Abstract
BACKGROUND:
The provincial formulary review processes in Canada lead to the slow and inequitable availability of new products. In 2004, the exploration of a national pharmaceuticals strategy (NPS) was announced. The pricing policies of New Zealand and Australia have been suggested as possible models for the NPS.
OBJECTIVE:
To compare health care indexes and health care use information from Canada, Australia and New Zealand.
METHODS:
The 2006 Organisation for Economic Co-operation and Development health data were used to compare health and health care indexes from Canada, Australia and New Zealand between 1994 and 2002 to 2004. The principal focus of the evaluation was cardiovascular and respiratory disorders.
RESULTS:
Although the mortality rate from acute myocardial infarction decreased in each country from 1994, it levelled off in New Zealand in 1997, 1998 and 1999. Between 1994 and 2003, the average length of hospital stay for any cause and for cardiovascular disorders was stable in Australia and Canada, but increased in New Zealand, while the rate of hospital discharges for cardiovascular diseases decreased in Canada and Australia, but strongly increased in New Zealand. Over the same period, sales of cardiovascular drugs decreased in New Zealand, while sharply increasing in Canada and Australia.
CONCLUSIONS:
Although only circumstantial, our results suggest an association between decreasing cardiovascular drug sales and markers of declining cardiovascular health in New Zealand. Careful consideration must be given to the potential consequences of any model for an NPS in Canada, as well as to opportunities provided for discussion and input from health care professionals and patients.
Keywords: Canadian health care system, Health outcomes, Health policy, Prescription drugs
Abstract
CONTEXTE:
Le processus de révision des listes provinciales de médicaments au Canada conduit à une arrivée lente et inéquitable des nouveaux produits. L’analyse exploratoire d’une stratégie pharmaceutique nationale (SPN) a été annoncée en 2004. On a suggéré d’appliquer les politiques d’établissement des prix en vigueur en Australie et en Nouvelle-Zélande comme modèles possibles de SPN.
BUT:
L’étude avait pour but de comparer les indices de soins de santé et les données sur l’utilisation des soins de santé entre le Canada, l’Australie et la Nouvelle-Zélande.
MÉTHODE:
Nous avons utilisé les données 2006 de l’Organisation de coopération et de développement économiques sur la santé pour comparer les indices de santé et de soins de santé entre le Canada, l’Australie et la Nouvelle-Zélande, de 1994 à 2002 jusqu’en 2004. Le principal point d’intérêt était les maladies respiratoires et cardiovasculaires.
RÉSULTATS:
Même si le taux de mortalité attribuable à l’infarctus du myocarde a diminué dans chacun des pays à partir de 1994, il a atteint un plateau en Nouvelle-Zélande en 1997, 1998 et 1999. La durée moyenne du séjour à l’hôpital, de 1994 à 2003, pour tous types de troubles et pour les troubles cardiovasculaires est restée stable en Australie et au Canada mais a augmenté en Nouvelle-Zélande, tandis que le taux de sortie de l’hôpital pour les maladies cardiovasculaires a diminué au Canada et en Australie mais a considérablement augmenté en Nouvelle-Zélande. Pendant la même période, les ventes de médicaments à action cardiovasculaire ont diminué en Nouvelle-Zélande mais ont fortement augmenté au Canada et en Australie.
CONCLUSIONS:
Bien qu’il ne s’agisse que d’éléments probants indirects, les résultats donnent à penser qu’il existe un lien entre la diminution des ventes de médicaments à action cardiovasculaire et les indicateurs de détérioration de la santé cardiovasculaire en Nouvelle-Zélande. Aussi faudra-t-il porter une attention particulière aux conséquences possibles des différents modèles de SPN au Canada et aux possibilités, pour les professionnels de la santé et pour les patients, de participer aux discussions et d’émettre leurs commentaires.
In Canada, the regulatory review of new prescription medications for efficacy and safety is performed by the federal health department, while reviews of new drugs for listing on formularies for public insurance are predominantly the responsibility of provincial and territorial governments (1). The federal evaluation of efficacy and safety, as well as the provincial formulary review processes, have been demonstrated to be slow (2–7), and the provincial system has also been shown to lead to flawed decision making (6,7). This results in inequitable coverage of prescription drugs across the country (1,6–9).
In an attempt to correct some of the problems with the provincial and territorial evaluation procedures, the Common Drug Review (CDR) was established in 2002 as a centralized process to assess new drugs for inclusion in the formularies of all provinces except Quebec (10). Based on reviews of clinical and pharmacoeconomic evidence, the CDR recommends whether a drug should be listed (with or without conditions), or whether the decision be deferred pending the clarification of information. In practice, the CDR “tends to be conservative, because it is hard to remove a drug once it has been approved, but it is relatively easy to conduct a re-evaluation” (11). The fact that 14 of the first 25 drugs (56%) to have completed the CDR process were not recommended for listing supports this statement (12), and this is particularly applicable to drugs for rare diseases (13).
Most CDR recommendations of drugs not to list are accepted by provincial and territorial formulary committees, while recommendations of drugs to list are revisited, which frequently leads to many months elapsing before a decision is made; it also leads to drugs being listed only with conditions, irrespective of the CDR decision (12). A principal aim of the CDR is to eliminate wasteful duplication (10). However, this is not being achieved, because the CDR regularly requests data already reviewed by Health Canada and multiple provincial reviews continue. Moreover, the persistence of inequity in access to new medications is of paramount concern (1).
One reason for the slow and inequitable inclusion of new products in drug plan formularies is the increasing cost of new drugs. Drugs continue to be the second largest and the fastest growing category of health care spending in Canada (14). Consequently, there is increasing pressure on public and private drug insurance payers to manage their budgets as tightly as possible. The form that this management has taken is predominantly one of cost-containment rather than one focusing on the overall value of drugs. Expenditure on drugs is a relatively easy target for governments because it is a potential vote winner, unlike constraints on hospitals or physician services, which tend to be viewed more negatively by constituents.
In September 2004, following a meeting of the First Ministers of the provincial and territorial governments, it was announced that a national pharmaceuticals strategy (NPS) would be explored (15). The NPS would include the following actions: (a) develop, assess and cost options for catastrophic pharmaceutical coverage; (b) establish a common national drug formulary for participating jurisdictions based on safety and cost-effectiveness; (c) accelerate access to breakthrough drugs for unmet health needs through improvements to the drug approval process; (d) strengthen evaluation of real-world drug safety and effectiveness; (e) pursue purchasing strategies to obtain best prices for Canadians for drugs and vaccines; (f) enhance action to influence the prescribing behaviour of health care professionals so that drugs are used only when needed and the right drug is used for the right problem; (g) broaden the practice of electronic prescribing through accelerated development and deployment of the electronic health record; (h) accelerate access to nonpatented drugs and achieve international parity on prices of nonpatented drugs; and (i) enhance analysis of cost drivers and cost-effectiveness, including best practices in drug plan policies. Actions b through d, as well as f and i, are appealing to health care providers and patients because they should improve patient health, while a, e, f, h and i are attractive to tax payers because they focus on cost-containment.
Subsequently, the federal, provincial and territorial governments identified five areas on which to focus to move the process forward. These are real-world drug safety and effectiveness, expensive drugs for rare diseases, drug pricing and purchasing, catastrophic drug coverage and a common formulary. An NPS similar to the approach in New Zealand, with a common formulary of essential drugs, tighter controls on drug prices and purchasing, and controls on prescribing and access, is receiving a favourable response in several quarters (16,17). The system would include a reference-based pricing (RBP) process. In RBP, a reference price or ceiling is established for a group of products regarded as comparable or interchangeable, and this becomes the maximum amount covered for the group (18). To obtain a drug in the category with a cost greater than the reference price, patients usually have to pay the difference between the two.
Health care policy analysts compared various health care indexes and health spending measures between New Zealand and Australia in 2005, and concluded that the system has had a negative effect on the cardiovascular health of the New Zealand population (19). A comparison of health care indexes in these countries with those in Canada is warranted. Australia, Canada and New Zealand have socialized health care systems based, to varying extents, on the United Kingdom’s National Health System model; each provides hospitalization and physician services for most emergency and noncosmetic requirements paid out of taxation. Prescription drugs are subsidized in the three countries, but the methods used and the breadth and depth of coverage vary.
In Australia, the Pharmaceutical Benefits Advisory Committee (PBAC) reviews new drugs and makes recommendations to the Pharmaceutical Benefits Pricing Authority and the federal government on their suitability for reimbursement through the Pharmaceutical Benefits Scheme. The PBAC assesses applications for new listings, price increases and new indications for already recorded drugs, all of which require a pharmacoeconomic evaluation. In making its recommendations, which are listed on the Australian government’s Web site (20), the PBAC considers the efficacy, safety, costs, cost-effectiveness and clinical place of the drug in relation to other already marketed products. Drugs approved for reimbursement are subject to an RBP policy, but Australia does not link a decision to put a drug on the subsidy list with an overall budget cap, nor is the decision determined by a tendering process for the least expensive among similar drugs (19).
In New Zealand, the Pharmacology and Therapeutics Advisory Committee performs a similar role as the PBAC, but its processes are more informal and less transparent. The country also has a strong cost-containment approach with RBP and a single purchaser, the Pharmaceutical Management Agency Limited (PHARMAC), which has been in effect since 1997. PHARMAC has received strong criticism from the country’s medical profession (21–35) because its decisions have led to more restrictions on which drugs are subsidized, to which the agency has responded vigorously (36–40).
METHODS
Using the latest (2006) version of the Organisation for Economic Co-operation and Development (OECD) health data (41), health care indexes and health care use information for Canada, Australia and New Zealand were compared. The principal focus of the evaluation was cardiovascular and respiratory disorders. Selected characteristics of the countries were examined to evaluate comparability and, subsequently, markers of cardiovascular and respiratory health compared using the total OECD population for 1980 as the reference for calculations of comparable rates of discharges and age-standardized mortality rates. Throughout, data were selected from 1994 until the latest year for which relevant information was available for at least two of the countries, resulting in some inconsistency in the last year in the analyses (2002 to 2004).
During the study period, the coding of diagnoses in the three countries changed from the International Classification of Diseases version 9 (ICD-9) to ICD-10. In ICD-9, cardiovascular and respiratory disorders were coded as 390 to 459 and 460 to 519, respectively, while in ICD-10, they were coded as I00 to I99 and J00 to J98, respectively. Acute myocardial infarction (AMI) was coded as 410 in ICD-9 and I21 or I22 in ICD-10.
Pharmaceutical sales data, limited to large therapeutic classes, were available for Australia and New Zealand in the OECD health data, and are known to underestimate the real figures for Australia (41). Total drug sales for Canada were obtained from the Canadian Institute for Health Information annual report on drug consumption (14), although these also include over-the-counter (OTC) medications that are not in the OECD health data. OTC medications are known to constitute approximately 20% of the total annual cost of all drugs in Canada and, thus, the Canadian Institute for Health Information figures were adjusted for comparability with the OECD health data. Data on drug sales by therapeutic class for Canada were obtained from Brogan Inc’s PharmaStat database (42), but only from 1998 onward. To allow comparability, all sales data were converted to sales per capita in USD purchasing power.
RESULTS
The age distribution in each country was similar in 2004: 24% to 29% were younger than 20 years of age, approximately 60% were 20 to 64 years of age and 12% to 13% were 65 years of age or older (Table 1). There have been approximately two practising physicians per 1000 population since 1997 in each country. The total expenditure on health as a percentage of the country’s GDP increased in all three countries from 1997 and, in 2003, was more than 9% in Australia and Canada but only 8% in New Zealand. The figures for public expenditure on health were comparable among the three countries.
TABLE 1.
Selected characteristics of Canada, Australia and New Zealand
| Characteristic | Canada | Australia | New Zealand |
|---|---|---|---|
| Age distribution in 1000s in 2004, n (%) | |||
| <20 years | 7802 (24.4) | 5374 (26.7) | 1185 (29.2) |
| 20–64 years | 20,003 (62.6) | 12,132 (60.3) | 2391 (58.9) |
| 65+ years | 4141 (13.0) | 2605 (13.0) | 486 (12.0) |
| Practising physicians per 1000 population in 2003, n | 2.1 | 2.6 | 2.2 |
| Total expenditure on health in 2003 (% of GDP) | 9.9 | 9.2 | 8.0 |
| Public expenditure on health in 2003 (% of GDP) | 6.9 | 6.2 | 6.3 |
| Life expectancy at birth, years | |||
| 1994 | 78.0 | 78.0 | 76.4 |
| 1998 | 78.8 | 78.7 | 77.8 |
| 2003 | 79.9 | 80.3 | 79.2 |
Data from reference 41
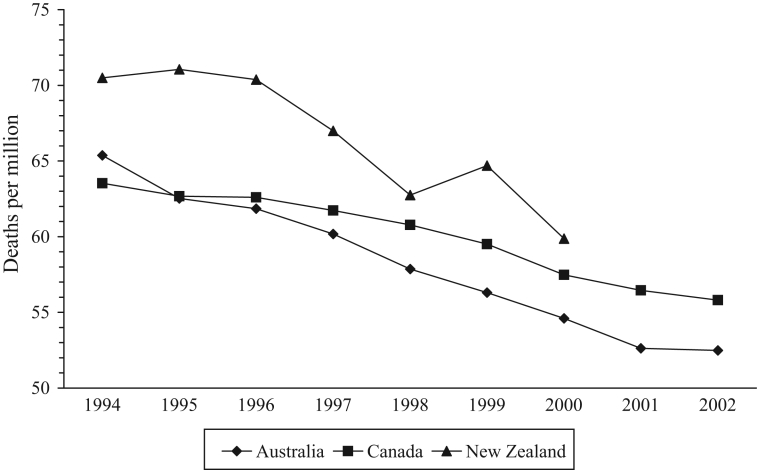
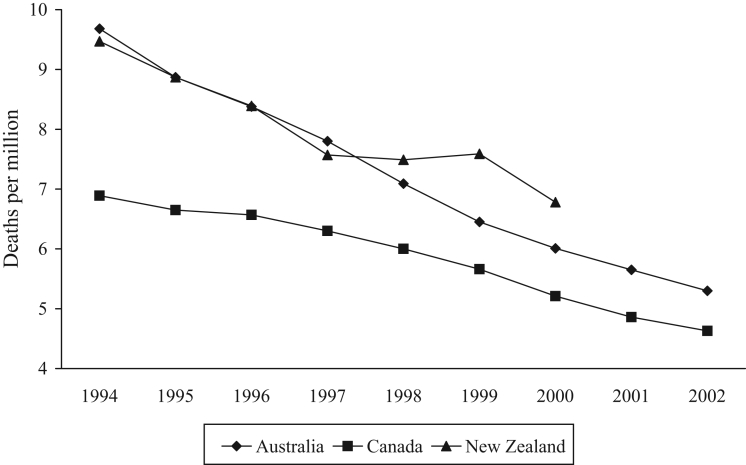
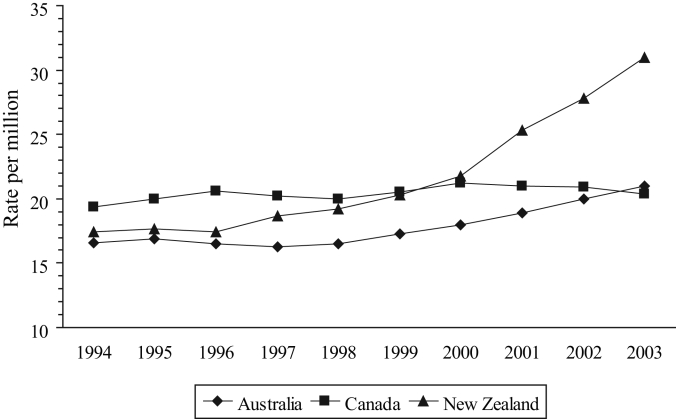
Life expectancy is a marker of national health and has increased by approximately one year in each country between 1994 and 1998 (Table 1). The same trend continued in Australia and Canada until 2003. In New Zealand, life expectancy levelled off at 78.7 years between 2000 and 2002, but increased to 79.2 years in 2003. Mortality from all causes decreased in each country, albeit with some variation in New Zealand (Figure 1). The gradient of the Canadian line was flatter and significantly different from the Australian and New Zealand slopes (P<0.05). The same trend was apparent in deaths from malignancies and cardiovascular disorders, the two main causes of death in all the countries (data not shown). The mortality rate from AMI in New Zealand levelled off in 1997, 1998 and 1999 before continuing to decline in 2000 (Figure 2), so that the rate in New Zealand in 2000 (6.8 per million) was 31% higher than the rate in Canada (5.2 per million).
Figure 1).
Mortality from all causes. Data from reference 41
Figure 2).
Mortality from acute myocardial infarction. Data from reference 41
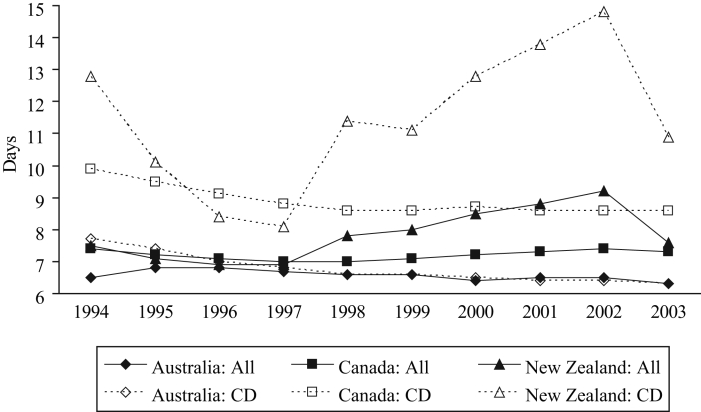
The average length of stay (ALOS) of a hospitalization reflects the seriousness of the illness being treated. The ALOS for all causes was similar in each country, at approximately seven days, until 1997 (Figure 3). Subsequently, it remained approximately the same in Canada, decreased somewhat in Australia, but increased to 9.2 days in New Zealand by 2002, with a substantial decrease to 7.6 days in 2003. The overall ALOS hides an even greater increase in the ALOS for cardiovascular disorders in New Zealand, from 8.1 days in 1997 to 14.8 days in 2002 (83% increase, Figure 3). The ALOS for these disorders in New Zealand decreased subsequently, but still remained 35% longer than in 1997. In contrast, over the same period, the ALOS for cardiovascular disorders in Canada remained stable (8.6 to 8.7 days). The ALOS for respiratory diseases in Canada increased by one day, from 6.1 days in 1997 to 7.1 days in 2002 (16% increase), compared with an increase of more than two days, from 4.7 to 7.2 days (53%), in New Zealand.
Figure 3).
Average length of hospital stay for all causes and cardiovascular disorders (CD). Data from reference 41
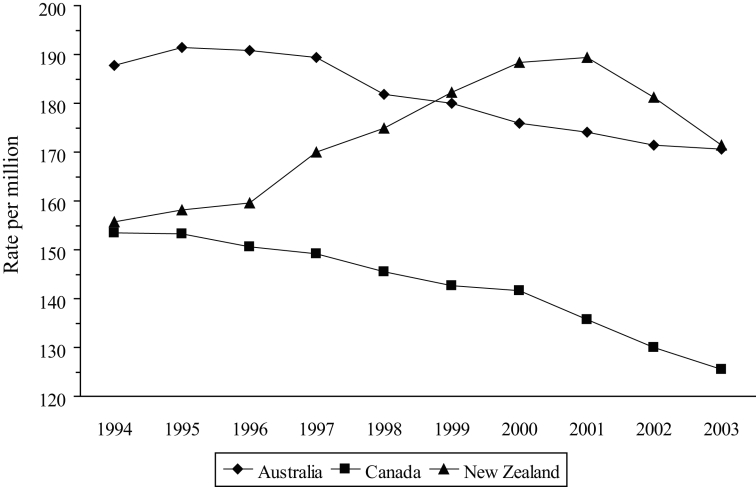
Another measure of hospital use is the rate of discharge, which can be considered to be a marker of hospital admissions (the OECD health data include deaths in hospital). Although the overall discharge rates are different (in 2002, these were 883, 1566 and 2076 per million in Canada, Australia and New Zealand, respectively), they have remained relatively stable in each country. However, the overall measure again disguises significant differences in individual disease areas. Most notably, the discharge rate for cardiovascular disorders has decreased in Canada and Australia (with similar gradients and a correlation of 0.946, Figure 4), while increasing in New Zealand with a slope that was significantly different from the other two (P<0.001). In 1994, the rate in New Zealand was slightly more than that in Canada (155.8 and 153.4 per million, respectively), but by 2003, the rates were 171.4 per million in New Zealand (10% increase) and 125.6 per million in Canada (18% decrease). Between 1996 and 2003, the rate of discharge for AMI in Canada varied from 20.0 to 21.2 per million, whereas in New Zealand, it increased by almost 14 per million, from 17.4 to 31.0 (Figure 5) – an increase of 78%. The discharge rate for AMI in Australia increased by 27% over the same period.
Figure 4).
Discharge rate for cardiovascular disorders. Data from reference 41
Figure 5).
Discharge rate for acute myocardial infarction. Data from reference 41
The discharge rate for respiratory disorders in New Zealand increased by approximately 20 per million, from 136.2 in 1998 to 152.1 in 2003 (12% increase). However, in Canada, the discharge rate decreased by a similar amount from 96.9 to 78.7 per million (19% decrease).
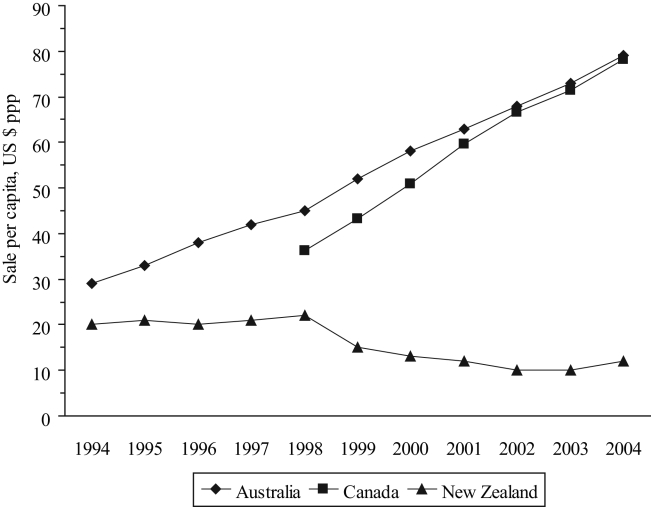
Between 1994 and 2004, total pharmaceutical sales per capita increased almost threefold in Australia, but remained reasonably stable in New Zealand (Table 2). The total sales per capita in Australia and Canada (after adjusting for OTC sales) increased by 76% and 60%, respectively, between 1998 and 2004. Sales of cardiovascular drugs decreased in New Zealand from $22 per capita in 1998 to $12 per capita in 2004 (45%), while sharply increasing in Canada and Australia (117% and 76%, respectively; Figure 6). Although the correlation between the Canadian and Australian figures was 0.997, the rate of increase in Canada between 1998 and 2004 was significantly greater than that in Australia over the same period (P<0.001). Sales of drugs for the respiratory system declined in New Zealand (from $15 per capita in 1997 to $10 per capita in 2004 – 33%) but increased in the other two countries (31% in Australia and 86% in Canada, Table 2).
TABLE 2.
Pharmaceutical sales per capita* in Canada, Australia and New Zealand
| Pharmaceuticals | Canada | Australia | New Zealand |
| Total | |||
| 1994 | NA | 97 | 92 |
| 1998 | 279 | 143 | 100 |
| 2004 | 447 | 252 | 103 |
| Cardiovascular system | |||
| 1994 | NA | 29 | 20 |
| 1998 | 36 | 45 | 22 |
| 2004 | 78 | 79 | 12 |
| Respiratory system | |||
| 1994 | NA | 10 | 18 |
| 1998 | 7 | 13 | 15 |
| 2004 | 13 | 17 | 10 |
Figure 6).
Sale of cardiovascular drugs per capita. ppp Purchasing power parity. Data from references 41 and 42
Table 3 shows the differences among the three countries in publicly funded access to five types of cardiovascular drugs commonly used in the ambulatory setting: beta-adrenergic blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs) and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins). Because Canada has a fragmented prescription drug support system, the data are presented in terms of the number of provincial schemes providing open access, restricted access requiring special authorization, or not listed. For each of the countries, the highest level of support is shown (there may be other forms of the medication with more restricted support). All or almost all the Canadian provinces provide open access to all the drugs in each of the five categories, while Australians have open access to five beta-blockers (42%), eight ACE inhibitors (80%), four ARBs (67%) and five CCBs (71%), together with restricted access to five statins (83%) and four additional beta-blockers (33%). However, in New Zealand, residents do not have open access to any of these drugs. Restricted access is available to 10 of the 12 beta-blockers (83%), seven of the 10 ACE inhibitors (70%) (one with a reduced subsidy), two of the six ARBs (33%), five of the seven CCBs (71%) and three of the six statins (50%), including one with a reduced subsidy.
TABLE 3.
Availability of five types of commonly used cardiovascular drugs
| Canadian provinces with
|
|||||
|---|---|---|---|---|---|
| Drug | OA | R | NL | Australia | New Zealand |
| Beta-adrenergic blockers | |||||
| Acebutolol | 10 | NL | R | ||
| Atenolol | 10 | OA | R | ||
| Bisoprolol | 8 | 2 | R | NL | |
| Carvedilol | 3 | 7 | R | R | |
| Labetalol | 10 | OA | R | ||
| Metoprolol | 10 | R | R | ||
| Nadolol | 10 | NL | R | ||
| Oxprenolol | 10 | OA | NL | ||
| Pindolol | 10 | OA | R | ||
| Propranolol | 10 | OA | R | ||
| Sotalol | 10 | R | R | ||
| Timolol | 10 | NL | R | ||
| Angiotensin-converting enzyme inhibitors | |||||
| Benazepril | 9 | 1 | NL | NL | |
| Captopril | 10 | OA | R | ||
| Cilazapril | 10 | NL | R | ||
| Enalapril | 10 | OA | R | ||
| Fosinopril | 10 | OA | NL | ||
| Lisinopril | 10 | OA | R | ||
| Perindopril | 10 | OA | R* | ||
| Quinapril | 10 | OA | R | ||
| Ramipril | 10 | OA | NL | ||
| Trandolapril | 10 | OA | R* | ||
| Angiotensin receptor blockers | |||||
| Candesartan | 9 | 1 | OA | R | |
| Eprosartan | 9 | 1 | OA | NL | |
| Irbesartan | 9 | 1 | OA | NL | |
| Losartan | 9 | 1 | NL | R | |
| Telmisartan | 9 | 1 | OA | NL | |
| Valsartan | 9 | 1 | NL | NL | |
| Calcium channel blockers | |||||
| Amlodipine | 10 | OA | R | ||
| Diltiazem | 10 | OA | R | ||
| Felodipine | 10 | OA | R | ||
| Nicardipine | 2 | 8 | NL | NL | |
| Nifedipine | 10 | OA | R | ||
| Nimodipine | 5 | 3 | 2 | NL | NL |
| Verapamil | 10 | OA | R | ||
| 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors | |||||
| Atorvastatin | 10 | R | R* | ||
| Fluvastatin | 10 | R | NL | ||
| Lovastatin | 10 | NL | NL | ||
| Pravastatin | 10 | R | R | ||
| Rosuvastatin | 10 | R | NL | ||
| Simvastatin | 10 | R | R | ||
NL Not listed; OA Open access; R Restricted access; R* Restricted access with reduced subsidy
DISCUSSION
The OECD health data are a useful resource for intercountry comparisons and have been used successfully in several projects, especially those focusing on expenditures on health (43-46). However, gaps remain with respect to international agreements on statistical methods, so that the same term may mean something different in each of the OECD countries; for example, hospital separation data may or may not include same-day discharges. Moreover, even if the same methods are used to obtain a particular measure, individual country policies may lead to differences; for instance, the ALOS may be shorter in countries that have developed strong posthospitalization services, although the actual time needed for the service may be similar.
Nevertheless, there are no significant differences among Australia, Canada and New Zealand reported in the OECD definitions, sources and methods for the measures used in this analysis, with the exception of the change from coding using the ICD-9 to ICD-10. This began in Australia in 1998, but did not commence in Canada and New Zealand until 2000. Although ICD-10 is one of the most significant modifications in the history of the system, the conversion of codes for cardiovascular conditions is relatively straightforward and does not prevent comparisons between the two versions (47). The changes in the coding of respiratory disorders are more profound, especially in asthma and chronic obstructive pulmonary disease, but the overall coding for respiratory conditions, which we used, remains reasonably consistent.
Canada, Australia and New Zealand each have socialized health care systems that serve similarly aged populations with similar life expectancy at birth and decreasing mortality rates. The three countries also have comparable estimated rates of hypertension (20% to 25%) and diabetes (4% to 5%). In addition, they have similar adult obesity rates (20% to 22% with a body mass index higher than 30 kg/m2), and 15% to 22% of the adult populations are daily smokers (41). Approximately two-thirds of each country’s residents live in an urban setting. Each country has an indigenous ethnic group (First Nations and Métis in Canada, Aborigines and Torres Strait Islanders in Australia, and Maoris and Pacific Islanders in New Zealand) that has a generally poorer health status than the majority Caucasian population. In Canada and Australia, indigenous people constitute only 2% to 3% of the total population, but they form more than 20% of the New Zealand population.
Our analysis suggests some important differences between the three countries in terms of health outcomes and hospitalization use in the cardiovascular and respiratory disease areas. In Canada and Australia, the ALOS for cardiovascular and respiratory disorders decreased or remained stable, whereas in New Zealand, it increased substantially until 2002. Similarly, the discharge rates for circulatory and respiratory diseases have declined in Canada and Australia, but increased in New Zealand. Increasing numbers of hospitalizations of longer duration for cardiovascular and respiratory disorders suggest that hospital costs have risen substantially in New Zealand. Together with the possibility of a reduction in the rate of the decrease in AMI mortality, these findings point to a decline in cardiovascular and respiratory health in New Zealand compared with stability or improvement in these disease areas in Australia and Canada.
The results of our analysis could be due to inherent limitations within the OECD health data as a result of countries providing incomplete, inaccurate or noncomparable data, or different policies in the countries leading to erroneous comparisons. An example of the latter would be differences in ALOS due to variation in the extent of available posthospitalization care, resulting in patients staying in hospital longer simply because there is nowhere for them to go. It is likely that, in all three countries, the socialized health care system is stretched to the limit (this is certainly true in Canada and New Zealand [48]), so that the length of stay in hospital is kept to a minimum unless it is absolutely necessary. Nevertheless, it should be remembered that the overall discharge rate in Canada in 2002 was approximately 50% and 40% of that in Australia and New Zealand, respectively.
Mortality rate is a harder measure of health status and care than ALOS and discharge rates. As in most industrialized countries, the mortality rates from coronary artery disease in Canada, Australia and New Zealand have declined since the 1960s and continue to do so, but at a slower pace (49–53). However, these decreases are not consistent across all parts of the three countries or in all groups of their populations. The differences among and within countries are due, in part, to variation in risk factors and access to services (53–63). Moreover, as people live longer but have more sedentary lifestyles leading to obesity, diabetes and other complications, the improvements seen in cardiovascular health in the last half of the 20th century will slow down and may be reversed to some extent.
Consistent with our finding that cardiovascular health appears to be declining in New Zealand, concern has been expressed by New Zealand health professionals about worsening cardiovascular health (64–66). For example, Elliott and Richards (65) reported a 6.6% increase in deaths due to coronary artery disease between 2000 and 2001, and an increase in the number of hospitalizations for AMI of more than 60% between 1997 to 1998 and 2002 to 2003. There is also evidence that the increase is somewhat higher in women and younger age groups, and is definitely higher in indigenous New Zealanders. Elliott and Richards believe that there is an “epidemic in acute coronary syndromes” in progress in New Zealand. They also believe that “low levels of appropriate investigations and evidence-based treatments compared with contemporary international guidelines”, together with insufficient angiography or cardiac catheterization facilities (63), are contributing to the problem. The National Heart Foundation of New Zealand is also concerned about “the possible emergence of a substantive and adverse cohort effect” leading to higher mortality rates from coronary artery disease in young and middle-aged adults from the 1951 birth cohort onward (66).
Although not strictly comparable, because different sources had to be used, sales data for cardiovascular and respiratory drugs demonstrate a strong and similar increasing trend in Canada and Australia. This contrasts sharply with the trend of tightly controlled sales in New Zealand from 1997, when the PHARMAC cost-containment process began functioning. These data suggest an association between decreased cardiovascular and respiratory drug sales and markers of declining cardiovascular and respiratory health in New Zealand. However, the evidence is only circumstantial. The association could be due to an ecological fallacy in which aggregate results do not apply to individuals (67). Correlation analyses of this type are among the weakest types of study design.
Nevertheless, potential lessons for the NPS being considered for Canada exist in these data. An overly tight cost-containment process may lead to the underuse of highly beneficial drugs, which, in turn, is likely to produce a negative effect on the health of Canadians. A proposed paradigm for the NPS is based heavily on the British Columbia model of restricting access to drugs, which is based in part on the New Zealand approach. Both models use RBP and therapeutic substitution to contain costs.
In New Zealand, prescription medicines are made available through a tendering process in which the government uses its single-buyer power to negotiate the lowest price for a drug through PHARMAC. The successful bid gets the tender and that is frequently the only drug available to patients, eg, only pravastatin and simvastatin are available through a restricted access process with full subsidy in New Zealand. However, the success of a bid does not seem to be always based on scientific evidence, but more on the price of the drug and often on ‘package deals’ offered by the manufacturer. ‘Package agreements’ and ‘ministerial direction’ are among the central influencers of drug coverage in New Zealand (19). This approach, rather than one of evidence-based decision making, led to the RBP statin in New Zealand changing from fluvastatin to ator-vastatin to simvastatin over a period of less than six years (28,35). Negative outcomes (increased lipid levels and thrombotic vascular events) of these decisions were demonstrated (68,69).
An RBP system is designed to contain costs and has several attractions to pharmaceutical insurers. However, international evidence suggests that it has limited effectiveness and places little value on individual patient’s needs and convenience (70). Patients’ convenience is often not a focus of formulary review committees, although adherence to therapy is likely to increase with convenience. Several countries have tried RBP, and at least two (Norway and Denmark) have decided that it is not an efficient method to control prescription drug costs without detrimentally affecting the health of its citizens (71). Interestingly, Lexchin (72) has recently reported that prices of drugs within a class in Canada are frequently relatively similar until there are four or more products in the class, at which point price reductions begin to occur. In other words, more drugs in the same class lead to lower prices.
New Zealand has a culture of extreme fiscal restraint in subsidizing prescription drugs due to limited resources. This has undoubtedly led to significant savings in drug costs, as demonstrated by the fact that PHARMAC has consistently generated surpluses within the capped drug budget. As a result of the cost-containment system for prescription drugs, fewer new products are available in New Zealand than in Australia and Canada. Eighty-five new drugs were released into the world market between 1994 and 1998, 56 of which were made available for sale in Canada, 43 in Australia and only 28 in New Zealand (73). Although these numbers are smaller than in the United Kingdom and the United States, and new drugs are not necessarily better than currently marketed products, patients in New Zealand are disadvantaged when it comes to access to the newest therapies. In contrast to the evidence that cardiovascular health improved significantly in New Zealand until the early 1990s (50,51), our analysis of the OECD health data points to declining cardiovascular health in New Zealand, which is supported by other findings (64–66).
Whether New Zealand’s drug cost-containment system is directly related to the negative impact on patient health or one of many contributing factors is debatable, but the disparities in cardiovascular mortality and the use of services for cardiovascular disorders among New Zealand and the other two countries, where cost-containment is not so restrictive, is remarkable. Further evaluation of the effects of the model on the health and welfare of New Zealanders and on the potential increasing costs in other areas of the health care system should be pursued. It is important that those who direct and manage the Canadian health care system learn from the experience of other countries, for “those who cannot remember the past are condemned to repeat it” (74). For Canadians, careful consideration must be given to the potential downstream consequences of any model for a NPS in this country, and to the opportunities provided for discussion and input from health care professionals and patients.
Acknowledgments
We thank Howard Gaskin, Oliver Johnston, Alex Lofthouse and Christian Ouellet from GlaxoSmithKline Canada for providing additional data for this work and their insightful comments on the results.
Footnotes
DISCLOSURE: Dr LeLorier has served as a consultant for, and received research grants from, Pfizer, Merck Frosst, AstraZeneca and Bristol-Myers Squibb. Dr Rawson is a full-time employee of GlaxoSmithKline. The views expressed are those of the authors and do not necessarily represent those of their employers.
REFERENCES
- 1.Marra CA, Lynd LD, Anis AH, Esdaile JM. Approval process and access to prescription drugs in Canada. Arthritis Rheum. 2006;55:9–11. doi: 10.1002/art.21709. [DOI] [PubMed] [Google Scholar]
- 2.Rawson NS, Kaitin KI, Thomas KE, Perry G. Drug review in Canada: A comparison with Australia, Sweden, the United Kingdom and the United States. Drug Inf J. 1998;32:1133–41. [Google Scholar]
- 3.Rawson NS. Time required for approval of new drugs in Canada, Australia, Sweden, the United Kingdom and the United States in 1996–1998. Can Med Assoc J. 2000;162:501–4. [PMC free article] [PubMed] [Google Scholar]
- 4.Rawson NS. Timeliness of review and approval of new drugs in Canada from 1999 through 2001: Is progress being made? Clin Ther. 2003;25:1230–47. doi: 10.1016/s0149-2918(03)80080-2. [DOI] [PubMed] [Google Scholar]
- 5.Rawson NSB. Issues in the approval of, access to, and post-marketing follow-up of new drugs in Canada: A personal viewpoint. Pharmacoepidemiol Drug Saf. 2002;11:335–40. doi: 10.1002/pds.740. [DOI] [PubMed] [Google Scholar]
- 6.West R, Borden EK, Collet JP, Rawson NS, Tonks RS. “Cost-effectiveness” estimates result in flawed decision-making in listing drugs for reimbursement”. Can J Public Health. 2002;93:421–5. doi: 10.1007/BF03405029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West R, Borden EK, Collet JP, Rawson NS, Tonks RS. Need for an improved submission process for listing drugs for reimbursement in Canadian provinces. Can J Clin Pharmacol. 2003;10:207–10. [PubMed] [Google Scholar]
- 8.Anis AH, Guh D, Wang X. A dog’s breakfast: Prescription drug coverage varies widely across Canada. Med Care. 2001;39:315–26. doi: 10.1097/00005650-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Grootendorst P. Beneficiary cost sharing under Canadian provincial prescription drug benefit programs: History and assessment. Can J Clin Pharmacol. 2002;9:79–99. [PubMed] [Google Scholar]
- 10.Canadian Agency for Drugs and Technologies in Health Common Drug Review. < www.cadth.ca/index.php/en/cdr> (Version current at June 7, 2007).
- 11.Roehr B. Data for evaluating drugs is often poor, say experts. BMJ. 2005;330:984. doi: 10.1136/bmj.330.7498.984-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahon M, Morgan S, Mitton C. The Common Drug Review: A NICE start for Canada? Health Policy. 2006;77:339–51. doi: 10.1016/j.healthpol.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Clarke JT. Is the current approach to reviewing new drugs condemning the victims of rare diseases to death? A call for a national orphan drug review policy. Can Med Assoc J. 2006;174:189–90. doi: 10.1503/cmaj.050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canadian Institute for Health Information Drug expenditure in Canada, 1985 to 2005. < secure.cihi.ca/cihiweb/dispPage.jsp?cw_page=AR_80_E> (Version current at June 7, 2007).
- 15.Health Canada A 10-year plan to strengthen health care. 2004 Sep 16; < www.hc-sc.gc.ca/hcs-sss/delivery-prestation/fptcollab/2004-fmm-rpm/nr-cp_9_16_2_e.html> (Version current at June 7, 2007).
- 16.Canadian Health Coalition More for less: A national pharmacare strategy. 2006 May 26; < www.healthcoalition.ca/moreforless.pdf> (Version current at June 7, 2007).
- 17.Hanley G, Morgan S. A national formulary for Canada: Lessons from New Zealand. May, 2006.
- 18.Ioannides-Demos LL, Ibrahim JE, McNeil JJ. Reference-based pricing schemes: Effect on pharmaceutical expenditure, resource utilisation and health outcomes. Pharmacoeconomics. 2002;20:577–91. doi: 10.2165/00019053-200220090-00002. [DOI] [PubMed] [Google Scholar]
- 19.Sundakov A, Sundakov V. New Zealand pharmaceutical policies: Time to take a fresh look. 2005 Aug; < www.castalia.fr/SITE_Default/x-files/14634.pdf> (Version current at June 7, 2007).
- 20.Department of Health and Ageing Pharmaceutical Benefits Scheme. < www.pbs.gov.au/html/home> (Version current at June 7, 2007).
- 21.Bagg W, Braatvedt G. Fluvastatin: What is the evidence? NZ Med J. 1997;110:321–2. [PubMed] [Google Scholar]
- 22.Allen P. Fluvastatin. NZ Med J. 1997;110:403. [PubMed] [Google Scholar]
- 23.Ellis CJ, McHaffie D, Elliott J, Wilkins G. Statins and PHARMAC. NZ Med J. 1998;111:38. [PubMed] [Google Scholar]
- 24.Mann J, Scott R. Lipid-modifying drugs. NZ Med J. 1998;111:285–7. [PubMed] [Google Scholar]
- 25.Ellis CJ, McHaffie D, Elliott J, Wilkins G. Statins and PHARMAC. NZ Med J. 1999;112:55–6. [PubMed] [Google Scholar]
- 26.Martin J, Begg E. Reference pricing – is it in the public interest? NZ Med J. 2000;113:422–5. [PubMed] [Google Scholar]
- 27.Swinburn B, Milne R, Richards M, Begg E, Foote S, Jackson R. Reimbursement of pharmaceuticals in New Zealand: Comments on PHARMAC’s processes. NZ Med J. 2000;113:425–7. [PubMed] [Google Scholar]
- 28.Begg E, Sidwell A, Gardiner S, Nicholls G, Scott R. The sorry saga of the statins in New Zealand – pharmacopolitics versus patient care. NZ Med J. 2003;116:U360. < www.nzma.org.nz/journal/116-1170/360/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 29.Beasley R, Masoli M. Asthma in New Zealand – time to get control. NZ Med J. 2003;116:U434. < www.nzma.org.nz/journal/116-1174/434/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 30.MacKay P. Is PHARMAC’s sole-supply tendering policy harming the health of New Zealanders? NZ Med J. 2005;118:U1433. < www.nzma.org.nz/journal/118-1214/1433/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 31.Gillies J, Brown J, Byrnes C, Farrell A, Graham D. PHARMAC and Ventolin in New Zealand. NZ Med J. 2005;118:U1616. < www.nzma.org.nz/journal/118-1220/1616/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 32.Mann S. Dihydropyridines, felodipine, and PHARMAC. NZ Med J. 2005;118:U1569. < www.nzma.org.nz/journal/118-1218/1569/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 33.Jones D. Long-acting inhaled bronchodilators for COPD – lack of logic continues. NZ Med J. 2005;118:U1669. < www.nzma.org.nz/journal/118-1222/1669/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 34.White H, Ellis C. PHARMAC and lack of funding for clopidogrel. NZ Med J. 2006;119:U1808. < www.nzma.org.nz/journal/119-1228/1808/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 35.Ellis C, White H. PHARMAC and the statin debacle. NZ Med J. 2006;119:U2033. < www.nzma.org.nz/journal/119-1236/2033/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 36.Moodie P, Metcalfe S, McNee W. Response from PHARMAC: Difficult choices. NZ Med J. 2003;116:U361. < www.nzma.org.nz/journal/116-1170/361/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 37.Davies A, Metcalfe S, Moodie P, McNee W. PHARMAC responds to Stewart Mann on dihydropyridine calcium channel antagonists. NZ Med J. 2005;118:U1621. < www.nzma.org.nz/journal/118-1220/1621/content.pdf> (Version curren at June 7, 2007). [PubMed] [Google Scholar]
- 38.Metcalfe S, Moodie P, Davies A, McNee W. PHARMAC responds on salbutamol. NZ Med J. 2005;118:U1644. < www.nzma.org.nz/journal/118-1221/1644/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 39.Metcalfe S, Dougherty S. PHARMAC responds on long-acting inhalers for COPD. NZ Med J. 2005;118:U1743. < www.nzma.org.nz/journal/118-1225/1743/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 40.Moodie P, Dougherty S. PHARMAC’s response on clopidogrel. NZ Med J. 2006;119:U1872. < www.nzma.org.nz/journal/119-1229/1872/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 41.Organisation for Economic Co-operation and Development Health Data 2006: Statistics and Indicators for 30 Countries. 2006 Jun 26; < www.oecd.org/document/30/0,2340,en_2649_34631_12968734_1_1_1_1,00.html> (Version current at June 7, 2007).
- 42.Brogan Inc Pharmaceutical Market Data: PharmaStat. < www.broganinc.com/english/products/pstat.html> (Version current at June 7, 2007).
- 43.Deber R, Swan B. Canadian health expenditures: Where do we really stand internationally? CMAJ. 1999;160:1730–4. [PMC free article] [PubMed] [Google Scholar]
- 44.Huber M, Orosz E. Health expenditure trends in OECD countries, 1990–2001. Health Care Financ Rev. 2003;25:1–22. [PMC free article] [PubMed] [Google Scholar]
- 45.Comanor WS, Frech HE, Miller RD. Is the United States an outlier in health care and health outcomes? A preliminary analysis. Int J Health Care Finance Econ. 2006;6:3–23. doi: 10.1007/s10754-006-6863-8. [DOI] [PubMed] [Google Scholar]
- 46.Leal J, Luengo-Fernández R, Gray A, Petersen S, Rayner M. Economic burden of cardiovascular diseases in the enlarged European Union. Eur Heart J. 2006;27:1610–9. doi: 10.1093/eurheartj/ehi733. [DOI] [PubMed] [Google Scholar]
- 47.Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM.Comparability of cause of death between ICD-9 and ICD-10: Preliminary estimates National vital statistics reports, vol 49, no 2. National Center for Health Statistics,2001. <www.cdc.gov/nchs/data/nvsr/nvsr49/nvsr49_02.pdf> (Version current at June 7, 2007). [PubMed]
- 48.Health services at breaking point ...don’t panic? NZ Med J. 2002;115:1. [PubMed] [Google Scholar]
- 49.Campbell NR, Onysko J, Johansen H, Gao RN. Changes in cardiovascular deaths and hospitalization in Canada. Can J Cardiol. 2006;22:425–7. doi: 10.1016/s0828-282x(06)70929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beaglehole R, Stewart AW, Jackson R, et al. Declining rates of coronary heart disease in New Zealand and Australia, 1983–1993. Am J Epidemiol. 1997;145:707–13. doi: 10.1093/aje/145.8.707. [DOI] [PubMed] [Google Scholar]
- 51.Hay DR. Cardiovascular disease in New Zealand, 2004: A summary of recent statistical information. 2004 Oct; < www.nhf.org.nz/files/NHF6949%20TechReport.pdf> (Version current at June 7, 2007).
- 52.Tobias M, Sexton K, Mann S, Sharpe N. How low can it go? Projecting ischaemic heart disease mortality in New Zealand to 2015. NZ Med J. 2006;119:U1932. < www.nzma.org.nz/journal/119-1232/1932/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 53.Sexton PT, Sexton TL. Excess coronary mortality among Australian men and women living outside the capital city statistical divisions. Med J Aust. 2000;172:370–4. doi: 10.5694/j.1326-5377.2000.tb124008.x. [DOI] [PubMed] [Google Scholar]
- 54.Tanuseputro P, Manuel DG, Leung M, Nguyen K, Johansen H. Risk factors for cardiovascular disease in Canada. Can J Cardiol. 2003;19:1249–59. [PubMed] [Google Scholar]
- 55.Naylor CD, Jaglal SB. Regional revascularization patterns after myocardial infarction in Ontario. Can J Cardiol. 1995;11:670–4. [PubMed] [Google Scholar]
- 56.Cox JL, Ramer SA, Lee DS, et al. Canadian Cardiovascular Outcomes Research Team Investigators Pharmacological treatment of congestive heart failure in Canada: A description of care in five provinces. Can J Cardiol. 2005;21:337–43. [PubMed] [Google Scholar]
- 57.Heller RF. Mortality from cardiovascular disease is too high outside capital cities. Med J Aust. 2000;172:360–1. doi: 10.5694/j.1326-5377.2000.tb124005.x. [DOI] [PubMed] [Google Scholar]
- 58.Leeder SR. Achieving equity in the Australian healthcare system. Med J Aust. 2003;179:475–8. doi: 10.5694/j.1326-5377.2003.tb05650.x. [DOI] [PubMed] [Google Scholar]
- 59.Carr J, Robson B, Reid P, Purdie G, Workman P. Heart failure: Ethnic disparities in morbidity and mortality in New Zealand. NZ Med J. 2002;115:15–7. [PubMed] [Google Scholar]
- 60.Sharpe N, Wilkins G. Quality and equity in cardiovascular health in New Zealand: The need for agreed achievable standards of care, cohesive planning, and action. NZ Med J. 2004;117:U951. < www.nzma.org.nz/journal/117-1197/951/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 61.Doolan-Noble F, Broad J, Riddell T, North D. Cardiac rehabilitation services in New Zealand: Access and utilisation. NZ Med J. 2004;117:U955. < www.nzma.org.nz/journal/117-1197/955/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 62.Ellis C, Gamble G, French J, et al. Management of patients admitted with an acute coronary syndrome in New Zealand: Results of a comprehensive nationwide audit. NZ Med J. 2004;117:U953. < www.nzma.org.nz/journal/117-1197/953/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 63.Ellis C, Devlin G, Matsis P, et al. New Zealand Acute Coronary Syndromes [NZACS] Audit Group Acute coronary syndrome patients in New Zealand receive less invasive management when admitted to hospitals without invasive facilities. NZ Med J. 2004;117:U954. < www.nzma.org.nz/journal/117-1197/954/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 64.Campbell DJ. Heart failure: How can we prevent the epidemic? Med J Aust. 2003;179:422–5. doi: 10.5694/j.1326-5377.2003.tb05620.x. [DOI] [PubMed] [Google Scholar]
- 65.Elliott J, Richards M. Heart attacks and unstable angina (acute coronary syndromes) have doubled in New Zealand since 1989: How do we best manage the epidemic? NZ Med J. 2005;118:U1674. < www.nzma.org.nz/journal/118-1223/1674/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 66.Sharpe N. Heart health has an adverse future forecast in New Zealand: An alarm call to action across the continuum. NZ Med J. 2006;119:U1936. < www.nzma.org.nz/journal/119-1232/1936/content.pdf> (Version current at June 7, 2007). [PubMed] [Google Scholar]
- 67.Spasoff RA. Epidemiologic Methods for Health Policy. New York: Oxford University Press; 1999. [Google Scholar]
- 68.Thomas MC, Mann J, Williams S. The impact of reference pricing on clinical lipid control. NZ Med J. 1998;111:292–4. [PubMed] [Google Scholar]
- 69.Thomas M, Mann J. Increased thrombotic vascular events after change of statin. Lancet. 1998;352:1830–1. doi: 10.1016/S0140-6736(05)79893-7. [DOI] [PubMed] [Google Scholar]
- 70.Bosanquet N. PHARMAC Mark 2: Towards agreed solutions? NZ Med J. 2000;113:409–10. [PubMed] [Google Scholar]
- 71.ECON Centre for Economic Analysis Evaluation of the reference pricing system for medicines. 2006 Jun 22; < www.econ.no> (Version current at June 7, 2007).
- 72.Lexchin J. Do manufacturers of brand-name drugs engage in price competition? An analysis of introductory prices. CMAJ. 2006;174:1120–1. doi: 10.1503/cmaj.051687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Danzon PM, Wang YR, Wang L. The impact of price regulation on the launch delay of new drugs – evidence from twenty-five major markets in the 1990s. Health Econ. 2005;14:269–92. doi: 10.1002/hec.931. [DOI] [PubMed] [Google Scholar]
- 74.Santayana G. New York: Scribner’s; 1905. The Life of Reason: Reason in Common Sense; p. 284. [Google Scholar]