Abstract
Purpose
The role of 18F FDG PET has been studied in ovarian carcinoma, but its sensitivity and specificity calculations are based on dedicated PET acquisition, not PET/CT in the majority of the published studies. Therefore, we were prompted to review our experience with PET/CT in the management of patients with ovarian carcinoma.
Materials and methods
This is a retrospective study of 43 women with ovarian carcinoma, 27–80 years old (average: 53.9 ± 7.8), who had whole-body PET/CT at our institution from Jan 1st, 2003 to Aug 31st, 2006. We reviewed the patients’ outcomes from medical records and compared them to the interpretation of the PET/CT scans. Sensitivity and specificity were calculated using a 2 × 2 table with pathology results (79.1% of the patients) or clinical follow-up (20.9% of the cases) as the gold standard. Confidence interval (CI) estimations were performed using the Wilson score method.
Results
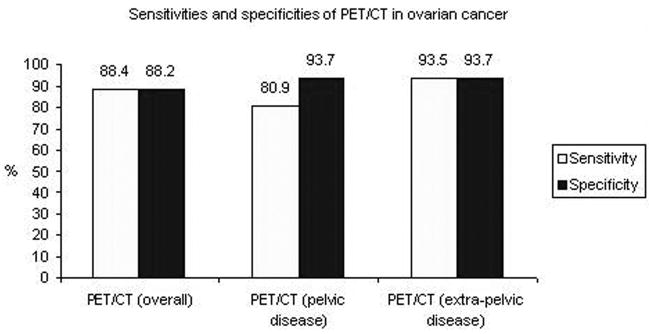
All patients had advanced stage ovarian cancer and the study was requested for re-staging. A total of 60 scans were performed: 30 pts had 1 scan, 9 pts had 2 scans and 4 pts had 3 scans. The administered doses of 18F FDG ranged 381.1 – 769.6 MBq (average: 569.8 ± 73.3). PET/CT had a sensitivity of 88.4% (95% CI: 75.1–95.4) and a specificity of 88.2% (95% CI: 64.4–97.9) for detection of ovarian cancer. The SUV max of the detected lesions ranged 3–27 (average: 9.4±5.9). The CA-125 tumor marker ranged 3–935 kU/ml (average: 265.2) in patients with positive scans and 4–139 kU/ml (average: 17.1) in patients with negative scans. This difference was statistically significant (P value: 0.0242).
Conclusion
This study confirms the good results of 18F FDG PET/CT for identification of residual/recurrent ovarian cancer, as well as for distant metastases localization. PET/CT should be an integral part in evaluation of patients with high risk ovarian cancer or rising values of tumor markers (CA-125), prior to selection of the most appropriate therapy.
Keywords: ovarian, carcinoma, FDG, PET, CT
Introduction
There are approximately 21,650 new cases of ovarian carcinoma and 15,520 deaths from ovarian carcinoma expected in 2008 in the United States (1). Epithelial ovarian cancer is usually diagnosed in an advanced stage and carries a poor prognosis. Imaging has become a significant part of the clinical management of patients with ovarian cancer. It is important for tumor detection, staging, treatment planning, and follow-up. Imaging findings assist in creating a specific treatment plan for each individual patient (2).
18F Fluoro-2-Deoxyglucose (18F FDG) positron emission tomography and computed tomography (PET/CT) combines the ability to detect active metabolic processes and their morphologic features in a single exam. The role of 18F FDG PET is proven in a variety of cancers, including lymphoma, colorectal carcinoma, lung cancer and melanoma, entities for which it changed the practice of oncology (3). In ovarian cancer, sequential imaging with PET has been shown to predict response to neoadjuvant chemotherapy (as early as after the first cycle). PET was more accurate than clinical or histopathologic response criteria including changes in tumor marker CA-125 alone in determining the prognosis (4). It is especially beneficial in conjunction with elevated serum CA-125 (5–7) or when CT or magnetic resonance imaging (MRI) show a suspected recurrence, but biopsy is infeasible. In this paper we review our experience with PET/CT in the management of patients with ovarian cancer.
Materials and methods
This is a retrospective study of 43 consecutive women with epithelial ovarian cancer, 27–80 years old (average: 53.9 ± 7.8), who had whole-body PET/CT at our institution from Jan 1st, 2003 to Aug 31st, 2006. The study was performed with the Institutional Review Board approval. The inclusion criteria were histopathologically proven diagnosis of ovarian cancer and availability of PET/CT scans. The reports of PET/CT and pathology examinations were reviewed and their results were recorded. A joint Nuclear Medicine - Radiology readout assures the accuracy of the findings on the CT portion of the exams during routine interpretation of the PET/CT exams. Reinterpretation of the studies by board certified Nuclear Medicine physicians was performed for consistency.
The 18F-FDG PET/CT scans were acquired using a Discovery LS PET/CT unit (GE Medical Systems, Milwaukee, WI). The patients fasted at least 6 hours prior to imaging and their blood glucose levels were less than 150 mg/dl at the time of the tracer injection. A standard dose of 555 MBq was prescribed for adult patients. However, due to variations in the daily clinical schedule, the administered doses of 18F FDG ranged 381.1 – 769.6 MBq (average: 569.8 ± 73.3). Approximately 60 minutes after tracer administration, a CT scan (5 mm contiguous axial cuts) was obtained in four integrated multi-slice helical non-contrast CT, from the skull base to the mid-thighs. The acquisition was obtained in helical mode, using 140 kV, 40 mAs and a 512×512 matrix size, acquiring a field of view (FOV) of 867 mm in 22.5 s. The CT scan was used for attenuation correction purposes and to help in anatomic localization of FDG. Immediately after the CT, an emission PET scan was acquired in 2D mode over the same anatomical regions starting at the level of the mid-thighs. The acquisition time was 4 minutes per bed position (35 slices/bed) in 6 beds, with a one-slice overlap at the borders of the FOV. The PET emission scan was corrected using segmented attenuation data of the CT scan. The PET images were reconstructed with a standard iterative algorithm (OSEM, two iterative steps, 28 subsets) using GE software release 5.0. All images were reformatted into axial, coronal, and sagittal views and viewed with the software provided by the manufacturer (eNtegra, GE Medical Systems, Haifa, Israel).
Semi-quantitative analysis of the 18F FDG uptake in the suspected lesions was based on calculation of standard uptake value (SUV), defined as the ratio of activity per milliliter of tissue to the activity in the injected dose corrected for decay and the patient’s body weight. Regions of interest were placed around the areas of increased FDG uptake for SUVmax determination. The cutoff SUVmax for malignant lesions was considered ≥2.5 (8).
Sensitivity and specificity for PET/CT in detection of ovarian cancer were calculated using the pathology results (79.1% of the patients) or clinical follow-up (20.9% of the cases) as the gold standard, using a 2 × 2 contingency table. For the 79.1% of patients with PET/CT findings correlated with tissue diagnosis, histological specimens were obtained after surgical re-exploration due to abnormal imaging findings in the abdomen/pelvis or from biopsy of lesions reported outside the abdominal cavity. Sites of disease progression on anatomic imaging (CT and/or MRI) at 6–12 months after PET/CT were recorded as true positive lesions for the remaining 20.9% of patients. Confidence interval (CI) estimations were performed using the Wilson score method (9).
Results
All patients had advanced stage ovarian cancer and the study was requested for disease re-staging. A total of 60 PET/CT scans were performed: 30 patients had 1 scan, 9 patients had 2 scans and 4 patients had 3 scans. Using the pathology results (79.1% of the patients) or clinical follow-up (20.9% of the cases) as the gold standard, PET/CT had an overall per patient sensitivity of 88.4% (95% CI: 75.1–95.4) and specificity of 88.2% (95% CI: 64.4–97.9) for detection of ovarian cancer. There were 5 false negative scans and 2 false positive scans (rectal uptake and colon uptake at the site of post-surgical anastomosis).
The results were also analyzed looking at lesions detected in the pelvis vs. those in other locations. PET/CT had a sensitivity of 80.9% (95% CI: 59.4–92.9) and specificity of 93.7% (95% CI: 69.7–99.9) for pelvic disease and a sensitivity of 93.5% (95% CI: 78.2–99.9) and specificity of 93.7% (95% CI: 69.7–99.9) for extra-pelvic lesions. The extra-pelvic lesions were located in the liver, lungs, omentum/peritoneum, bones and lymph nodes and are detailed in Table 1.
Table 1.
Summary of the extra-pelvic lesions detected in the studied population.
| Patient # | Extra-pelvic lesions |
|---|---|
| 2 | Mediastinal lymph nodes, peritoneum |
| 6 | Falciform ligament, peritoneum |
| 7 | Mediastinal lymph nodes, liver |
| 8 | Peritoneum, omentum, liver |
| 10 | Omentum |
| 13 | Liver, retroperitoneal lymph nodes |
| 14 | Peritoneum |
| 16 | Lung, liver |
| 18 | Left supraclavicular lymph node |
| 20 | Lung |
| 21 | Liver |
| 22 | Liver, retroperitoneal lymph nodes |
| 23 | Liver, peritoneum |
| 24 | Mediastinal lymph nodes, thoracic spine |
| 26 | Peritoneum |
| 27 | Liver, peritoneum, mediastinal lymph nodes |
| 29 | Lung, peritoneum |
| 38 | Liver, retroperitoneal lymph nodes |
| 43 | Liver, peritoneum, lumbar spine |
SUV max ranged from 3–27 (average: 9.4±5.9). The two false positive lesions had SUVmax of 5.2 and 5.3, respectively. The CA-125 tumor marker measurements were available at the time of 55 of the 60 PET/CT scans. CA-125 values ranged 3–935 U/ml (average: 265.2) in patients with positive scans and 4–139 U/ml (average: 17.1) in patients with negative scans. This difference was statistically significant (P value: 0.0242). The normal value for CA-125 is <35 U/ml at our institution. A total of 16 patients had CA-125 >35 U/ml: 15 had positive PET/CT (all true positive) and 1 had negative PET/CT (false negative after pathology exam).
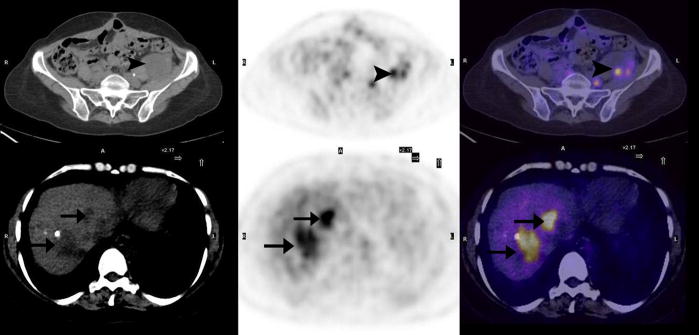
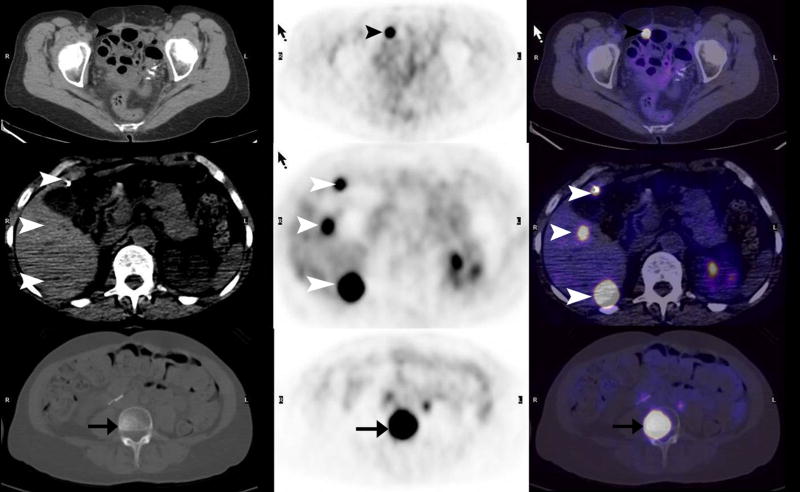
The sensitivities and specificities of PET/CT in ovarian cancer are presented in Figure 1, based on overall, pelvic and extra-pelvic lesion per patient analysis. In Figure 2 we present PET/CT images of recurrent disease in the pelvis (arrows), as well as liver metastases (arrowheads). Peritoneal (black arrowheads), liver (white arrowheads) and vertebral (arrows) metastases are seen in Figure 3 on the PET/CT scan of a 63-year-old woman with ovarian cancer.
Figure 1.
The sensitivities and specificities of PET/CT in ovarian cancer, based on overall, pelvic and extra-pelvic lesion per patient analysis.
Figure 2.
40-year-old woman with ovarian cancer. Transaxial PET, CT and fused PET/CT images demonstrate metastases in the pelvis (arrowheads) and liver (arrows).
Figure 3.
63-year-old woman with ovarian cancer. Transaxial PET, CT and fused PET/CT images show peritoneal (black arrowheads), liver (white arrowheads) and vertebral (arrows) metastases.
Discussion
Accurate diagnosis of recurrence and re-staging in ovarian cancer is important in an effort to improve the poor prognosis. Re-staging radiologic studies are complicated by alterations to the anatomy and physiology of the abdomen/pelvis from surgery and radiation. Moreover, the ability to fully evaluate the patient in one combined imaging modality rather than multiple tests is advantageous if the performance of that test is equal to or better than the others.
There has long been interest in the role of PET in ovarian carcinoma, but the majority of studies in the current literature are primarily based on dedicated PET acquisitions (10–26). Both early (15) as well as recent studies (13) with dedicated PET have shown comparable or higher sensitivity, specificity, and diagnostic accuracy in comparison to conventional imaging or tumor markers.
However, there is evidence that PET plus CT performs better than either alone in various solid cancers including ovarian cancer (27, 28). There are relatively few studies published on concurrent PET/CT in gynecologic malignancy (29–31). A recent prospective study (32) in 97 patients, evaluating the utility of PET/CT for the evaluation of a pelvic mass of unknown origin (with post-surgical histopathologic follow-up) found that the sensitivity and specificity for PET/CT in diagnosing a malignant pelvic tumor were 100% and 93%, respectively. Other studies evaluating PET/CT in recurrent ovarian cancer also show promising results. Three retrospective studies (29, 33, 34) evaluating PET/CT in recurrent ovarian cancer provide sensitivity and specificity values of 83–95%, and 71–100%, respectively, which are, again, superior to that reported in the literature for conventional imaging. And, another (35) showed that a change in the clinical management was observed in 44% of cases when PET/CT information was added to conventional follow-up findings.
With these considerations in mind, PET/CT shows promise in the restaging evaluation of ovarian cancer. Our study confirms this with an overall per patient sensitivity of 88% and specificity of 88% for detection of ovarian cancer at restaging. In comparison, conventional imaging has a reported sensitivity of 53–70% and specificity of 82–83% (13, 29).
One group has demonstrated improved sensitivity and specificity of PET/CT for the evaluation of recurrent ovarian carcinoma when their early experience was compared to later. Sensitivity improved from78% to 93% and specificity from 75% to 100% (30, 31). This indicates that as experience grows with this relatively new modality, the accuracy will increase.
The results in our study showed a lower sensitivity of 81% for lesions in the pelvis compared to 96% for disease in other sites. This difference is likely due to post-surgical and post-treatment induced inflammatory uptake within the pelvis. This hypothesis is supported by a study (32) showing that staging with PET/CT (before surgery) has sensitivity and specificity of 100%, and 92%, respectively, in comparison to subsequent histopathologic findings. Nonetheless, conventional imaging is also complicated by post-therapy changes, such that the reported sensitivity and specificity remains higher with PET/CT. Another contributing factor to these results may be to post-therapy anatomical changes accounting for challenges in interpretation of non-contrast enhanced CT images. Using i.v. contrast-enhanced pelvic CT may result in improved sensitivity in this location for patients with ovarian cancer. The specificities were equal at 94% for pelvic and extra-pelvic lesions.
Outside the pelvis, both the sensitivity and specificity are high, indicating the very good performance of PET/CT in identifying more distant lesions. Extra-pelvic lesions were located in the liver, lungs, omentum, bones, and lymph nodes. This is significant in terms of altering the management, as shown in one study which found that a change in clinical management was observed in 44% of cases when PET/CT information was added to conventional follow-up findings (35).
The limitations in this study acknowledge that patient selection in referrals for PET/CT is important for the results of the scan. However, this is difficult to overcome in any retrospective review.
Taken together, the results of this study confirm a high sensitivity and specificity of PET/CT in ovarian cancer staging both within and outside the pelvis. The values reported in this study are in accord with those reported in the literature thus far and add to the limited number of studies on this important subject. The main advantages of PET/CT for ovarian cancer are in restaging, finding extra-pelvic lesions that can effect patient management, and in evaluation of those patients with increased CA-125 levels. The sensitivity of PET/CT is lower within the pelvis, probably due to post-operative changes and lack of i.v. contrast, but remains higher than conventional imaging. The latter, however, is established and remains important for its improved resolution. Therefore, the combined use of conventional imaging and PET/CT in the evaluation of ovarian cancer is recommended.
Conclusions
This study confirms the good results of 18F FDG PET/CT for identification of residual/recurrent ovarian cancer, as well as distant metastases. PET/CT should be an integral part in evaluation of patients with high-risk ovarian cancer or rising CA-125 values.
Acknowledgments
this study was supported in part by grant NCI ICMIC P50 CA114747 (SSG).
Contributor Information
Andrei Iagaru, Stanford Hospital and Clinics, Division of Nuclear Medicine, 300 Pasteur Dr, Room H-0101, Stanford, CA 94305, Phone: 650 736 2859, Fax: 650 498 5047, E-mail: aiagaru@stanford.edu.
Erik S. Mittra, Stanford Hospital and Clinics, Division of Nuclear Medicine.
I. Ross McDougall, Stanford Hospital and Clinics, Division of Nuclear Medicine.
Andrew Quon, Stanford Hospital and Clinics, Division of Nuclear Medicine.
Sanjiv Sam Gambhir, Stanford Hospital and Clinics, Departments of Radiology and Bioengineering, Division of Nuclear Medicine.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar–Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Mironov S, Akin O, Pandit-Taskar N, Hann LE. Ovarian cancer. Radiol Clin North Am. 2007 Jan;45(1):149–66. doi: 10.1016/j.rcl.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002 Sep;2(9):683–93. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 4.Avril N, Sassen S, Schmalfeldt B, Naehrig J, Rutke S, Weber WA, et al. Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluorodeoxyglucose positron emission tomography in patients with advanced-stage ovarian cancer. Clin Oncol. 2005 Oct 20;23(30):7445–53. doi: 10.1200/JCO.2005.06.965. [DOI] [PubMed] [Google Scholar]
- 5.Jiménez-Bonilla J, Maldonado A, Morales S, Salud A, Zomeño M, Román J, et al. Clinical Impact of 18F-FDG-PET in the Suspicion of Recurrent Ovarian Carcinoma Based on Elevated Tumor Marker Serum Levels. Clin Positron Imaging. 2000 Nov;3(6):231–236. doi: 10.1016/s1095-0397(01)00053-x. [DOI] [PubMed] [Google Scholar]
- 6.Chang WC, Hung YC, Kao CH, Yen RF, Shen YY, Lin CC. Usefulness of whole body positron emission tomography (PET) with 18F-fluoro-2-deoxyglucose (FDG) to detect recurrent ovarian cancer based on asymptomatically elevated serum levels of tumor marker. Neoplasma. 2002;49(5):329–33. [PubMed] [Google Scholar]
- 7.Menzel C, Döbert N, Hamscho N, Zaplatnikov K, Vasvatekis S, Matic V, et al. The influence of CA 125 and CEA levels on the results of (18)F-deoxyglucose positron emission tomography in suspected recurrence of epithelial ovarian cancer. Strahlenther Onkol. 2004 Aug;180(8):497–501. doi: 10.1007/s00066-004-1208-3. [DOI] [PubMed] [Google Scholar]
- 8.Giorgetti A, Sorace O, Pisani P, Salvadori PA, Mariani G. Accuracy of Qualitative and Semiquantitative Analysis of 18FDG Positron Emission Tomography Scans in the Evaluation of Primary and Metastatic Lesions. Clin Positron Imaging. 2000 Jul;3(4):182. doi: 10.1016/s1095-0397(00)00094-7. [DOI] [PubMed] [Google Scholar]
- 9.Newcombe RG. Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med. 1998 Nov 30;17(22):2635–50. [PubMed] [Google Scholar]
- 10.Belhocine T, De Barsy C, Hustinx R, Willems-Foidart J. Usefulness of (18)F-FDG PET in the post-therapy surveillance of endometrial carcinoma. Eur J Nucl Med Mol Imaging. 2002 Sep;29(9):1132–9. doi: 10.1007/s00259-002-0878-2. [DOI] [PubMed] [Google Scholar]
- 11.Chao A, Chang TC, Ng KK, Hsueh S, Huang HJ, Chou HH, et al. 18F-FDG PET in the management of endometrial cancer. Eur J Nucl Med Mol Imaging. 2006 Jan;33(1):36–44. doi: 10.1007/s00259-005-1876-y. [DOI] [PubMed] [Google Scholar]
- 12.Cho SM, Ha HK, Byun JY, Lee JM, Kim CJ, Nam-Koong SE, et al. Usefulness of FDG PET for assessment of early recurrent epithelial ovarian cancer. AJR Am J Roentgenol. 2002 Aug;179(2):391–5. doi: 10.2214/ajr.179.2.1790391. [DOI] [PubMed] [Google Scholar]
- 13.García-Velloso MJ, Jurado M, Ceamanos C, Aramendía JM, Garrastachu MP, López-García G, et al. Diagnostic accuracy of FDG PET in the follow-up of platinum-sensitive epithelial ovarian carcinoma. Eur J Nucl Med Mol Imaging. 2007 Sep;34(9):1396–405. doi: 10.1007/s00259-007-0366-9. [DOI] [PubMed] [Google Scholar]
- 14.Havrilesky LJ, Kulasingam SL, Matchar DB, Myers ER. FDG-PET for management of cervical and ovarian cancer. Gynecol Oncol. 2005 Apr;97(1):183–91. doi: 10.1016/j.ygyno.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Hubner KF, McDonald TW, Niethammer JG, Smith GT, Gould HR, Buonocore E. Assessment of primary and metastatic ovarian cancer by positron emission tomography (PET) using 2-[18F]deoxyglucose (2-[18F]FDG) Gynecol Oncol. 1993 Nov;51(2):197–204. doi: 10.1006/gyno.1993.1272. [DOI] [PubMed] [Google Scholar]
- 16.Israel O, Mor M, Guralnik L, Hermoni N, Gaitini D, Bar-Shalom R, et al. Is 18F-FDG PET/CT useful for imaging and management of patients with suspected occult recurrence of cancer? J Nucl Med. 2004 Dec;45(12):2045–51. [PubMed] [Google Scholar]
- 17.Khan N, Oriuchi N, Yoshizaki A, Kanuma T, Higuchi T, Endo K. Diagnostic accuracy of FDG PET imaging for the detection of recurrent or metastatic gynecologic cancer. Ann Nucl Med. 2005 Apr;19(2):137–45. doi: 10.1007/BF03027393. [DOI] [PubMed] [Google Scholar]
- 18.Kubik-Huch RA, Dörffler W, von Schulthess GK, Marincek B, Köchli OR, Seifert B. Value of (18F)-FDG positron emission tomography, computed tomography, and magnetic resonance imaging in diagnosing primary and recurrent ovarian carcinoma. Eur Radiol. 2000;10(5):761–7. doi: 10.1007/s003300051000. [DOI] [PubMed] [Google Scholar]
- 19.Kumar R, Chauhan A, Jana S, Dadparvar S. Positron emission tomography in gynecological malignancies. Expert Rev Anticancer Ther. 2006 Jul;6(7):1033–44. doi: 10.1586/14737140.6.7.1033. [DOI] [PubMed] [Google Scholar]
- 20.Makhija S, Howden N, Edwards R, Kelley J, Townsend DW, Meltzer CC. Positron emission tomography/computed tomography imaging for the detection of recurrent ovarian and fallopian tube carcinoma: a retrospective review. Gynecol Oncol. 2002 Apr;85(1):53–8. doi: 10.1006/gyno.2002.6606. [DOI] [PubMed] [Google Scholar]
- 21.Nakamoto Y, Saga T, Ishimori T, Mamede M, Togashi K, Higuchi T, et al. Clinical value of positron emission tomography with FDG for recurrent ovarian cancer. AJR Am J Roentgenol. 2001 Jun;176(6):1449–54. doi: 10.2214/ajr.176.6.1761449. [DOI] [PubMed] [Google Scholar]
- 22.Saga T, Higashi T, Ishimori T, Mamede M, Nakamoto Y, Mukai T, et al. Clinical value of FDG-PET in the follow up of post-operative patients with endometrial cancer. Ann Nucl Med. 2003 May;17(3):197–203. doi: 10.1007/BF02990022. [DOI] [PubMed] [Google Scholar]
- 23.Takekuma M, Maeda M, Ozawa T, Yasumi K, Torizuka T. Positron emission tomography with 18F-fluoro-2-deoxyglucose for the detection of recurrent ovarian cancer. Int J Clin Oncol. 2005 Jun;10(3):177–81. doi: 10.1007/s10147-005-0489-6. [DOI] [PubMed] [Google Scholar]
- 24.Torizuka T, Nobezawa S, Kanno T, Futatsubashi M, Yoshikawa E, Okada H, et al. Ovarian cancer recurrence: role of whole-body positron emission tomography using 2-[fluorine-18]-fluoro-2-deoxy- D-glucose. Eur J Nucl Med Mol Imaging. 2002 Jun;29(6):797–803. doi: 10.1007/s00259-001-0750-9. [DOI] [PubMed] [Google Scholar]
- 25.Yen RF, Sun SS, Shen YY, Changlai SP, Kao A. Whole body positron emission tomography with 18F-fluoro-2-deoxyglucose for the detection of recurrent ovarian cancer. Anticancer Res. 2001 Sep-Oct;21(5):3691–4. [PubMed] [Google Scholar]
- 26.Zimny M, Siggelkow W. Positron emission tomography scanning in gynecologic and breast cancers. Curr Opin Obstet Gynecol. 2003 Feb;15(1):69–75. doi: 10.1097/00001703-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Antoch G, Saoudi N, Kuehl H, Dahmen G, Mueller SP, Beyer T, et al. Accuracy of whole-body dual-modality fluorine-18-2-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol. 2004 Nov 1;22(21):4357–68. doi: 10.1200/JCO.2004.08.120. [DOI] [PubMed] [Google Scholar]
- 28.Eubank WB, Mankoff DA, Schmiedl UP, Winter TC, 3rd, Fisher ER, Olshen AB, et al. Imaging of oncologic patients: benefit of combined CT and FDG PET in the diagnosis of malignancy. AJR Am J Roentgenol. 1998 Oct;171(4):1103–10. doi: 10.2214/ajr.171.4.9763005. [DOI] [PubMed] [Google Scholar]
- 29.Picchio M, Sironi S, Messa C, Mangili G, Landoni C, Gianolli L, et al. Advanced ovarian carcinoma: usefulness of [(18)F]FDG-PET in combination with CT for lesion detection after primary treatment. Q J Nucl Med. 2003 Jun;47(2):77–84. [PubMed] [Google Scholar]
- 30.Sironi S, Messa C, Mangili G, Zangheri B, Aletti G, Garavaglia E, et al. Integrated FDG PET/CT in patients with persistent ovarian cancer: correlation with histologic findings. Radiology. 2004 Nov;233(2):433–40. doi: 10.1148/radiol.2332031800. [DOI] [PubMed] [Google Scholar]
- 31.Sironi S, Picchio M, Landoni C, Galimberti S, Signorelli M, Bettinardi V, et al. Post-therapy surveillance of patients with uterine cancers: value of integrated FDG PET/CT in the detection of recurrence. Eur J Nucl Med Mol Imaging. 2007 Apr;34(4):472–9. doi: 10.1007/s00259-006-0251-y. [DOI] [PubMed] [Google Scholar]
- 32.Risum S, Høgdall C, Loft A, Berthelsen AK, Høgdall E, Nedergaard L, et al. The diagnostic value of PET/CT for primary ovarian cancer-A prospective study. Gynecol Oncol. 2007 Apr;105(1):145–9. doi: 10.1016/j.ygyno.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Nanni C, Rubello D, Farsad M, De Iaco P, Sansovini M, Erba P, et al. (18)F-FDG PET/CT in the evaluation of recurrent ovarian cancer: a prospective study on forty-one patients. Eur J Surg Oncol. 2005 Sep;31(7):792–7. doi: 10.1016/j.ejso.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Thrall MM, DeLoia JA, Gallion H, Avril N. Clinical use of combined positron emission tomography and computed tomography (FDG-PET/CT) in recurrent ovarian cancer. Gynecol Oncol. 2007 Apr;105(1):17–22. doi: 10.1016/j.ygyno.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 35.Mangili G, Picchio M, Sironi S, Viganò R, Rabaiotti E, Bornaghi D, et al. Integrated PET/CT as a first-line re-staging modality in patients with suspected recurrence of ovarian cancer. Eur J Nucl Med Mol Imaging. 2007 May;34(5):658–66. doi: 10.1007/s00259-006-0306-0. [DOI] [PubMed] [Google Scholar]