Discussion for details).
DISCUSSION
HPV is an established carcinogen in humans. Abundant epidemiologic and experimental evidence has led to the recognition of HPV as causative agent in 90–98% of cases of squamous cell carcinoma of the cervix (Psyrri and DiMaio, 2008), which is the second most common cancer in women worldwide with an annual incidence and mortality of 500,000 and 200,000, respectively (Ellenson and Wu, 2004; Waggoner,2003). HPV types 16 and 18 account for approximately 70% of cervical cancer cases (Psyrri and DiMaio, 2008) with other high-risk types accounting for the majority of the remaining cases. Moreover, HPV infection is thought to be causally related to other squamous cell carcinomas, including vulvar, anal, penile and oropharyngeal cancers (Psyrri and DiMaio, 2008).
In HPV-associated cancer, HPV DNA is typically integrated into the cellular genomic DNA, and most of the viral genome is deleted except for the E6 and E7 genes (Psyrri and DiMaio, 2008). E7 is known for its ability to bind to and inhibit pRB function, whereas E6 is most notorious for its role in ubiquitination and degradation of p53 (Narisawa-Saito et al., 2007). However, p53-independent functions of E6 are well described, and, in fact, p53 elimination is not required for cellular immortalization by E6 (Kiyono et al., 1998). For example, E6 mediates the degradation of the pro-apoptotic protein BAK and the TSC1 tumor suppressor and activates cellular telomerase (Klingelhutz et al., 1996; Narisawa-Saito et al., 2007). Here, we describe a role for the HPV-encoded E6 protein in a biochemical response to hypoxia. Specifically, through its ability to induce sustained NF-κB activation in response to reduced ambient oxygen tension, E6 mediates a cellular adaptation to prolonged hypoxia. Importantly, we found that prolonged hypoxia-induced NF-κB activation is not a generalized phenomenon amongst cancers, but rather is limited to cancer cells that are infected with high risk HPV. The mechanism underlying hypoxia-induced NF-κB activation involves E6- mediated ubiquitination and degradation the CYLD K63 deubiquitinase, which releases components in the NF-κB signaling cascade from the inhibitory effects of CYLD. Although the E6-mediated effects on CYLD and the NF-κB pathway are intensified under hypoxic conditions, we did consistently observe similar but less pronounced effects in normoxia (Figures 2B and I). Thus, in ambient oxygen tension, E6 gene silencing in HPV-positive cells led to NF-κB and IKKβ activation and increased CYLD expression, whereas ectopic expression of E6 in HPV-negative cells resulted in NF-κB activation and CYLD degradation. These latter effects are consistent with the results of the protein-protein interactions studies, which demonstrated that E6 does in fact bind to CYLD under normoxic conditions, albeit to a lesser degree than under hypoxia.
The heightened ubiquitination and degradation of CYLD are recapitulated by hydroxylase inhibition, which suggests that post-translational hydroxylation plays a central role in E6-dependent, hypoxia-induced of CYLD degradation. In a preliminary analysis of the CYLD primary amino acid sequence, we did not detect any consensus prolyl hydroxylation sites (LXXLAP). CYLD does harbor a potential asparagine hydroxylation site, wherein the asparagine at position 646 is preceded by valine (position 643) in the context of leucine(s) located seven to eight positions amino-terminal to the asparagine (Cockman et al., 2006). This juxtaposition of valine to asparagine is required for optimal substrate recognition by the asparaginyl hydroxylase known as factor inhibitor of HIF (FIH) (Linke et al., 2004). However, these amino acids are not found within an ankyrin repeat domain, which is the basis for substrate recognition by FIH (Cockman et al., 2006). Similarly, the E6 sequence does not contain either prolyl or asparaginyl hydroxylation sites. Thus, the details of hypoxia mediated post-translational regulation of the E6-CYLD interaction remain to elucidated, and may involve a previously unidentified oxygen-sensing hydroxylase. It is also plausible that post-translational hydroxylation may take place on a protein involved in formation of a protein complex that includes E6 and CYLD, whereby hydroxylation impairs complex formation and, by extension, the ability of E6 to target CYLD for ubiquitination.
Interactions between p53 and NF-κB have been reported. On the one hand, mutual transcriptional repression has been reported between p53 and NF-κB, yet p53 has also been shown to activate NF-κB in some contexts (Ikeda et al., 2000; Wadgaonkar et al., 1999; Webster and Perkins, 1999; Huang et al., 2007; Ryan et al., 2000). Thus, in the interpretation of the experiments involving RNAi of E6, we considered that the inhibition of constitutive and hypoxia-induced NF-κB through suppression of endogenous E6 protein could potentially be attributed to p53-dependent effects on NF-κB. Specifically, restoration of p53 expression by E6 RNAi could result in reduced NF-κB transcriptional activity if mutual transcriptional repression between p53 and NF-κB were operative in HPV-positive cells. However, it has recently been shown that in models in which p53 suppresses NF-κB transcriptional activity, NF-κB DNA binding is actually enhanced and DNA bound NF-κB undergoes p53-dependent post-translational modifications that result in transcriptional inhibition of NF-κB (Kawauchi et al., 2008). Yet we observed decreased NF-κB DNA binding in response to E6 RNAi in HPV-positive cells (Figures 2B and S3B). Moreover, since p53-dependent transcriptional inhibition of NF-κB does not appear to involve TRAF6 or CYLD (Ikeda et al., 2000; Wadgaonkar et al., 1999), and p53 expression remains undetectable under hypoxic conditions due to the presence of E6 (Figure 2A), we cannot indict p53 as a mediator of hypoxia-induced NF-κB activation in HPV-positive cells.
In addition to the prolonged hypoxia-induced NF-κB activation observed in HPV-positive cells, we observed that some HPV-negative tumor models are capable of transient NF-κB activation during hypoxia (Figures 1F and S2B). A mechanism for E6-independent NF-κB activation is suggested by a recent report in which IKKβ was shown to be negatively regulated by prolyl hydroxylase 1 (PHD-1), such that under conditions in which oxygen is a limiting substrate (i.e. hypoxia), PHD-1 activity is diminished and IKKβ activity is augmented (Cummins et al., 2006). To account for the varied hypoxia-induced NF-κB responses that we observed, we propose the following model (Figure 8D). In what we term Type I cells, HPV infection and more specifically the E6 protein, perhaps in conjunction with a hydroxylase and other cellular proteins, results in prolonged hypoxia-induced NF-κB activation. Type II cells are HPV-negative and manifest transient hypoxia-induced NF-κB activation mediated by hydroxylases (e.g. PHD-1) and possibly other cellular factors. In Type III cells, which are also HPV-negative, augmentation of NF-κB activity is either entirely prevented or hypoxia induces a decrease in NF-κB activity. Because the tumor microenvironment is typified by chronic hypoxia, the prolonged nature of the NF-κB activation observed in the setting of HPV infection may represent a more suitable adaptive response to hypoxia in vivo.
The clinical evidence that hypoxia is associated with poor outcomes (e.g. increased local tumour recurrence, the development of systemic metastases, resistance to radiation and chemotherapy and reduced overall survival) is actually most well-established in two HPV-associated cancers: cervical and head and neck cancers, although ample clinical data also exist for sarcomas (Subarsky and Hill, 2003). Importantly, frequent NF-κB activation has been observed in cervix and head and neck patient specimens (Mishra et al., 2006; Nair et al., 2003). A recent study highlights the HPV--NF-κB connection: the degree of NF-κB activation was reported to correlate with HPV status in head and neck cancer (Mishra et al., 2006). A biological role for hypoxia-induced NF-κB activation is supported by our studies demonstrating that HPV-positive cervical cancer cells are dependent upon heightened NF-κB activity for optimal anchorage-independent growth under hypoxic conditions. Since NF-κB transcriptional activity results in expression of proteins that promote angiogenesis, proliferation, drug resistance, invasion and metastasis, the aggressive tumor biology characteristic of hypoxic tumors could in large part be recapitulated by hypoxia-induced NF-κB activation. However, NF-κB transcriptional activity cannot account for the entire hypoxic gene expression profile, and other transcription factors, such as HIFα, are known to play a central role in cellular responses to hypoxia (Denko et al., 2003). Nevertheless, prolonged hypoxia-induced NF-κB activation is apt to promote tumor progression in HPV-infected malignancies and has important implications for the carcinogenesis and potential therapeutic targeting of the relatively common malignancies associated with HPV infection.
METHODS
In vitro hypoxia treatment
All hypoxia experiments were carried out in 1% oxygen (O2) in a chamber in which CO2 and nitrogen are independently regulated (Biospherix, Redfield, NY). Oxygen and CO2 concentrations were continuously monitored with probes that reside within the chamber. Calibration of the oxygen probe to ambient oxygen concentrations (21% O2) and 0% oxygen (100% nitrogen) was performed periodically. For protein extractions, cells were removed from the hypoxia chamber, medium was rapidly aspirated, and ice-cold phosphate buffered saline (PBS) was immediately added for washing.
In vivo hypoxia
All animal studies were performed in accordance with institutional and national guidelines and were approved by the Animal Research Committee of the VA Greater Los Angeles Healthcare System-West Los Angeles. HeLa or CAL27 cells (2 × 106 cells in 200 µl of saline) were injected subcutaneously into the right flank of 6-week old female nude mice. Tumors were grown until a maximum dimension of ~ 1 cm was reached. Sixty minutes prior to euthanasia, mice were injected intraperitoneally with 1 mg/kg of pimonidazole (NPI Inc., Burlington, MA), a 2-nitroimidazole that forms adducts with thiol groups on cellular proteins and peptides at a pO2 < 10 mm Hg.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the NIH and the Department of Veterans Affairs (to M.B.R.). We thank Chris Logg and Nori Kasahara (UCLA) for helpful advice and the UCLA Vector Core (NIH grant 2P30DK041301) for production of viral vectors. We thank Drs. Genhong Cheng and Alan Lichtenstein for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J. Clin. Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- Butz K, Ristriani T, Hengstermann A, Denk C, Scheffner M, Hoppe-Seyler F. siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene. 2003;22:5938–5945. doi: 10.1038/sj.onc.1206894. [DOI] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockman ME, Lancaster DE, Stolze IP, Hewitson KS, McDonough MA, Coleman ML, Coles CH, Yu X, Hay RT, Ley SC, et al. Posttranslational hydroxylation of ankyrin repeats in IkappaB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH) Proc. Natl. Acad. Sci. U. S. A. 2006;103:14767–14772. doi: 10.1073/pnas.0606877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denko NC, Fontana LA, Hudson KM, Sutphin PD, Raychaudhuri S, Altman R, Giaccia AJ. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22:5907–5914. doi: 10.1038/sj.onc.1206703. [DOI] [PubMed] [Google Scholar]
- Ellenson LH, Wu TC. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5:533–538. doi: 10.1016/j.ccr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Favre-Bonvin A, Reynaud C, Kretz-Remy C, Jalinot P. Human papillomavirus type 18 E6 protein binds the cellular PDZ protein TIP-2/GIPC, which is involved in transforming growth factor beta signaling and triggers its degradation by the proteasome. J. Virol. 2005;79:4229–4237. doi: 10.1128/JVI.79.7.4229-4237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Nolan GP, Liou HC, Scott ML, Baltimore D. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-kappa B p50 homodimers. Genes Dev. 1993;7:1354–1363. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Hirota K, Semenza GL. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem. Biophys. Res. Commun. 2005;338:610–616. doi: 10.1016/j.bbrc.2005.08.193. [DOI] [PubMed] [Google Scholar]
- Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- Huang WC, Ju TK, Hung MC, Chen CC. Phosphorylation of CBP by IKKalpha promotes cell growth by switching the binding preference of CBP from p53 to NF-kappaB. Mol. Cell. 2007;26:75–87. doi: 10.1016/j.molcel.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Sun X, Li Y, Zhang Y, Eckner R, Doi TS, Takahashi T, Obata Y, Yoshioka K, Yamamoto K. p300/CBP-dependent and -independent transcriptional interference between NF-kappaB RelA and p53. Biochem. Biophys. Res. Commun. 2000;272:375–379. doi: 10.1006/bbrc.2000.2786. [DOI] [PubMed] [Google Scholar]
- Jiang M, Milner J. Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference. Oncogene. 2002;21:6041–6048. doi: 10.1038/sj.onc.1205878. [DOI] [PubMed] [Google Scholar]
- Jono H, Lim JH, Chen LF, Xu H, Trompouki E, Pan ZK, Mosialos G, Li JD. NF-kappaB is essential for induction of CYLD, the negative regulator of NF-kappaB: evidence for a novel inducible autoregulatory feedback pathway. J. Biol. Chem. 2004;279:36171–36174. doi: 10.1074/jbc.M406638200. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Kawauchi K, Araki K, Tobiume K, Tanaka N. Activated p53 induces NF-kappaB DNA binding but suppresses its transcriptional activation. Biochem. Biophys. Res. Commun. 2008;372:137–141. doi: 10.1016/j.bbrc.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res. 1994;54:1425–1430. [PubMed] [Google Scholar]
- Krappmann D, Scheidereit C. A pervasive role of ubiquitin conjugation in activation and termination of IkappaB kinase pathways. EMBO Rep. 2005;6:321–326. doi: 10.1038/sj.embor.7400380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23:293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl. Acad. Sci. U. S. A. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke S, Stojkoski C, Kewley RJ, Booker GW, Whitelaw ML, Peet DJ. Substrate requirements of the oxygen-sensing asparaginyl hydroxylase factor-inhibiting hypoxia-inducible factor. J. Biol. Chem. 2004;279:14391–14397. doi: 10.1074/jbc.M313614200. [DOI] [PubMed] [Google Scholar]
- Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125:665–677. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Mishra A, Bharti AC, Varghese P, Saluja D, Das BC. Differential expression and activation of NF-kappaB family proteins during oral carcinogenesis: Role of high risk human papillomavirus infection. Int. J. Cancer. 2006;119:2840–2850. doi: 10.1002/ijc.22262. [DOI] [PubMed] [Google Scholar]
- Munger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, Grace M, Huh K. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Venkatraman M, Maliekal TT, Nair B, Karunagaran D. NF-kappaB is constitutively activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene. 2003;22:50–58. doi: 10.1038/sj.onc.1206043. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Handa K, Yugawa T, Ohno S, Fujita M, Kiyono T. HPV16 E6-mediated stabilization of ErbB2 in neoplastic transformation of human cervical keratinocytes. Oncogene. 2007;26:2988–2996. doi: 10.1038/sj.onc.1210118. [DOI] [PubMed] [Google Scholar]
- Psyrri A, DiMaio D. Human papillomavirus in cervical and head-and-neck cancer. Nat. Clin. Pract. Oncol. 2008;5:24–31. doi: 10.1038/ncponc0984. [DOI] [PubMed] [Google Scholar]
- Reynolds TY, Rockwell S, Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res. 1996;56:5754–5757. [PubMed] [Google Scholar]
- Royds JA, Dower SK, Qwarnstrom EE, Lewis CE. Response of tumour cells to hypoxia: role of p53 and NFkB. Mol. Pathol. 1998;51:55–61. doi: 10.1136/mp.51.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KM, Ernst MK, Rice NR, Vousden KH. Role of NF-kappaB in p53-mediated programmed cell death. Nature. 2000;404:892–897. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Whitaker NJ. Human papillomavirus-induced carcinogenesis and the ubiquitin-proteasome system. Semin. Cancer Biol. 2003;13:59–67. doi: 10.1016/s1044-579x(02)00100-1. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ. Signalling hypoxia by HIF hydroxylases. Biochem. Biophys. Res. Commun. 2005;338:617–626. doi: 10.1016/j.bbrc.2005.08.111. [DOI] [PubMed] [Google Scholar]
- Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin. Exp. Metastasis. 2003;20:237–250. doi: 10.1023/a:1022939318102. [DOI] [PubMed] [Google Scholar]
- Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int. J. Radiat. Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P, Briest S, Hockel M. Hypoxia in breast cancer: pathogenesis, characterization and biological/therapeutic implications. Wien. Med. Wochenschr. 2002;152:334–342. doi: 10.1046/j.1563-258x.2002.02032.x. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: role of hypoxia and anemia. Med. Oncol. 2001;18:243–259. doi: 10.1385/MO:18:4:243. [DOI] [PubMed] [Google Scholar]
- Wadgaonkar R, Phelps KM, Haque Z, Williams AJ, Silverman ES, Collins T. CREB-binding protein is a nuclear integrator of nuclear factor-kappaB and p53 signaling. J. Biol. Chem. 1999;274:1879–1882. doi: 10.1074/jbc.274.4.1879. [DOI] [PubMed] [Google Scholar]
- Waggoner SE. Cervical cancer. Lancet. 2003;361:2217–2225. doi: 10.1016/S0140-6736(03)13778-6. [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Iwamura T, Shinoda T, Fujita T. Regulation of NFKB1 proteins by the candidate oncoprotein BCL-3: generation of NF-kappaB homodimers from the cytoplasmic pool of p50-p105 and nuclear translocation. EMBO J. 1997;16:3609–3620. doi: 10.1093/emboj/16.12.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GA, Perkins ND. Transcriptional cross talk between NF-kappaB and p53. Mol. Cell Biol. 1999;19:3485–3495. doi: 10.1128/mcb.19.5.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SD, Marshall RS, Hill RP. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc. Natl. Acad. Sci. U. S. A. 1988;85:9533–9537. doi: 10.1073/pnas.85.24.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.