Abstract
The prevalence of human papillomavirus (HPV) infection in cervical cell scrapes from young women was determined by polymerase chain reaction (PCR) by using general primer pairs localized within the L1 region. With a one-step general PCR, 5.9% (35 of 590) of young women in a population-based study were found to contain HPV DNA. The proportion of HPV-positive women increased with age, from 1.4% (1 of 69) among women aged 19 years to 9.2% (13 of 142) among women aged 25 years. Among the cervical scrapes from women with normal cytology, 5.6% (30 of 539) harbored HPV DNA. A total of 5 of 19 (26.3%) of the women with pathological signs were positive for HPV DNA. By a two-step PCR, using nested general primers, 20.3% (118 of 581) of all women were shown to contain HPV DNA. The proportion of HPV-positive women also increased with age, from 17.4% (12 of 69) among women aged 19 years to 31.9% (43 of 135) among women aged 25 years, when the two-step PCR was used. Some 19.2% (102 of 530) of cervical scrapes from women with normal cytology contained HPV DNA. Among the women with pathological signs, 16 of 19 (84.2%) were positive for HPV DNA. The HPV DNA-positive specimens were demonstrated to contain HPV type 6, 11, 16, 18, 31, 33, 35, 39, 40, 45, 55, or 56. The most prevalent HPV types were 6 (2.0%) and 16 (2.7%). More than one type was found in 16 specimens. Sixty HPV-positive samples could not be typed.
Full text
PDF

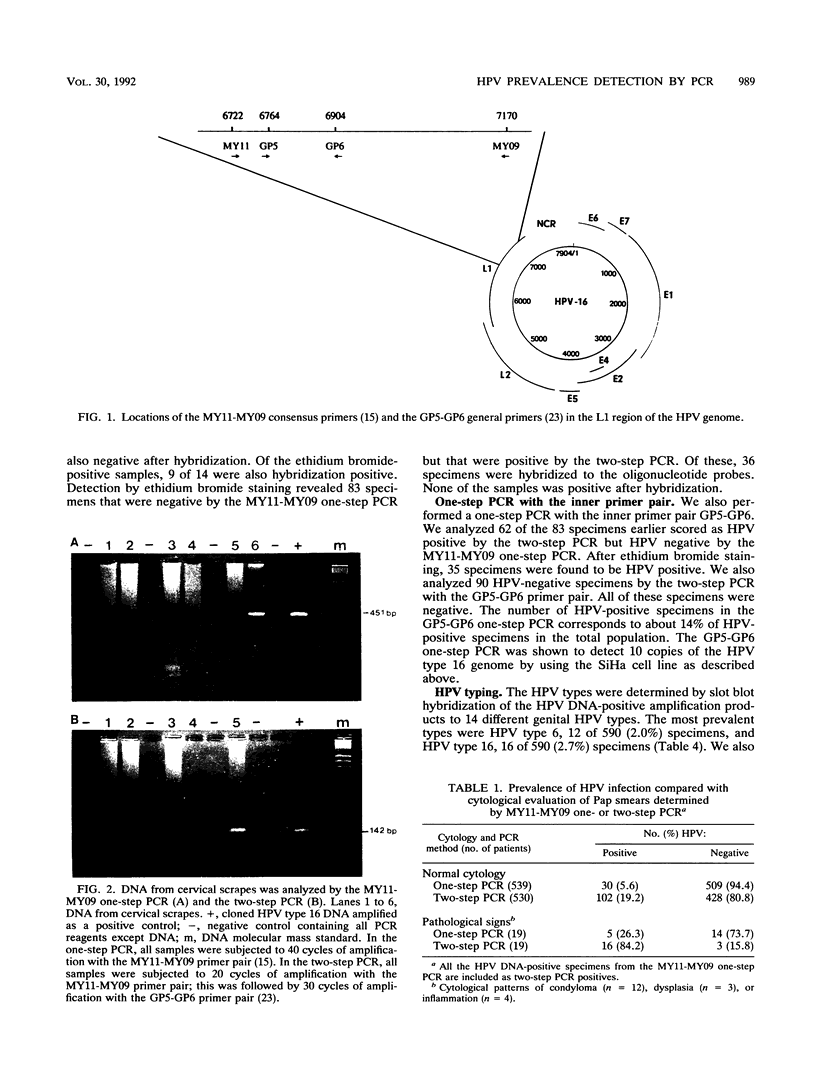



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer H. M., Ting Y., Greer C. E., Chambers J. C., Tashiro C. J., Chimera J., Reingold A., Manos M. M. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991 Jan 23;265(4):472–477. [PubMed] [Google Scholar]
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Nasseri M., Wolinsky S. M., Broker T. R. Human papillomavirus types 6 and 11 mRNAs from genital condylomata acuminata. J Virol. 1987 Aug;61(8):2581–2588. doi: 10.1128/jvi.61.8.2581-2588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evander M., Bodén E., Bjersing L., Rylander E., Wadell G. Oligonucleotide primers for DNA amplification of the early regions 1, 6, and 7 from human papillomavirus types 6, 11, 16, 18, 31, and 33. Arch Virol. 1991;116(1-4):221–233. doi: 10.1007/BF01319244. [DOI] [PubMed] [Google Scholar]
- Evander M., Wadell G. A general primer pair for amplification and detection of genital human papillomavirus types. J Virol Methods. 1991 Feb-Mar;31(2-3):239–250. doi: 10.1016/0166-0934(91)90162-s. [DOI] [PubMed] [Google Scholar]
- Gregoire L., Arella M., Campione-Piccardo J., Lancaster W. D. Amplification of human papillomavirus DNA sequences by using conserved primers. J Clin Microbiol. 1989 Dec;27(12):2660–2665. doi: 10.1128/jcm.27.12.2660-2665.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer S. K., de Villiers E. M., Haugaard B. J., Christensen R. B., Teisen C., Møller K. A., Poll P., Jensen H., Vestergaard B. F., Lynge E. Human papillomavirus, herpes simplex virus and cervical cancer incidence in Greenland and Denmark. A population-based cross-sectional study. Int J Cancer. 1988 Apr 15;41(4):518–524. doi: 10.1002/ijc.2910410408. [DOI] [PubMed] [Google Scholar]
- Koutsky L. A., Galloway D. A., Holmes K. K. Epidemiology of genital human papillomavirus infection. Epidemiol Rev. 1988;10:122–163. doi: 10.1093/oxfordjournals.epirev.a036020. [DOI] [PubMed] [Google Scholar]
- Lorincz A. T., Lancaster W. D., Temple G. F. Cloning and characterization of the DNA of a new human papillomavirus from a woman with dysplasia of the uterine cervix. J Virol. 1986 Apr;58(1):225–229. doi: 10.1128/jvi.58.1.225-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lörincz A. T., Temple G. F., Patterson J. A., Jenson A. B., Kurman R. J., Lancaster W. D. Correlation of cellular atypia and human papillomavirus deoxyribonucleic acid sequences in exfoliated cells of the uterine cervix. Obstet Gynecol. 1986 Oct;68(4):508–512. [PubMed] [Google Scholar]
- Macnab J. C., Walkinshaw S. A., Cordiner J. W., Clements J. B. Human papillomavirus in clinically and histologically normal tissue of patients with genital cancer. N Engl J Med. 1986 Oct 23;315(17):1052–1058. doi: 10.1056/NEJM198610233151703. [DOI] [PubMed] [Google Scholar]
- Meisels A., Morin C., Casas-Cordero M. Human papillomavirus infection of the uterine cervix. Int J Gynecol Pathol. 1982;1(1):75–94. doi: 10.1097/00004347-198201000-00009. [DOI] [PubMed] [Google Scholar]
- Melchers W., van den Brule A., Walboomers J., de Bruin M., Burger M., Herbrink P., Meijer C., Lindeman J., Quint W. Increased detection rate of human papillomavirus in cervical scrapes by the polymerase chain reaction as compared to modified FISH and southern-blot analysis. J Med Virol. 1989 Apr;27(4):329–335. doi: 10.1002/jmv.1890270413. [DOI] [PubMed] [Google Scholar]
- Pfister H. Biology and biochemistry of papillomaviruses. Rev Physiol Biochem Pharmacol. 1984;99:111–181. doi: 10.1007/BFb0027716. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schiffman M. H., Bauer H. M., Lorincz A. T., Manos M. M., Byrne J. C., Glass A. G., Cadell D. M., Howley P. M. Comparison of Southern blot hybridization and polymerase chain reaction methods for the detection of human papillomavirus DNA. J Clin Microbiol. 1991 Mar;29(3):573–577. doi: 10.1128/jcm.29.3.573-577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders P. J., van den Brule A. J., Schrijnemakers H. F., Snow G., Meijer C. J., Walboomers J. M. The use of general primers in the polymerase chain reaction permits the detection of a broad spectrum of human papillomavirus genotypes. J Gen Virol. 1990 Jan;71(Pt 1):173–181. doi: 10.1099/0022-1317-71-1-173. [DOI] [PubMed] [Google Scholar]
- Stanley M. Genital papillomaviruses, polymerase chain reaction and cervical cancer. Genitourin Med. 1990 Dec;66(6):415–417. doi: 10.1136/sti.66.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P., Parry G. N., Yule R., Coleman D. V., Malcolm A. D. Human papillomavirus subtype 16a. Lancet. 1989 Jul 15;2(8655):170–170. doi: 10.1016/s0140-6736(89)90240-7. [DOI] [PubMed] [Google Scholar]
- Williamson A. L., Rybicki E. P. Detection of genital human papillomaviruses by polymerase chain reaction amplification with degenerate nested primers. J Med Virol. 1991 Mar;33(3):165–171. doi: 10.1002/jmv.1890330305. [DOI] [PubMed] [Google Scholar]
- Young L. S., Bevan I. S., Johnson M. A., Blomfield P. I., Bromidge T., Maitland N. J., Woodman C. B. The polymerase chain reaction: a new epidemiological tool for investigating cervical human papillomavirus infection. BMJ. 1989 Jan 7;298(6665):14–18. doi: 10.1136/bmj.298.6665.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers E. M. Heterogeneity of the human papillomavirus group. J Virol. 1989 Nov;63(11):4898–4903. doi: 10.1128/jvi.63.11.4898-4903.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers E. M., Wagner D., Schneider A., Wesch H., Miklaw H., Wahrendorf J., Papendick U., zur Hausen H. Human papillomavirus infections in women with and without abnormal cervical cytology. Lancet. 1987 Sep 26;2(8561):703–706. doi: 10.1016/s0140-6736(87)91072-5. [DOI] [PubMed] [Google Scholar]
- van den Brule A. J., Claas E. C., du Maine M., Melchers W. J., Helmerhorst T., Quint W. G., Lindeman J., Meijer C. J., Walboomers J. M. Use of anticontamination primers in the polymerase chain reaction for the detection of human papilloma virus genotypes in cervical scrapes and biopsies. J Med Virol. 1989 Sep;29(1):20–27. doi: 10.1002/jmv.1890290105. [DOI] [PubMed] [Google Scholar]
- van den Brule A. J., Meijer C. J., Bakels V., Kenemans P., Walboomers J. M. Rapid detection of human papillomavirus in cervical scrapes by combined general primer-mediated and type-specific polymerase chain reaction. J Clin Microbiol. 1990 Dec;28(12):2739–2743. doi: 10.1128/jcm.28.12.2739-2743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brule A. J., Snijders P. J., Gordijn R. L., Bleker O. P., Meijer C. J., Walboomers J. M. General primer-mediated polymerase chain reaction permits the detection of sequenced and still unsequenced human papillomavirus genotypes in cervical scrapes and carcinomas. Int J Cancer. 1990 Apr 15;45(4):644–649. doi: 10.1002/ijc.2910450412. [DOI] [PubMed] [Google Scholar]