Abstract
OBJECTIVES:
To determine whether myocardial contrast echocardiography (MCE) can quickly and accurately assess myocardial perfusion and infarct-related artery (IRA) patency before emergency angiography during acute myocardial infarction (AMI).
BACKGROUND:
Despite encouraging experimental and clinical studies, the reliability and practicality of MCE in predicting IRA patency during AMI before angiography has not been proven.
METHODS:
Two-dimensional echocardiography and MCE were performed in 51 patients with AMI just before emergency angiography. With knowledge of the electrocardiogram findings and regional wall motion, myocardial perfusion was assessed to predict IRA patency.
RESULTS:
Myocardial perfusion studies were adequate for interpretation in 40 patients. An occluded IRA was predicted in 28 patients; the artery was occluded in 22 patients, and six patients had Thrombolysis In Myocardial Infarction (TIMI) grade 2 flow or less. A patent IRA was predicted in 12 patients; eight patients had TIMI grade 3 flow, one patient had TIMI grade 2 flow and the IRA was occluded in three patients. In one of the three patients, the appropriate view was not obtained. In another patient, collateral flow was adequate for near-normal regional wall motion, and in the last, the findings suggested reperfusion of the proximal artery with distal embolic occlusion. Taken together, MCE accurately predicted either TIMI grade 2 flow or less, or TIMI grade 3 flow in 36 of 40 patients. Sensitivity was 87.5%, specificity and positive predictive value were 100% and negative predictive power was 66.7% (P<0.001).
CONCLUSIONS:
MCE, together with the electrocardiogram and regional wall motion, can be used to quickly and reliably predict IRA patency early during AMI and may be useful to facilitate a management strategy.
Keywords: Acute myocardial infarction, Echocardiography, Myocardial contrast perfusion
Abstract
OBJECTIFS :
Déterminer si l’échocardiographie myocardique de contraste (ÉMC) peut évaluer avec rapidité et précision la perfusion myocardique et la perméabilité de l’artère reliée à l’infarctus (ARI) avant l’angiographie d’urgence pendant un infarctus aigu du myocarde (IAM).
HISTORIQUE :
Malgré des études expérimentales et cliniques encourageantes, on n’a pas encore démontré la fiabilité et la valeur concrète de l’ÉMC à prédire la perméabilité de l’ARI pendant l’IAM avant l’angiographie.
MÉTHODOLOGIE :
On a procédé à une échocardiographie bidimensionnelle et à une ÉMC chez 51 patients atteints d’un IAM juste avant qu’ils subissent une angiographie d’urgence. Compte tenu des résultats de l’électrocardiogramme et du mouvement régional de la paroi, les auteurs ont évalué la perfusion myocardique pour prédire la perméabilité de l’ARI.
RÉSULTATS :
Les études de perfusion myocardique ont permis l’interprétation chez 40 patients. On a prédit une occlusion de l’ARI chez 28 patients. Cette occlusion a été vérifiée chez 22 patients, et six patients ont eu une thrombolyse dans l’infarctus du myocarde (TIMI) avec débit de grade 2 ou moins. On a prédit une ARI perméable chez 12 patients. Huit patients ont subi une TIMI avec débit de grade 3, un patient a subi une TIMI avec débit de grade 2, et on a constaté une occlusion de l’ARI chez trois patients. Chez l’un des trois patients, on n’a pu obtenir la vue pertinente. Chez un autre patient, le débit collatéral assurait un mouvement quasinormal de la paroi, et chez le dernier, les résultats laissaient supposer une reperfusion de l’artère proximale avec occlusion embolique distale. Dans l’ensemble, l’ÉMC permet de prédire avec précision la TIMI avec débit de grade 2 ou moins ou la TIMI avec débit de grade 3 chez 36 des 40 patients. La sensibilité s’élevait à 87,5 %, la spécificité et la valeur prédictive positive, à 100 %, et la puissance prédictive négative, à 66,7 % (p < 0,001).
CONCLUSIONS :
L’ÉMC, conjointement avec l’électrocardiogramme et le mouvement régional de la paroi, peut être utilisée pour prédire avec rapidité et précision la perméabilité de l’ARI au début de l’IAM et peut être utile pour faciliter une stratégie de prise en charge.
Optimal therapy during acute myocardial infarction (AMI) includes prompt reestablishment of flow in the infarct-related artery (IRA) and effective reperfusion (1–3). Early, spontaneous reperfusion occurs in a small but significant proportion of IRAs (4,5), and the majority are reperfused with thrombolytic therapy. It is difficult to determine whether patency has been achieved at a given time in most patients without cardiac catheterization. The availability of a noninvasive method to rapidly assess myocardial perfusion has important implications in the emergency management of patients presenting with a known or suspected AMI, or after administration of a thrombolytic agent. However, no technique has yet been demonstrated to quickly and reliably predict the effectiveness of reperfusion or status of the IRA.
Myocardial contrast echocardiography (MCE) uses an injection of microbubbles to assess myocardial perfusion. MCE can be used to reliably assess myocardial reperfusion following primary percutaneous coronary intervention (PCI) for AMI and to predict improvement in left ventricular (LV) function, as well as to assess the degree of collateral support to the infarcted territory (6–17). However, due to time constraints and the technical demands of performing contrast echocardiography before angiography during AMI, few clinical studies have assessed the accuracy of this technique in determining the status of the IRA. Thus, despite promising human and animal studies (14, 18–22), it remains to be shown whether the technique is practical and accurate in the clinical setting.
We performed a study on patients undergoing immediate angiography and intended primary PCI during AMI to determine whether MCE can be used to reliably assess myocardial perfusion in the infarct territory. We hypothesized that combined with analysis of the electrocardiogram (ECG) and the infarct region wall motion assessed echocardiographically, MCE can be used to reliably detect myocardial perfusion and, thus, IRA patency.
METHODS
Study design
The present study included patients undergoing primary PCI for a first AMI at the Foothills Medical Centre (Calgary, Alberta). Two-dimensional echocardiography and MCE were performed immediately before angiography to assess wall motion and microvascular perfusion, respectively. Patients were eligible for the study if they arrived in the catheterization laboratory within 6 h of symptom onset, had ongoing chest pain and had ST segment elevation in at least three contiguous ECG leads. Patients were excluded if they had a history of previous infarction or were hemodynamically unstable, or if the ECG suggested that the risk area was likely to be very small (only in leads II, III and aVF, or only two precordial leads) and, thus, likely difficult to assess accurately. The study protocol was approved by the Conjoint Health Research Ethics Board of the University of Calgary and the Calgary Health Region, and all patients gave informed consent.
Echocardiography
Echocardiography was performed while the patients were being prepared for angiography using a Sonos 5500 ultrasound system (Philips Ultrasound, USA) and a phased-array transducer with transmit and receive frequencies of 1.3 MHz and 3.6 MHz, respectively. Wall motion was assessed using harmonic imaging before the injection of a contrast agent. MCE was performed using either an intravenous bolus or continuous infusion of Optison (Mallinckrodt Inc, USA) in six patients, Levovist (Schering, Germany) in 15 patients or Definity (Bristol Meyers Squibb, USA) in 30 patients. Images displaying the infarcted territory were obtained from the apical four-, three- and two-chamber views using intermittent ultraharmonic or power Doppler imaging. A dynamic range of approximately 80 dB was used. The focus was set at the level of the mitral annulus, unless there was a concern about apical destruction artifact, in which case it was moved to the apex. Lateral and time-gain compensation settings were optimized and kept constant throughout the studies. Systolic frames (triggered to the downslope of the T wave) were captured at pulsing intervals of one, three, five and eight beats, and were stored on video tape.
Coronary angiography and PCI
All patients received oral acetylsalicylic acid (160 mg) and intravenous heparin. Coronary angiography was performed using standard views. Images were digitized and stored on compact disc. The decision to perform immediate PCI was at the discretion of the interventional cardiologist.
Data analysis
Patient characteristics are listed as mean ± SD. Echocardiographic images were later evaluated by three echocardiographers blinded to the angiographic findings. The infarct territory was first defined by reviewing the ECG. Wall motion in the IRA territory was then graded using a scale of 1 to 5 (1 – normal; 2 – hypokinetic; 3 –severely hypokinetic; 4 – akinetic; 5 – dyskinetic) and compared with the ECG findings. An initial prediction of IRA patency was based on the whether there was a mismatch between the ECG findings and wall motion. For example, we predicted that the IRA was patent if wall motion in the infarct territory was normal or hypokinetic, or if a small area of akinesis was associated with extensive ECG changes that suggested involvement of a much larger area. Myocardial perfusion in the infarct territory was then graded as being normal, patchy or absent. Consensus was reached in cases in which there was initial disagreement, with the MCE results considered to be the most important predictor of IRA flow. IRA patency was then predicted based on synthesis of the ECG, regional myocardial function and myocardial perfusion. Coronary angiograms were subsequently reviewed independently by another investigator (an interventional cardiologist). Flow in the IRA before PCI was graded according to the Thrombolysis In Myocardial Infarction (TIMI) classification (23). Fisher’s exact test was used to assess accuracy of the technique.
RESULTS
Patient characteristics
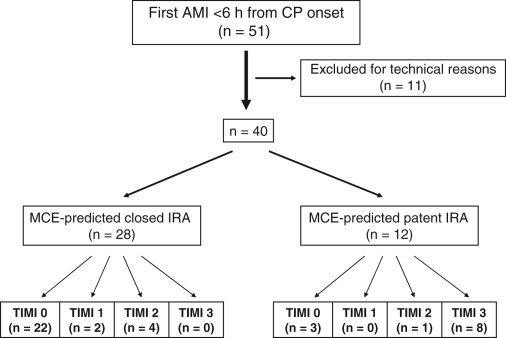
A total of 51 patients were enrolled in the study (Figure 1). Without knowledge of the angiographic results, the technical quality of the contrast perfusion study was considered to be unsatisfactory for analysis in 11 patients; the data presented are from the remaining 40 patients. Patient demographics and clinical variables are listed in Table 1. Mean age was 60±12 years. The IRA was the right coronary artery in nine patients, the left anterior descending artery in 27 patients and the left circumflex artery in four patients. Time from symptom onset to performance of the echocardiographic studies was 198±107 min. Delay to initiating cardiac catheterization due to the echocardiographic study was always less than 5 min.
Figure 1).
Predicted versus angiographic findings. AMI Acute myocardial infarction; CP Chest pain; IRA Infarct-related artery; MCE Myocardial contrast echocardiography; TIMI 3 Thrombolysis In Myocardial Infarction grade 3 flow
TABLE 1.
Patient characteristics (n=40)
| Age, years (mean ± SD) | 60±12 |
| Female sex, n (%) | 10 (25) |
| Male sex, n (%) | 30 (75) |
| Smoking history, n (%) | 28 (70) |
| Hypercholesterolemia, n (%) | 27 (68) |
| Diabetes, n (%) | 9 (23) |
| Infarct-related artery, n (%) | |
| Left anterior descending | 26 (65) |
| Right coronary | 10 (25) |
| Left circumflex | 4 (10) |
| Symptom onset to PCI, hour:min (mean ± SD) | 3:37±1:37 |
PCI Percutaneous coronary intervention
Infarct region function
Table 2 lists the predicted status of the IRA and the angiographic findings. Based on a qualitative match between the extent of the wall motion abnormality in the infarct region and the number of leads with ST segment elevation, an occluded IRA was predicted in 28 patients; of these, 22 patients had an occluded artery, four patients had TIMI grade 2 flow and two patients had TIMI grade 3 flow. Better than expected wall motion in relation to the ECG findings predicted a patent IRA in 10 patients; of these, six patients had TIMI grade 3 flow, one patient had TIMI grade 2 flow and three patients had an occluded artery. There was no clear explanation for the incorrect prediction in two of these patients; one patient with near-normal wall motion had excellent collateral flow. Wall motion could not be assessed in two patients because of poor endocardial definition before contrast injection.
TABLE 2.
Predicted infarct-related artery (IRA) status versus angiographic findings
| IRA flow grade | MCE-predicted occluded IRA (n=28) | Wall motion-predicted occluded IRA (n=28) | MCE-predicted patent IRA (n=12) | Wall motion-predicted patent IRA (n=10) |
|---|---|---|---|---|
| TIMI 0 | 22 | 22 | 3 | 3 |
| TIMI 1 | 2 | – | – | – |
| TIMI 2 | 4 | 4 | 1 | 1 |
| TIMI 3 | – | 2 | 8 | 6 |
MCE Myocardial contrast echocardiography; TIMI 3 Thrombolysis In Myocardial Infarction grade 3 flow
MCE
The perfusion studies predicted an occluded IRA in 28 patients; the artery was occluded in 22 patients, two patients had TIMI grade 1 flow and four patients had TIMI grade 2 flow. Thus, observation of patchy or absent perfusion by MCE accurately predicted suboptimal or absent flow in the IRA in all 28 patients. The perfusion studies predicted a patent IRA in 12 patients; eight patients had TIMI grade 3 flow and one patient had TIMI grade 2 flow. The IRA was occluded in three patients; one patient had excellent collateral flow that explained the findings (wall motion was near normal), and the appropriate view was not obtained in a second patient. In the remaining patient, both the ECG and extent of the wall motion abnormality suggested a large infarct, but only a small branch of the left circumflex artery was occluded, which suggests that there was distal embolization from the more proximal patent artery. Overall, in patients with interpretable contrast studies (40 of 51), MCE had a sensitivity of 87.5%, specificity of 100%, positive predictive value of 100% and negative predictive power of 66.7% (P<0.001).
The findings in the five patients with TIMI grade 2 flow are also interesting. A perfusion defect was absent in one patient, and the IRA was predicted to be open (wall motion was normal). However, the other four patients with TIMI grade 2 flow had a perfusion defect, and the IRA was predicted to be closed. These patients also had a wall motion abnormality in keeping with the extent of ischemia implied by the ECG, suggesting that microvascular perfusion was indeed abnormal, despite flow in the epicardial artery. If one considers that these latter patients might have benefited from improved perfusion, MCE correctly identified 29 of 32 patients who might have benefited from additional intervention. In two of the remaining patients (the one with excellent collateral flow and the one with presumed distal embolization to a small branch of the large proximal IRA), PCI might have had limited benefit. Overall, MCE correctly predicted microvascular perfusion status in 37 of 40 patients, resulting in an overall diagnostic accuracy of 92.5%. Accuracy was similar in anterior (25 of 26) and inferior (eight of 10) infarctions.
DISCUSSION
In the present study, we demonstrated that MCE, combined with knowledge of the ECG findings and regional wall motion, can be used to quickly and reliably predict the status of the IRA in most patients presenting within 6 h of symptom onset with ST segment elevation AMI. With minimal advance notice, we were prepared for the studies before patient arrival in the catheterization laboratory. Wall motion and perfusion studies took 5 min to 10 min while the patient was being prepared for angiography, resulting in little delay. When absent or poor perfusion was observed, the culprit vessel was either occluded or there was suboptimal (TIMI grade 2 or less) flow in the IRA. When MCE suggested adequate perfusion, the assessment was accurate in two-thirds of patients. However, in the remaining patients, one had TIMI grade 2 flow (with possible adequate reperfusion) and there were good explanations for the findings in the other three patients, which may otherwise be considered to be misleading (we suggest that PCI had limited potential benefit in two of the three patients).
Prompt re-establishment of myocardial perfusion is the primary treatment goal in patients with AMI. Early restoration of IRA patency is associated with reduced long-term mortality and greater myocardial salvage; TIMI grade 3 flow is optimal, and lower flow grades are associated with worse outcomes (24–26).
A rapid, reliable, noninvasive assessment of myocardial perfusion and IRA patency may facilitate decision making during the initial assessment of patients with AMI. The ability to evaluate the status of the IRA at any time is particularly advantageous following administration of a lytic agent, when rescue PCI may be a consideration. Assessment of wall motion by echocardiography is an excellent technique to identify the risk area, but persistent myocardial stunning after effective reperfusion precludes reliable prediction of vessel patency – abnormal wall motion does not indicate the absence of myocardial salvage. MCE is particularly well suited for determining the integrity of the microcirculation and, therefore, the status of the IRA during AMI (6–17). The small number of patients with predicted occluded IRAs who had patent vessels precludes a quantitative expression of the added value of MCE in identifying such patients. The intent of the present study was to assess the value of using all the readily available data, including the echocardiographic findings, to predict IRA status.
The most appropriate use of MCE to facilitate prompt decision making requires validation in clinical trials. However, regardless of whether lysis has been attempted, if myocardial perfusion is shown to be satisfactory, the potential value of immediate angiography in such a select subgroup of patients has not been tested. In fact, one can interpret the early angioplasty trials to suggest that the opposite is true – early, routine angioplasty in such patients may even be harmful (27–29).
The finding of absent perfusion in the infarct region implies that the microvasculature has been disrupted, either due to an occluded IRA, obstruction at the capillary level or irreversible myocardial damage. Early presentation associated with absent perfusion would favour the presence of viable myocardium. In the present study, patients were studied relatively early (mean time from symptom onset was approximately 3 h). Thus, absent or poor perfusion was more likely to be associated with an occluded IRA or TIMI grade 2 flow or less, rather than being confounded by patients with largely irreversible damage and patent arteries, as one would predict if the studies had been performed later, after symptom onset (23,30,31). When MCE suggests that perfusion is good in the infarct region, either reperfusion has been achieved or there is sufficient collateral flow to maintain viability, as was true in one of our patients in whom wall motion was also near-normal despite an occluded IRA. When perfusion in the risk area is good, the role of immediate angiography remains to be defined. Patients with absent or poor perfusion are most likely to benefit from early intervention. The availability of MCE makes it possible to test reperfusion strategies faster and more directly than was previously possible in clinical trials. Given that a recent study (32) confirmed that rescue PCI was beneficial (in which 90 min ST segment resolution was used to assess reperfusion following thrombolysis), the potential value of a more rapid and accurate technique deserves further study.
Clinical assessment of reperfusion
Clinical findings that may suggest reperfusion have not been proven to be reliable predictors of IRA patency. Chest pain resolution is not sufficiently accurate for decision making (33,34). Accelerated idioventricular rhythm is a relatively specific but insensitive predictor of patency (35). Numerous studies have assessed ST segment resolution as a predictor of reperfusion and outcomes (26, 36–44). Although ST segment resolution is relatively sensitive for detecting a patent IRA, specificity is only modest. More importantly, the technique takes 60 min to 90 min, during which time myocardial necrosis may be ongoing in the absence of effective reperfusion. It is therefore very difficult to select appropriate patients for urgent intervention in a timely fashion – in the Middlesbrough Early Revascularization to Limit INfarction (MERLIN) study, 41% of patients who underwent angiography with intended rescue angioplasty had patent IRAs (45). Up to 50% of patients with persistent ST segment elevation may have a patent artery reflecting inadequate microvascular reperfusion or the ‘no reflow’ phenomenon (46). Persistent ST segment elevation associated with a patent artery is more common when reperfusion therapy is delayed beyond 3 h, likely due to greater microvascular disruption (47).
Previous studies
In a multicentre study involving 59 patients, Kamp et al (23) performed perfusion studies before angiography and predicted IRA occlusion with a high sensitivity in anterior infarction, but the sensitivity in inferior infarction was 28%. In a similar 30-patient study by Balcells et al (30), perfusion early after PCI correlated with subsequent recovery of function. However, the investigators did not predict IRA patency based on the perfusion studies. Moir et al (31) assessed perfusion qualitatively and offline quantitatively to predict patency in 34 patients with suspected AMI before angiography. The qualitative method was sensitive for the detection of a perfusion defect, but specificity was only 50%. The quantitative method (offline and time consuming) predicted lower than TIMI grade 3 flow, with a sensitivity of 73% and a specificity of 67%.
In the present study, the perfusion studies, combined with assessment of the ECG and regional wall motion, accurately predicted IRA status in 90% of patients, with no apparent difference between anterior and inferior infarctions. In the remaining four patients, the findings were explained by one technical error, excellent collateral flow, a small occluded branch in a large risk area and TIMI 2 flow, which might have been adequate to maintain viability. Thus, MCE appears to be highly reliable when carefully performed and interpreted. Our results may be related to improved imaging technology and to early enrolment of patients who are most likely to have sufficient viable myocardium detected if present. Encouraging results were also obtained by Main et al (48), who recently studied 29 patients using real-time perfusion imaging and Definity. They found that the location of the perfusion defect correlated well with the artery involved, and that infarct size (assessed by peak serum creatine kinase levels) correlated with the extent of the perfusion defect. However, no attempt was made to predict IRA patency.
In the study by Moir et al (31), MCE was not a better a predictor of IRA patency than wall motion. A regional wall motion abnormality accurately reflects perfusion status if the IRA is occluded or if there is irreversible myocardial injury. However, if the IRA becomes patent early after occlusion, myocardial stunning may cause a persistent wall motion abnormality despite adequate perfusion. In the present study, all 24 patients correctly predicted by MCE to have an occluded IRA also had a wall motion abnormality (Table 2). However, two patients who were predicted to have an occluded IRA based on wall motion had a patent artery, consistent with stunned myocardium – both had good perfusion as determined by MCE. Although based on small numbers, we suggest that myocardial perfusion predicts patency more reliably than wall motion alone, particularly when identifying patients who may not require urgent intervention. In addition, two patients had uninterpretable wall motion studies due to poor images, which is not uncommon when studying patients in such a setting. We conclude that there was a modest but important incremental benefit of MCE over regular imaging that can be accomplished quickly and at minimal cost. The technique may be even more useful after lytic therapy; a greater proportion of patients have successful reperfusion and may therefore not require urgent angiography.
It is interesting that four of the five patients with TIMI grade 2 flow in the present study had poor or absent perfusion. MCE has been proven to be an excellent method for the detection of microvascular dysfunction following angioplasty during infarction and for predicting subsequent recovery of function (6,8,9,12,13). TIMI grade 2 flow may be a less accurate indicator of ineffective perfusion than myocardial contrast perfusion (49); a recent study (50) suggested that MCE is superior to other modalities for assessing myocardial reperfusion after infarction. We suggest that patients with poor perfusion and TIMI grade 2 flow have reduced microvascular flow and that some may benefit from improved myocardial perfusion, if it can be achieved. Identification of these patients may be important if measures can be taken to improve perfusion and, consequently, outcomes.
Limitations
The present study was performed during normal working hours, when study personnel were immediately available. Sufficient resources to provide full coverage before direct PCI would constitute substantial commitment and is therefore generally not practical. However, MCE may be more practical when rescue PCI is being considered. We have full echocardiography coverage in our region, so that only upgrading of technical skills is required to provide this service.
The most significant limitation of our study was that perfusion was not interpretable for technical reasons in 22% of patients. We believe it is critical to recognize technically inadequate studies, because they depend on good acoustic windows, and they may be difficult to obtain quickly with patients in the supine position and often in pain. Two-thirds of our uninterpretable studies occurred early in our experience using first-generation contrast agents. The relatively small number of studies with each of the contrast agents does not allow for a valid comparison between agents. However, accuracy was similar with all three agents when the studies were considered to be adequate.
It is worth emphasizing that patients who appeared to be having a relatively small AMI by ECG and those with a previous infarction were not studied. We anticipate that if all patients with AMI were to undergo MCE, the reliability of the technique would be lower in those with small risk areas, simply because of imaging limitations – small perfusion defects cannot be as confidently identified as larger ones. It is also possible that previously infarcted areas make it more difficult to assess perfusion defects in at least some patients. Until the accuracy of this technique in such patients is determined, the applicability of MCE in patients with small or previous infarctions remains uncertain.
There were too few patients in the present study to make definitive comments regarding TIMI grade 2 flow. We also did not have data to relate our findings to myocardial salvage. However, the study was designed to determine whether assessment of regional wall motion and perfusion can be used to quickly and reliably predict the status of the IRA. In this respect, we demonstrated that the technique can be used effectively. Our results relate closely to the issues faced early during AMI and are not limited by a lack of long-term follow-up, given the extensive literature supporting the close associations between IRA patency, myocardial perfusion, recovery of myocardial function and outcomes.
Few of our patients presented relatively late after symptom onset. One would expect that absent or decreased perfusion would be observed more often in patients who present late with more irreversible damage.
Interpretation of the perfusion and wall motion studies was reached by consensus and therefore might not have reflected the accuracy that can be achieved quickly in the catheterization laboratory. Because of the detailed discussion during analysis sessions of selected portions of each of the echocardiograms, a true assessment of inter- and intraobserver variability was not possible. However, in the last 17 studies, the angiographers were informed of the prediction before angiography and were correct in all cases, except the one in which we suspect that there was distal embolization of thrombus from the proximal IRA. It is therefore clear to us that with experience, it is reasonable to use this technique with confidence when the studies are performed.
CONCLUSIONS
MCE accurately predicts IRA occlusion or less than TIMI grade 3 flow early during AMI. The technique is also reliable in identifying patients with adequate myocardial perfusion, provided that it is performed correctly. Thus, with experience, modern contrast agents and current imaging technology, interpretable images can be rapidly obtained in most patients, and the technique is sufficiently accurate to facilitate decision making early during infarction.
Acknowledgments
The authors express their appreciation for the support of the Foothills Hospital Interventional Cardiology Research Group (Calgary, Alberta), the technical support of the staff of the cardiac catheterization and echocardiography laboratories, as well as Dr YC Sun MD PhD.
Footnotes
FUNDING: Supported by grants from Hoffman-LaRoche Canada and Bristol Myers Squibb USA.
REFERENCES
- 1.Belenkie I, Knudtson ML, Roth DL, et al. Relation between flow grade after thrombolytic therapy and the effect of angioplasty on left ventricular function: A prospective randomized trial. Am Heart J. 1991;121:407–16. doi: 10.1016/0002-8703(91)90706-n. [DOI] [PubMed] [Google Scholar]
- 2.Sheehan FH, Mathey DG, Schofer J, Dodge HT, Bolson EL. Factors that determine recovery of left ventricular function after thrombolysis in patients with acute myocardial infarction. Circulation. 1985;71:1121–8. doi: 10.1161/01.cir.71.6.1121. [DOI] [PubMed] [Google Scholar]
- 3.Belenkie I, Thompson CR, Manyari DE, et al. Importance of effective, early and sustained reperfusion during acute myocardial infarction. Am J Cardiol. 1989;63:912–6. doi: 10.1016/0002-9149(89)90138-0. [DOI] [PubMed] [Google Scholar]
- 4.DeWood MA, Spores J, Notske R, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980;303:897–902. doi: 10.1056/NEJM198010163031601. [DOI] [PubMed] [Google Scholar]
- 5.Ross AM, Coyne KS, Reiner JS, et al. A randomized trial comparing primary angioplasty with a strategy of short-acting thrombolysis and immediate planned rescue angioplasty in acute myocardial infarction: The PACT trial. PACT investigators. Plasminogen-activator Angioplasty Compatibility Trial. J Am Coll Cardiol. 1999;34:1954–62. doi: 10.1016/s0735-1097(99)00444-1. [DOI] [PubMed] [Google Scholar]
- 6.Ito H, Tomooka T, Sakai N, et al. Lack of myocardial perfusion immediately after successful thrombolysis. A predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation. 1992;85:1699–705. doi: 10.1161/01.cir.85.5.1699. [DOI] [PubMed] [Google Scholar]
- 7.Ito H, Okamura A, Iwakura K, et al. Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation. 1996;93:1993–9. doi: 10.1161/01.cir.93.11.1993. [DOI] [PubMed] [Google Scholar]
- 8.Lepper W, Hoffmann R, Kamp O, et al. Assessment of myocardial reperfusion by intravenous myocardial contrast echocardiography and coronary flow reserve after primary percutaneous transluminal coronary angioplasty [correction of angiography] in patients with acute myocardial infarction Circulation 20001012368–74.(Erratum in 2000;102:482). [DOI] [PubMed] [Google Scholar]
- 9.Lepper W, Kamp O, Vanoverschelde JL, et al. Intravenous myocardial contrast echocardiography predicts left ventricular remodeling in patients with acute myocardial infarction. J Am Soc Echocardiogr. 2002;15:849–56. doi: 10.1067/mje.2002.121277. [DOI] [PubMed] [Google Scholar]
- 10.Sabia PJ, Powers ER, Jayaweera AR, Ragosta M, Kaul S. Functional significance of collateral blood flow in patients with recent acute myocardial infarction. A study using myocardial contrast echocardiography. Circulation. 1992;85:2080–9. doi: 10.1161/01.cir.85.6.2080. [DOI] [PubMed] [Google Scholar]
- 11.Swinburn JM, Lahiri A, Senior R. Intravenous myocardial contrast echocardiography predicts recovery of dysynergic myocardium early after acute myocardial infarction. J Am Coll Cardiol. 2001;38:19–25. doi: 10.1016/s0735-1097(01)01317-1. [DOI] [PubMed] [Google Scholar]
- 12.Lepper W, Sieswerda GT, Vanoverschelde JL, et al. Predictive value of markers of myocardial reperfusion in acute myocardial infarction for follow-up left ventricular function. Am J Cardiol. 2001;88:1358–63. doi: 10.1016/s0002-9149(01)02113-0. [DOI] [PubMed] [Google Scholar]
- 13.Bolognese L, Carrabba N, Parodi G, et al. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–6. doi: 10.1161/01.CIR.0000118496.44135.A7. [DOI] [PubMed] [Google Scholar]
- 14.Coggins MP, Sklenar J, Le DE, Wei K, Lindner JR, Kaul S. Noninvasive prediction of ultimate infarct size at the time of acute coronary occlusion based on the extent and magnitude of collateral-derived myocardial blood flow. Circulation. 2001;104:2471–7. doi: 10.1161/hc4501.098954. [DOI] [PubMed] [Google Scholar]
- 15.Janardhanan R, Burden L, Senior R. Usefulness of myocardial contrast echocardiography in predicting collateral blood flow in the presence of a persistently occluded acute myocardial infarction-related coronary artery. Am J Cardiol. 2004;93:1207–11. doi: 10.1016/j.amjcard.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 16.Asanuma T, Fujihara T, Otani K, Miki A, Ishikura F, Beppu S. Noninvasive vessel-selective perfusion imaging with intravenous myocardial contrast echocardiography. J Am Soc Echocardiogr. 2004;17:654–8. doi: 10.1016/j.echo.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Ragosta M, Camarano G, Kaul S, Powers ER, Sarembock IJ, Gimple LW. Microvascular integrity indicates myocellular viability in patients with recent myocardial infarction. New insights using myocardial contrast echocardiography. Circulation. 1994;89:2562–9. doi: 10.1161/01.cir.89.6.2562. [DOI] [PubMed] [Google Scholar]
- 18.Iwakura K, Ito H, Kawano S, et al. Predictive factors for development of the no-reflow phenomenon in patients with reperfused anterior wall acute myocardial infarction. J Am Coll Cardiol. 2001;38:472–7. doi: 10.1016/s0735-1097(01)01405-x. [DOI] [PubMed] [Google Scholar]
- 19.Zoghbi WA. Evaluation of myocardial viability with contrast echocardiography. Am J Cardiol. 2002;90:65J–71J. doi: 10.1016/s0002-9149(02)02950-8. [DOI] [PubMed] [Google Scholar]
- 20.Villanueva FS. Myocardial contrast echocardiography in acute myocardial infarction. Am J Cardiol. 2002;90:38J–47J. doi: 10.1016/s0002-9149(02)02947-8. [DOI] [PubMed] [Google Scholar]
- 21.Ohmori K, Cotter B, Leistad E, et al. Assessment of myocardial postreperfusion viability by intravenous myocardial contrast echocardiography: Analysis of the intensity and texture of opacification. Circulation. 2001;103:2021–7. doi: 10.1161/01.cir.103.15.2021. [DOI] [PubMed] [Google Scholar]
- 22.Lafitte S, Higashiyama A, Masugata H, et al. Contrast echocardiography can assess risk area and infarct size during coronary occlusion and reperfusion: Experimental validation. J Am Coll Cardiol. 2002;39:1546–54. doi: 10.1016/s0735-1097(02)01771-0. [DOI] [PubMed] [Google Scholar]
- 23.Kamp O, Lepper W, Vanoverschelde JL, et al. Serial evaluation of perfusion defects in patients with a first acute myocardial infarction referred for primary PTCA using intravenous myocardial contrast echocardiography. Eur Heart J. 2001;22:1485–95. doi: 10.1053/euhj.2001.2604. [DOI] [PubMed] [Google Scholar]
- 24.Anderson JL, Karagounis LA, Becker LC, Sorensen SG, Menlove RL. TIMI perfusion grade 3 but not grade 2 results in improved outcome after thrombolysis for myocardial infarction. Ventriculographic, enzymatic, and electrocardiographic evidence from the TEAM-3 Study. Circulation. 1993;87:1829–39. doi: 10.1161/01.cir.87.6.1829. [DOI] [PubMed] [Google Scholar]
- 25.Fath-Ordoubadi F, Huehns TY, Al-Mohammad A, Beatt KJ. Significance of the Thrombolysis in Myocardial Infarction scoring system in assessing infarct-related artery reperfusion and mortality rates after acute myocardial infarction. Am Heart J. 1997;134:62–8. doi: 10.1016/s0002-8703(97)70107-8. [DOI] [PubMed] [Google Scholar]
- 26.Shah A, Wagner GS, Granger CB, et al. Prognostic implications of TIMI flow grade in the infarct related artery compared with continuous 12-lead ST-segment resolution analysis. Reexamining the “gold standard” for myocardial reperfusion assessment. J Am Coll Cardiol. 2000;35:666–72. doi: 10.1016/s0735-1097(99)00601-4. [DOI] [PubMed] [Google Scholar]
- 27.Immediate vs delayed catheterization and angioplasty following thrombolytic therapy for acute myocardial infarction. TIMI II A results. The TIMI Research Group. JAMA. 1988;260:2849–58. doi: 10.1001/jama.1988.03410190097031. [DOI] [PubMed] [Google Scholar]
- 28.Topol EJ, Califf RM, George BS, et al. A randomized trial of immediate versus delayed elective angioplasty after intravenous tissue plasminogen activator in acute myocardial infarction. N Engl J Med. 1987;317:581–8. doi: 10.1056/NEJM198709033171001. [DOI] [PubMed] [Google Scholar]
- 29.SWIFT trial of delayed elective intervention v conservative treatment after thrombolysis with anistreplase in acute myocardial infarction. SWIFT (Should We Intervene Following Thrombolysis?) Trial Study Group. BMJ. 1991;302:555–60. doi: 10.1136/bmj.302.6776.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balcells E, Powers ER, Lepper W, et al. Detection of myocardial viability by contrast echocardiography in acute infarction predicts recovery of resting function and contractile reserve. J Am Coll Cardiol. 2003;41:827–33. doi: 10.1016/s0735-1097(02)02962-5. [DOI] [PubMed] [Google Scholar]
- 31.Moir S, Haluska B, Leung D, Lim R, Garrahy P, Marwick TH. Quantitative myocardial contrast echocardiography for prediction of thrombolysis in myocardial infarction flow in acute myocardial infarction. Am J Cardiol. 2004;93:1212–7. doi: 10.1016/j.amjcard.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Gershlick AH, Stephens-Lloyd A, Hughes S, et al. REACT Trial Investigators Rescue angioplasty after failed thrombolytic therapy for acute myocardial infarction. N Engl J Med. 2005;353:2758–68. doi: 10.1056/NEJMoa050849. [DOI] [PubMed] [Google Scholar]
- 33.Califf RM, O’Neil W, Stack RS, et al. Failure of simple clinical measurements to predict perfusion status after intravenous thrombolysis. Ann Intern Med. 1988;108:658–62. doi: 10.7326/0003-4819-108-5-658. [DOI] [PubMed] [Google Scholar]
- 34.Morrow DA, Antman EM, Sayah A, et al. Evaluation of the time saved by prehospital initiation of reteplase for ST-elevation myocardial infarction: Results of the Early Retavase-Thrombolysis in Myocardial Infarction (ER-TIMI) 19 trial. J Am Coll Cardiol. 2002;40:71–7. doi: 10.1016/s0735-1097(02)01936-8. [DOI] [PubMed] [Google Scholar]
- 35.Shah PK, Cercek B, Lew AS, Ganz W. Angiographic validation of bedside markers of reperfusion. J Am Coll Cardiol. 1993;21:55–61. doi: 10.1016/0735-1097(93)90716-e. [DOI] [PubMed] [Google Scholar]
- 36.Cura FA, Roffi M, Pasca N, et al. Global Use of Strategies to Open Occluded Arteries V investigators ST-segment resolution 60 minutes after combination treatment of abciximab with reteplase or reteplase alone for acute myocardial infarction (30-day mortality results from the resolution of ST-segment after reperfusion therapy substudy) Am J Cardiol. 2004;94:859–63. doi: 10.1016/j.amjcard.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Giugliano RP, Sabatine MS, Gibson CM, et al. Combined assessment of thrombolysis in myocardial infarction flow grade, myocardial perfusion grade, and ST-segment resolution to evaluate epicardial and myocardial reperfusion. Am J Cardiol. 2004;93:1362–7. doi: 10.1016/j.amjcard.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 38.French JK, Ramanathan K, Stewart JT, Gao W, Theroux P, White HD. A score predicts failure of reperfusion after fibrinolytic therapy for acute myocardial infarction. Am Heart J. 2003;145:508–14. doi: 10.1067/mhj.2003.184. [DOI] [PubMed] [Google Scholar]
- 39.Gibson CM, Karha J, Giugliano RP, et al. INTEGRITI Study Group Association of the timing of ST-segment resolution with TIMI myocardial perfusion grade in acute myocardial infarction. Am Heart J. 2004;147:847–52. doi: 10.1016/j.ahj.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Syed MA, Borzak S, Asfour A, et al. Single lead ST-segment recovery: A simple, reliable measure of successful fibrinolysis after acute myocardial infarction. Am Heart J. 2004;147:275–80. doi: 10.1016/j.ahj.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Brodie BR, Stuckey TD, Hansen C, et al. Relation between electrocardiographic ST-segment resolution and early and late outcomes after primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2005;95:343–8. doi: 10.1016/j.amjcard.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 42.French JK, Hyde TA, Straznicky IT, et al. Relationship between corrected TIMI frame counts at three weeks and late survival after myocardial infarction. J Am Coll Cardiol. 2000;35:1516–24. doi: 10.1016/s0735-1097(00)00577-5. [DOI] [PubMed] [Google Scholar]
- 43.Maas AC, Wyatt CM, Green CL, et al. Combining baseline clinical descriptors and real-time response to therapy: The incremental prognostic value of continuous ST-segment monitoring in acute myocardial infarction. Am Heart J. 2004;147:698–704. doi: 10.1016/j.ahj.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 44.McLaughlin MG, Stone GW, Aymong E, et al. Prognostic utility of comparative methods for assessment of ST-segment resolution after primary angioplasty for acute myocardial infarction: The Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J Am Coll Cardiol. 2004;44:1215–23. doi: 10.1016/j.jacc.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 45.Sutton AG, Campbell PG, Graham R, et al. A randomized trial of rescue angioplasty versus a conservative approach for failed fibrinolysis in ST-segment elevation myocardial infarction: The Middlesbrough Early Revascularization to Limit INfarction (MERLIN) trial. J Am Coll Cardiol. 2004;44:287–96. doi: 10.1016/j.jacc.2003.12.059. [DOI] [PubMed] [Google Scholar]
- 46.de Lemos JA, Antman EM, Giugliano RP, et al. ST-segment resolution and infarct-related artery patency and flow after thrombolytic therapy. Thrombolysis in Myocardial Infarction (TIMI) 14 investigators. Am J Cardiol. 2000;85:299–304. doi: 10.1016/s0002-9149(99)00736-5. [DOI] [PubMed] [Google Scholar]
- 47.Wehrens XH, Doevendans PA, Ophuis TJ, Wellens HJ. A comparison of electrocardiographic changes during reperfusion of acute myocardial infarction by thrombolysis or percutaneous transluminal coronary angioplasty. Am Heart J. 2000;139:430–6. doi: 10.1016/s0002-8703(00)90086-3. [DOI] [PubMed] [Google Scholar]
- 48.Main ML, Kusnetzky LL, Dillon D, Daniel WC. Reperfusion assessment using myocardial contrast echocardiography in patients with ST-segment elevation acute myocardial infarction. Am J Cardiol. 2004;93:1401–3. A9. doi: 10.1016/j.amjcard.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto K, Ito H, Iwakura K, et al. Two different coronary blood flow velocity patterns in thrombolysis in myocardial infarction flow grade 2 in acute myocardial infarction: Insight into mechanisms of microvascular dysfunction. J Am Coll Cardiol. 2002;40:1755–60. doi: 10.1016/s0735-1097(02)02486-5. [DOI] [PubMed] [Google Scholar]
- 50.Greaves K, Dixon SR, Fejka M, et al. Myocardial contrast echocardiography is superior to other known modalities for assessing myocardial reperfusion after acute myocardial infarction. Heart. 2003;89:139–44. doi: 10.1136/heart.89.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]