Abstract
During infection of a new host, the first surfaces encountered by herpes simplex viruses are the apical membranes of epithelial cells of mucosal surfaces. These cells are highly polarized, and the protein composition of their apical and basolateral membranes are very different, so that different viral entry pathways have evolved for each surface. To determine whether the viral glycoprotein G (gG) is specifically required for efficient infection of a particular surface of polarized cells, apical and basal surfaces were infected with wild-type virus or a gG deletion mutant. After infection of polarized cells in culture, the gG− virus was deficient in infection of apical surfaces but was able to infect cells through basal membranes, replicate, and spread into surrounding cells. The gG-dependent step in apical infection was a stage beyond attachment. After in vivo infection of apical surfaces of epithelial cells of nonscarified mouse corneas, infection by glycoprotein C− or gG− virus was considerably reduced as compared with that observed after infection with wild-type virus. In contrast, when corneas were scarified, allowing virus access to other cell surfaces, the gG and glycoprotein C deletion mutants infected eyes as efficiently as wild-type viruses. A secondary mutation allowing infection of apical surfaces by gG− virus arose readily during passage of the virus in nonpolarized cells, indicating that either the gG-dependent step of apical infection can be bypassed or that another viral protein can acquire the same function.
In the course of a natural infection, in the absence of any wound to the epithelium, herpes simplex viruses (HSVs) first must infect the apical surfaces of the epithelial cells of mucosal membranes. After replication in the epithelium, the virus infects the peripheral endings of sensory neurons, is transported through axons to the neuronal nuclei, and establishes a latent infection. Reactivation of latent virus results in axonal transport of virus back to the epithelium and recurrent infection of epithelial cells near the site of initial infection. Both epithelial cells and neurons are highly polarized cells, sorting membrane and secreted proteins very specifically to either the apical or basolateral domains, resulting in very different protein compositions of the two surfaces (reviewed in ref. 1). Therefore, completion of this cycle by HSV requires the infection of three very different cell surfaces: apical surfaces of epithelial cells and peripheral endings of sensory neurons during primary infection, and basolateral surfaces of epithelial cells during recurrent infection. Because infection first involves interaction with cell surface components, it seems likely that HSV infection of different surfaces of polarized cells may require different complements of viral proteins.
The first indication that HSV-1 encodes proteins specifically required for multiple infection pathways came from studies of glycoprotein C (gC). With a few exceptions, cultured cells generally have lost the ability to polarize and exhibit most membrane proteins mixed fairly homogeneously on all surfaces. Although attachment to nonpolarized cells in culture is primarily accomplished by glycoprotein B (2), gC also was shown to play a secondary role in attachment of HSV-1 to nonpolarized cells (3, 4). This apparent redundancy was resolved when polarized epithelial cells were infected with a recombinant virus containing a deletion of the gC gene; the gC− virus was unable to attach to or infect apical surfaces, but was able to attach to and infect basal membranes, apparently via an interaction between a different viral protein and a second cell surface receptor (5). After basal infection of polarized epithelial cells, the gC− virus replicated and spread to surrounding cells (5). Nonpolarized cells in culture apparently express the proteins necessary for both gC-dependent and gC-independent attachment, so that attachment of gC− virus is impaired but not ablated (3).
The function of glycoprotein G (gG) also has been difficult to identify. Recombinant viruses with deletions of or insertions into the gG gene replicate normally in nonpolarized cells in culture (6, 7) and exhibit little or no attenuation of replication in vivo (7). However, previous studies, both in cell culture and in vivo, have used models that bypassed the need for the virus to infect apical cell surfaces. We show here that a recombinant virus lacking gG is defective in infection of the apical surfaces of polarized epithelial cells in culture. In addition, both gG and gC are required for efficient infection in vivo of the apical surfaces of corneal epithelial cells.
Materials and Methods
Cells and Viruses.
Vero and Madin-Darby canine kidney (MDCK) cells were obtained from the American Type Culture Collection. Rabbit skin cells and HSV1(F), a wild-type clinical isolate, passaged a limited number of times in culture, were gifts from Bernard Roizman, University of Chicago. Details of the construction of R6012 (a gC deletion mutant) and R6016 (a repair of R6012) have been published previously (5). MDCK cells were maintained in DMEM supplemented with 10% FBS and were cultured as polarized monolayers on 3-μm microporous filters (Falcon). To ensure that polarized cultures on filters were free of clumps or multilayered regions of nonpolarized cells, considerable care was taken when the cells were plated. Filters were equilibrated in PBS containing 0.9 mM CaCl2 and 0.5 mM MgCl2 for 4 hr before to adding cells (3 × 106 MDCK cells/filter), and medium was changed daily before use. All MDCK cultures were used 3 days postseeding; these conditions reproducibly resulted in polarized cultures as assayed by transepithelial resistance (over 1,000 Ohm⋅cm2), and without clumps or multilayered regions as assessed by confocal microscopy.
Infection of Cells on Filters.
Vero and MDCK cells were grown on 3.0-μm pore size filters (Falcon) suspended in 6-well plates and infected immediately after confluency (Vero) or 48 hr after confluency (MDCK). Virus was added to either the apical or basal compartments. Cells were fixed 48 hr after infection and immunoperoxidase-stained by using antibody directed against glycoprotein D (gD) to visualize plaques.
Plasmids and Antibodies.
pRB4006 contains a PstI–SacI fragment of HSV-1(F) DNA, spanning the gG gene, with a deletion of approximately 50% of the gG coding sequences. mAb #1107 directed against gG was purchased as ascites fluid from the Goodwin Institute for Cancer Research (Plantation, FL) and was used at a dilution of 1:500.
Infection of Mice.
Female CBA/J mice were purchased at 3 weeks of age (The Jackson Laboratory) and housed for 2½ weeks in suspended caging to prevent any abrasion of the eyes from bedding materials. Mice then were anaesthetized before inoculation. Scarification, when performed, was accomplished by scratching the cornea lightly 10 times with a 30-gauge needle. Inocula were centrifuged for 5 min at 1,500 × g to remove any particulate matter that could cause abrasion, and eyes were infected with 106 plaque-forming units (pfu) in 10 μl/eye. For infection of nonscarified eyes, care was taken not to touch the surface of the eye with pipet tips. At various times after infection, mice were anaesthetized and euthanized, and eyes were removed and placed in 1 ml of medium, frozen, thawed, and homogenized. Homogenates were sonicated and centrifuged at 1,500 × g for 10 min, and virus in supernatants was titered on Vero cells.
Results
Construction of Recombinant Viruses.
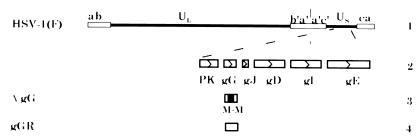
A virus with a deletion of the gG coding sequences was constructed by cotransfection of rabbit skin cells with wild-type HSV-1(F) DNA and a plasmid containing the gG gene with a deletion of approximately 50% of the coding sequences (Fig. 1). Stocks of viruses from the transfections were plated on Vero cells, and live infected cells were immunoperoxidase-stained by using an antibody directed against gG (8). Plaques generated by viruses not expressing gG were identified by the lack of staining, and gG− viruses were isolated and purified. The genomic structure of the deletion mutant (RAS104) was confirmed by Southern blot analysis.
Figure 1.
Genome arrangements of HSV-1(F), RAS104, and RAS105. Line 1 shows the genomic structure of HSV-1(F). Line 2 is an expansion of part of Us. Open boxes designate ORFs as defined by McGeoch et al. (20). PK: protein kinase; gG, gJ, gD, gE, and gI: glycoproteins G, J, D, E, and I. Line 3 indicates the location of the deletion contained in RAS104. M: MboI restriction endonuclease. Line 4 indicates the restoration of deleted sequences in RAS105.
As a control to ensure that any phenotype observed after infection with RAS104 was caused by the loss of the gG gene, and not by a secondary mutation in the viral genome, the deletion in the RAS104 genome was repaired. RAS104 viral DNA was cotransfected with a plasmid containing an intact gG gene. The repaired virus (RAS105) was isolated and purified by selection of positively staining plaques in immunoperoxidase assays using anti-gG antibody. In no case did the phenotype of RAS105 differ from that of the wild-type parental strain, HSV-1(F).
gG Is Required for Efficient Infection of Apical But Not Basal Surfaces of Polarized Epithelial Cells in Culture.
Previous studies of the role of gG in infection of a variety of cell types in culture and in vivo have failed to identify a step of infection requiring gG (6, 7). Those studies, however, did not examine the role of gG in infection of specific surfaces of polarized epithelial cells. To determine whether gG was required for infection of apical or basal membranes, MDCK cells were grown on microporous filters and infected from either the apical or basal compartments with wild-type virus, the gG deletion mutant RAS104, or the gG repaired virus RAS105. Cell polarity was assayed by transepithelial resistance; confluent monolayers exhibited resistances of greater than 1,000 Ohm⋅cm2. R6012, a recombinant virus with a deletion in gC, and R6016, with a repair of the R6012 deletion, were included as additional controls; R6012 previously has been shown to attach to and infect basal but not apical membranes of MDCK cells. After apical infection of cells on filters, the efficiency of plating (MDCK titer/Vero titer) for RAS104 was 300-fold lower than those of the wild-type viruses (Table 1). After basal infection, the efficiency of plating of RAS104 was slightly reduced as compared with wild-type viruses (Table 1).
Table 1.
Apical and basal efficiency of plating of viruses on cells grown on filters
| Virus | Apical
|
Basal
|
||||
|---|---|---|---|---|---|---|
| MDCK | Vero | MDCK/Vero | MDCK | Vero | MDCK/Vero | |
| HSV-1(F) | 2.8 × 104 | 5.5 × 108 | 3.1 × 10−5 | 3.3 × 102 | 4.1 × 107 | 8.0 × 10−6 |
| RAS104 | 1.0 × 102 | 7.8 × 108 | 1 × 10−7 | 4.8 × 101 | 8.8 × 107 | 5.0 × 10−7 |
| RAS105 | 2.0 × 105 | 6.4 × 109 | 3.1 × 10−5 | 9.2 × 102 | 4.3 × 108 | 2.1 × 10−6 |
| R6012 | <10 | 1.3 × 109 | <7.7 × 10−9 | 2.3 × 102 | 1.4 × 108 | 1.6 × 10−6 |
Titer for each virus calculated after apical or basal infection of MDCK or Vero cells grown on 3.0-μm pore filters. Efficiency of plating on MDCK vs. Vero cells was calculated as the ratio of titers obtained on each cell line.
After basal infection of MDCK cells, RAS104 spread from cell to cell and formed plaques indistinguishable from those of the wild-type viruses (data not shown). gG was therefore necessary for efficient infection of apical membranes, but not for infection of basal membranes, for replication in MDCK cells, or for cell-to-cell spread.
gG Enhances But Is Not Required for Attachment to Apical Membranes.
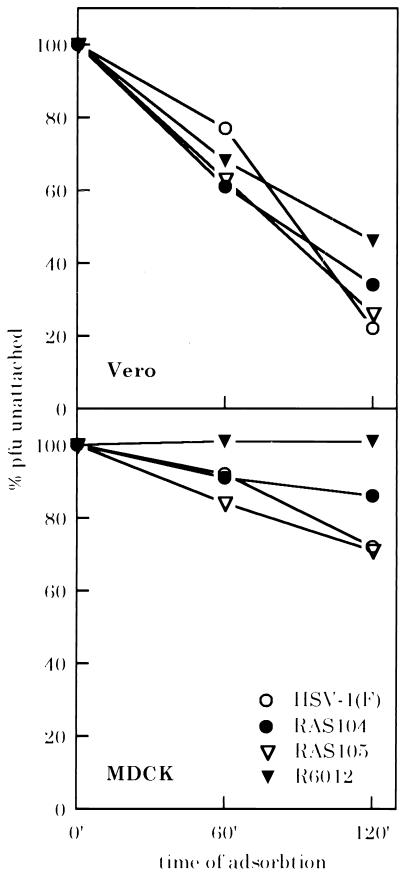
To determine what stage of infection of apical membranes was blocked by the absence of gG, the ability of wild-type and mutant viruses to attach to MDCK and Vero cells was assayed. RAS104, RAS105, HSV-1(F), and R6012 were added to confluent monolayers of Vero or MDCK cells grown on plastic plates. Zero, 60, or 120 min after addition of the virus to the cells, portions of the inoculum were removed and titered on Vero cells to determine the amount of virus remaining unattached (5). Attachment of RAS104 to Vero cells was as efficient as that of the wild-type control viruses (Fig. 2). A slight decrease in the level of attachment of R6012 to Vero cells as compared with the wild-type controls was observed, consistent with previous reports that gC enhances levels of attachment even to nonpolarized cells (3). One hundred percent of R6012 remained unattached to MDCK cells 60 and 120 min after infection, indicating that the cells were polarized, and that the only pathway of entry functioning during apical infection was the gC-dependent pathway. Final levels of attachment of HSV-1(F) virus and RAS105 were similar (28% and 27%, respectively), whereas only 14% of RAS104 was removed from the inoculum. This 2-fold difference in levels of attachment was reproducible in several experiments (data not shown); although gG was not required for attachment to apical membranes, it appeared to enhance gC-dependent attachment.
Figure 2.
Attachment of gG and wild-type viruses to MDCK and Vero cells. % pfu unattached: percent of original inoculum remaining in medium after adsorbtion. (Upper) Vero cells. (Lower) MDCK cells.
The Defect in Infection Caused by the Loss of gG Can Be Complemented by a Second Site Mutation in the Viral Genome.
Plaques that were formed in MDCK cells apically infected with RAS104 could have arisen from contaminating wild-type virus, from a low level of infectivity of RAS104 itself, or from viruses with a compensatory mutation at a locus other than the gG gene. To distinguish between these possibilities, plaques were isolated from MDCK cells after apical infection with RAS104, and stocks of the viruses were grown on Vero cells. These viruses then were tested for the presence of the gG sequences deleted from RAS104, production of the gG protein, and their ability to infect MDCK cells from the apical surface. Southern blot analysis, as well as immunoperoxidase stains of infected cells with anti-gG antibody, indicated that the viral genomes of these viruses still retained the deletion in gG, and that the viruses did not produce any gG protein (data not shown). However, after apical infection of MDCK cells, the efficiency of plating of these reversion mutants (designated gGrev) was approximately 10-fold better than that of wild-type virus (Table 2). This reversion was not simply caused by passage of virus through MDCK cells, as plaques picked from MDCK cells infected apically with HSV-1(F) or stocks of HSV1-(F) passaged through MDCK cells did not exhibit the same increase in efficiency of plating (data not shown). Plaques formed by the gGrev viruses were not syncytial in either MDCK or Vero cells. The gGrev viruses acquired a second-site mutation that allowed them to bypass the gG-dependent step of apical infection.
Table 2.
Efficiency of plating of viruses with mutations allowing gG-independent apical infection
| Virus | MDCK/Vero |
|---|---|
| HSV-1(F) | 2 × 10−5 |
| RAS104 | 1 × 10−7 |
| gGrev | 1 × 10−4 |
Efficiency of plating on MDCK vs. Vero cells was calculated as the ratio of titers obtained on each cell line.
gG and gC Are Required in Vivo for Efficient Infection of Apical Surfaces of Mouse Corneal Epithelial Cells.
Infection of apical surfaces in cell culture is most likely reflective of the first stage of infection of a new host in vivo, i.e., infection of the apical surfaces of mucosal epithelial cells. Because most currently used animal models involve scarification of epithelial surfaces, allowing access of virus to basolateral membranes or deposition of virus s.c. at or near the basal layer of cells, a model was devised to assess the ability of wild-type and recombinant viruses to infect apical membranes in vivo. In these assays, 106 pfu of virus was deposited onto corneal epithelial cells with or without prior scarification. For infection of nonscarified eyes, extreme care was taken to avoid accidental abrasion of the eyes by bedding materials, particulate debris in the inocula, or pipets. Eyes were harvested 36, 60, or 84 hr after infection, and the tissue was homogenized to recover infectious virus.
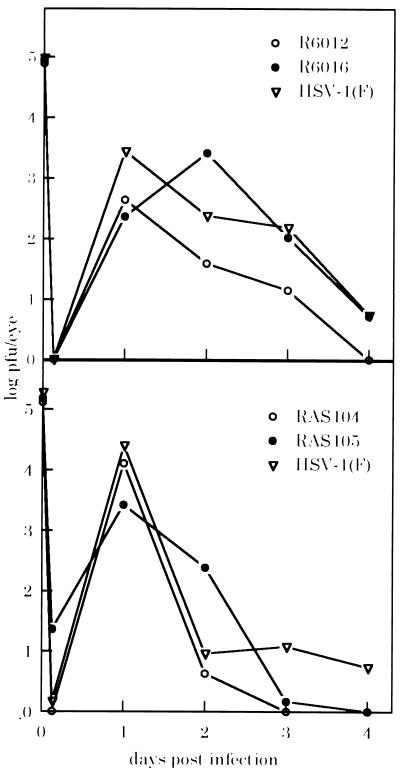
After infection of nonscarified eyes, a considerable decrease was observed in the ability of recombinant viruses R6012 (gC−) and RAS104 (gG−) to infect and replicate in the epithelium (Table 3). Only one eye of 14 infected with each virus yielded detectable levels of virus. In contrast, the wild-type viruses were recovered from 4- to 8-fold more nonscarified eyes (P < 0.05 for either RAS104 or R6012 compared with wild-type viruses). Virus yields from the two positive eyes infected with R6012 and RAS104 were comparable to those recovered from wild-type virus-infected eyes, indicating that as in cell culture, once these viruses did infect polarized cells they replicated normally. When the same viruses were used to infect scarified eyes, 100% of the eyes yielded infectious virus, and no significant differences were seen in the mean titers of recovered mutant or wild-type viruses (Fig. 3), indicating that once infection was accomplished all of the viruses were equally capable of replication in the mouse eye.
Table 3.
Growth of virus in nonscarified eyes
| Virus | # Positive eyes/total tested
|
Mean* | Peak† | %Positive | ||
|---|---|---|---|---|---|---|
| 36 hpi | 60 hpi | 84 hpi | ||||
| HSV-1(F) | 2/6 | 1/6 | 2/4 | 1.2 | 1.4 | 31 |
| R6012 | 0/6 | 1/6 | 0/2 | 1.8 | 1.8 | 7‡ |
| R6016 | 3/6 | 1/6 | 2/2 | 2.3 | 2.5 | 43 |
| RAS104 | 0/6 | 0/6 | 1/2 | 1.7 | 1.7 | 7‡ |
| RAS105 | 3/6 | 3/6 | 2/2 | 2.0 | 2.0 | 57 |
| WT total | 8/18 | 6/18 | 6/8 | 1.8 | 44‡ | |
hpi, hours postinfection. WT, wild type.
Log geometric mean titer of virus in all eyes positive at all times indicated.
Log geometric mean titer of virus in all positive eyes at time of highest mean titer.
P < 0.05 comparing either R6012 or RAS104 to sum of wild-type viruses.
Figure 3.
Growth of viruses in scarified mouse eyes. Virus recovered from scarified eyes at 6 hr, 1, 2, 3, or 4 days postinfection was titered on Vero cells. Log pfu/eye: log10 geometric mean titer of virus recovered.
Discussion
The normal life cycle of HSV involves infection of at least three different cell surfaces. The virus first must enter a new host through the apical surfaces of mucosal epithelial cells, and then infect and establish latency in sensory neurons. After reactivation from latency, virus travels via neuronal axons back to the mucosal epithelia and infects basal or lateral surfaces of epithelial cells.
Infection of different cell surfaces requires different viral glycoproteins. During infection of nonpolarized cells in culture, the initial stages of infection are accomplished by glycoprotein B (responsible for most attachment to nonpolarized cells, ref. 2) and gD (required for a postattachment stage of entry, via binding to one of several different cell surface receptors, refs. 9–12). We previously have demonstrated that HSV-1 contains at least one protein, gC, that is specifically required for efficient attachment to and infection of the apical membranes (5). Viral entry therefore appears to occur by at least two different pathways: the gC-dependent, apical pathway, and a gC-independent route, exemplified by the surfaces of nonpolarized cells, and requiring glycoprotein B and gD.
Data presented here indicate that gG also functions specifically in the gC-dependent, apical pathway of infection. A recombinant virus with a deletion of the gG coding sequences was able to attach to apical membranes, but infection was greatly reduced compared with wild-type viruses. After exposure of basal membranes to the same virus, the gG deletion mutant was able to infect the cells, replicate, and spread from cell to cell as well as wild-type viruses. Although it is possible that this is reflective of a third pathway of entry, the most likely explanation is that gG is necessary for some postattachment step of entry in the gC-dependent route, possibly similar to that role carried out by gD on nonpolarized surfaces.
The precise role of gG in the gC-dependent entry pathway is unclear. Attempts to bypass the requirement for gG by polyethylene glycol fusion of attached virus to apical cell surfaces were unsuccessful (data not shown), making it unlikely that the function of gG is simply to fuse virion membranes with apical cell surface membranes. Attachment of gG− virus to apical surfaces was reproducibly less efficient than that of wild-type virus. At least two possible functions for gG could explain this result: gG may directly enhance gC-dependent attachment to the cell surface by functioning cooperatively with gC in binding to the cell surface receptor, or gG may be necessary for a second, independent step in entry that renders gC-dependent attachment irreversible, resulting in higher apparent levels of attachment.
Previous analyses of HSV-1 gC and gG deletion mutants during in vivo infection have failed to identify a role for either protein during viral replication in mice (7, 13, 14), although gC has been shown to be necessary for HSV-1 virulence in human skin implanted into severe combined immunodeficient (SCID) mice (15). gC binds the complement component C3b and protects virions and infected cells from complement mediated lysis (16–18). gC binds murine C3b poorly, and it has been postulated that the lack of phenotype observed with gC deletion mutants in murine models is caused by its inability to affect the murine immune response. However, a role for gC in immune evasion does not rule out the possibility that it also functions directly in the viral replication cycle.
Previous studies have used models of HSV infection that involve scarification of epithelial tissues: ear pinnas in the case of gG or corneas in the case of gC. These routes of inoculation result in deposition of virus near the basal layer of epithelial cells and also can result in infection of the supporting fibroblasts. Wounding of the epithelium also results in the replication and migration into the wound area of dividing, nondifferentiated epithelial cells from the basal epithelial layer; during migration these cells do not form tight junctions with surrounding cells and are not polarized (19). Finally, stocks of the gG recombinant virus used by other investigators could not be tested for the presence of virus compensatory mutations (7) and may have contained high enough percentages of such second site reversions to obscure the role of gG during infection.
In the analyses described here, when infection by gG or gC deletion mutants was limited to the apical surfaces of corneal epithelial cells, significantly fewer eyes were infected by the mutants than by wild-type viruses. In contrast, when scarified corneas were infected with the same viruses, no differences could be detected in infection or replication of the gC or gG deletion mutants and wild-type viruses. Infection of nonscarified corneas allows infection only of apical surfaces. This work then has shown that HSV-1 encodes at least two glycoproteins, gG and gC, that function specifically to allow efficient infection of the first cell surface that is encountered by HSV during infection of a new host, i.e., the apical surface of polarized epithelial cells.
Viruses containing a mutation in a non-gG locus that allowed apical infection by gG− virus were easily isolated. The mutation (gGrev) arose frequently during passage of the gG deletion mutant in nonpolarized Vero cells, and the percentage of gGrev virus increased with repeated passage of RAS104 on Vero cells (data not shown). The presence of this mutation in stocks of RAS104 passaged only on nonpolarized cells indicates that even in nonpolarized cells the mutation confers a selective advantage, and that the loss of gG is somewhat detrimental to virus growth. The second-site mutation may allow the virus to bypass the gG-dependent step of entry either by allowing viral entry via a totally different mechanism than that requiring gG, or by the acquisition of the role of gG by another viral protein; mapping and analysis of the gGrev mutation therefore may help to elucidate the function of gG.
Acknowledgments
We thank Yelena Besnovataya for expert technical assistance. This work was supported by Public Health Service Grants AI32145 and AI24009 from the National Institutes of Health.
Abbreviations
- HSV
herpes simplex virus
- gC
glycoprotein C
- gD
glycoprotein D
- gG
glycoprotein G
- MDCK
Madin-Darby canine kidney
- pfu
plaque-forming unit
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.020510297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.020510297
References
- 1.Mostov K E, Cardone M H. BioEssays. 1995;17:129–138. doi: 10.1002/bies.950170208. [DOI] [PubMed] [Google Scholar]
- 2.Herold B C, Visalli R J, Susmarski N, Brandt C R, Spear P G. J Gen Virol. 1994;75:1211–1222. doi: 10.1099/0022-1317-75-6-1211. [DOI] [PubMed] [Google Scholar]
- 3.Herold B C, WuDunn D, Soltys N, Spear P G. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume G, Stripe D, Boscaro A, Avitable E, Foa-Tomasi L, Barker D, Roizman B. Virology. 1990;178:213–222. doi: 10.1016/0042-6822(90)90396-9. [DOI] [PubMed] [Google Scholar]
- 5.Sears A E, McGwire B S, Roizman B. Proc Natl Acad Sci USA. 1991;88:5087–5091. doi: 10.1073/pnas.88.12.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber P C, Levine M, Glorioso J C. Science. 1987;236:576–579. doi: 10.1126/science.3033824. [DOI] [PubMed] [Google Scholar]
- 7.Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 8.Kousoulas K G, Pellet P E, Pereira L, Roizman B. Virology. 1984;135:379–395. doi: 10.1016/0042-6822(84)90194-6. [DOI] [PubMed] [Google Scholar]
- 9.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, et al. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krummenacher C, Nicola A V, Whitbeck J C, Lou H, Hou W, Lambris J D, Geraghty R J, Spear P G, Cohen G H, Eisenberg R J. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson D C, Ligas M W. J Virol. 1988;62:4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ligas M W, Johnson D C. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roizman B, Sears A E. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- 14.Sunstrum J C, Chrisp C E, Levine M, Glorioso J C. Virus Res. 1988;11:17–32. doi: 10.1016/0168-1702(88)90064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moffat J F, Zerboni L, Kinchington P R, Grose C, Kaneshima H, Arvin A M. J Virol. 1998;72:965–974. doi: 10.1128/jvi.72.2.965-974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee G T Y, Para M F, Spear P G. J Virol. 1982;43:41–49. doi: 10.1128/jvi.43.1.41-49.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNearny T A, Odell C, Holers V M, Spear P G, Atkinson J P. J Exp Med. 1987;66:448–457. doi: 10.1084/jem.166.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris S L, Frank I, Yee A, Cohen G H, Eisenberg R J, Friedman H M. J Infect Dis. 1990;162:331–337. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- 19.Cotsarelis G, Cheng S, Dong G, Sun T, Lavker R M. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 20.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]