Abstract
Studies of the general population have suggested that high homocysteine levels are associated with cardiovascular morbidity and mortality. In chronic kidney disease, homocysteine levels rise, and cardiovascular risk increases with declining kidney function. While some studies in this population have found an association between elevated homocysteine and cardiovascular risk, others have noted that this association is largely attenuated by adjustment for kidney function, and several studies of patients with kidney failure have found that lower homocysteine levels predict mortality.
Homocysteine levels can be lowered with folate, vitamin B6 and vitamin B12. Three large, randomized, controlled trials of patients with pre-existing cardiovascular disease and two smaller, randomized, controlled trials of patients with kidney failure failed to detect any cardiovascular benefit from homocysteine-lowering vitamins. Several more interventional trials are ongoing, but the available data thus far do not support screening for or treatment of hyperhomocysteinemia.
Keywords: Cardiovascular risk factors, Chronic kidney disease, Homocysteine
Abstract
D’après des études menées dans la population en général, un taux élevé d’homocystéine serait associé à la morbidité et à la mortalité cardiovasculaires. Or, dans les néphropathies chroniques, le taux d’homocystéine s’élève et le risque de maladie cardiovasculaire (MCV) augmente au fur et à mesure que diminue le fonctionnement rénal. Tandis que, dans certaines études menées dans la population touchée, on a établi une association entre un taux élevé d’homocystéine et le risque de MCV, dans d’autres études, on a constaté un amoindrissement marqué de cette association après rajustement pour tenir compte du fonctionnement rénal et, d’après plusieurs études menées chez des insuffisants rénaux, une diminution du taux d’homocystéine est un prédicteur de mortalité.
Le taux d’homocystéine peut être abaissé par les folates, la vitamine B6 et la vitamine B12. Dans trois essais comparatifs, de grande taille, menés avec répartition aléatoire chez des patients atteints d’une cardiopathie préexistante et dans deux essais comparatifs, de moindre taille, menés avec répartition aléatoire chez des patients atteints d’insuffisance rénale, il a été impossible de dégager un bienfait cardiovasculaire de l’emploi des vitamines pour abaisser le taux d’homocystéine. Plusieurs essais internationaux sont en cours et, d’après les données recueillies jusqu’à maintenant, rien ne justifie le dépistage ou le traitement de l’hyperhomocystéinémie.
Elevated plasma total homocysteine has been proposed as a risk factor for cardiovascular morbidity and mortality. The public health implications of homocysteine as a cardiovascular risk factor are particularly far-reaching, because homocysteine can be lowered effectively, inexpensively and safely by treatment with folic acid and other B vitamins. In patients with chronic kidney disease (CKD), homocysteine levels rise and cardiovascular risk increases as glomerular filtration rate declines, raising the question of whether hyperhomocysteinemia may be responsible, in part, for the high cardiovascular disease burden in this population. The reasons for increased homocysteine levels in CKD are not entirely understood, but probably relate to decreased renal clearance and metabolism of homocysteine.
In the present paper, the following are reviewed: possible biological mechanisms underlying the role for homocysteine as a cardiovascular risk factor; observational studies examining the association between elevated homocysteine and cardiovascular risk in the general population and in patients with CKD; interventional studies of homocysteine-lowering therapy; possible reasons for the largely negative results from these trials; and the ongoing trials of homocysteine-lowering therapy.
Pathophysiology of cardiovascular risk from elevated homocysteine levels
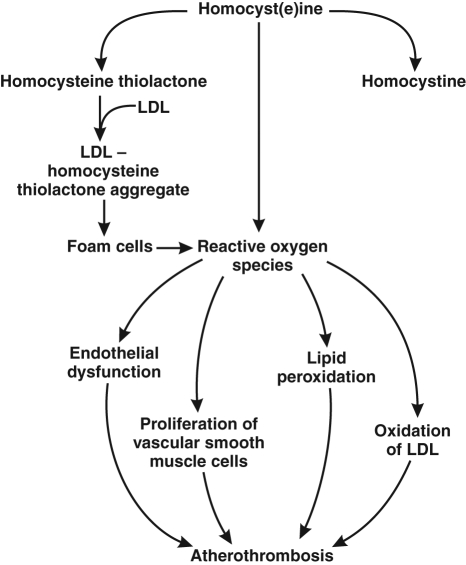
Hyperhomocysteinemia is believed to promote atherogenesis and atherothrombosis via several mechanisms (Figure 1) (1). Homocysteine metabolism generates reactive oxygen species that can directly injure the endothelium. Homocysteine has been shown to inhibit nitric oxide synthase activity, leading to endothelial dysfunction. Other proatherogenic mechanisms attributed to hyperhomocysteinemia include dysregulated methylation of proteins and DNA resulting in abnormal vascular smooth muscle cell proliferation, as well as increased lipid peroxidation.
Figure 1).
Postulated adverse vascular effects of homocysteine. The postulated effects involve oxidative damage to vascular endothelial cells and increased proliferation of vascular smooth muscle cells after oxidative metabolism of homocysteine to homocystine and homocysteine thiolactone. Oxidative modification of low-density lipoprotein (LDL) promotes the formation of foam cells, which in turn yields another source of reactive oxygen species. Reproduced with permission from reference 1
Observational studies of homocysteine levels and cardiovascular disease
Data from observational studies examining the association between elevated homocysteine levels and cardiovascular morbidity and mortality in the general population have been inconsistent. Homocysteine was an independent risk factor for all-cause mortality in the Physicians’ Health Study (2). Male physicians with the highest 5% of homocysteine levels demonstrated a five-year RR of 3.4 for acute myocardial infarction (MI) or death due to coronary disease compared with subjects with the lowest 90% of homocysteine levels. Two meta-analyses of observational data demonstrated a similar association. Wald et al (3) evaluated 20 prospective studies and concluded that lowering homocysteine concentrations by 3 μmol/L decreased the risk of ischemic heart disease by 16% and stroke by 24%. A second meta-analysis of 18 retrospective and 12 prospective studies (4) determined that a 25% reduction in homocysteine levels was associated with an 11% lower ischemic heart disease risk and a 19% lower stroke risk. Conversely, investigators were unable to demonstrate an association between homocysteine and cardiovascular disease in the Atherosclerosis Risk In Communities (ARIC) (5) study and the Multiple Risk Factor Intervention Trial (MRFIT) (6).
There are limited data evaluating homocysteine as a risk factor for cardiovascular disease in patients with CKD before reaching kidney failure. In a subset of 93 subjects from a cohort of 147 patients with creatinine clearances between 20 mL/min/1.73 m2 and 55 mL/min/1.73 m2 (7), a high homocysteine level was an independent risk factor for incident cardiovascular disease. Two studies of kidney transplant recipients found an association between homocysteine and a composite outcome of fatal and nonfatal cardiovascular events and all-cause mortality. In contrast, in a cohort of patients with stage 3 or 4 nondiabetic CKD, there was no relationship between high homocysteine levels and all-cause and cardiovascular mortality after adjustment for iothalamate glomerular filtration rate, an accurate measure of kidney function (8).
Several studies have examined the association between elevated levels of homocysteine and outcomes in patients with kidney failure, with contradictory findings. A few studies have found high homocysteine to be a risk factor for outcomes; for example, the Cardiovascular Risk Extended Evaluation in Dialysis patients (CREED) (9) investigators followed 175 patients with kidney failure for 29 months and found that a 10 μmol/L increase in homocysteine was associated with 20% increased risk for cardiovascular events. In contrast, some studies have described an inverse relationship between homocysteine levels and outcomes. In a cohort of 367 patients with kidney failure and a 94% prevalence of hyperhomocysteinemia (defined as levels higher than 13.5 μmol/L), low, rather than high, homocysteine levels were associated with a greater than twofold increase in the risk of hospitalizations and death during a one-year follow-up period (10). One potential explanation for the latter finding is that homocysteine is directly correlated with indicators of nutritional status such as creatinine and albumin. Hence, in kidney failure, low homocysteine levels may denote malnutrition and, therefore, a poor prognosis.
Randomized controlled trials of homocysteine lowering and cardiovascular disease
Several randomized controlled trials have been conducted to study whether homocysteine lowering with folate and vitamins B6 and B12 reduces the risk of cardiovascular disease (Table 1). The Vitamin Intervention for Stroke Prevention (VISP) trial (11) was a multicentre, randomized nonplacebo-controlled trial of 3680 patients with a history of ischemic stroke who were randomly assigned to receive either low or high doses of folate and B vitamins. The study was terminated early because at two years, an interim analysis showed no difference in cardiovascular outcomes between the high- and low-dose groups. In the Heart Outcomes Prevention Evaluation (HOPE) 2 study (12), 5522 patients with pre-existing cardiovascular disease were randomly assigned to homocysteine-lowering therapy or placebo. After a five-year follow-up, there were no differences between the treatment and placebo groups in MI or cardiovascular death, although there was a 24% RR reduction in stroke. The Norwegian Vitamin Intervention Trial (NORVIT) (13) randomly assigned 3749 subjects with history of acute MI to receive either folate plus vitamins B12 and B6, folate plus vitamin B12, vitamin B6 only or placebo. No significant differences in MI, stroke or sudden cardiac death were detected among any of the four groups, and there was a trend toward an increased event rate in subjects receiving folate and vitamins B12 and B6.
TABLE 1.
Completed and ongoing randomized controlled trials investigating the effect of homocysteine-lowering vitamins on cardiovascular outcomes
| Trial (reference) | Population | Primary clinical outcome | Results (or study status if not completed) |
|---|---|---|---|
| Vitamin Intervention for Stroke Prevention (VISP) trial (n=3680) (11) | Nondisabling stroke | Recurrent stroke | RR 1.0 (0.8–1.1) |
| NORwegian Vitamin Intervention Trial (NORVIT) (n=3749) (13) | Acute myocardial infarction | Composite of new nonfatal and fatal myocardial infarction, nonfatal and fatal stroke, and sudden death attributed to coronary artery disease | RR 1.22 (1.00–1.50) |
| Heart Outcomes Prevention Evaluation 2 (HOPE 2) study (n=5522) (12) | Vascular disease or diabetes | Composite mortality from cardiovascular causes, myocardial infarction and stroke | RR 0.95 (0.84–1.07) |
| AtheroSclerosis and Folic Acid Supplementation Trial (ASFAST) (n=315) (15) | Kidney failure | Composite of myocardial infarction, stroke, and cardiovascular death | RR 0.98 (0.66–1.47) |
| Wrone et al (n=510) (14) | Kidney failure | Composite of cardiovascular events and death | No difference in treatment versus control arms |
| WEstern Norway B-vitamin Intervention Trial (WENBIT) (n=3088) (22) | Undergoing coronary angiography | Cardiovascular death, nonfatal myocardial infarction, unstable angina and nonfatal thromboembolic stroke | Completed enrolment |
| Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) (n=12,064) (23) | Prior myocardial infarction | Nonfatal myocardial infarction, coronary death, stroke or arterial revascularization | Completed enrolment |
| HOmocysteinemia in kidney and end-stage renal disease STudy (HOST) (n=2006) (25) | Advanced chronic kidney disease or kidney failure | All-cause mortality | Completed enrolment |
| VITAmins TO Prevent Stroke (VITATOPS) (n=8000) (24) | Recent stroke or transient ischemic attack | Nonfatal stroke, nonfatal myocardial infarction, death from vascular causes | Enrolling |
| Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) study (n=4000) (26) | Chronic renal transplant recipients | Composite fatal and nonfatal vascular events (coronary, cerebrovascular and peripheral vascular) | Enrolling |
There are no published interventional studies to date of homocysteine lowering in patients in the earlier stages of CKD. Two small, randomized, controlled trials have investigated the effects of homocysteine-lowering therapy in patients with kidney failure. Wrone et al (14) randomly assigned patients with kidney failure to receive 1 mg, 5 mg or 15 mg of folic acid plus standard doses of vitamins B6 and B12. There were no observed differences in cardiovascular events or mortality after a median follow-up of 24 months. Moreover, there was an inverse relationship between baseline homocysteine levels and cardiovascular event rate, with the highest event rate occurring in the lowest quartile of homocysteine. The AtheroSclerosis and Folic Acid Supplementation Trial (ASFAST) investigators (15) randomly assigned 315 patients either with kidney failure or who were nearing the need for dialysis (creatinine clearance of less than 25 mL/min) to receive 15 mg of folic acid or placebo. Patients were followed for four years and monitored for MI, stroke, death and carotid artery intima-media thickness. Despite a robust homocysteine-lowering response in the folic acid group compared with placebo, there were no differences in the clinical and surrogate end points.
Possible reasons for lack of benefit in interventional studies
While several observational studies point to an association between high homocysteine levels and risk for cardiovascular disease, the randomized controlled trials addressing this issue have been largely negative. There are several potential reasons for this failure in demonstrating cardiovascular benefit with homocysteine-lowering therapy, which have been reviewed in several recent editorials (16–18).
First, as described in an analysis from the B-Vitamin Trialists’ Collaboration (19), the cardiovascular benefits of homocysteine lowering may have been too small for the trials to detect. The interventional trials were designed with the assumption that folic acid and B vitamins could reduce cardiac and cerebrovascular risk by approximately 30%. However, these estimates might have been too optimistic. As described in a previous section, two meta-analyses concluded that ischemic heart disease risk could be reduced by 16% with a 3 μmol/L decrease in homocysteine levels (3) and by 11% with a 25% decrease in homocysteine levels (4). Therefore, many of the recent interventional trials were powered to detect larger cardiovascular risk reductions than would be expected from the degree of homocysteine lowering achieved.
Second, established cardiovascular disease may be more difficult to reverse with folic acid and other B vitamins, regardless of the plasma homocysteine level. All of the negative homocysteine-lowering trials performed thus far have addressed secondary prevention, while there may be a more important role for homocysteine lowering in the primary prevention of cardiovascular disease. In essence, a large-scale natural experiment has already commenced with the mandatory fortification of grain with folate in Canada and the United States beginning in 1998. A recent report (20) identified a decrease in stroke-related mortality in Canada and the United States since mandatory grain fortification was initiated. However, a randomized controlled trial of homocysteine lowering for primary prevention would entail a very large sample size.
Third, folate fortification of grain in Canada and the United States may have diminished the benefit of high-dose vitamins in the VISP trial. However, a HOPE 2 trial subgroup analysis of subjects from countries lacking folate fortification failed to demonstrate a benefit of homocysteine-lowering vitamins, and NORVIT was performed in a country without folate fortification.
Fourth, although folate and vitamins B6 and B12 lower homocysteine, they may simultaneously increase cardiovascular risk through homocysteine-independent mechanisms. A recent editorial in the New England Journal of Medicine postulated that folic acid and other B vitamins may promote atherosclerosis by increasing cell proliferation in atherosclerotic plaques, enhancing methylation of DNA (leading to gene activation) and augmenting levels of asymmetric dimethylarginine (16). Asymmetric dimethylarginine is an endogenous inhibitor of nitric oxide synthase, and has been implicated in the pathogenesis of endothelial dysfunction and accelerated vascular disease. Thus, it is biologically plausible that folate and vitamins B6 and B12 could have negative effects on the endothelium that offset their potential benefit from homocysteine lowering.
Fifth, total plasma homocysteine may be a less important measure of homocysteine toxicity compared with its aminothiol constituents, especially in kidney failure. The free reduced form of homocysteine typically only comprises less than 3% of the total plasma homocysteine level, yet it is likely the component most injurious to endothelium. The ratio of reduced to total homocysteine level increases dramatically in kidney failure and may be a more sensitive measure of effectiveness of homocysteine-lowering therapy and cardiovascular risk (21).
Sixth, in the interventional trials performed in patients with kidney failure, homocysteine levels remained elevated despite treatment, which could partially explain the lack of detected benefit. Compared with the general population, higher doses of B vitamins are needed to normalize homocysteine levels in patients with CKD before kidney failure, while normal homocysteine levels are rarely attainable in patients with kidney failure.
Finally, there is a possibility of homocysteine not being related to cardiovascular risk, but rather existing as a marker for other cardiovascular risk factors, such as reduced kidney function and severity of kidney disease (8), or for the presence of pre-existing or subclinical cardiovascular disease. As previously noted, in patients with kidney failure, homocysteine may be more associated with malnutrition than with cardiovascular risk (10).
Ongoing trials
Ongoing and recently concluded randomized controlled trials of B vitamins for the prevention of cardiovascular events (Table 1) include the WEstern Norway B-vitamin Intervention Trial (WENBIT) (22) in patients undergoing coronary angiography, the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) trial (23) in patients with MI, and the VITAmins TO Prevent Stroke (VITATOPS) trial (24) in patients with stroke. Two trials are being conducted in patients with CKD. The HOmocysteinemia in kidney and end-stage renal disease STudy (HOST) (25) is testing extremely high doses of folate, vitamin B6 and vitamin B12 in patients with kidney failure, and the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial (26) will evaluate the cardiovascular effects of high-dose folate, vitamin B6 and vitamin B12 in the kidney transplant population.
CONCLUSIONS
Despite observational data suggesting a relationship between elevated homocysteine levels and cardiovascular risk, several recent randomized controlled trials have failed to demonstrate a reduction in cardiovascular risk with homocysteine-lowering therapy. Homocysteine does not appear to be a direct mediator of cardiovascular risk in patients with CKD. In the earlier stages of CKD, high homocysteine may reflect the severity of kidney disease; in kidney failure, low homocysteine levels may be a marker for malnutrition and, thus, are associated with poor outcomes. The current state of knowledge does not support a strategy to screen for or treat high homocysteine levels in patients with or without CKD.
REFERENCES
- 1.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042–50. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 2.Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992;268:877–81. [PubMed] [Google Scholar]
- 3.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ. 2002;325:1202–6. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homocysteine Studies Collaboration Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA. 2002;288:2015–22. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 5.Folsom AR, Nieto FJ, McGovern PG, et al. Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related genetic polymorphisms, and B vitamins: The Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1998;98:204–10. doi: 10.1161/01.cir.98.3.204. [DOI] [PubMed] [Google Scholar]
- 6.Evans RW, Shaten BJ, Hempel JD, Cutler JA, Kuller LH. Homocyst(e)ine and risk of cardiovascular disease in the Multiple Risk Factor Intervention Trial. Arterioscler Thromb Vasc Biol. 1997;17:1947–53. doi: 10.1161/01.atv.17.10.1947. [DOI] [PubMed] [Google Scholar]
- 7.Jungers P, Massy ZA, Khoa TN, et al. Incidence and risk factors of atherosclerotic cardiovascular accidents in predialysis chronic renal failure patients: A prospective study. Nephrol Dial Transplant. 1997;12:2597–02. doi: 10.1093/ndt/12.12.2597. [DOI] [PubMed] [Google Scholar]
- 8.Menon V, Sarnak MJ, Greene T, et al. Relationship between homocysteine and mortality in chronic kidney disease. Circulation. 2006;113:1572–7. doi: 10.1161/CIRCULATIONAHA.105.570127. [DOI] [PubMed] [Google Scholar]
- 9.Mallamaci F, Zoccali C, Tripepi G, et al. CREED Investigators Hyperhomocysteinemia predicts cardiovascular outcomes in hemodialysis patients. Kidney Int. 2002;61:609–14. doi: 10.1046/j.1523-1755.2002.00144.x. [DOI] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K, Block G, Humphreys MH, McAllister CJ, Kopple JD. A low, rather than a high, total plasma homocysteine is an indicator of poor outcome in hemodialysis patients. J Am Soc Nephrol. 2004;15:442–53. doi: 10.1097/01.asn.0000107564.60018.51. [DOI] [PubMed] [Google Scholar]
- 11.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–75. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 12.Lonn E, Yusuf S, Arnold MJ, et al. Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. (Erratum in 2006;355:746). [DOI] [PubMed] [Google Scholar]
- 13.Bønaa KH, Njølstad I, Ueland PM, et al. NORVIT Trial Investigators Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 14.Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004;15:420–6. doi: 10.1097/01.asn.0000110181.64655.6c. [DOI] [PubMed] [Google Scholar]
- 15.Zoungas S, McGrath BP, Branley P, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: A multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47:1108–16. doi: 10.1016/j.jacc.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 16.Loscalzo J. Homocysteine trials – clear outcomes for complex reasons. N Engl J Med. 2006;354:1629–32. doi: 10.1056/NEJMe068060. [DOI] [PubMed] [Google Scholar]
- 17.Hoffer LJ. Testing the homocysteine hypothesis in end-stage renal disease: Problems and a possible solution. Kidney Int. 2006;69:1507–10. doi: 10.1038/sj.ki.5000279. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg IH, Mulrow CD. Trials that matter: Should we routinely measure homocysteine levels and “treat” mild hyperhomocysteinemia. Ann Intern Med. 2006;145:226–7. doi: 10.7326/0003-4819-145-3-200608010-00011. (Erratum in 2006;145:475). [DOI] [PubMed] [Google Scholar]
- 19.B-Vitamin Treatment Trialists’ Collaboration Homocysteine-lowering trials for prevention of cardiovascular events: A review of the design and power of the large randomized trials. Am Heart J. 2006;151:282–7. doi: 10.1016/j.ahj.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Botto LD, Erickson JD, et al. Improvement in stroke mortality in Canada and the United States, 1990 to 2002. Circulation. 2006;113:1335–43. doi: 10.1161/CIRCULATIONAHA.105.570846. [DOI] [PubMed] [Google Scholar]
- 21.Sjöberg B, Anderstam B, Suliman M, Alvestrand A. Plasma reduced homocysteine and other aminothiol concentrations in patients with CKD. Am J Kidney Dis. 2006;47:60–71. doi: 10.1053/j.ajkd.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov <clinicaltrials.gov/show/nct00354081> (Version current at June 21, 2007).
- 23.MacMahon M, Kirkpatrick C, Cummings CE, et al. A pilot study with simvastatin and folic acid/vitamin B12 in preparation for the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Nutr Metab Cardiovasc Dis. 2000;10:195–203. (Erratum in 2001;11:III). [PubMed] [Google Scholar]
- 24.VITATOPS Trial Study Group The VITATOPS (Vitamins to Prevent Stroke) Trial: rationale and design of an international, large, simple, randomised trial of homocysteine-lowering multivitamin therapy in patients with recent transient ischaemic attack or stroke. Cerebrovasc Dis. 2002;13:120–6. doi: 10.1159/000047761. [DOI] [PubMed] [Google Scholar]
- 25.Jamison RL, Hartigan P, Gaziano JM, et al. Design and statistical issues in the homocysteinemia in kidney and end stage renal disease (HOST) study. Clin Trials. 2004;1:451–60. doi: 10.1191/1740774504cn038oa. [DOI] [PubMed] [Google Scholar]
- 26.Bostom AG, Carpenter MA, Kusek JW, et al. FAVORIT Investigators Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J. 2006;152:448.e1–7. doi: 10.1016/j.ahj.2006.03.004. [DOI] [PubMed] [Google Scholar]