Abstract
Background
The survival of Salmonella enterica within the intracellular host niche requires highly co-ordinated expression of virulence effectors predominantly regulated by the SsrAB two-component regulatory system. S. enterica serovar Typhimurium mutants lacking the ssrAB genes are avirulent in mice, highlighting the importance of this regulatory system in vivo. Mutants lacking the gene encoding the alternative sigma factor σE (rpoE) are also highly attenuated for intracellular survival, pointing to a potential connection with the SsrAB regulatory system.
Results
In this study we demonstrate that RpoE is involved in fine-tuning the expression of a subset of SsrB-regulated genes found in the Salmonella pathogenicity island-2 (SPI-2) genetic locus that encodes a horizontally acquired type III secretion system, and unlinked genes integrated into this regulon that are required for virulence in host animals.
Conclusion
These data point to a potential connection between the virulence phenotype of strains lacking ssrB and rpoE, and highlight new transcriptional regulation that might be essential for appropriate temporal and spatial control of the virulence-associated type III secretion system during host infection.
Background
Salmonella enterica are enteric pathogens that acquired a type III secretion system (T3SS) through horizontal gene transfer of a genomic island termed Salmonella Pathogenicity Island 2 (SPI-2) [1,2]. The SPI-2-encoded T3SS and its translocated effectors modify the intracellular host niche for Salmonella replication [3-5]. SPI-2 also has genes, ssrA and ssrB, which code for SsrAB, a two-component regulatory system needed for expression of the T3SS [6,7]. SsrB regulates the expression of SPI-2 encoded substrate effectors including ssaB, as well as several integrated virulence effectors such as sseL [8] and srfN [9] that are encoded elsewhere on the chromosome but that have integrated into the SsrB regulon. Mutants lacking ssrAB are unable to survive within macrophages and are avirulent in mice [1].
Alternative sigma factors coordinate gene expression in response to environmental cues sensed by the bacterium. Sigma factors have a specific recognition motif at the -35 and -10 positions and function to concentrate RNA polymerase at a subset of promoters [10]. One alternative sigma factor, RpoE (σE) responds to envelope stress at the cell surface. Release of σE from its inner membrane anchored anti-sigma factor, RseA, leads to induction of genes required to maintain cell envelope integrity [11]. SsrB-regulated translocated effectors protect S. Typhimurium against host cell defences such as oxidative stress and antimicrobial peptides that perturb bacterial membrane integrity and provide a stimulus for σE release [4,12-15]. Although proficient at cellular invasion, rpoE or ssrB mutants are highly attenuated for intracellular survival in both cultured cells and animal hosts [16]. In addition, the expression of rpoE and ssrB is up-regulated within macrophages [17]. Links between RpoE and virulence gene expression is evident in other bacterial systems as well. Deletion of rseA in Yersinia pseudotuberculosis causes elevated virulence effector synthesis and secretion [18], establishing links between alternative sigma factors and virulence-specific regulators. Taken together, a connection between σE and SsrB is suggested from the available literature, however the role of σE in activating SsrB-regulated genes has not been studied.
We tested the hypothesis that RpoE is involved in expression of genes that use the SsrB response regulator for activation. By testing six promoters representing four classes of SsrB-regulated promoters ((i) two type III secretion structural operons in SPI-2, (ii) the effector operon in SPI-2, (iii) two effector genes unlinked with SPI-2, and (iv) an integrated virulence gene unlinked with SPI-2) we demonstrate that RpoE elicits an effect on a subset of SsrB-regulated genes. This effect was bidirectional depending on the promoter and was downstream of ssrB expression itself, since deletion of rpoE had no effect on SsrB levels in the mutant cells. These data help unite the virulence phenotypes of strains lacking SsrB and RpoE, and highlight new transcriptional regulation that might be essential for appropriate temporal and spatial control of the virulence-associated type III secretion system during host infection.
Results
Deletion of rpoE affects a subset of SsrB-regulated virulence genes
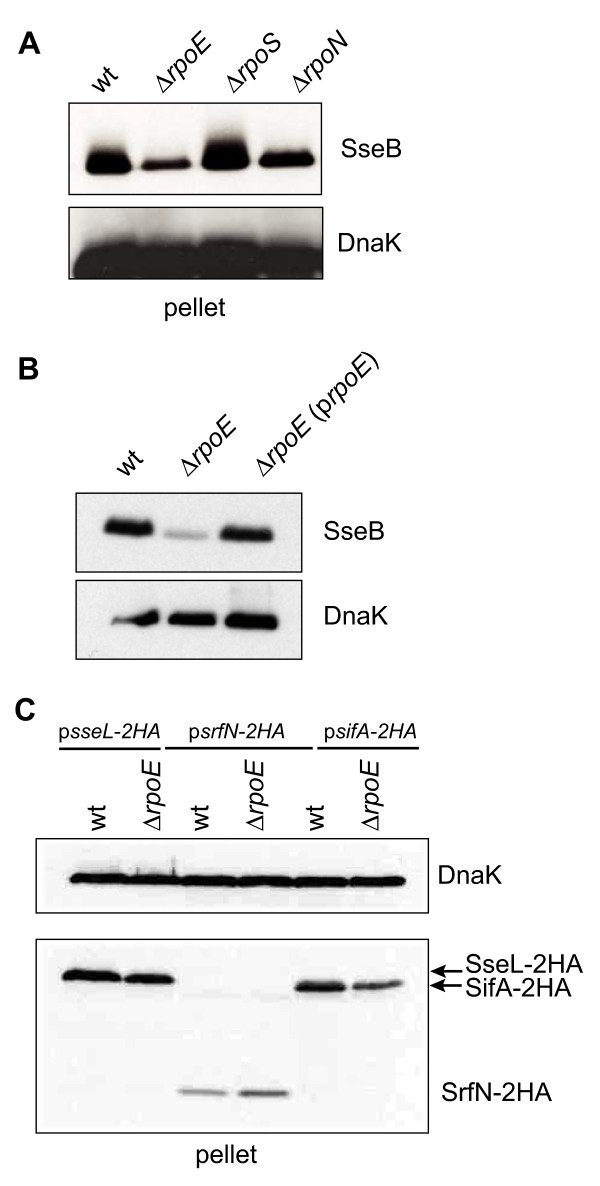
Salmonella virulence gene expression is coordinated in vivo and may be regulated, in part, by alternative sigma factor(s) in order to quickly respond to the host environment. To date, no sigma factor has been identified as regulating SsrB-dependent virulence genes. To start, we first screened four alternative sigma factor mutants of S. Typhimurium (rpoS, rpoN, rpoE, rpoH) for their ability to express a key virulence gene, sseB, that requires SsrB for expression and whose gene product is essential for intracellular pathogenesis. For an rpoH deletion, this strain was only viable at temperatures below 30°C. Since SPI-2 gene activation is integrated into a thermosensing circuit [19] we were unable to test the role of σH in this study (data not shown). In this screen, rpoS deletion resulted in a slight increase in SseB levels (Figure 1A) indicating a role for RpoS in the repression of SPI-2. Both rpoE and rpoN deletions resulted in decreased SseB levels with a more pronounced effect in the rpoE deletion. Since we were predominantly interested in sigma factors that activate SPI-2 and which could be linked to the previous observation that rpoE mutants are highly attenuated in vivo we choose to focus on RpoE in the current study, which had the most influence on SseB levels in the cell.
Figure 1.
Loss of rpoE changes the abundance of virulence factors in Salmonella. (A) wild type (wt), ΔrpoE, ΔrpoS, and ΔrpoN S. Typhimurium 14028s were grown for 6 hours under SsrB-inducing conditions. Lysates were probed by western blot for SseB, a component of the T3SS needle complex. (B) Western blot was performed as above and lysates probed for SseB in wild type (wt) S. Typhimurium SL1344, ΔrpoE and in ΔrpoE complemented with pWSK29 carrying full length rpoE with endogenous promoters. (C) Wild type S. Typhimurium SL1344 and ΔrpoE cells were immunoblotted as above and lysates probed for SseL-2HA, SrfN-2HA and SifA-2HA which were expressed from their endogenous promoters in pWSK29. Blots were probed for DnaK as a control. The experiment was performed three times with similar results.
An unmarked in-frame deletion of rpoE was then generated in S. Typhimurium strain SL1344 and we verified that this in-frame deletion had the same effect on SseB as the rpoE::cat mutant used previously (Figure 1B). A low-copy plasmid containing full-length rpoE and the three endogenous promoters that can drive its expression [20] was able to restore wild type levels of SseB to ΔrpoE cells (Figure 1B) demonstrating that the results were specific to the rpoE deletion. In these complementation experiments, attempts were made to examine the levels of SseB secreted into the culture supernatant [21], however consistent with previous observations [22,23] perturbations to the rpoE pathway increased cell lysis resulting in contamination of secreted fractions with cytosolic proteins which precluded accurate interpretation (data not shown).
In order to examine the effect of σE (rpoE) on the expression of a broad range of SsrB-regulated virulence genes, we tested whether or not the effect of rpoE deletion was specific to sseB or if it extended to other SsrB-regulated genes. To do this we examined the levels of SseL-2HA, SifA-2HA and SrfN-2HA expressed from their endogenous promoters under SPI-2 inducing conditions (Figure 1C). Consistent with the results for SseB, there was a decrease in SifA-2HA levels in ΔrpoE compared to wild type, although deletion of rpoE did not have an effect on SseL-2HA. Relative to its expression in wild type cells, the level of SrfN-2HA was reproducibly increased in the ΔrpoE cells, suggesting a role for σE in the repression of SrfN, although it is unlikely that this is through a direct mechanism.
RpoE is involved in transcriptional activity of a subset of virulence genes
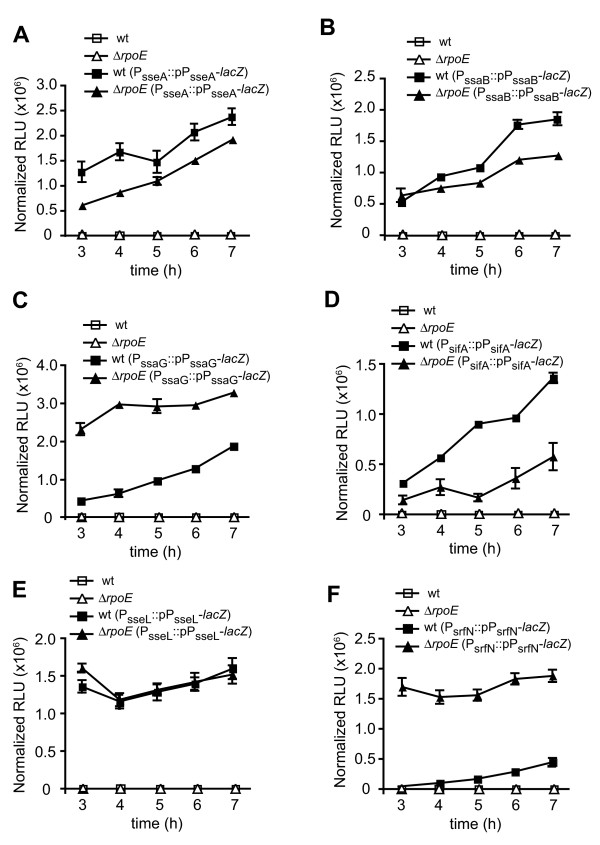
In order to confirm the effect of σE on the expression of a broad range of SsrB-regulated virulence genes, we used wild type and ΔrpoE cells and integrated into the chromosome individually six single-copy transcriptional fusions representing promoters for four classes of SsrB-dependent genes or operons ((i) type III secretion effector operon (sseA); (ii) structural operon I (ssaB); (iii) structural operon II (ssaG); (iv and v) effectors encoded outside of SPI-2 (sseL and sifA); and (vi) integrated virulence genes unlinked to SPI-2 (srfN) [9]. Transcriptional fusions to lacZ of the sseA, ssaB, ssaG, sseL, sifA and srfN promoters (mapped previously; [9,24] were integrated into the chromosome of wild type and ΔrpoE cells and then grown in the SsrB-activating medium LPM. The activity of each promoter was measured using a β-galactosidase assay during exponential growth. Although ΔrpoE was observed to have a slightly prolonged lag phase relative to wild type cells under these experimental conditions, at later time points the mutant grew similarly to wild type. To account for any differences in growth kinetics of the cultures, all data was normalized to the optical density at 600 nm of the culture, which permitted direct comparisons.
In wild type cells, promoter activity from all the transcriptional fusions was high, as expected, because LPM medium is highly inducing for SsrB activity [21]. In contrast, promoter activity for sseA, ssaB, and sifA decreased in the rpoE mutant compared to wild type cells (Figure 2A, B and 2D), whereas promoter activity from the ssaG and srfN reporters was upregulated in the rpoE mutant (Figure 2C and 2F). β-galactosidase activity observed from the sseL reporter was unaltered in the rpoE deletion compared to that in wild type cells (Figure 2E). These data are consistent with the protein levels detected for these gene products. Together, these data indicate that σE can have a variable and bidirectional effect on SsrB-regulated virulence genes.
Figure 2.
The transcriptional activity of SsrB-regulated virulence genes is affected by an rpoE deletion. Wild type and ΔrpoE cells carrying single-copy chromosomal transcriptional reporters of (A) PsseA::pPsseA-lacZ, (B) PssaB::pPssaB-lacZ, (C) PssaG::pPssaG-lacZ, (D) PsifA::pPsifA-lacZ, (E) PsseL::pPsseL-lacZ and (F) PsrfN::pPsrfN-lacZ were grown in LPM (pH 5.8). At the indicated time β-galactosidase activity was measured and expressed as relative light units (RLU) normalized to optical density of the culture. Wild type and ΔrpoE cells lacking the transcriptional reporters were used as controls in each experiment. Data are the means with standard error from triplicate determinations from three independent experiments.
The effect of RpoE on virulence genes is downstream of ssrB expression
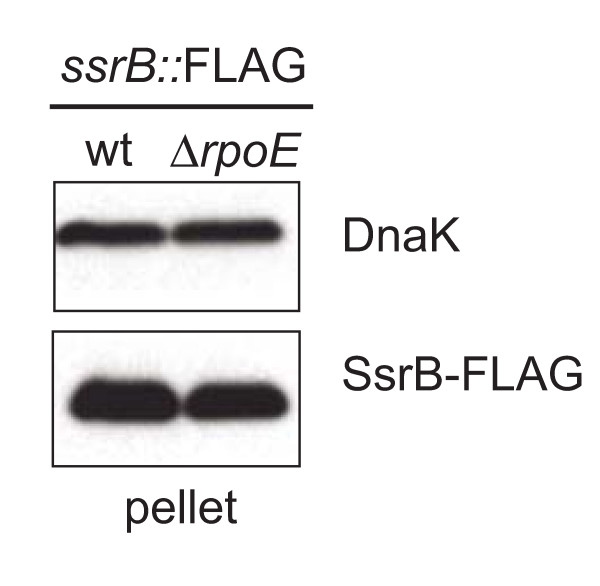
The variable effects of rpoE deletion on SsrB-regulated effectors suggested that RpoE might direct transcription downstream of ssrB expression. To test this, we replaced the ssrB gene in ΔrpoE and wild type cells with an ssrB::FLAG allele [19] and examined the levels of SsrB protein in the strains by western blot. There was no change in the levels of SsrB-FLAG between wild type and ΔrpoE cells (Figure 3), indicating that the effects of RpoE on the four classes of virulence gene promoters examined here was not mediated through changes to SsrB protein levels. Together these data establish a role for RpoE in the fine-tuning of virulence gene expression in S. Typhimurium.
Figure 3.
The effect of RpoE on SsrB-regulated genes is downstream of ssrAB expression. The ssrB gene in wild type and ΔrpoE cells was replaced with an ssrB-FLAG allele in its native location on the chromosome. Cells were grown under SsrB-activating conditions for six hours and lysates were probed by western blot to detect SsrB-FLAG and intracellular DnaK as a control. The data shown are representative of two experiments performed independently with identical results.
Discussion
In this work we found that the alternative sigma factor, σE, is involved in fine tuning the expressing of a subset of SsrB-regulated virulence genes required for Salmonella pathogenesis. Although the effect of rpoE deletion on promoter activity in some cases was mild, we have previously shown that gene regulators providing only modest transcriptional input have a profound influence on bacterial fitness in a host animal [25]. In cases where the regulator is deleted, the loss of genetic fine-tuning causes incongruous changes in the timing and magnitude of virulence gene expression, leading to fitness loss and strong attenuation. We predict that RpoE confers a similar fine-tuning effect on Salmonella virulence gene expression that is required for optimal within-host fitness during infection.
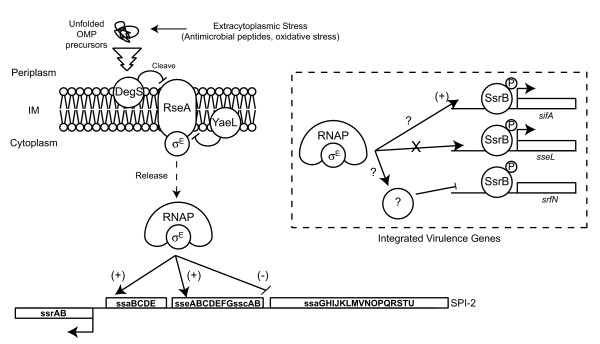
When we examined the -10 and -35 positions of the promoters studied here relative to the transcriptional start sites identified previously [24], these promoters did not appear to contain σE consensus sequences. Instead they appeared to have consensus sites for σ70. Although a bioinformatics screen identified σE consensus sequences upstream of the SPI-2 genes ssaU, ssaJ, sscA and ssaC [26], these genes were not tested for σE-dependence in the present study because the identified consensus sites are in coding sequence within operons, and as a result may not be directly relevant. Due to the high degree of conservation in σ factor binding sequences, σE may not be directly regulating SsrB-dependent promoters. The lack of a canonical σE sequence at these promoters suggests that another regulatory gene may be epistatic to σE or that these promoters encode functional, but non-canonical σE-binding sites due to their horizontal acquisition and gradual integration into the σE regulatory network. This integration may help Salmonella coordinate expression of the virulence-associated T3SS in response to host factors that compromise bacterial membrane integrity (Figure 4). This mechanism would activate a restorative σE pathway, which is consistent with the enhanced susceptibility of rpoE mutants to oxidative stress and antimicrobial peptides [13,15,16], both of which perturb membrane integrity in vivo. Although there is no evidence that σE can directly repress transcription, the negative effect on two promoters observed here might be due to an intermediate RpoE-regulated repressor or compensatory effect where loss of rpoE increases the relative abundance of another sigma factor that can directly activate the ssaG and srfN promoters. Future work will be required to resolve these possibilities.
Figure 4.
Model for σE-dependent regulation of the SsrB regulon. Membrane-targeting host defences including reactive oxygen stress and antimicrobial peptides cause an accumulation of unfolded outer membrane proteins (OMPs) and stimulate the cleavage of the anti-sigma factor RseA consequently releasing σE into the cytoplasm where it directs RNA polymerase to a subset of SPI-2 promoters. RpoE can positively or negatively regulate SsrB-regulated genes including integrated virulence genes unlinked with SPI-2 but has no effect on some effector genes such as sseL. This regulatory pathway may have evolved to coordinate virulence gene expression with host infection by responding to host-specific defence pathways that perturb the bacterial outer membrane.
Our results indicate that rpoE deletion has no effect on SsrB levels under SPI-2 inducing conditions suggesting that the σE pathway regulates effector expression downstream of ssrAB transcription. Unlinking ssrAB transcription from the σE regulon would be advantageous to the cell to prevent commitment to a virulence gene expression program in response to envelope stress not associated with infection. The results from this study demonstrate that σE has the ability to affect expression of SsrB-regulated virulence genes and offers potential insight into the virulence attenuation of rpoE mutants. Although when considered individually, each promoter was modestly affected by deletion of rpoE, the cumulative effects of mild rewired inputs on multiple virulence promoters has been shown to severely compromise in-host fitness and virulence ability [25].
Conclusion
Based on these and other data [4,12-15], the genetic interaction between σE and a subset of SsrB-regulated genes may serve to coordinate the spatial and temporal activation of virulence genes in a host setting, likely in response to membrane damage resulting from oxidative anti-microbial systems and membrane-targeted host defence peptides.
Methods
Strains and Growth Conditions
Bacteria were propagated at 37°C with aeration in Luria-Bertani (LB) broth. S. enterica serovar Typhimurium (S. Typhimurium) strain 14028s with inactivating mutations in rpoE, rpoS, rpoN and rpoH were provided by Ferric Fang (University of Washington, Seattle, WA) [27]. ΔrpoH was grown at 30°C and ΔrpoN was supplemented with 2 mM L-glutamine. An unmarked, in-frame deletion of rpoE was made in S. Typhimurium strain SL1344 by λ Red recombination [28] using primers BKC187 and BKC188. Mutants were screened for loss of rpoE using primers BKC193 and BKC194. To generate an ssrB::FLAG allele in ΔrpoE, the ssrB::FLAG allele from wild type SL1344 [19] was transduced into ΔrpoE by P22-mediated transduction. All plasmids and strains used in this work are described in Table 1. Primer sequences for mutant and plasmid construction are listed in Table 2.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or description a | Reference |
|---|---|---|
| Plasmids | ||
| pKD46 | repA101ts, oriR101, araC-ParaB-gam-bet-exo, bla | [28] |
| pCP20 | FLP+, λ cl857+ λ pR Repts, AmpR, CmR | [28] |
| pKD3 | pANTSy derivative, FRT-flanked cat from pSC140, CmR | [28] |
| prpoE | rpoE with endogenous promoters in pWSK29, AmpR | This work |
| psrfN-2HA | srfN with endogenous promoter and C-term. tandem HA fusion in pWSK129, KanR | Our collection |
| psseL-2HA | sseL with endogenous promoter and C-term. tandem HA fusion in pWSK129, KanR | [8] |
| psseL-2HA | sifA with endogenous promoter and internal tandem HA fusion in pACYC184 | [37] |
| pIVET5n | tnpR-lacZ, sacB, R6K ori, bla, AmpR | [29] |
| pPsseA-lacZ | sseA promoter fused to tnpR-lacZ in pIVET5n, AmpR | [29] |
| pPsseL-lacZ | sseA promoter fused to tnpR-lacZ in pIVET5n, AmpR | This work |
| pPsrfN-lacZ | srfN promoter fused to tnpR-lacZ in pIVET5n, AmpR | This work |
| pPsifA-lacZ | sifA promoter fused to tnpR-lacZ in pIVET5n, AmpR | This work |
| pPssaB-lacZ | ssaB promoter fused to tnpR-lacZ in pIVET5n, AmpR | This work |
| pPssaG-lacZ | ssaG promoter fused to tnpR-lacZ in pIVET5n, AmpR | This work |
| Strains | ||
| SL1344 | wild type S. enterica sv. Typhimurium, SmR | [38] |
| 14028s | wild type S. enterica sv. Typhimurium | [27] |
| 14028s rpoE::cat | rpoE deletion, CmR | [27] |
| 140282 rpoS::Tn10dCm | rpoS deletion, CmR | [27] |
| 14028s rpoN::Ap | rpoN deletion, AmpR | [27] |
| 14028s rpoH::PCN | rpoH deletion, temperature sensitive, AmpR | [27] |
| SL1344 ΔrpoE | Unmarked, in-frame deletion of rpoE, SmR | This work |
| SL1344 ssrB::3FLAG | ssrB-FLAG replacement allele on chromosome | This work |
| SL1344 PsseA::pPsseA-lacZ | Merodiploid containing integrated PsseA-lacZ reporter | [25] |
| SL1344 PsseL::pPsseL-lacZ | Merodiploid containing integrated PsseL-lacZ reporter | This work |
| SL1344 PsrfN::pPsrfN-lacZ | Merodiploid containing integrated PsrfN-lacZ reporter | This work |
| SL1344 PsifA::pPsifA-lacZ | Merodiploid containing integrated PsifA-lacZ reporter | This work |
| SL1344 PssaB::pPssaB-lacZ | Merodiploid containing integrated PssaB-lacZ reporter | This work |
| SL1344 PssaG::pPssaG-lacZ | Merodiploid containing integrated PssaG-lacZ reporter | This work |
| ΔrpoE PsseA::pPsseA-lacZ | Merodiploid containing integrated PsseA-lacZ reporter | This work |
| ΔrpoE PsseL::pPsseL-lacZ | Merodiploid containing integrated PsseL-lacZ reporter | This work |
| ΔrpoE PsrfN::pPsrfN-lacZ | Merodiploid containing integrated PsrfN-lacZ reporter | This work |
| ΔrpoE PsifA::pPsifA-lacZ | Merodiploid containing integrated PsifA-lacZ reporter | This work |
| ΔrpoE PssaB::pPssaB-lacZ | Merodiploid containing integrated PssaB-lacZ reporter | This work |
| ΔrpoE PssaG::pPssaG-lacZ | Merodiploid containing integrated PssaG-lacZ reporter | This work |
aSm, streptomycin; Amp, ampicillin; Cm, chloramphenicol; Kan, kanamycin
Table 2.
Primers used in this study
| Primer | Sequenceab |
|---|---|
| BKC183 | GCTCGAGTTTACCGCGCCGGATAACATACCG |
| BKC184 | CACCAATTGTTAGCTGAATGAAGCAACCGTTGCCAG |
| BKC185 | CCTCGAGCGATTGCCGTCAAAGGTATTC |
| BKC186 | CACCAATTGTTACTGATCACCGTTCTCTACGGCGCT |
| BKC187 | GGTTTGGGGAGACATTACCTCGGATGAGCGAGCAGTTAGTGTAGGCTGGAGCTGCTTCG |
| BKC188 | CCTAATACCTTTTCCAGTATCCCGCTATCGTCAACGCATATGAATATCCTCCTTA |
| BKC195 | CAGCTCACTATCCAACGTTT |
| BKC196 | TGCTTGCTCATAGTGCGGCTT |
| BKC205 | CACCAATTGTTACTATAGTAATCGGCATATTAA |
| BKC206 | CCGCTCGAGTATGGCGCTGATCGCCAC |
| SEO005 | CCGCTCGAGGTGCCATCCTTTGCCGTTT |
| SEO006 | CACCAATTGTTACATGAATCCCTCCTCAGACAT |
| SEO011 | CCGCTCGAGATATGGAGAGTGGTAGAATAG |
| SEO012 | CACCAATTGTTATAATTGTGCAATATCCATAA |
| SEO095 | GCTCTAGATCAACGCCTGATAAGCGGTT |
| SEO096 | ACGCGTCGACCCACTCTTTATTGCGATTCCA |
aunderlined nucleotides indicate restriction enzyme sites; XhoI (CTCGAG), MfeI (CAATTG)
b bolded nucleotides indicate complementarity to rpoE
Plasmid Construction
A complementation construct for rpoE was generated that included the three endogenous promoters (rpoEp1, rpoEp2, rpoEp3) identified in Salmonella [20] plus the wild type rpoE gene. This construct was cloned into the low-copy plasmid pWSK29 using primers SEO095 and SEO096 as a SalI and XbaI fragment. Constructs were verified by sequencing and transformed into S. Typhimurium SL1344 ΔrpoE and selected on LB agar with appropriate antibiotics. The promoters for ssaB (SEO005 and SEO006), ssaG (SEO011 and SEO012), sifA (SEO205 and SEO206), sseL (BKC185 and BKC186) and srfN (BKC183 and BKC184) were cloned into pIVET5n [29] to generate single-copy transcriptional fusions to lacZ. Reporters were transformed into E. coli SM10 λpir, conjugated into SL1344 and merodiploid cells were selected on LB agar with appropriate antibiotics. Transcriptional fusions, including a previously constructed reporter for the sseA promoter [30], were integrated into the chromosome of wild type and ΔrpoE cells using homologous recombination. The promoters we chose use the SsrB response regulator for expression of the downstream gene or operon, and include both SPI-2-encoded and non-SPI-2-encoded virulence effectors representing structural apparatus genes and effector substrates of the type III secretion system [8,30-35]
Chemiluminescent β-galactosidase Assay
Reporter strains were inoculated from an overnight culture into culture medium (LPM pH 5.8) that induces SsrB-dependent gene expression [21,36]. Cultures were propagated at 37°C for 7 hours and samples were taken hourly to measure β-galactosidase activity using a chemiluminescence assay described previously [25]. Data was expressed as relative light units (RLU) and was normalized to the optical density (OD600 nm) of the parent culture.
Immunoblotting
To examine the protein levels of SseB, SseL, SrfN and SifA under SPI-2 inducing conditions, we used plasmids psifA-2HA, psseL-2HA and psrfN-2HA that were published previously (Table 1) [8,37]. These constructs express the given gene under the control of the endogenous promoter. Wild type and ΔrpoE cells were transformed with these plasmids and grown in LPM pH 5.8 at 37°C for 6 hours. Whole cell lysates were collected and analyzed by immunoblotting using anti-SseB (1:1000) [21] and anti-HA (1:1000, Covance) antibodies. Blots were probed for DnaK (1:3500, Stressgen) as a control.
Authors' contributions
SEO designed and performed research, interpreted data and wrote the paper. BKC designed and interpreted research and wrote the paper. Both authors read and approved the final manuscript.
Contributor Information
Suzanne E Osborne, Email: osborns@mcmaster.ca.
Brian K Coombes, Email: coombes@mcmaster.ca.
Acknowledgements
We would like to thank Jose Puente for providing λ Red recombination plasmids, Ferric Fang for providing sigma factor mutants in the 14028s strain background, and members of the Coombes laboratory for helpful comments on this work. This work was funded by a grant to BKC from the Canadian Institute for Health Research (MOP-82704). SEO is the recipient of an Ontario Graduate Scholarship. BKC is the recipient of a New Investigator Award from the CIHR, a Young Investigator Award from the American Society of Microbiology, and an Early Researcher Award from the Ontario Ministry of Research and Innovation.
References
- Shea JE, Hensel M, Gleeson C, Holden DW. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H, Soncini FC, Solomon F, Groisman EA. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo DM, Valdivia RH, Monack DM, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- Hensel M. Salmonella pathogenicity island 2. Mol Microbiol. 2000;36:1015–1023. doi: 10.1046/j.1365-2958.2000.01935.x. [DOI] [PubMed] [Google Scholar]
- Hensel M, Shea JE, Waterman SR, Mundy R, Nikolaus T, Banks G, Vazquez-Torres A, Gleeson C, Fang FC, Holden DW. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- Garmendia J, Beuzon CR, Ruiz-Albert J, Holden DW. The roles of SsrA-SsrB and OmpR-EnvZ in the regulation of genes encoding the Salmonella typhimurium SPI-2 type III secretion system. Microbiology. 2003;149:2385–2396. doi: 10.1099/mic.0.26397-0. [DOI] [PubMed] [Google Scholar]
- Worley MJ, Ching KH, Heffron F. Salmonella SsrB activates a global regulon of horizontally acquired genes. Mol Microbiol. 2000;36:749–761. doi: 10.1046/j.1365-2958.2000.01902.x. [DOI] [PubMed] [Google Scholar]
- Coombes BK, Lowden MJ, Bishop JL, Wickham ME, Brown NF, Duong N, Osborne S, Gal-Mor O, Finlay BB. SseL is a Salmonella-specific translocated effector integrated into the SsrB-controlled salmonella pathogenicity island 2 type III secretion system. Infect Immun. 2007;75:574–580. doi: 10.1128/IAI.00985-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne S, Walthers D, Tomljenovic AM, Mulder D, Silphaduang U, Duong N, Lowden M, Wickham ME, Waller R, Kenney LJ. Pathogenic adaptation of intracellular bacteria by rewiring a cis-regulatory input function. Proc Natl Acad Sci USA. 2009. in press . [DOI] [PMC free article] [PubMed]
- Browning DF, Busby SJ. The regulation of bacterial transcription initiation. Nat Rev Microbiol. 2004;2:57–65. doi: 10.1038/nrmicro787. [DOI] [PubMed] [Google Scholar]
- Alba BM, Gross CA. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol Microbiol. 2004;52:613–619. doi: 10.1111/j.1365-2958.2003.03982.x. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, Mastroeni P, Fang FC. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- Testerman TL, Vazquez-Torres A, Xu Y, Jones-Carson J, Libby SJ, Fang FC. The alternative sigma factor sigmaE controls antioxidant defences required for Salmonella virulence and stationary-phase survival. Mol Microbiol. 2002;43:771–782. doi: 10.1046/j.1365-2958.2002.02787.x. [DOI] [PubMed] [Google Scholar]
- Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Crouch ML, Becker LA, Bang IS, Tanabe H, Ouellette AJ, Fang FC. The alternative sigma factor sigma is required for resistance of Salmonella enterica serovar Typhimurium to anti-microbial peptides. Mol Microbiol. 2005;56:789–799. doi: 10.1111/j.1365-2958.2005.04578.x. [DOI] [PubMed] [Google Scholar]
- Humphreys S, Stevenson A, Bacon A, Weinhardt AB, Roberts M. The alternative sigma factor, sigmaE, is critically important for the virulence of Salmonella typhimurium. Infect Immun. 1999;67:1560–1568. doi: 10.1128/iai.67.4.1560-1568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol. 2003;47:103–118. doi: 10.1046/j.1365-2958.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- Carlsson KE, Liu J, Edqvist PJ, Francis MS. Extracytoplasmic-stress-responsive pathways modulate type III secretion in Yersinia pseudotuberculosis. Infect Immun. 2007;75:3913–3924. doi: 10.1128/IAI.01346-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong N, Osborne S, Bustamante VH, Tomljenovic AM, Puente JL, Coombes BK. Thermosensing coordinates a cis-regulatory module for transcriptional activation of the intracellular virulence system in Salmonella enterica serovar Typhimurium. J Biol Chem. 2007;282:34077–34084. doi: 10.1074/jbc.M707352200. [DOI] [PubMed] [Google Scholar]
- Miticka H, Rowley G, Rezuchova B, Homerova D, Humphreys S, Farn J, Roberts M, Kormanec J. Transcriptional analysis of the rpoE gene encoding extracytoplasmic stress response sigma factor sigmaE in Salmonella enterica serovar Typhimurium. FEMS Microbiol Lett. 2003;226:307–314. doi: 10.1016/S0378-1097(03)00600-1. [DOI] [PubMed] [Google Scholar]
- Coombes BK, Brown NF, Valdez Y, Brumell JH, Finlay BB. Expression and secretion of Salmonella pathogenicity island-2 virulence genes in response to acidification exhibit differential requirements of a functional type III secretion apparatus and SsaL. J Biol Chem. 2004;279:49804–49815. doi: 10.1074/jbc.M404299200. [DOI] [PubMed] [Google Scholar]
- Nitta T, Nagamitsu H, Murata M, Izu H, Yamada M. Function of the sigma(E) regulon in dead-cell lysis in stationary-phase Escherichia coli. J Bacteriol. 2000;182:5231–5237. doi: 10.1128/JB.182.18.5231-5237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir MS, Yamashita D, Koyama S, Oshima T, Kurokawa K, Maeda M, Tsunedomi R, Murata M, Wada C, Mori H. Cell lysis directed by sigmaE in early stationary phase and effect of induction of the rpoE gene on global gene expression in Escherichia coli. Microbiology. 2005;151:2721–2735. doi: 10.1099/mic.0.28004-0. [DOI] [PubMed] [Google Scholar]
- Walthers D, Carroll RK, Navarre WW, Libby SJ, Fang FC, Kenney LJ. The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Mol Microbiol. 2007;65:477–493. doi: 10.1111/j.1365-2958.2007.05800.x. [DOI] [PubMed] [Google Scholar]
- Coombes BK, Wickham ME, Lowden MJ, Brown NF, Finlay BB. Negative regulation of Salmonella pathogenicity island 2 is required for contextual control of virulence during typhoid. Proc Natl Acad Sci USA. 2005;102:17460–17465. doi: 10.1073/pnas.0505401102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodius VA, Suh WC, Nonaka G, West J, Gross CA. Conserved and variable functions of the sigmaE stress response in related genomes. PLoS Biol. 2006;4:e2. doi: 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang IS, Frye JG, McClelland M, Velayudhan J, Fang FC. Alternative sigma factor interactions in Salmonella : sigma and sigma promote antioxidant defences by enhancing sigma levels. Mol Microbiol. 2005;56:811–823. doi: 10.1111/j.1365-2958.2005.04580.x. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NF, Vallance BA, Coombes BK, Valdez Y, Coburn BA, Finlay BB. Salmonella pathogenicity island 2 is expressed prior to penetrating the intestine. PLoS Pathog. 2005;1:e32. doi: 10.1371/journal.ppat.0010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes BK, Brown NF, Kujat-Choy S, Vallance BA, Finlay BB. SseA is required for translocation of Salmonella pathogenicity island-2 effectors into host cells. Microbes Infect. 2003;5:561–570. doi: 10.1016/S1286-4579(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Beuzon CR, Meresse S, Unsworth KE, Ruiz-Albert J, Garvis S, Waterman SR, Ryder TA, Boucrot E, Holden DW. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000;19:3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumell JH, Tang P, Zaharik ML, Finlay BB. Disruption of the Salmonella-containing vacuole leads to increased replication of Salmonella enterica serovar typhimurium in the cytosol of epithelial cells. Infect Immun. 2002;70:3264–3270. doi: 10.1128/IAI.70.6.3264-3270.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Albert J, Mundy R, Yu XJ, Beuzon CR, Holden DW. SseA is a chaperone for the SseB and SseD translocon components of the Salmonella pathogenicity-island-2-encoded type III secretion system. Microbiology. 2003;149:1103–1111. doi: 10.1099/mic.0.26190-0. [DOI] [PubMed] [Google Scholar]
- Zurawski DV, Stein MA. SseA acts as the chaperone for the SseB component of the Salmonella Pathogenicity Island 2 translocon. Mol Microbiol. 2003;47:1341–1351. doi: 10.1046/j.1365-2958.2003.03373.x. [DOI] [PubMed] [Google Scholar]
- Rytkonen A, Poh J, Garmendia J, Boyle C, Thompson A, Liu M, Freemont P, Hinton JC, Holden DW. SseL, a Salmonella deubiquitinase required for macrophage killing and virulence. Proc Natl Acad Sci USA. 2007;104:3502–3507. doi: 10.1073/pnas.0610095104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiwick J, Nikolaus T, Erdogan S, Hensel M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol Microbiol. 1999;31:1759–1773. doi: 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- Brumell JH, Goosney DL, Finlay BB. SifA, a type III secreted effector of Salmonella typhimurium, directs Salmonella-induced filament (Sif) formation along microtubules. Traffic. 2002;3:407–415. doi: 10.1034/j.1600-0854.2002.30604.x. [DOI] [PubMed] [Google Scholar]
- Wray C, Sojka WJ. Experimental Salmonella typhimurium infection in calves. Res Vet Sci. 1978;25:139–143. [PubMed] [Google Scholar]