Abstract
Background
Phthalates are synthetic chemicals that are ubiquitous in our society and may have adverse health effects in humans. Detectable concentrations of phthalate metabolites have been found in adults and children, but no studies have examined the relationship between maternal and infant phthalate metabolite concentrations.
Objective
We investigated the relationship between maternal and infant urinary phthalate metabolite concentrations.
Methods
We measured nine phthalate metabolites in urine samples from 210 mother/infant pairs collected on the same study visit day (1999–2005) and obtained demographic history from questionnaires. Using multivariate linear regression analyses, we examined the degree to which maternal urine phthalate metabolite concentration predicted infant phthalate metabolite concentration. All analyses were adjusted for infant age, creatinine concentration, and race.
Results
Correlation coefficients between phthalate metabolite concentrations in the urine of mothers and their infants were generally low but increased with decreasing age of infant. In multivariate analyses, mother’s phthalate metabolite concentrations were significantly associated with infants’ concentrations for six phthalate metabolites: monobenzyl phthalate, monoethyl phthalate, monoisobutyl phthalate, and three metabolites of di(2-ethylhexyl) phthalate: mono(2-ethylhexyl) phthalate, mono(2-ethyl-5-hydroxy-hexyl) phthalate and mono(2-ethyl-5-oxo-hexyl) phthalate (p-values for all coefficients <0.05).
Discussion
Mother’s urine phthalate metabolite concentration is significantly associated with infant urine phthalate metabolite concentration for six phthalate metabolites. It is plausible that shared exposures to phthalates in the immediate surrounding environment accounted for these relationships, but other unidentified sources may also contribute to infants’ phthalate exposures. This study indicates the importance of further identifying infant phthalate exposures that may be distinct from maternal exposures in order to decrease overall infant phthalate exposures.
Keywords: Phthalate, Infant, Maternal, Environmental Health
Introduction
Phthalates are synthetic chemicals some of which have been shown to have toxic effects on developing endocrine, reproductive, and immune systems in animal models. Phthalates can be found in a variety of household and industrial products including: foods/beverages, children’s plastic toys, lubricants, baby care products, chemical stabilizers in cosmetics, personal care products, vinyl flooring, and polyvinyl chloride (PVC) tubing (Hauser and Calafat, 2005). Humans can be exposed to phthalates through ingestion (dietary exposures), inhalation (dust/air), dermal absorption, and percutaneous exposures (IV tubing) (Schettler, 2006).
Phthalates act through an anti-androgenic, and possibly estrogenic, mechanism leading to known endocrine and reproductive tract abnormalities (Gray et al., 2006). In animal models, prenatal exposures to some phthalates, particularly di(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), and benzyl butyl phthalate (BBzP)) cause male reproductive anomalies including decreased anogenital distance (a marker of in utero androgenization), hypospadias, cryptorchidism, and decreased fertility as mature adults (Barlow et al., 2004; Carruthers and Foster, 2005; Gray et al., 2000). This cluster of effects has been termed the “phthalate syndrome”. In humans, prenatal phthalate exposure has been associated with decreased anogenital distance in male infants (Swan et al., 2005), and postnatal breast milk phthalate exposure has been associated with altered reproductive hormone concentrations in male infants (Main et al., 2006). Phthalate exposure in early childhood has also been associated with an increased incidence of atopic disease including allergic rhinitis, wheezing, and eczema (Bornehag et al., 2004; Kolarik et al., 2008).
Food is thought to be an important source of phthalate exposure in the general population due to unintentional contamination from processing and packaging (Castle et al., 1988; Chou Karen, 2006). Potential home phthalate exposures include household dust and indoor air environments (Afshari A, 2004; Bornehag et al., 2005; Fromme et al., 2004; Uhde et al., 2001). Several studies document phthalates in personal care and other common consumer products. (Harm, 2002; Schettler, 2006). Therefore, families may have shared exposures from the surrounding environment in addition to exposure from foods and individual products such as personal care products and cosmetics (Hauser and Calafat, 2005; Schettler, 2006).
Phthalates are ubiquitous in human biological samples, but characterization of distributions within families is poorly understood. Several studies have estimated human phthalate exposures attributable to home environmental exposure (Wormuth et al., 2006), but only one human study has measured the relationship between maternal and cord blood phthalate concentrations (Latini et al., 2003). This study focused solely on DEHP in premature neonates and their mothers at birth. Our study examines the relationship between maternal and infant phthalate metabolite concentrations in urine in a population of healthy mother/infant pairs to assess whether maternal phthalate concentration predicts infant phthalate concentration.
Methods
Study Participants
Infants in our study were born to healthy women originally recruited in the first phase of the Study for Future Families (SFFI), a multicenter pregnancy cohort study, at prenatal clinics in Los Angeles, California (Harbor-UCLA and Cedars-Sinai), Minneapolis, Minnesota (University of Minnesota Health Center), Iowa City, Iowa (University of Iowa Medical Center) and Columbia, Missouri (University Physicians), from September 1999 through August 2005. Methods are described in detail elsewhere (Swan et al., 2003; Swan et al., 2005). Briefly, couples whose pregnancy was not medically assisted were eligible unless the woman or her partner was < 18 years of age, either partner did not read and speak Spanish or English, or the father was unavailable or unknown. All participants completed a questionnaire, gave blood samples, and after urine collection was added midway through the study, also gave a urine sample.
Eighty-five percent of SFFI participants agreed to be re-contacted, and we invited these mothers to take part in a follow-up study (SFFII). The family was eligible for this follow-up study if the pregnancy ended in a live birth, the baby was 2–36 months of age at the time of re-contact, and the mother lived within 50 miles of the clinic and could attend at least one study visit. Infants were eligible for this study if data existed on mother’s urine phthalate measurement, infant urine phthalate measurements, and demographic characteristics. This analysis includes 210 infants from California, Missouri, Iowa and Minnesota and represents 61% of all infants born to SFFI participants at those centers. We obtained infant demographic from the SFFII questionnaire as well as from the study visit at which all infants were weighed, measured, and given a physical exam. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory in this study was limited and was determined not to constitute engagement in human subjects research. For all other participating institutions, human subject committees approved SFFI and SFFII, and all participants signed informed consents for each study.
Phthalate Metabolite Measurements
We obtained maternal and infant urine samples on the same study visit day. Mothers were asked to provide a urine sample in a standard urine collection cup. Mothers were all given the same type of study diaper and asked to bring in a wet diaper (no stool) on the day of the infant study visit. Samples of all urine collection materials, pre-screened for monoester phthalate metabolites at CDC, did not contain phthalate monoester metabolites at concentrations above the limit of detection (LOD). The presence of oxidative metabolites in urine cannot result from contamination (Koch et al., 2006). Infant urine samples were obtained by squeezing the diaper and collecting urine in containers. All urine samples were frozen and shipped in dry ice to the Division of Laboratory Sciences, National Center for Environmental Health, CDC; and stored frozen at or below −40 °C until analysis.. Urinary phthalate metabolite measurements were carried out at the Division of Laboratory Sciences, National Center for Environmental Health, CDC; CDC researchers received coded samples without subject identifiers and had no access to participant data. The analytical method involved the enzymatic deconjugation of the phthalate metabolites from their glucuronidated form, followed by concentration of the analytes of interest by solid-phase extraction, separation with high-performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry (Kato et al., 2005; Silva et al., 2004). This approach allows for the simultaneous quantification in human urine of the following phthalate metabolites reported in this work: monoethyl phthalate (MEP) , monobutyl phthalate (MBP), mono(3-carboxypropyl) phthalate (MCPP), a metabolite of dioctyl phthalate and a minor metabolite of DBP, monobenzyl phthalate (MBzP), monoisobutyl phthalate (MiBP), mono(2-ethylhexyl) phthalate (MEHP), and two oxidative metabolites of DEHP, mono(2-ethyl-5-hydroxy-hexyl) phthalate (MEHHP), and mono(2-ethyl-5-oxo-hexyl) phthalate (MEOHP). Limits of detection (LOD) are in the low µg/L range. Isotopically labeled internal standards and conjugated internal standards were used to increase precision and accuracy of the measurements. Quality control and reagent blank samples were analyzed along with the study samples to monitor the performance of the method.
Statistical Analysis
We first examined descriptive statistics for concentration and distribution of maternal and infant phthalates. Most metabolite concentrations were above the LOD, which was between 0.95 – 1.07 µg/L for all phthalate metabolites. Concentrations below the LOD were assigned the specific metabolite LOD value divided by the square root of 2 for statistical analyses, as has been recommended (Hornung RW, 1990). All phthalate metabolite concentrations were logarithmically transformed to normalize distributions. MEHP, MEOHP, and MEHHP are all metabolites of a single parent compound (DEHP). Therefore, we used the sum of these metabolites to reflect total DEHP exposure.
We used the Pearson correlation coefficient to first examine individual correlations between maternal and infant phthalate concentrations and then to examine correlations between quartiles of exposure. Because infants likely have different sources and routes of exposure at different ages, we examined the correlation of quartiles of exposure based on age. We then used linear regression to explore the associations between infant individual log phthalate metabolite concentrations and maternal phthalate concentration adjusting for covariates. We back transformed (inverse logarithm) all coefficients and confidence intervals obtained from the regression models.
We examined several potentially confounding factors including maternal and infant age, infant sex, infant weight, maternal and infant urinary creatinine, geographic location, race/ethnicity, socioeconomic factors, and mother’s report of ever breastfeeding and current breastfeeding practice. Infant age, race, and maternal and infant urinary creatinine concentrations were associated with the exposures of interest and urine phthalate metabolite concentrations; these variables were included as covariates in all regression analyses.
Because urinary creatinine can be a measure of both dilution and muscle breakdown in the body, we decided to present results for two models: one model adjusts for creatinine using maternal and infant urine phthalate/ square root urine creatinine and the other model includes maternal and infant urine square root creatinine values as covariates. We plotted the square root of creatinine against urine phthalate metabolite concentration which showed a nearly linear relationship; therefore, we used the square root of creatinine in these models.
Results
Table 1 shows demographic characteristics for the 210 mother/infant pairs in the study. Approximately 37% of mother/infant pairs lived in Minnesota, 27% in Missouri, 22% in California, and 15% in Iowa. Participants were predominantly white (80%) with 11% reporting Hispanic/Latino ethnicity. The majority of mothers reported having health insurance (91%). Mother’s ages ranged from 25 – 40 years, and the majority of infants were between 1 – 16 months of age (mean of 13 months).
Table 1.
Demographic Characteristics for Mother/Infant Pairs (N=210)
| Mothers | ||
|---|---|---|
| N | % | |
| Center | ||
| California | 46 | 21.9 |
| Minnesota | 77 | 36.7 |
| Missouri | 56 | 26.7 |
| Iowa | 31 | 14.8 |
| Race | ||
| White | 168 | 80.0 |
| Black | 5 | 2.4 |
| Native American | 2 | 1.0 |
| Asian American | 12 | 5.7 |
| Hispanic/Latino | 22 | 10.5 |
| Other | 0 | 0.0 |
| Maternal Smoking Around Baby | ||
| Yes | 6 | 2.9 |
| No | 202 | 96.2 |
| Ever Breastfed | ||
| Yes | 202 | 96.2 |
| No | 7 | 3.3 |
| Insurance | ||
| With Health Insurance | 190 | 90.5 |
| No Health Insurance | 17 | 8.1 |
| Mother's Age (years) | ||
| <25 | 27 | 12.9 |
| 25 – 30 | 54 | 25.7 |
| >30 – 35 | 73 | 34.8 |
| >35 | 56 | 26.7 |
| Infants | ||
| Gender | ||
| Male | 116 | 55.2 |
| Female | 94 | 44.8 |
| Age (months) | ||
| 1–8 | 77 | 36.7 |
| 9–16 | 84 | 40.0 |
| 17 – 24 | 37 | 17.6 |
| 24 – 37 | 12 | 5.7 |
Missing data not shown
For infants, MCPP concentrations were above the LOD in 58% of samples, and MiBP in 81% of samples. All other phthalate concentrations were above the LOD in 92% or greater of all samples. For the mothers, MEHP concentrations were detected in 56% of samples, and all other phthalate metabolite concentrations were above the LOD in 80% or greater of all samples. Pearson correlation coefficients varied from value between −0.03 to 0.32 for individual, uncategorized maternal and infant creatinine adjusted phthalate metabolite concentrations. Table 2 shows Pearson correlation coefficients for creatinine adjusted quartiles of phthalate metabolite concentrations by infant age. Correlation coefficient values for the DEHP and MEP metabolites were much stronger for the younger infants (0.34 – 0.45) compared to the older infants (0.07 – 0.20). MiBP and MBzP correlation coefficient values did not vary significantly between age groups (0.32 – 0.47)
Table 2.
Correlation Coefficients for Creatinine Adjusted Maternal and Infant Quartile of Phthalate Metabolite Urinary Concentration by Infant Age (N=210)
| All (N=210) | N=63 | N=75 | N=135 | |
|---|---|---|---|---|
| Correlation | <=4 months | <=6 months | >6 months | |
| MCPP | 0.12 | 0.02 | 0.03 | 0.16 |
| MEHP | 0.17 | 0.27 | 0.24 | 0.07 |
| MEOHP | 0.24 | 0.35 | 0.31 | 0.19 |
| MEHHP | 0.25 | 0.45 | 0.34 | 0.20 |
| MEP | 0.22 | 0.34 | 0.28 | 0.17 |
| MBP | 0.12 | 0.08 | 0.00 | 0.19 |
| MBzP | 0.38 | 0.41 | 0.44 | 0.34 |
| MiBP | 0.40 | 0.37 | 0.32 | 0.47 |
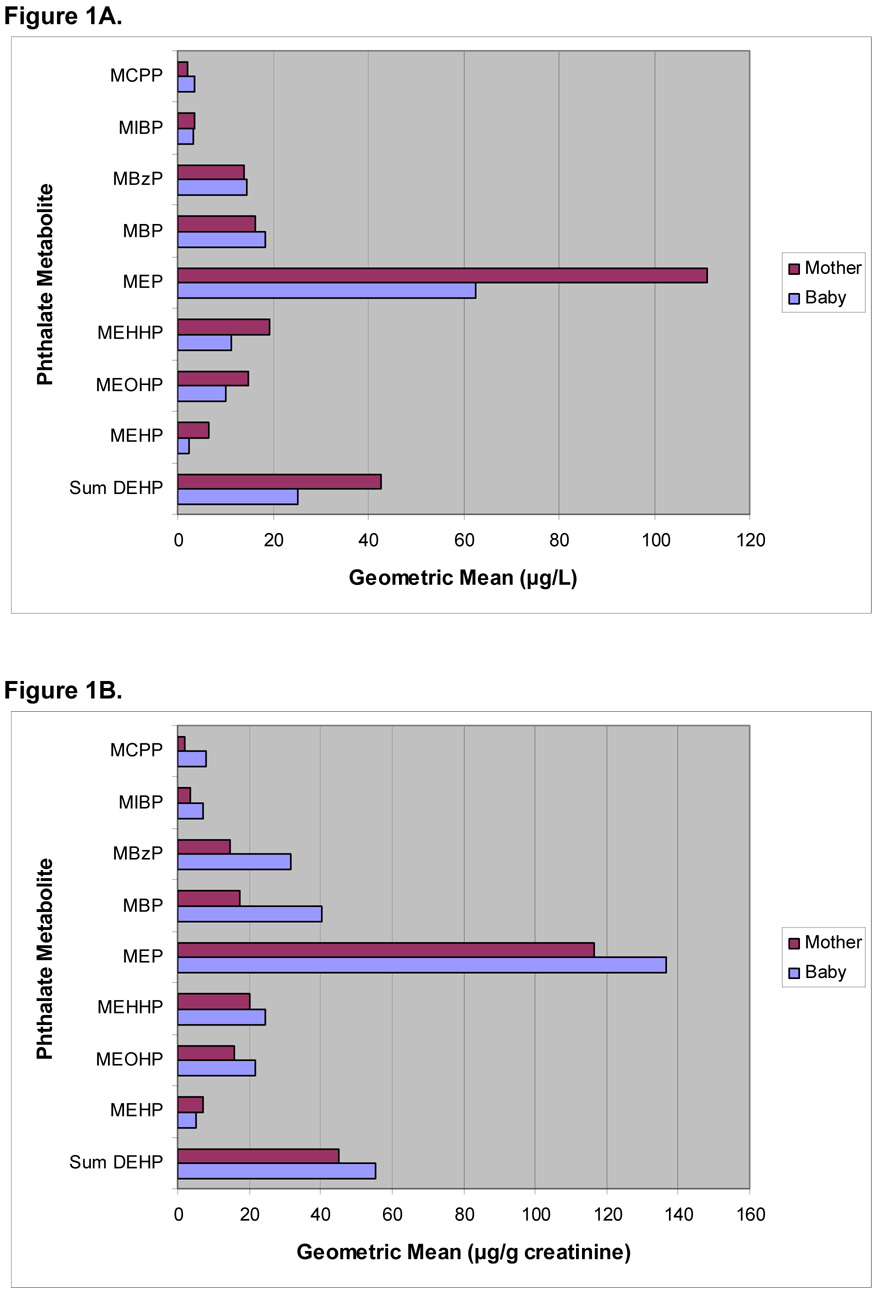
Figure 1A shows the geometric mean comparisons of unadjusted mother and infant urine phthalate metabolite concentrations. Mother’s phthalate metabolite concentrations were higher than infant phthalate metabolite concentrations for MEP, MiBP, and all DEHP metabolites. Infant phthalate metabolite concentrations were higher than mother’s metabolite concentrations for MBP, MBzP, and MCPP. Figure 1B shows the geometric mean comparisons of square root creatinine adjusted mother and infant phthalate metabolite concentrations. Creatinine-adjusted infant phthalate metabolite concentrations were higher on average than creatinine-adjusted mother’s phthalate metabolite concentrations for most compounds measured while MEHP was higher in mothers as compared to infants.
Figure 1.
Unadjusted (A) and Creatinine Adjusted (B) Geometric Mean Phthalate Metabolite Urinary Concentrations for 210 Mother/Infant Pairs
In Table 3, we show results of two linear regression models used to predict infant phthalate metabolite concentrations: model 1 includes square root of creatinine included as a covariate and model 2 includes creatinine adjusted phthalate concentrations (phthalate/sqrt creatinine). All values are listed as back transformed values from the log scale. Mother’s phthalate metabolite concentration significantly predicted infant phthalate metabolite concentrations for MBzP, MEHP, MEHHP, MEOHP, sum of DEHP metabolites, MEP, and MiBP, with p-values all less than 0.05 in these models. The r-squared values for the metabolites in model 1 ranged from 0.30 – 0.62 while those for model 2 were much lower, 0.08 – 0.36. Regression coefficient values for each model were similar for each phthalate metabolite. For every natural log unit increase in mother’s MBzP concentration, a 1.48 µg/L (95% CI, 1.27 – 1.70) and 1.43 µg/g creatinine (95% CI, 1.25 – 1.65) increase is observed in infant MBzP concentration in models 1 and 2, respectively. All infant DEHP metabolites were significantly predicted by the corresponding maternal DEHP metabolite concentration, and mother’s sum of DEHP metabolites did predict infant DEHP metabolites concentrations, 1.26 µg/L (95% CI 1.12 – 1.42) and 1.32 µg/g creatinine (95% CI 1.16 – 1.51) in models 1 and 2, respectively.
Table 3.
Regression Coefficients for Mother's Phthalate Metabolite Urinary Concentration in Two Models Predicting Infant Phthalate Metabolite Urinary Concentration, N=210 Mother/Infant Pairs
| Coefficient | 95% CI | p-value | Adjusted r squared | |
|---|---|---|---|---|
| Sum DEHP1 | 1.26 | 1.12 – 1.42 | 0.00 | 0.62 |
| Sum DEHP2 | 1.32 | 1.16 – 1.51 | 0.00 | 0.31 |
| MEHP1 | 1.15 | 1.01 – 1.31 | 0.04 | 0.30 |
| MEHP2 | 1.16 | 1.02 – 1.32 | 0.03 | 0.08 |
| MEOHP1 | 1.23 | 1.09 – 1.39 | 0.00 | 0.60 |
| MEOHP2 | 1.30 | 1.15 – 1.48 | 0.00 | 0.26 |
| MEHHP1 | 1.23 | 1.09 – 1.40 | 0.00 | 0.62 |
| MEHHP2 | 1.35 | 1.15 – 1.49 | 0.00 | 0.36 |
| MEP1 | 1.21 | 1.09 – 1.34 | 0.00 | 0.38 |
| MEP2 | 1.23 | 1.12 – 1.36 | 0.00 | 0.15 |
| MBP1 | 1.09 | 0.97 – 1.23 | 0.15 | 0.50 |
| MBP2 | 1.11 | 0.98 – 1.25 | 0.09 | 0.04 |
| MBzP1 | 1.48 | 1.27 – 1.70 | 0.00 | 0.42 |
| MBzP2 | 1.43 | 1.25 – 1.65 | 0.00 | 0.15 |
| MIBP1 | 1.42 | 1.26 – 1.60 | 0.00 | 0.48 |
| MIBP2 | 1.38 | 1.22 – 1.55 | 0.00 | 0.19 |
| MCPP1 | 1.11 | 0.94 – 1.28 | 0.22 | 0.51 |
| MCPP2 | 1.14 | 0.97 – 1.35 | 0.11 | 0.08 |
Results reported in µg/L and sqrt creatinine, infant age, and race included as covariates
Results reported in µg/g creatinine and infant age and race included as covariates
We also examined infant personal care products (PCP) use in the models and found that mother’s reported use of infant PCPs was a significant covariate in the MEP model but not in any of the other phthalate metabolite models. The addition of PCP use did not change the overall point estimate for MEP prediction by mother’s phthalate metabolite concentration in either the creatinine adjusted or creatinine unadjusted models. We included an interaction term for exposure by infant age and added this to the models listed above but did not observe a significant change in predictions.
Discussion
While maternal and infant phthalate metabolite concentrations in urine samples taken on the same day differed, many were significantly correlated when controlling for confounding by creatinine and age. These analyses show that, for several phthalate metabolites, maternal concentration in urine is significantly associated with infant concentration. The correlation between maternal and infant phthalate metabolite urinary concentrations may reflect shared environmental exposures to the corresponding parent phthalate diesters inside and outside the home, but other sources may also contribute to infant phthalate exposure distinct from maternal exposures. This conclusion is supported by the finding that correlation coefficients were low overall (all below 0.45) and were stronger for younger infants but weaker for older infants suggesting that older infants may have separate, distinct sources of exposure from their mothers.
Sources of phthalate exposure in the environment are numerous and include: food, dust, indoor air, cosmetic products, personal care products, and a variety of industrial products including vinyl flooring and IV medical tubing. Wormuth and colleagues conducted an analysis estimating sources of phthalate exposure for the general European population. They used measured concentrations of eight phthalates in a variety of media including: indoor/outdoor air, foods, dust, and personal care products and estimated exposures through various routes of exposure (Wormuth et al., 2006). They found that exposure occurred primarily through indoor air for dimethyl phthalate, from personal care products use for DEP, and through the diet for DBP, diisobutyl phthalate and DEHP. These results were consistent across age groups. Interestingly, they found that infants/toddlers had the highest DEHP exposures from dust compared to other age groups (Wormuth et al., 2006).
In our study, we found that DEHP metabolites and MEP were more strongly correlated in younger infants. This likely reflects limited sources and routes of exposure for the younger infant leading to a similar diet (if mothers were breast feeding only, phthalates could be passed from mother to infant through breast milk) (Main et al., 2006) and shared environment by mom and baby. Our questionnaire asked about breast feeding, bottle feeding with breast milk, formula feeding, and cow’s milk exposure in the past week. These data were not significantly associated with infant urinary phthalate metabolite concentrations in our models and did not change overall point estimates. It may be that our sample size for each different type of feeding exposure was too small to detect between-group differences, or that milk/formula may not be significant sources of phthalate exposure in young infants. In adults, food seems to be the major source of exposure to DEHP. In a study involving 50 volunteers over 7 days, estimated DEHP intakes from the urinary metabolites data correlated significantly with dietary DEHP intake from the day before and the dietary intake calculated from food duplicates could explain the internal DEHP exposure (Fromme et al., 2007). It is likely that food is also a major source of exposure to DEHP for children. Mothers and infants share similar food exposures when an infant is breast feeding only and likely do not share the same foods when children start eating solid foods.
As infants get older, they begin to crawl, walk and have increased hand to mouth behaviors. At this time, they have much floor and close to the ground play. From the linear regression models in Table 3, the r-squared values are much higher for MEOHP, MEHPP, and the sum of the DEHP metabolites as compared to the other metabolites showing that 60–62% of the variance in infant phthalate metabolite concentrations is accounted for by the model. Because DEHP is a component of flexible plastics and many industrial and building materials as well as commonly used household products (Schettler, 2006), it may be that DEHP concentrations predominate in the background environment that is shared such as dust and indoor air. Bornehag et al. analyzed indoor dust from 346 bedrooms and found DEHP and BBzP concentrations to be highest compared to other phthalate compounds measured (Bornehag et al., 2005). Several other studies have also detected DEHP in house or building dust samples (Becker et al., 2004; Fromme et al., 2004; Rudel et al., 2003). Becker et al. did not find a significant correlation between concentrations of DEHP in dust and in urine in children ages 3– 14 years but comments that infants and toddlers who are crawling and walking likely have the highest dust exposures because their play is much closer to the ground (Becker et al., 2004) which may explain some of the discrepancy between higher correlation coefficients for younger infants and lower correlation coefficients for older infants observed in our study. Few studies have examined phthalate concentrations in indoor air, and found DBP concentrations to be highest as compared to other phthalate compounds measured (Fromme et al., 2004; Rudel et al., 2003; Wormuth et al., 2006).
The MEP model had an r-squared value of only 0.38, which is much lower than for the other phthalate metabolites although we did see increased correlation coefficient values for quartiles of exposure in the younger infants. MEP is the main metabolite of diethyl phthalate (DEP), the phthalate used primarily in cosmetics and personal care products. In a previous analysis of the infant urine samples examined in the present study, we found that mother’s report of personal care product use on the infant was significantly associated with increased concentrations of MEP, MMP, and MiBP (Sathyanarayana et al., 2008). Mother and infant are not necessarily exposed to similar cosmetics and personal care products as the infant gets older, and this may explain the weaker association. When we added the infant personal care product use into the model, the r-squared increased to 0.40, and the personal care product use variable was significant in the model.
To date, no other studies have examined the relationship between maternal and infant phthalate exposures. Latini et al. measured maternal and cord blood phthalate concentrations for DEHP only, but this only reflects fetal exposures from the in utero environment (Latini et al., 2003). Our study is the first to show that mother’s urinary concentrations of specific phthalate metabolites are significantly associated with infant phthalate metabolite concentrations in urine.
There are several limitations in the current study. We could not measure home environmental phthalate or dietary phthalate exposures which are likely significant predictors of infant phthalate metabolite concentrations. Therefore, we could not easily parse out what exposures may be specific to individual mother and infant. In addition, although widely used, urinary creatinine may not be an ideal measurement for urine dilution. Urinary creatinine concentrations in infants tend to be extremely variable and in the low µg/L range, much lower than for adults (Jacobs DS, 2001). These values tend to shift overall concentrations even with square root or log transformations. In addition, our model fit was much worse (lower r-squared value) when we adjusted phthalate concentrations by dividing by urinary creatinine as compared to using it as a covariate in regression analyses. We did not have specific gravity measures or 24 hour urine measurements and could therefore not control for urinary dilution in any other manner. From Table 3, we found that urine creatinine tends to explain much of the variance related to infant phthalate metabolite concentrations which would be expected given that urine creatinine is one measure of urinary dilution and glomerular filtration.
Conclusions
The finding that maternal urinary concentrations of some phthalate metabolites are associated with infant phthalate metabolite urinary concentrations likely reflects shared environmental exposures of mother and infant to the parent phthalates. Given that correlation coefficients were quite low overall and were increased in the youngest subset of infants, it is likely that age related behaviors and differences in diet contribute to separate sources of exposure for older infants. In order to decrease early childhood phthalate exposures, further research is required to determine environmental sources of exposure that contribute to measurable concentrations of phthalate metabolites. Phthalates are ubiquitous in our environment, and additional research is needed to determine potential health effects of these exposures, especially to young children because infants are more susceptible to the effects of phthalates given their developing endocrine and reproductive systems.
Acknowledgements
The authors gratefully acknowledge the technical assistance of Manori Silva, Jack Reidy, Ella Samandar and Jim Preau (Centers for Disease Control and Prevention [CDC], Atlanta, GA) in measuring the urinary concentrations of phthalate metabolites. We also thank the health care providers and study participants at University Physicians Clinic, Columbia, MO; Fairview Riverside Women’s Clinic, Minneapolis, MN; Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center; Cedars-Sinai Medical Center; and University of Iowa Hospitals and Clinics.
Grant Information: Grants from the USEPA and NIH grants R01-ES09916 to the University of Missouri, MO1-RR00400 to the University of Minnesota, MO1-RR0425 to Harbor-UCLA Medical Center, and Grant 18018278 from the State of Iowa to the University of Iowa, National Institutes of Health K12 HD053984-02, NICHD/NICHD, Male Reproductive Health Research Center Development Program
Abbreviations
- CDC
Centers for Disease Control and Prevention
- BBzP
benzyl butyl phthalate (CAS Number: 85-68-7)
- DEHP
di(2-ethylhexyl) phthalate (CAS Number: 117-81-7)
- DEP
diethyl phthalate (CAS Number: 84-66-2)
- DBP
dibutyl phthalate (CAS Number: 84-74-2)
- LOD
limit of detection
- MEP
monoethyl phthalate
- MBP
monobutyl phthalate
- MBzP
monobenzyl phthalate
- MiBP
monoisobutyl phthalate
- MCPP
mono(3-carboxypropyl) phthalate
- MEHP
mono(2-ethylhexyl) phthalate
- MEOHP
mono(2-ethyl-5-oxohexyl) phthalate
- MEHHP
mono(2-ethyl-5-hydroxyhexyl) phthalate
- PVC
polyvinyl chloride
- SFF
Study For Future Families
- µg/L
micrograms/liter
- µg/g
micrograms/gram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
References
- Afshari A, G L, Clausen PA, Hansen V. Emission of phthalates from PVC and other material. Indoor Air. 2004;14:120–128. doi: 10.1046/j.1600-0668.2003.00220.x. [DOI] [PubMed] [Google Scholar]
- Barlow NJ, McIntyre BS, Foster PM. Male reproductive tract lesions at 6, 12, and 18 months of age following in utero exposure to di(n-butyl) phthalate. Toxicol Pathol. 2004;32:79–90. doi: 10.1080/01926230490265894. [DOI] [PubMed] [Google Scholar]
- Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, Rosskamp E, Schluter C, Seifert B, Ullrich D. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207:409–417. doi: 10.1078/1438-4639-00309. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Lundgren B, Weschler CJ, Sigsgaard T, Hagerhed-Engman L, Sundell J. Phthalates in indoor dust and their association with building characteristics. Environ Health Perspect. 2005;113:1399–1404. doi: 10.1289/ehp.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, Weschler CJ, Sigsgaard T, Lundgren B, Hasselgren M, Hagerhed-Engman L. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environ Health Perspect. 2004;112:1393–1397. doi: 10.1289/ehp.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers CM, Foster PM. Critical window of male reproductive tract development in rats following gestational exposure to di-n-butyl phthalate. Birth Defects Res B Dev Reprod Toxicol. 2005;74:277–285. doi: 10.1002/bdrb.20050. [DOI] [PubMed] [Google Scholar]
- Castle L, Mercer AJ, Startin JR, Gilbert J. Migration from plasticized films into foods. 3. Migration of phthalate, sebacate, citrate and phosphate esters from films used for retail food packaging. Food Addit Contam. 1988;5:9–20. doi: 10.1080/02652038809373657. [DOI] [PubMed] [Google Scholar]
- Chou Karen WR. Phthalates in Food and Medical Devices. Journal of Medical Toxicology. 2006;2 doi: 10.1007/BF03161027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Lahrz T, Piloty M, Gebhart H, Oddoy A, Ruden H. Occurrence of phthalates and musk fragrances in indoor air and dust from apartments and kindergartens in Berlin (Germany) Indoor Air. 2004;14:188–195. doi: 10.1111/j.1600-0668.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, Howdeshell K, Ankley GT, Guillette L. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int J Androl. 2006;29:96–104. doi: 10.1111/j.1365-2605.2005.00636.x. discussion 105–108. [DOI] [PubMed] [Google Scholar]
- Harm HCW. Pretty Nasty: Phthalates in European Cosmetics. 2002. [Google Scholar]
- Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW RL. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygeine. 1990;5 [Google Scholar]
- Jacobs DS, O D, DeMott WR. Laboratory Test Handbook. Hudson, OH: Lexi-Comp.; 2001. [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77:2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure-- an update and latest results. Int J Androl. 2006;29:155–165. doi: 10.1111/j.1365-2605.2005.00607.x. discussion 181–185. [DOI] [PubMed] [Google Scholar]
- Kolarik B, Naydenov K, Larsson M, Bornehag CG, Sundell J. The association between phthalates in dust and allergic diseases among Bulgarian children. Environ Health Perspect. 2008;116:98–103. doi: 10.1289/ehp.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111:1783–1785. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, Schmidt IM, Suomi AM, Virtanen HE, Petersen DV, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol. 2003;37:4543–4553. doi: 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Karr CJ, Lozano P, Brown E, Calafat AM, Liu F, Swan SH. Baby care products: possible sources of infant phthalate exposure. Pediatrics. 2008;121:e260–e268. doi: 10.1542/peds.2006-3766. [DOI] [PubMed] [Google Scholar]
- Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29:134–139. doi: 10.1111/j.1365-2605.2005.00567.x. discussion 181–185. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Slakman AR, Reidy JA, Preau JL, Jr, Herbert AR, Samandar E, Needham LL, Calafat AM. Analysis of human urine for fifteen phthalate metabolites using automated solid-phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;805:161–167. doi: 10.1016/j.jchromb.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Swan SH, Kruse RL, Liu F, Barr DB, Drobnis EZ, Redmon JB, Wang C, Brazil C, Overstreet JW. Semen quality in relation to biomarkers of pesticide exposure. Environ Health Perspect. 2003;111:1478–1484. doi: 10.1289/ehp.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhde E, Bednarek M, Fuhrmann F, Salthammer T. Phthalic esters in the indoor environment--test chamber studies on PVC-coated wallcoverings. Indoor Air. 2001;11:150–155. doi: 10.1034/j.1600-0668.2001.011003150.x. [DOI] [PubMed] [Google Scholar]
- Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006;26:803–824. doi: 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]