Abstract
Behavioural studies widely implicate sleep in memory consolidation in the learning of a broad range of behaviours1-4. During sleep, brain regions are reactivated5,6, and specific patterns of neural activity are replayed7-10, consistent with patterns observed in prior waking behaviour. Birdsong learning is a paradigmatic model system for skill learning11-14. Song development in juvenile zebra finches is characterised by sleep-dependent circadian fluctuations in singing behaviour, with immediate post-sleep deterioration in song structure followed by recovery later in the day15. In sleeping adult birds, spontaneous bursting activity of forebrain premotor neurones in the robust nucleus of the arcopallium (RA) carries information about daytime singing16. Here we show that in juvenile zebra finches, playback during the day of an adult “tutor” song induced profound and tutor song-specific changes in bursting activity of RA neurones during the following night of sleep. The night-time neuronal changes preceded tutor song-induced changes in singing, first observed the following day. Interruption of auditory feedback greatly reduced sleep bursting and prevented the tutor song-specific neuronal remodelling. Thus, night-time neuronal activity is shaped by the interaction of the song model (sensory template) and auditory feedback, with changes in night-time activity proceeding the onset of practice associated with vocal learning. We hypothesise that night-time bursting induces adaptive changes in premotor networks during sleep as part of vocal learning. By this hypothesis, plastic changes are driven by replay of sensory information at night and evaluation of sensory feedback during the day, with the interaction between the two leading to complex circadian patterns such as are seen early in vocal development.
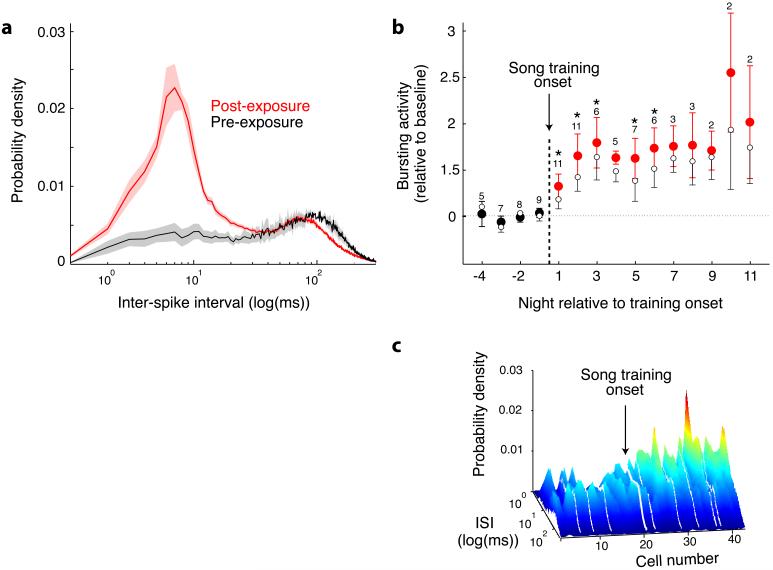
To explore the role of sleep in the early phases of song learning, we characterised the properties of single RA neurones in head-fixed, sleeping juvenile zebra finches during nights surrounding the onset of tutor song exposure. Sleep was defined based on behavioural and electroencephalographic criteria (see Supplementary Information)17. During sleep, RA neurones tended to discharge irregularly or burst, as seen in distributions of inter-spike intervals (ISIs) (Fig. 1a, black curve) 16. Starting on the night following the first day of exposure to the tutor song, there was a sharp increase in the amount of high-frequency spiking activity (Fig. 1a, red curve). Across all birds we quantified the effect of tutor song exposure as a normalised change in the percentage of ISIs ≤ 40 ms, which showed a significant increase starting on the night following the first day of tutor song exposure and persisted thereafter (Fig. 1b). A significant result was also obtained considering firing rates that were normalized by linearly scaling the ISIs for each cell (p=0.02, repeated measures ANOVA, alpha=0.05, Fig. 1b, open circles). This verified that the increase in high frequency activity was not dependent on changes in mean spike rates following tutoring, but was the result of a specific increase in high-frequency activity including bursting. Within each bird there was some variation in the amount of high-frequency activity of RA cells on nights after the onset of song learning, but the tendency towards shorter ISIs was apparent in most cells (Fig. 1c).
Figure 1.
High-frequency bursting in RA. a, ISI distributions for all cells for each night from bird S6 were averaged; each curve (± SEM) shows global average of all nights pre-tutor song exposure (black; n = 19 cells, 3 nights, post-hatch days 43-47), post-exposure (red; n = 59 cells, 12 nights, PHD 49-62). b, Tutor song triggered increase in high-frequency bursting (13 birds, n = 489 cells, 37.4 ± 56.4 min/cell, 5.6 cells/bird/night). Number of birds contributing to each point shown above point. *p < 0.05 by sign test comparing mean pre and post-exposure values within birds, alpha = 0.05. Open circles: spike-rate normalised data. (See Methods, Supplementary Information). c, ISI distributions for all 44 consecutively recorded cells from bird S9. White lines: daytime.
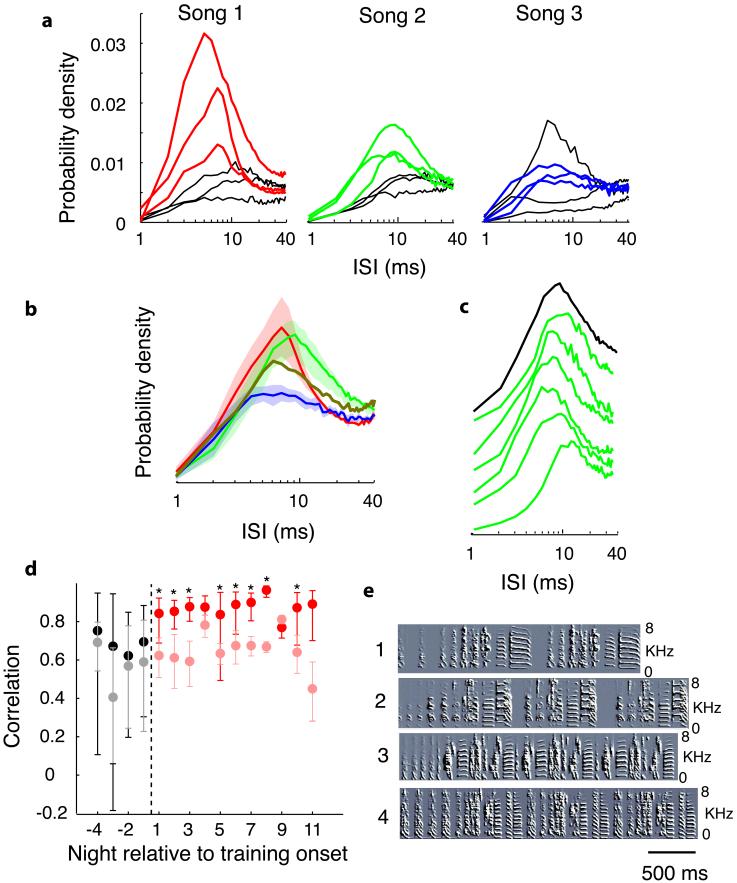
Emerging RA bursting activity, furthermore, was shaped by the specific tutor song a bird heard. Nightly mean ISI distributions were calculated for all RA neurones recorded for each bird following tutor song exposure (which showed little difference from night to night; Fig. 2c), and nightly mean distributions were averaged together to generate one mean curve per bird. For the resulting post-exposure curves, within the high frequency range (ISIs ≤ 40 ms), the shapes (as assessed using Pearson correlation coefficients) were more similar in birds hearing the same tutor song than birds hearing different tutor songs (see Supplementary Information). This grouping of ISI distribution shapes by tutor song can be visualized by comparing the average ISI distributions for individual birds on nights prior to tutor song exposure as compared to following (Fig. 2a, black and coloured lines, respectively). The differences between groups can be visualized by comparing global average ISI distributions, one for each group of birds hearing a given tutor song (Fig. 2b).
Figure 2.
Tutor songs shape RA bursting. a, ISI distributions averaged across all post-exposure nights, one per bird, by tutor song. Black lines are pre-exposure, coloured lines post exposure. Song 1, n=3 birds, 59 cells (only a representative 3 of 7 birds are shown to reduce clutter, see Supplementary Fig. 4 for all birds); Song 2, n=3 birds, 85 cells; Song 3, n=3 birds, 38 cells. b, Global mean (±SEM) ISI distributions following tutor song exposure, one curve for each tutor song group. For Song 1, two birds with the largest high frequency peaks were removed from the average so that curves have comparable peak heights for comparison. Brown line is the single Song 4 bird. c, Green lines are averages for each of the six post-exposure nights in one bird; black line is Song 2 global mean (from Fig. 2b). d, ISI distributions change upon tutor song exposure. Pearson correlation coefficients comparing nightly curves for given bird with global post-exposure means (see text). Nightly means (±SD) comparing birds hearing the same (pre- and post-exposure, black and red dots, respectively) or different (grey and pink dots, respectively) tutor songs. e, Tutor song spectrographs.
Once a bird was exposed to a tutor song, a prototypical post-exposure ISI distribution shape was quickly obtained and then maintained. To quantify this, we compared (Pearson correlations) the nightly ISI distributions (≤ 40 ms) for each bird before and after song exposure to the corresponding global mean curve (Fig. 2b), excluding data from the bird being analysed from the global mean distributions.. Prior to tutor song exposure, both the within and between group comparisons (Fig. 2d, black and grey dots, respectively) had large variability and were not significantly different from each other on any night (p = 0.25 to p = 0.73). By the first night after tutor song exposure, ISI distributions had already assumed their post-exposure shape, showing increased Pearson correlations with much lower variability (Fig. 2d, red dots), while the across-group correlations did not increase (Fig. 2d, pink dots). These differences were statistically significant starting from the first night (p = 0.0082, 2-tailed t-test, alpha = 0.05), and continued to show a significant difference throughout the post-exposure period (p < 0.05 for 8 of 11 post-exposure nights) (see also Supplementary Fig. 2), emphasising that stable changes in ISI distributions were rapidly achieved and then maintained in the days following tutor song exposure.
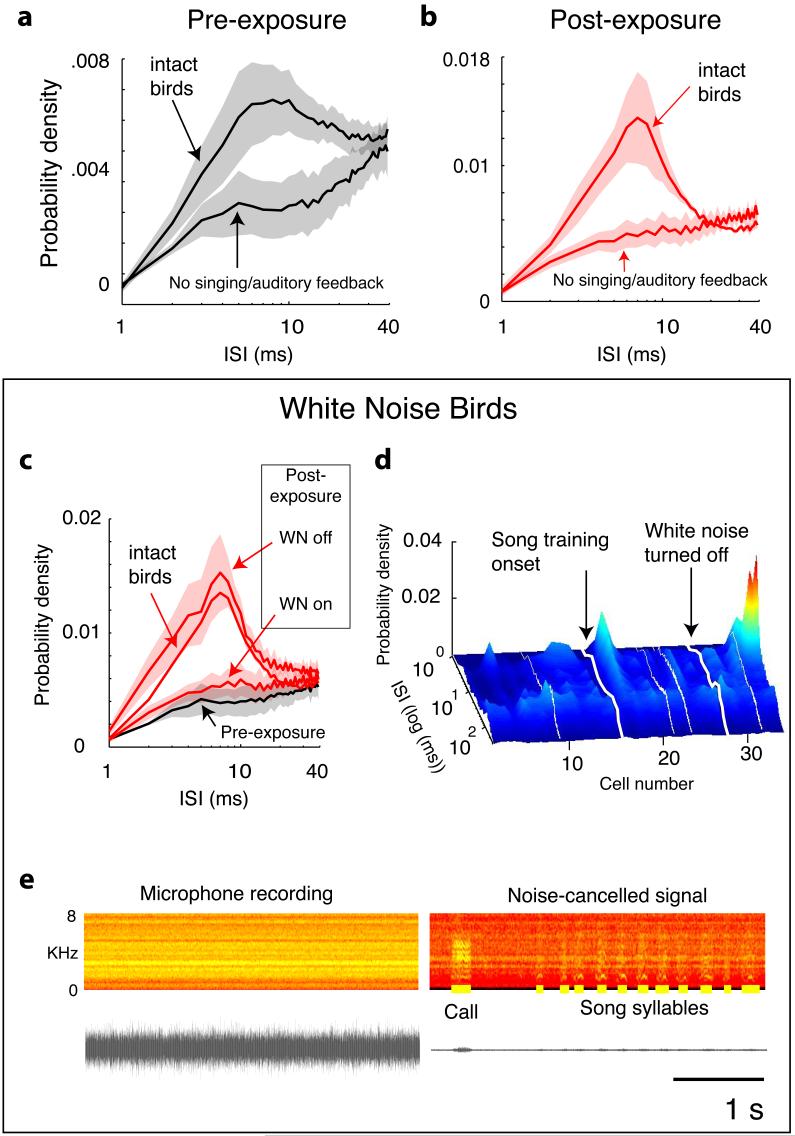
Zebra finches begin singing as early as 25 days of age18, so our ≈40 day-old birds had extensive sensorimotor experience prior to neural recordings. To explore the influence of singing and auditory feedback on the structure of RA neuronal bursting, we performed two additional experiments. We prevented singing by surgically muting two birds (a third bird, M2, sang in spite of the surgery), and placed a second group of four birds in a continuous 100 dB white noise (WN) environment to suppress auditory feedback. All seven birds cued tutor song playback, which for WN birds also briefly eliminated the masking noise. A noise cancellation technique allowed us to qualitatively assess the amount of singing in WN (Fig. 3e).
Figure 3.
RA sleep activity in absence of auditory feedback (WN or muted). a, ISI distributions prior to tutor song exposure, averaged across two muted birds and four birds raised in WN (lower black line), and averaged (for comparison) across 13 birds with normal feedback (upper black line). b, WN and muted birds fail to show tutor song-specific ISI distributions after song exposure. Lower red line: mean post-exposure distribution from muted and WN birds (5/6 birds heard Song 1). Upper red line: mean distribution from intact Song 1 birds (same as in Fig. 2c,). c, Bursting returns when feedback is restored. WN birds (n=4) both pre-exposure (black line) and post-exposure but in WN (lower red line) show suppression of bursting; bursting recovers after WN is turned off (upper red line). Compare with distribution for intact Song 1 birds (middle red line). d, Profound suppression of bursting for all cells in a WN bird. The second night following withdrawal of WN, bursting appears. White lines represent daytime. e, Recording of singing in presence of 100 dB WN without and with noise cancellation (left and right, respectively). Right, bold yellow lines mark vocalisations.
Absence of auditory feedback had profound effects on RA sleep bursting. Even prior to the onset of tutor song exposure, experimental birds exhibited dramatically reduced levels of bursting activity compared to birds with intact auditory feedback. ISI distributions showed suppressed short-duration ISIs for all six feedback-deprived birds (that is, excluding M2) (14 nights, 65 cells; Fig. 3a, lower black line) compared with birds with normal feedback (n = 13 birds, 38 nights 163 cells; Fig. 3a, upper black line). Considering ISIs ≤ 40 ms, these differences were significant (p = 0.03, 2-tailed t-test on arcsin-transformed data, alpha = 0.05).
The absence of feedback also disrupted the changes in RA induced by sensory exposure. In feedback-deprived birds, tutor song exposure did not induce an increase in bursting activity (comparing ISIs ≤ 40 ms pre vs. post exposure, p = 0.45, 2-tailed t-test on arcsin-transformed data, alpha = 0.05, n = 6 birds, 31 nights, 151 cells). Furthermore, on the nights after tutor song exposure, high-frequency bursting was greatly suppressed in feedback-deprived birds (n = 6 birds, 18 nights, 98 cells; Fig. 3b, lower red line) compared with normal birds (Fig. 3b, upper red line) (p = 0.04, 2-tailed t-test on arcsin-transformed data, alpha = 0.05).
Subsequent recovery of sensorimotor feedback released RA neurones to rapidly recover to normal patterns. After exposure to Song 1, but before cessation of the masking noise, the four WN birds showed suppressed high-frequency activity compared to intact birds (comparing ISIs ≤ 40 ms, p = 0.04, 2-tailed t-test on arcsin-transformed data, alpha = 0.05, n=12 nights, 56 cells), with similar ISI distributions to those observed prior to tutor song exposure (n=8 nights, 44 cells; Fig. 3c, black curve). The WN was turned off for a portion of the fourth day of tutor song exposure (turned off for first three hours, n = 2 birds; turned off for last five hours, n = 2 birds) and then turned off completely starting on the fifth day. During sleep on the night following the fourth day of exposure, in two birds that sang robustly during WN exposure (one released from WN at the start of the day, the other at the end of the day), for the first time RA neurones expressed the expected tutor-song-specific ISI distributions. A third robust singer also showed the expected shift, but only on the following night (Fig. 3d). Singing was suppressed in the fourth bird (WN4) throughout the period of WN exposure and showed a robust increase only by the fourth day after WN was turned off. Concomitantly, sleep bursting of RA neurones also remained suppressed for those three additional nights, and then on the night following that first day of singing, RA neurones expressed bursting with an ISI distribution appropriate for Song 1 (r = 0.92 +0.04 / - 0.08 within-group on the 2 nights post-singing onset vs. r = 0.16 + 0.39 / -0.46 for the 3 nights prior to singing onset). The mean ISI curve for all four birds (n = 17 nights, 84 cells), representing only the data collected after WN was turned off (n = 3 birds), or WN was turned off and singing commenced (for WN4), was typical of Song 1 birds with normal feedback (Fig. 3c, upper red curve).
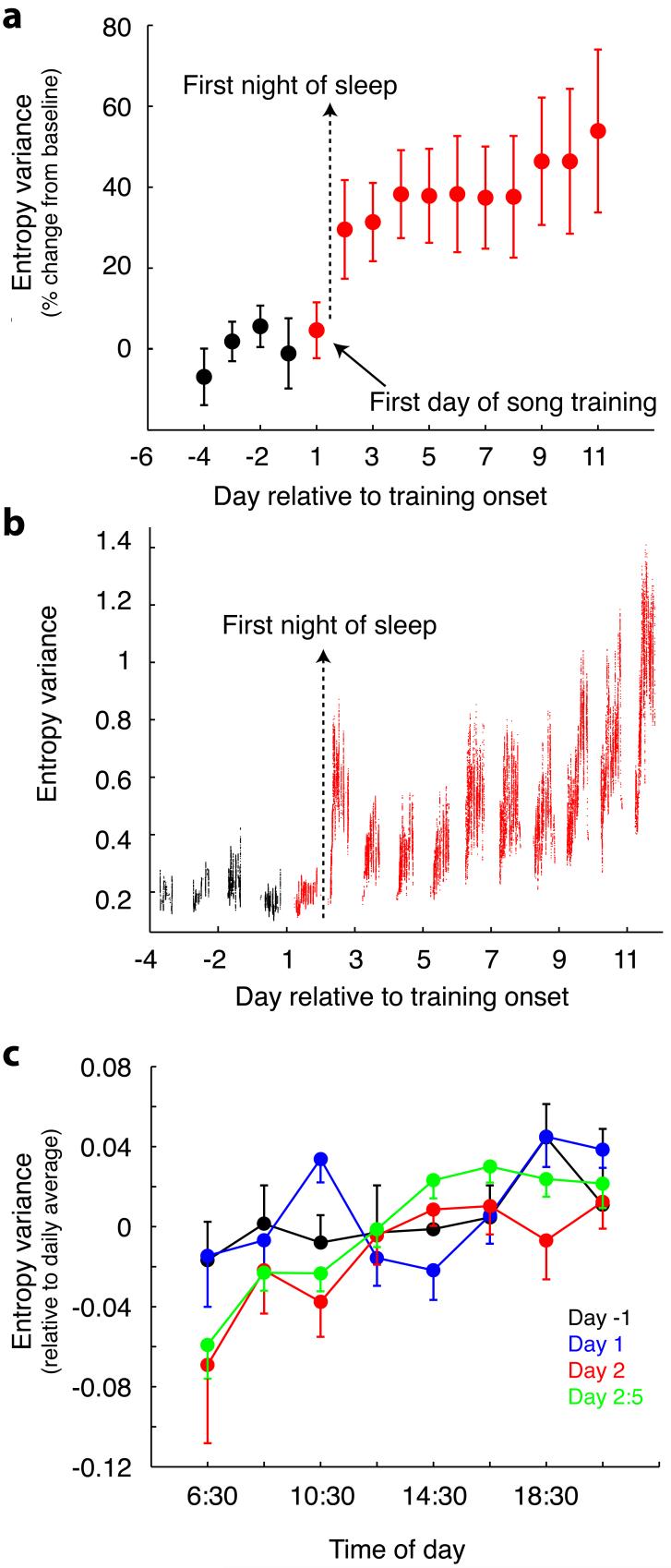
Analysis of the fine structure of vocal development further illuminated the tight temporal correlation between song development and night-time bursting. We examined the entropy variance (EV) - a measure of song complexity which exhibits an upward developmental trend tracking song learning15 - for all song segments each bird sang each day. By this measure, songs gained structure in a saltatory fashion on the second day of tutor song exposure (p=0.006, repeated measures ANOVA, alpha=0.05, Fig. 4a)19. The increase in EV on the second day of training was not present with the first vocalisations of that day. Rather, the increase seen on the second day was achieved across singing in the early part of the day. On days prior to tutor song exposure and on the first day of exposure, there was no significant increase in EV across morning singing (Fig. 4b, 4c, see Supplementary Information for statistical analysis). However, starting on the second day of exposure and on the days thereafter, birds showed a clear increase in EV across the early part of the day (Fig. 4b, 4c), similar to what has been reported previously15,20, and this was significant (see Supplementary Information). Since birds vocalised on the first day of song tutoring before and after they cued tutor song playback, however, this could confound the implications of changes in night-time RA bursting if the birds modified their songs on the first day but only after tutor song exposure, and this was not reflected in mean changes for that day. Analysis of singing at the end of the day, of the most complex songs, or of singing immediately following tutoring, however, all failed to support this alternative explanation (Supplementary Information).
Figure 4.
Entropy variance (EV) changes after a night of sleep. a, Mean daily EV for bird vocalisations. Data for each bird were normalised by dividing within-day means by mean of the 4 nights preceding exposure. b, EV of all sounds produced by one bird across 15 days, smoothed by 40 data points within each day. EV increase across second day of training is achieved across morning singing. c, EV daily trends, averaged across all (non-manipulated) birds in 2-hour bins (± SEM) for the day before training onset (black), first day of training (blue), second day of training (red) and the average of the 2nd through 5th days of training (green).
In birdsong learning, memories of conspecific songs are acquired early in development and act as a referent to guide subsequent auditory feedback-dependent sensorimotor learning12-14,21-24. In an influential account of this process, the acquired memory of song was envisioned to act as a “sensory template”, directly matching auditory feedback arising from singing with the sensory representation of the memorised song guiding changes in vocal output13,25. We found that the effects of tutor song exposure were immediate, profound, and distributed - expressed as rapid, fundamental, and long-lasting changes in song-specific high-frequency spiking activity in a forebrain nucleus one synapse from the motor nucleus innervating the syrinx. The magnitude of these effects is likely to have been enhanced by the isolation rearing our birds experienced prior to tutor song exposure beyond what would otherwise be seen in normally raised birds26. Nevertheless, the timing and song-type specificity of the changes are consistent with the action of a song (sensory) memory influencing the functional organisation of a motor circuit27, albeit a causal link has yet to be established. The rapidity of changes in RA sleep activity and its subsequent stability bolsters the idea that the sensory representation was quickly established, opening a “gate” or enabling a dormant circuit, and that song development proceeded with the sensory representation expressed at night guiding changes in daytime premotor patterns. This motivates a new hypothesis, that sensory memories can act offline (indirectly) on sensorimotor performance via spontaneous activity.
We speculate that during sleep changes in the RA network (“consolidation”) reflect updating of sensory memories. Night-time changes would establish a new configuration in the RA network. The following morning, plastic responses released by singing subsequent to night-time reconfiguration might first drive the network away from stability before it recovered stability with additional iterations; this could explain the non-monotonic changes in singing observed each morning during development15. A larger magnitude of non-monotonic singing behaviour - which is positively correlates with eventual degree of song copying15 - would result from stronger or more accurate sensory influences on premotor networks. As the differences between sensory (night-time) and motor (daytime) network configurations decrease during development (improved sensorimotor integration), the magnitude of the circadian singing patterns would decrease, as is actually observed15. The plastic changes we have described are released by the confluence of two sensory signals - the model and feedback. We further speculate this could occur at the level of RA itself, which receives inputs from the descending motor pathway and the cortico-basal ganglia pathway.
Spontaneous activity guides early development of the nervous system. Here, high-frequency burst-mode firing, a common feature of vertebrate forebrain neurones, is structured by sleep-mediated learning mechanisms, emerging strikingly late in development as the organism experiences appropriate environmental cues. It remains to be seen if bursting sleep activity represents the activation of sensory traces in other forms of skill learning, and perhaps more generally. Structured spontaneous discharge is observed broadly in the forebrain during sleep28, and has been associated with recall29,30.
Methods Summary
Birds were bred in-house in sound isolation boxes, and were female-raised to isolate them from song beginning on or before post-hatch day (PHD) 15. Males (n = 21) were isolated in individual sound isolation boxes between PHD 30 - 35, and received a preparatory surgery between PHD 37 - 40. Song was recorded continuously starting on or before the first day post-surgery until at least PHD 90, and neural recordings began two to three days later. Birds were permitted to self-elicit song playback by pulling a string in the cage after 3.3 ± 1.6 nights of neuronal recordings.
Bursting increase: We quantified the increase in high-frequency neuronal activity as a normalised change in the percentage of the inter-spike-interval (ISI) distribution ≤ 40 ms, which captures short intervals associated with bursting in these juvenile birds and the difference in bursting before and after song exposure (Fig. 1a). Nightly values were calculated by first taking the mean ISI distribution across cells for a given night for a given bird. The proportion of the mean distribution ≤ 40 ms was found and nightly values were normalised within birds by the mean from the 4 nights preceding tutor song exposure onset (Fig. 1b). We also performed the same calculation but first applying a linear transformation of ISIs so that the mean spike rate was the same for all cells before and after tutor song exposure, finding a similar increase in bursting following song exposure (Fig. 1b, open circles).
Correlation coefficients: For statistical analysis of Pearson correlation coefficients, Fisher’s r to z’ transformation was performed on the correlation values. Where the transformed data are plotted (Fig. 2d), the r values have asymmetrical standard deviations as the transformation yields values between -1 and 1.
Supplementary Material
Acknowledgments
We thank Makoto Fukushima for extensive discussions, and Jan-Marino Ramirez, Howard C. Nusbaum, S. Murray Sherman, and Mazakazu Konishi for valuable comments on the manuscript. Amish S. Dave and M. F. designed and implemented the white noise recording/cancellation environment.
Appendix
Methods
Birds were maintained on a reverse light schedule (light: 6:30 pm - 10:30 am, L:D 16h:8h) to permit experiments during daytime hours. In a single surgery around PHD 38, subjects received a single surgery, including implantation of a head-restraint pin and dural EEG electrodes, and for some birds, muting.
Neural recordings began two to three days following the surgery, in the same boxes which housed the birds. Birds were wrapped in a cloth and the head was immobilised, connectors were attached to EEG electrode leads, and a high impedance electrode was lowered into the brain above RA. The box was then closed for the duration of the 8-hour night. Birds were illuminated with infrared light and EEG signals and video were recorded for the duration of the night while single-cell recordings in RA were obtained. Birds experienced normal levels of rapid eye movement (REM) and non-REM sleep as that which has been reported in songbirds previously1,2, and brief periods of wakefulness. RA was identified stereotaxically and by the readily identifiable activity patterns of RA neurones.
After 3.3 ± 1.6 nights in the recording apparatus (range 1 - 6 nights), when sufficient pre-exposure data had been obtained (2.6 ± 1.1 nights of successful recordings, range: 1 - 5 nights), birds were allowed to self-elicit playback of a song model by pulling a string, until PHD 90. Birds were limited to no more than 10 renditions of the tutor song each morning and afternoon training session. Neuronal recordings were collected over 5 - 16 nights (except one bird for which we only succeeded in collecting data for nights - 2, -1 and 1 relative to song exposure).
Because clearly identifiable repeated syllables do not begin to emerge until several days after tutor song exposure onset3, rather than try to cluster song elements in proto-syllables, we examined the EV of all song elements produced to assess daily trends before and after the onset of tutor song exposure. Data collection and analysis used Sound Analysis Pro, Matlab, and in-house software.
Surgical muting
For some birds, a muting procedure4 was performed at the same time as surgery described above. A small opening was made into the intraclavicular air sac. A small fenestra was made in the trachea just rostral to the syrinx and in the bronchi immediately caudal to the syrinx within the intraclavicular air sac. The external opening was sutured closed. This procedure likely eliminates the ability to generate pressure across the syrinx. Birds M1 and M3 were successfully muted but bird M2 continued to vocalise immediately following the surgery (presumably, the vocal tract holes sealed). Both muted birds eventually recovered the ability to sing. On days 9 and 11 post-surgery, M1 and M3 began making very sparse, soft vocalisations composed of simple harmonic stacks of various length. Over the following few weeks, birds developed normal plastic song and both eventually achieved a final copy which showed learning in the range of the non-devocalised birds.
White noise cancellation
White noise was played continuously, during the subjective day, starting on the day before the first night of neural recordings. In order to assess whether birds were singing we employed an active noise cancellation technique5. Briefly, probe auditory signals were played and recorded to estimate the transfer function of the recording chamber. Using the known input to the speaker and the transfer function, the white noise (WN) signal broadcast from the speaker was subtracted from the overall signal reaching the microphone, allowing us to observe song (albeit with low fidelity). Based on this technique we observed that birds WN1, WN2, and WN3 sang frequently while exposed to continuous WN (and they also sang immediately after the WN was turned off). Bird WN4 failed to sing in WN and also only recovered singing four days after WN exposure was terminated.
Song similarity measures
Similarity of a bird’s song to its tutor song was computed as described previously6-8 using Sound Analysis Pro software. Briefly, means and variances of acoustic features, principally duration, pitch, Weiner entropy and mean frequency, were calculated for each syllable produced by each bird. Similarity between a given song and the tutor song a bird heard was computed by a quantitative comparison of this array of acoustic features.
References
- 1.Low PS, Shank SS, Sejnowski TJ, Margoliash D. Mammalian-like features of sleep structure in zebra finches. Proc. Natl. Acad. Sci. U.S.A. 2008;105(26):9081–9086. doi: 10.1073/pnas.0703452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rattenborg NC, et al. Migratory sleeplessness in the white-crowned sparrow (Zonotrichia leucophrys gambelii) PLoS Biol. 2004;2:E212. doi: 10.1371/journal.pbio.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derégnaucourt S, Mitra PP, Feher O, Pytte C, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433:710–716. doi: 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- 4.Pytte CL, Suthers RA. A bird’s own song contributes to conspecific song perception. Neuroreport. 1999;10:1773–1778. doi: 10.1097/00001756-199906030-00027. [DOI] [PubMed] [Google Scholar]
- 5.Leonardo A. Experimental test of the birdsong error-correction model. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16935–16940. doi: 10.1073/pnas.0407870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001;291:2564–2569. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- 7.Derégnaucourt S, Mitra PP, Feher O, Pytte C, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433:710–716. doi: 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- 8.Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim. Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
Footnotes
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265(5172):679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 2.Fenn KM, Nusbaum HC, Margoliash D. Consolidation during sleep of perceptual learning of spoken language. Nature. 2003;425:614–616. doi: 10.1038/nature01951. [DOI] [PubMed] [Google Scholar]
- 3.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427(6972):352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 4.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 5.Peigneux P, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 7.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 8.Qin YL, McNaughton BL, Skaggs WE, Barnes CA. Memory reprocessing in corticocortical and hippocampocortical neuronal ensembles. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1997;352:1525–1533. doi: 10.1098/rstb.1997.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennevin E, Huetz C, Edeline JM. Neural representations during sleep: from sensory processing to memory traces. Neurobiol. Learn. Mem. 2007;87:416–440. doi: 10.1016/j.nlm.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Marler P, Tamura M. Culturally transmitted patterns of vocal behavior in sparrows. Science. 1964;146:1483–1486. doi: 10.1126/science.146.3650.1483. [DOI] [PubMed] [Google Scholar]
- 12.Marler P. A comparative approach to vocal learning: song development in white-crowned sparrows. J. Comp. Physiol. Psychol. 1970;71(2):1–25. Part 2. [Google Scholar]
- 13.Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z. Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- 14.Zeigler HP, Marler PR, editors. Behavioral Neurobiology of Birdsong. Annals of the N.Y. Academy of Sciences; New York, NY: 2004. [Google Scholar]
- 15.Derégnaucourt S, Mitra PP, Feher O, Pytte C, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433:710–716. doi: 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- 16.Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- 17.Low PS, Shank SS, Sejnowski TJ, Margoliash D. Mammalian-like features of sleep structure in zebra finches. Proc. Natl. Acad. Sci. U.S.A. 2008;105(26):9081–9086. doi: 10.1073/pnas.0703452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roper A, Zann R. The onset of song learning and song tutor selection in fledgling zebra finches. Ethology. 2006;112:458–470. [Google Scholar]
- 19.Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001;291:2564–2569. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- 20.Crandall SR, Adam M, Kinnischtzke AK, Nick TA. HVC neural sleep activity increases with development and parallels nightly changes in song behavior. J. Neurophysiol. 2007;98:232–240. doi: 10.1152/jn.00128.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marler P, Peters S. Sparrows learn adult song and more from memory. Science. 1981;213:780–782. doi: 10.1126/science.213.4509.780. [DOI] [PubMed] [Google Scholar]
- 22.Hultsch H, Todt D. Memorization and reproduction of songs in nightingales (Luscinia megarhynchos): evidence for package formation. J. Comp. Physiol. A. 1989;165(2):197–203. [Google Scholar]
- 23.Funabiki Y, Konishi M. Long memory in song learning by zebra finches. J. Neurosci. 2003;23:6928–6935. doi: 10.1523/JNEUROSCI.23-17-06928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonardo A, Konishi M. Decrystallization of adult birdsong by perturbation of auditory feedback. Nature. 1999;399(6735):466–470. doi: 10.1038/20933. [DOI] [PubMed] [Google Scholar]
- 25.Konishi M. In: Perception and Experience. Walk RD, Pick HLJ, editors. Plenum; New York: 1978. pp. 105–118. [Google Scholar]
- 26.Adret P, Margoliash D. Metabolic and neural activity in the song system nucleus robustus archistriatalis: effect of age and gender. J. Comp. Neurol. 2002;454:409–423. doi: 10.1002/cne.10459. [DOI] [PubMed] [Google Scholar]
- 27.Nick TA, Konishi M. Neural song preference during vocal learning in the zebra finch depends on age and state. J. Neurobiol. 2005;62:231–242. doi: 10.1002/neu.20087. [DOI] [PubMed] [Google Scholar]
- 28.Evarts EV, Bental E, Bihari B, Huttenlocher PR. Spontaneous discharge of single neurons during sleep and waking. Science. 1962;135:726–728. doi: 10.1126/science.135.3505.726. [DOI] [PubMed] [Google Scholar]
- 29.Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.