Abstract
BACKGROUND:
Practice guidelines support an early invasive strategy in patients with non-ST segment elevation acute coronary syndromes, particularly in those at higher risk.
OBJECTIVES:
To compare North American rates of invasive cardiac procedure use stratified by risk.
METHODS:
Use of invasive cardiac procedures and other care patterns in patients with non-ST segment elevation acute coronary syndromes from the United States (US) Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines (CRUSADE) National Quality Improvement Initiative (n=88,097; 465 hospitals) and Canadian ACS Registries I (n=1270; 51 hospitals) and II (n=1473; 36 hospitals) were compared after dividing patients into different risk categories based on predicted risk of in-hospital mortality.
RESULTS:
While the overall use of invasive procedures was higher in the US, high-risk patients were least likely to undergo coronary angiography (41% versus 64% in Canada [P<0.0001] and 53% versus 76% in the United States [P<0.0001]) and percutaneous coronary intervention (14% versus 32% in Canada [P<0.0001] and 28% versus 51% in the US [P<0.0001]) compared with low-risk patients in both countries, and had longer median waiting times for these procedures (120 h versus 96 h in Canada [P<0.0001] and 34 h versus 23 h in the US [P<0.0001] for coronary angiography).
CONCLUSIONS:
The inverse relationship between risk level and the use of invasive cardiac procedures for patients in the US and Canada suggests that a risk stratification-guided approach for triaging patients to an early invasive management strategy is paradoxically used. This incongruous relationship holds true regardless of resource availability or overall rates of cardiac catheterization.
Keywords: Acute coronary syndromes, Guidelines
Abstract
HISTORIQUE:
Les directives cliniques étayent une stratégie effractive précoce chez les patients atteints d’un syndrome coronarien aigu sans élévation du segment ST, notamment chez les personnes les plus vulnérables.
OBJECTIFS:
Comparer les taux nord-américains de recours aux interventions cardiaques effractives, stratifiées selon le risque.
MÉTHODOLOGIE:
Les auteurs ont comparé le recours aux interventions cardiaques effractives et d’autres modes de soins chez les patients atteints de syndromes coronariens aigus sans élévation du segment ST participant à l’initiative nationale d’amélioration de la qualité (n=88 097, 465 hôpitaux) CRUSADE des États-Unis – pour établir si la stratification rapide du risque des patients atteints d’angine instable peut supprimer les issues négatives lorsqu’elle s’accompagne de l’implantation précoce des lignes directrices de l’ACC et de l’AHA – au recours à ces interventions selon les registres canadiens de SCA I (n=1 270, 51 hôpitaux) et II (n=1 473, 36 hôpitaux) d’après le risque prévu de mortalité en milieu hospitalier.
RÉSULTATS:
Le recours global aux interventions effractives était plus élevé aux États-Unis, mais les patients à haut risque étaient les moins susceptibles de subir une coronarographie (41 % par rapport à 64 % au Canada [p < 0,0001] et 53 % par rapport à 76 % aux États-Unis [p < 0,0001]) et une intervention coronaire percutanée (14 % par rapport à 32% au Canada [p < 0,0001] et 28 % par rapport à 51 % aux États-Unis [p < 0,0001]) par rapport aux patients à faible risque dans les deux pays, et leur temps médian d’attente était plus long pour ces interventions (120 heures par rapport à 96 heures au Canada [p < 0,0001] et 34 heures par rapport à 23 heures aux États-Unis [p < 0,0001] pour la coronarographie).
CONCLUSIONS:
La relation inversement proportionnelle entre le taux de risque et le recours aux interventions cardiaques effractives des patients des États-Unis et du Canada laisse supposer l’utilisation paradoxale d’une démarche orientée par la stratification du risque au moment du triage des patients en vue d’une prise en charge effractive précoce. Cette relation incongrue demeure, quelle que soit la disponibilité des ressources ou le taux global de cathétérisme cardiaque.
Acute coronary syndromes continue to be one of the major causes of morbidity and mortality in North America (1). There is mounting evidence suggesting that an early invasive strategy for treating patients with high-risk non-ST segment elevation acute coronary syndromes (NSTE ACS) is associated with reduced morbidity and mortality (2–6). Practice guidelines for the management of patients with unstable angina or non-ST segment elevation myocardial infarction (NSTEMI), the two conditions that collectively make up NSTE ACS, were jointly published by the American College of Cardiology (ACC) and the American Heart Association (AHA) in September 2000 (7), and were subsequently updated with new evidence in March 2002 (8). The ACC/AHA guidelines give a class IA recommendation for early invasive treatment for patients with high-risk features such as elevated cardiac markers, ischemic electrocardiogram (ECG) changes, recurrent ischemia, signs of heart failure and signs of hemodynamic instability. Similar recommendations have been made regarding the risk stratification and management of NSTE ACS patients in Canada (9–11). However, recent studies have suggested that early invasive management strategies are selectively targeted for lower-risk patients in the United States (US) and Canada (12, 13).
Multiple prior studies have demonstrated that patients with STEMI and NSTE ACS in the US are more likely to undergo cardiac catheterization and revascularization than in Canada, perhaps due to differences in the availability of facilities for performing invasive cardiac procedures between the US and Canada (14–21). However, these studies did not evaluate how risk stratification influenced triage decisions for the use of invasive cardiac procedures and other care processes.
Thus, we analyzed contemporary care patterns for patients with NSTE ACS in both the US and Canada to determine how patient risk status influenced the use of medications and invasive procedures recommended by practice guidelines in the two countries.
METHODS
Study descriptions
The Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines (CRUSADE) National Quality Improvement Initiative is an ongoing quality improvement program that collects data on high-risk NSTE ACS patients from over 400 participating centres in the US. The institutional review board of each site approved participation in CRUSADE, and because data were collected only during initial hospitalization in an anonymous fashion, informed consent was waived. Patients in CRUSADE presented with acute ischemic symptoms (lasting 10 min or longer) at rest within 24 h before hospital arrival and had at least one of the following high-risk features: ST segment depression of 0.5 mm or greater, transient ST segment elevation of 0.5 mm to 1.0 mm (lasting for less than 10 min) or positive cardiac markers (elevated troponin I or T and/or creatine kinase [muscle-brain] greater than the upper limit of normal for the local laboratory assay used at each institution).
Details of the Canadian ACS Registry I were reported elsewhere (22). The registry included 5312 patients from 51 hospitals in nine provinces in Canada between September 1999 and June 2001 if they were 18 years of age or older, admitted to hospital with a suspected ACS (defined by symptoms consistent with acute cardiac ischemia within 24 h of onset), and if the qualifying ACS was not deemed to have been precipitated by a significant comorbidity such as trauma or gastrointestinal bleeding. The Canadian ACS Registry I contained patients with unstable angina, NSTEMI and STEMI, but did not require specific ischemic ECG changes for the inclusion of patients with unstable angina. The Canadian ACS Registry II focused specifically on NSTE ACS. It included 2356 patients from 36 hospitals in seven provinces in Canada between January and December 2003 if they were: 18 years of age or older, diagnosed with NSTE ACS as defined by symptoms thought to be consistent with acute cardiac ischemia within 24 h of symptom onset, and the qualifying ACS must not have been precipitated or accompanied by a significant comorbidity (eg, trauma, gastrointestinal bleeding, perioperative or periprocedural myocardial infraction). Likewise, specific ischemic ECG changes were not required for inclusion of patients with unstable angina. In both Canadian ACS registries, the local hospital research ethics board approved the study, and all patients who were followed after discharge provided informed consent.
Data collection
In all studies, a standardized case report form was used by the designated physician or study coordinator to record demographic and clinical characteristics, medical history, selected laboratory results, ECG findings, use of cardiac medications, in-hospital procedures, and cardiovascular events and outcomes. In the case of the Canadian ACS Registries I and II, the case report form was forwarded to the coordinating centre (the Canadian Heart Research Centre) and scanned directly into an electronic database (TeleForm, Version 7.0, Cardiff, USA). Data checks were performed centrally to ensure accuracy, and for key variables, sites were queried and corrected or clarified in case of missing or incomplete data. Details regarding data collection for CRUSADE and a description of the data collection form have been previously published (23). In brief, data collected included clinical characteristics, the use of acute medications (within 24 h of hospital arrival), the use and timing of invasive cardiac procedures, laboratory results, clinical outcomes, and discharge therapies and interventions. Data collection did not continue after hospital transfer due to current privacy regulations, which prohibit collection of anonymous data after hospital transfer. Any contraindications to guideline-recommended therapies were also recorded.
Analysis population
Patients with NSTE ACS (both unstable angina and NSTEMI) from CRUSADE and patients with NSTEMI from the Canadian ACS Registries I and II were analyzed, because patients with unstable angina in the Canadian registries did not require ischemic ECG changes for inclusion (as was required in CRUSADE). NSTE ACS patients in CRUSADE (n=88,097) who were treated at 465 hospitals (90% with onsite cardiac catheterization) in 45 US states from January 2001 through December 2003 were analyzed. Within the Canadian ACS Registry I, 1270 of 5312 patients had NSTEMI, while 1473 of 2356 patients had NSTEMI in the Canadian ACS Registry II. Thus, a total of 2743 patients with NSTEMI were included from both Canadian ACS registries. Of the 51 and 36 hospitals participating in the Canadian ACS Registries I and II, respectively, 29% and 33% had onsite cardiac catheterization. In a secondary analysis, the patient population was restricted to patients presenting to hospitals with onsite cardiac catheterization facilities.
Statistical analysis
Categorical variables are presented as frequencies or percentages, and continuous variables as medians with 25th and 75th percentiles. The Platelet glycoprotein IIb-IIIa in Unstable angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) mortality risk model for unstable angina/NSTEMI was used in the present study, and was adapted from the original model that was developed to predict 30-day mortality to, in turn, predict the risk of in-hospital mortality (24). Variables included in this model are age, sex, signs of congestive heart failure on presentation, admission heart rate, admission systolic blood pressure, ST depression and elevated cardiac markers. The original PURSUIT population was divided into tertiles of patients (low-risk, moderate-risk and high-risk) based on the predicted risk of in-hospital mortality for each patient. The ranges of in-hospital mortality rates within each risk tertile were used to establish low-risk, moderate-risk and high-risk categories for predicted in-hospital mortality rates for patients in the present analysis population.
Baseline characteristics, use of guideline-recommended acute (less than 24 h) medications, use and timing of invasive cardiac procedures, and in-hospital clinical outcomes were compared among the three risk groups separately in the US and Canadian populations. Comparisons between patient risk groups were made using the χ2 test for categorical variables and the Kruskal-Wallis test for continuous variables. The logistic generalized estimating equations and ordinary logistic regression methods were used to obtain the unadjusted OR for treatments and in-hospital outcomes by risk groups. The generalized estimating equations method produces estimates similar to those from ordinary logistic regression, but the estimated variances are adjusted for within-hospital clustering of responses, because patients admitted in the same hospital tend to be more similar to each other than to those in different hospitals (25). Direct comparisons were not made between the US and Canadian populations. Statistical analyses were performed by the Canadian Heart Research Centre and the Duke Clinical Research Institute using the SAS software package version 8.2 (SAS Institute, USA). P<0.05 was established as the level of statistical significance for all tests.
RESULTS
Patients
Among the 88,097 US patients in CRUSADE, 3814 patients were excluded due to missing data that precluded the classification of risk status. Among the 2743 Canadian patients with NSTEMI in the ACS Registries I and II, 44 patients were excluded due to missing data.
In the analyzable population of 84,283 US patients from CRUSADE, 50.0% of patients were classified as high-risk based on predicted in-hospital mortality rates, 24.3% were classified as moderate-risk and 22.7% were classified as low-risk. Among the 2699 Canadian patients in the analyzable population, 39.7% were classified as high-risk, 28.3% were classified as moderate-risk and 32.0% were classified as low-risk.
Baseline clinical characteristics stratified by risk are shown in Table 1. In both Canada and the US, patients in the high-risk groups were older, less commonly male, more likely to present with ST segment depression, and more likely to have signs of heart failure on presentation, renal insufficiency, diabetes mellitus, prior myocardial infarction, prior stroke and prior coronary artery bypass grafting surgery than patients in the moderate-risk and low-risk groups.
TABLE 1.
Baseline demographics and presenting characteristics by risk group for the United States and Canada
| Variable | United States
|
Canada
|
||||||
|---|---|---|---|---|---|---|---|---|
| Low risk (n=19,154) | Moderate risk (n=20,456) | High risk (n=44,673) | P | Low risk (n=863) | Moderate risk (n=764) | High risk (n=1072) | P | |
| Age (years) | 51 (45, 57) | 63 (56, 70) | 77 (70, 83) | <0.0001 | 54 (48, 60) | 67 (59, 72) | 76 (70, 82) | <0.0001 |
| Male sex | 69.1 | 63.4 | 54.5 | <0.0001 | 74.0 | 66.9 | 63.3 | <0.0001 |
| Heart rate (beats/min) | 73 (64, 84) | 77 (66, 89) | 90 (76, 108) | <0.0001 | 69 (60, 80) | 75 (63, 88) | 88 (74, 104) | <0.0001 |
| Systolic blood pressure (mmHg) | 151 (133, 172) | 148 (129, 169) | 140 (119, 160) | <0.0001 | 150 (133, 171) | 150 (130, 171) | 141 (122, 164) | <0.0001 |
| ST depression/transient ST elevation | 31.4 | 44.8 | 56.1 | <0.0001 | 11.9 | 23.3 | 48.2 | <0.0001 |
| Signs of congestive heart failure on presentation* | 4.2 | 9.6 | 36.7 | <0.0001 | 5.7 | 13.7 | 31.2 | <0.0001 |
| Renal insufficiency† | 6.4 | 9.4 | 18.2 | <0.0001 | 2.1 | 3.8 | 11.2 | <0.0001 |
| Hypertension | 59.7 | 67.8 | 72.8 | <0.0001 | 44.3 | 51.7 | 62.0 | <0.0001 |
| Diabetes mellitus | 24.6 | 32.3 | 36.3 | <0.0001 | 18.7 | 27.1 | 35.3 | <0.0001 |
| Current/recent smoking | 47.1 | 32.0 | 16.5 | <0.0001 | 42.1 | 24.1 | 15.1 | <0.0001 |
| Dyslipidemia | 47.5 | 52.4 | 44.2 | <0.0001 | 47.7 | 46.6 | 45.1 | 0.50 |
| Prior myocardial infarction | 23.3 | 28.6 | 34.5 | <0.0001 | 21.1 | 27.0 | 43.2 | <0.0001 |
| Percutaneous coronary intervention | 20.7 | 24.1 | 20.5 | <0.0001 | 12.2 | 15.2 | 12.8 | 0.18 |
| Prior coronary artery bypass grafting | 12.0 | 20.3 | 23.2 | <0.0001 | 7.0 | 12.0 | 16.3 | <0.0001 |
| Prior stroke | 4.3 | 7.9 | 14.8 | <0.0001 | 2.6 | 8.5 | 15.2 | <0.0001 |
Values are presented as percentages or median (25th and 75th percentiles) for age, heart rate and systolic blood pressure.
*Defined as jugular venous distension, rales, a third heart sound or pulmonary edema on initial chest x-ray documented by a physician on the initial history review and physical examination;
†Defined as creatinine > 177 μmol/L, calculated creatinine clearance <0.5 mL/s or the need for chronic renal dialysis
Acute medications
In the Canadian and the US populations, high-risk patients were least likely to have received acute (less than 24 h) acetylsalicylic acid, clopidogrel or ticlopidine, glycoprotein IIb/IIIa inhibitors and heparin than patients in the low- and moderate-risk groups (Tables 2 and 3). Patients in the US more commonly received clopidogrel and glycoprotein IIb/IIIa inhibitors, whereas patients in Canada more commonly received heparin.
TABLE 2.
Acute (<24 h) medications by risk group for the United States and Canada
| Medication | United States
|
Canada
|
||||
|---|---|---|---|---|---|---|
| Low risk (n=19,154) | Moderate risk (n=20,456) | High risk (n=44,673) | Low risk (n=863) | Moderate risk (n=764) | High risk (n=1072) | |
| Acetylsalicylic acid (%) | 94.2 | 93.2 | 90.3 | 96.1 | 93.7 | 89.3 |
| Glycoprotein IIb/IIIa inhibitor (%) | 47.5 | 43.1 | 28.9 | 14.1 | 12.7 | 7.1 |
| Clopidogrel (%) | 46.1 | 44.5 | 36.2 | 35.5 | 35.2 | 30.2 |
| Any heparin (%) | 86.2 | 85.8 | 81.6 | 95.3 | 92.3 | 88.1 |
| Unfractionated heparin (%) | 54.2 | 53.1 | 47.3 | 40.8 | 40.1 | 39.7 |
| Low-molecular-weight heparin (%) | 38.8 | 39.8 | 40.4 | 54.5 | 52.2 | 48.2 |
TABLE 3.
Unadjusted OR and 95% CI for medication use by risk group
| Medication | Total, n | Risk group | OR (95% CI) | P |
|---|---|---|---|---|
| Acetylsalicylic acid | 78,700 | Moderate versus low | 0.83 (0.77–0.90) | <0.0001 |
| High versus low | 0.57 (0.53–0.62) | <0.0001 | ||
| Glycoprotein IIb/IIIa Inhibitors | 72,326 | Moderate versus low | 0.84 (0.80–0.88) | <0.0001 |
| High versus low | 0.47 (0.45–0.50) | <0.0001 | ||
| Clopidogrel | 82,132 | Moderate versus low | 0.92 (0.88–0.95) | <0.0001 |
| High versus low | 0.66 (0.63–0.69 | <0.0001 | ||
| Any heparin | 79,525 | Moderate versus low | 0.94 (0.89–1.00) | 0.043 |
| High versus low | 0.69 (0.64–0.74) | <0.0001 | ||
| Intravenous or unfractionated heparin | 79,525 | Moderate versus low | 0.95 (0.91–0.98) | 0.004 |
| High versus low | 0.76 (0.73–0.80) | <0.0001 | ||
| Low-molecular-weight heparin | 79,525 | Moderate versus low | 1.04 (1.00–1.08) | 0.035 |
| High versus low | 1.05 (1.00–1.09) | 0.030 |
Invasive cardiac procedures
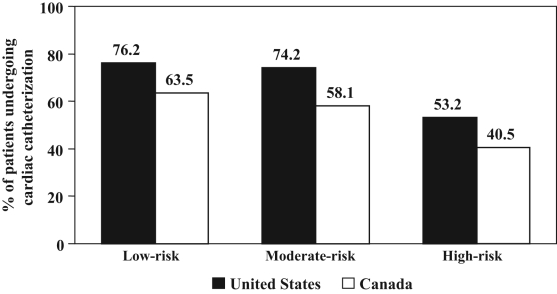
In Canada and the US, high-risk patients had lower rates of cardiac catheterization and catheterization within 48 h, and were less likely to receive percutaneous coronary intervention than moderate- and low-risk patients (Figure 1, Tables 4 and 5). High-risk patients also had longer median times from presentation to catheterization and percutaneous coronary intervention than moderate- and low-risk patients (Table 6). Overall, rates of catheterization, percutaneous coronary intervention and bypass surgery were higher in the US. When restricting the patient population to only those presenting to a hospital with onsite cardiac catheterization facilities, similar results were observed among the US (n=78,886) and Canadian (n=845) patients, with high-risk patients being least likely to undergo cardiac catheterization, catheterization within 48 h and percutaneous coronary intervention (all P<0.001; data not shown).
Figure 1).
Rates of cardiac catheterization by risk group in the United States and Canada
TABLE 4.
In-hospital invasive cardiac procedures by risk group
| Procedure | United States
|
Canada
|
||||
|---|---|---|---|---|---|---|
| Low risk (n=19,154) | Moderate risk (n=20,456) | High risk (n=44,673) | Low risk (n=863) | Moderate risk (n=764) | High risk (n=1072) | |
| Cardiac catheterization (%) | 76.2 | 74.2 | 53.2 | 63.5 | 58.1 | 40.5 |
| Catheterization <48 h (%) | 58.9 | 54.2 | 33.7 | 19.4 | 19.2 | 10.0 |
| Percutaneous coronary intervention (%) | 51.0 | 46.8 | 28.2 | 32.3 | 25.3 | 14.4 |
| Percutaneous coronary intervention <48 h (%) | 40.6 | 35.2 | 18.5 | 10.0 | 6.7 | 3.9 |
| Coronary artery bypass grafting (%) | 11.2 | 14.3 | 10.4 | 6.8 | 9.2 | 7.7 |
TABLE 5.
Unadjusted OR and 95% CI for procedures by risk group
| Procedure | Total, n | Risk group | OR (95% CI) | P |
|---|---|---|---|---|
| Diagnostic catheterization | 81,915 | Moderate versus low | 0.89 (0.85–0.93) | <0.0001 |
| High versus low | 0.42 (0.38–0.45) | <0.0001 | ||
| Catheterization within 48 h of arrival | 78,552 | Moderate versus low | 0.81 (0.78–0.84 | <0.0001 |
| High versus low | 0.37 (0.35–0.40) | <0.0001 | ||
| Percutaneous coronary intervention | 71,519 | Moderate versus low | 0.83 (0.80–0.87) | <0.0001 |
| High versus low | 0.42 (0.39–0.45) | <0.0001 | ||
| Percutaneous coronary intervention within 48 h of arrival | 70,095 | Moderate versus low | 0.77 (0.74–0.81) | <0.0001 |
| High versus low | 0.35 (0.32–0.37) | <0.0001 | ||
| Coronary artery bypass grafting | 70,882 | Moderate versus low | 1.34 (1.24–1.44) | <0.0001 |
| High versus low | 0.98 (0.89–1.07) | 0.65 |
TABLE 6.
Time to in-hospital invasive cardiac procedures by risk group
| Procedure | United States
|
Canada† |
||||||
|---|---|---|---|---|---|---|---|---|
| Low risk (n=19,154) | Moderate risk (n=20,456) | High risk (n=44,673) | P | Low risk (n=863) | Moderate risk (n=764) | High risk (n=1072) | P | |
| Time to catheterization* (h) | 22.7 (9.4, 43.3) | 24.4 (11.5, 47.7) | 34.3 (16.1, 65.0) | <0.0001 | 96 (48, 144) | 96 (48, 144) | 120 (60, 168) | 0.0002 |
| Time to PCI* (h) | 20.7 (6.0, 41.1) | 22.5 (6.6, 45.8) | 30.3 (11.9, 64.1) | <0.0001 | 96 (48, 168) | 120 (48, 168) | 120 (48, 192) | 0.21 |
| Time to CABG* (h) | 64.4 (39.5, 102.6) | 69.9 (41.5, 110.6) | 83.4 (45.6, 132.4) | <0.0001 | 240 (168, 360) | 300 (192, 432) | 300 (216, 432) | 0.31 |
*Values are presented as median (25th and 75th percentiles) for time to cardiac procedures;
†Time to procedures in Canadian ACS registries were reported as days, which were converted to hours for Canadian patients for this table. CABG Coronary artery bypass grafting; PCI Percutaneous coronary intervention
Unadjusted in-hospital clinical outcomes
Rates of death, reinfarction and death or reinfarction were progressively higher from the low- and moderate- to high-risk groups in both the US and Canada (Tables 7 and 8). Compared with the US, mortality rates by risk category were slightly lower in Canadian patients, but reinfarction rates were higher.
TABLE 7.
In-hospital outcomes by risk group
| Event | United States
|
Canada
|
||||
|---|---|---|---|---|---|---|
| Low risk n=19,154 | Moderate risk n=20,456 | High risk n=44,673 | Low risk n=863 | Moderate risk n=764 | High risk n=1072 | |
| Death (%) | 0.9 | 1.8 | 7.8 | 0.5 | 0.9 | 6.2 |
| Reinfarction (%) | 2.2 | 2.6 | 3.7 | 5.6 | 6.4 | 7.3 |
| Death or reinfarction (%) | 2.9 | 4.1 | 10.5 | 5.9 | 7.2 | 11.3 |
TABLE 8.
Unadjusted OR and 95% CI for in-hospital outcomes by risk group
| Event | Total, n | Risk group | OR (95% CI) | P |
|---|---|---|---|---|
| Death | 73,594 | Moderate versus low | 2.08 (1.76–2.47) | <0.0001 |
| High versus low | 8.57 (7.34–10.01) | <0.0001 | ||
| Postadmission infarction | 72,631 | Moderate versus low | 1.16 (1.02–1.31) | 0.0193 |
| High versus low | 1.58 (1.41–1.77) | <0.0001 | ||
| Death or myocardial infarction | 72,837 | Moderate versus low | 1.40 (1.27–1.56) | <0.0001 |
| High versus low | 3.50 (3.15–3.88) | <0.0001 |
DISCUSSION
We performed an analysis of the contemporary treatment of US and Canadian patients with NSTE ACS. Our study was unique, in that it dealt specifically with NSTE ACS using a much larger sample size than previous reports. In addition, our data were drawn from an era in which a significant number of patients were being treated after the emergence of evidence favouring an early invasive strategy. Moreover, we have incorporated the impacts of risk on choice of a management strategy in the real world. Our analysis revealed a paradoxical relationship between risk and treatment. Specifically, in both Canada and the US, the highest risk patients with NSTE ACS were least likely to receive guideline-recommended medical therapies and invasive cardiac procedures. The lower use of glycoprotein IIb/IIIa inhibitors in these patients can be explained, in part, by the lower rate of invasive cardiac procedures, because the greatest benefit of glycoprotein IIb/IIIa inhibitor therapy is in the context of an invasive procedure (8). Overall, catheterization and revascularization rates were higher in the US than in Canada. These results suggest that resource availability does not appear to influence triage decision making for NSTE ACS patients based on patient risk stratification.
Risk stratification
The differences observed in our analysis suggest that longstanding treatment biases continue to limit the adoption of a risk stratification approach to NSTE ACS management in the US and Canada. While practice guidelines strongly advocate targeting aggressive management approaches for patients with the highest expected risk of mortality, studies have consistently shown that patients with NSTE ACS and high-risk features, such as advanced age, diabetes mellitus, signs of congestive heart failure and renal insufficiency, less commonly receive evidence-based medications and less commonly undergo catheterization and revascularization (13, 26–28). However, early invasive management strategies have been shown to have preserved benefit in these populations, so safety concerns regarding the risks of invasive procedures in high-risk patients may be counterbalanced by the benefits offered by aggressive treatment strategies (29–31). It is possible that there were additional comorbidities and contraindications for invasive management that were not adequately accounted for in our analysis, but consistent findings across multiple observational studies have demonstrated that patients with NSTE ACS who are at the highest risk of mortality and have the greatest potential benefit from aggressive management are least likely to receive evidence-based care.
Efficiency of invasive management practices
Availability of resources would be expected to influence decisions regarding the triaging of patients with NSTE ACS to invasive cardiac procedures in Canada, where access to catheterization and revascularization is less available than in the US, such that higher-risk patients would be preferentially triaged to invasive management strategies (32). However, we have demonstrated that an inverse relationship between patient risk status and likelihood of undergoing catheterization and percutaneous coronary intervention persists in both Canada and the US, which suggests that treatment disparities for high-risk patients are not explained by access to cardiac services or the availability of procedural facilities. Similar findings from a prior analysis of cardiac catheterization rates in patients with STEMI showed that the predicted risk of severe coronary artery disease had no relationship to the frequency of catheterization in both countries (21). Additionally, the prevalence of severe coronary disease identified during catheterization after STEMI was similar in the US and Canada, but the higher frequency of catheterization in the US led to a greater than twofold higher rate of identification of severe coronary disease (20). Thus, more aggressive patterns of catheterization in the US can likely identify more patients with NSTE ACS who had severe coronary artery disease and who would be expected to benefit from revascularization, but the selective referral of low- to moderate-risk patients for invasive procedures in both the US and Canada suggests that the efficiency of invasive management strategies is not ideal in either country. The observed outcome differences among different risk groups verify that the risk model used in our study is appropriate for our patient population. However, a cause-and-effect relationship cannot be reliably made based on these observed differences alone.
Limitations
There are several limitations to the present study. First, these analyses are retrospective and based on observational data. Second, site selection in CRUSADE and the Canadian ACS registries was not random or specifically population-based. Furthermore, access to cardiac catheterization may be inherently limited for those patients presenting to a hospital without these facilities, and this might have impacted our findings. In an effort to address this issue, secondary analyses were performed in just those patients who presented to hospitals with on-site cardiac catheterization; the findings were consistent with the overall results. Third, we compared patients with unstable angina and NSTEMI from CRUSADE with patients with NSTEMI from the Canadian ACS registries as detailed in the methods, but approximately 90% of patients in CRUSADE have positive cardiac markers, so the comparison populations were similar (13). Fourth, we used the PURSUIT model to risk-stratify our patients, which has inferior prognostic calibration to other models in predicting in-hospital mortality. However, this model has previously been shown to have good discriminatory performance in predicting in-hospital mortality rates among the overall population in the Canadian ACS I Registry (33). Fifth, reinfarction rates were not adjudicated by a central committee, and hence, may be under-reported. Sixth, the objectives of our analysis were not to compare treatments and outcomes between the US and Canada, so we did not assess the impact of treatment differences between the countries on patient outcomes. Seventh, outcome data are limited to in-hospital events and do not evaluate longer-term differences in outcomes. Eighth, 3814 (4.3%) and 44 (1.6%) patients were excluded from the CRUSADE and Canadian ACS registries, respectively, due to missing data that precluded risk status classification. Ninth, because increasing age is a major contributor to the risk status and is often accompanied by comorbidities and different functional capacity and quality of life expectations that were not adequately captured by our case report forms, we may be overestimating the number of patients in the higher-risk group who were truly eligible for cardiac catheterization. Finally, the time frames for the generation of both the US and Canadian data were not entirely congruous and overlapped with the initial publication of the ACC/AHA guidelines and Canadian recommendations for NSTE ACS, as well as their subsequent updates in 2002 (7–10). It is therefore possible that changes in practice due to the ACC/AHA guidelines and Canadian recommendations were not fully captured by our analysis.
CONCLUSIONS
More appropriate triage decision making is needed to improve the use of guideline-recommended medical therapies and invasive cardiac procedures in patients with NSTE ACS in the US and Canada. Because improved adherence to guidelines has been shown to be associated with lower mortality in this population, increased use of guidelines recommendations in patients with high-risk NSTE ACS is expected to have a significant impact on ACS mortality rates in both the US and Canada (6,34).
Acknowledgments
The Canadian ACS Registries I and II were sponsored by the Canadian Heart Research Centre and Key Pharmaceuticals, Division of Schering Canada Inc (ACS I), Pfizer Canada Inc (ACS II), sanofi-Synthelabo Canada Inc (ACS II) and Bristol-Myers Squibb Canada Inc (ACS II). CRUSADE is funded by Millennium Pharmaceuticals, Inc USA and Schering Corporation, USA. Bristol-Myers Squibb/SanofiPharmaceuticals Partnership provides an unrestricted grant in support of the program.
REFERENCES
- 1.Kochanek KD, Smith BL. Deaths: Preliminary data for 2002. Natl Vital Stat Rep. 2004;52:1–47. [PubMed] [Google Scholar]
- 2.Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators. Lancet. 1999;354:708–15. [PubMed] [Google Scholar]
- 3.Cannon CP, Weintraub WS, Demopoulos LA, et al. TACTICS (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy)-Thrombolysis in Myocardial Infarction 18 Investigators Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–87. doi: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 4.Fox KA, Poole-Wilson PA, Henderson RA, et al. Randomized Intervention Trial of unstable Angina Investigators Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: The British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of unstable Angina. Lancet. 2002;360:743–51. doi: 10.1016/s0140-6736(02)09894-x. [DOI] [PubMed] [Google Scholar]
- 5.Wallentin L, Lagerqvist B, Husted S, Kontny F, Ståhle E, Swahn E. Outcome at 1 year after an invasive compared with a non-invasive strategy in unstable coronary-artery disease: The FRISC II invasive randomised trial. FRISC II Investigators. Fast Revascularisation during Instability in Coronary artery disease. Lancet. 2000;356:9–16. doi: 10.1016/s0140-6736(00)02427-2. [DOI] [PubMed] [Google Scholar]
- 6.Allen LA, O’Donnell CJ, Giugliano RP, Camargo CA, Jr, Lloyd-Jones DM. Care concordant with guidelines predicts decreased long-term mortality in patients with unstable angina pectoris and non-ST-elevation myocardial infarction. Am J Cardiol. 2004;93:1218–22. doi: 10.1016/j.amjcard.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 7.Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: Executive summary and recommendations A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina) Circulation 20001021193–209.(Erratum in 2000;102:1739). [DOI] [PubMed] [Google Scholar]
- 8.Braunwald E, Antman EM, Beasley JW, et al. American College of Cardiology; American Heart Association Committee on the Management of Patients With Unstable Angina. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction –summary article: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina) J Am Coll Cardiol. 2002;40:1366–74. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 9.Fitchett D, Goodman S, Langer A. New advances in the management of acute coronary syndromes: 1. Matching treatment to risk. CMAJ. 2001;164:1309–16. [PMC free article] [PubMed] [Google Scholar]
- 10.Fitchett D, Goodman SG, Gupta M, Langer A. Preventing thrombosis: Update of first-line therapy in the management of non-ST segment elevation acute coronary syndromes. Can J Cardiol. 2002;18:1179–90. [PubMed] [Google Scholar]
- 11.O’Neill BJ, Brophy JM, Simpson CS, et al. Canadian Cardiovascular Society Access to Care Working Group Treating the right patient at the right time: Access to care in non-ST segment elevation acute coronary syndromes. Can J Cardiol. 2005;21:1149–55. [PubMed] [Google Scholar]
- 12.Shamiss Y, Khaykin Y, Papastergiou J, et al. Risk stratification, management and outcomes of patients with non-ST elevation acute coronary syndrome: A Canadian teaching hospital perspective. Can J Cardiol. 2003;19:1033–9. [PubMed] [Google Scholar]
- 13.Bhatt DL, Roe MT, Peterson ED, et al. CRUSADE Investigators Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: Results from the CRUSADE Quality Improvement Initiative. JAMA. 2004;292:2096–104. doi: 10.1001/jama.292.17.2096. [DOI] [PubMed] [Google Scholar]
- 14.Rouleau JL, Moyé LA, Pfeffer MA, et al. A comparison of management patterns after acute myocardial infarction in Canada and the United States. The SAVE investigators. N Engl J Med. 1993;328:779–84. doi: 10.1056/NEJM199303183281108. [DOI] [PubMed] [Google Scholar]
- 15.Mark DB, Naylor CD, Hlatky MA, et al. Use of medical resources and quality of life after acute myocardial infarction in Canada and the United States. N Engl J Med. 1994;331:1130–5. doi: 10.1056/NEJM199410273311706. [DOI] [PubMed] [Google Scholar]
- 16.Tu JV, Pashos CL, Naylor CD, et al. Use of cardiac procedures and outcomes in elderly patients with myocardial infarction in the United States and Canada N Engl J Med 19973361500–5.(Erratum in 1997;337:139). [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Flather M, Pogue J, et al. Variations between countries in invasive cardiac procedures and outcomes in patients with suspected unstable angina or myocardial infarction without initial ST elevation. OASIS (Organisation to Assess Strategies for Ischaemic Syndromes) Registry Investigators. Lancet. 1998;352:507–14. doi: 10.1016/s0140-6736(97)11162-x. [DOI] [PubMed] [Google Scholar]
- 18.Pilote L, Racine N, Hlatky MA. Differences in the treatment of myocardial infarction in the United States and Canada. A comparison of two university hospitals. Arch Intern Med. 1994;154:1090–6. [PubMed] [Google Scholar]
- 19.Fu Y, Chang WC, Mark D, et al. Canadian-American differences in the management of acute coronary syndromes in the GUSTO IIb trial: One-year follow-up of patients without ST-segment elevation. Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) II Investigators. Circulation. 2000;102:1375–81. doi: 10.1161/01.cir.102.12.1375. [DOI] [PubMed] [Google Scholar]
- 20.Batchelor WB, Peterson ED, Mark DB, et al. A comparison of U.S. and Canadian cardiac catheterization practices in detecting severe coronary artery disease after myocardial infarction: Efficiency, yield and long-term implications. J Am Coll Cardiol. 1999;34:12–9. doi: 10.1016/s0735-1097(99)00174-6. [DOI] [PubMed] [Google Scholar]
- 21.Batchelor WB, Mark DB, Knight JD, et al. Development and validation of a simple model to predict severe coronary artery disease after myocardial infarction: Potential impact on cardiac catheterization use in the United States and Canada. Am Heart J. 2003;145:349–55. doi: 10.1067/mhj.2003.111. [DOI] [PubMed] [Google Scholar]
- 22.Yan AT, Yan RT, Tan M, et al. Canadian Acute Coronary Syndromes (ACS) Registry Investigators Troponin is more useful than creatine kinase in predicting one-year mortality among acute coronary syndrome patients. Eur Heart J. 2004;25:2006–12. doi: 10.1016/j.ehj.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Hoekstra JW, Pollack CV, Jr, Roe MT, et al. Improving the care of patients with non-ST-elevation acute coronary syndromes in the emergency department: The CRUSADE initiative. Acad Emerg Med. 2002;9:1146–55. doi: 10.1111/j.1553-2712.2002.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 24.Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation. 2000;101:2557–67. doi: 10.1161/01.cir.101.22.2557. [DOI] [PubMed] [Google Scholar]
- 25.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 26.Steg PG, Dabbous OH, Feldman LJ, et al. Global Registry of Acute Coronary Events Investigators Determinants and prognostic impact of heart failure complicating acute coronary syndromes: Observations from the Global Registry of Acute Coronary Events (GRACE) Circulation. 2004;109:494–9. doi: 10.1161/01.CIR.0000109691.16944.DA. [DOI] [PubMed] [Google Scholar]
- 27.Malmberg K, Yusuf S, Gerstein HC, et al. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: Results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000;102:1014–9. doi: 10.1161/01.cir.102.9.1014. [DOI] [PubMed] [Google Scholar]
- 28.Masoudi FA, Plomondon ME, Magid DJ, Sales A, Rumsfeld JS. Renal insufficiency and mortality from acute coronary syndromes. Am Heart J. 2004;147:623–9. doi: 10.1016/j.ahj.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Bach RG, Cannon CP, Weintraub WS, et al. The effect of routine, early invasive management on outcome for elderly patients with non-ST-segment elevation acute coronary syndromes. Ann Intern Med. 2004;141:186–95. doi: 10.7326/0003-4819-141-3-200408030-00007. [DOI] [PubMed] [Google Scholar]
- 30.Keeley EC, Kadakia R, Soman S, Borzak S, McCullough PA. Analysis of long-term survival after revascularization in patients with chronic kidney disease presenting with acute coronary syndromes. Am J Cardiol. 2003;92:509–14. doi: 10.1016/s0002-9149(03)00716-1. [DOI] [PubMed] [Google Scholar]
- 31.Januzzi JL, Cannon CP, DiBattiste PM, Murphy S, Weintraub W, Braunwald E, TACTICS-TIMI 18 Investigators Effects of renal insufficiency on early invasive management in patients with acute coronary syndromes (The TACTICS-TIMI 18 Trial) Am J Cardiol. 2002;90:1246–9. doi: 10.1016/s0002-9149(02)02844-8. [DOI] [PubMed] [Google Scholar]
- 32.Alter DA, Naylor CD, Austin PC, Chan BT, Tu JV. Geography and service supply do not explain socioeconomic gradients in angiography use after acute myocardial infarction. CMAJ. 2003;168:261–4. [PMC free article] [PubMed] [Google Scholar]
- 33.Yan AT, Jong P, Yan RT, et al. Canadian Acute Coronary Syndromes registry investigators Clinical trial-derived risk model may not generalize to real-world patients with acute coronary syndrome. Am Heart J. 2004;148:1020–7. doi: 10.1016/j.ahj.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee D, Fang J, Chetcuti S, Moscucci M, Kline-Rogers E, Eagle KA. Impact of combination evidence-based medical therapy on mortality in patients with acute coronary syndromes. Circulation. 2004;109:745–9. doi: 10.1161/01.CIR.0000112577.69066.CB. [DOI] [PubMed] [Google Scholar]