Abstract
Background
Visuospatial ability is a multifactorial process commonly impaired in chronic alcoholism. Identification of which features of visuospatial processing are affected and which are spared in alcoholism, however, has not been clearly determined. We used a global–local paradigm to assess component processes of visuospatial ability and MR diffusion tensor imaging (DTI) to examine whether alcoholism-related microstructural degradation of the corpus callosum contributes to disruption of selective lateralized visuospatial and attention processes.
Methods
A hierarchical letter paradigm was devised, where large global letters were composed of small local letters. The task required identification of target letters among distractors presented at global, local, both or neither level. Attention was either selectively directed to global or local levels or divided between levels. Participants were 18 detoxified chronic alcoholics and 22 age-matched healthy controls. DTI provided quantitative assessment of the integrity of corpus callosal white matter microstructure.
Results
Alcoholics generally had longer reaction times than controls but obtained similar accuracy scores. Both groups processed local targets faster than global targets and showed interference from targets at the unattended level. Alcoholics exhibited moderate compromise in selectively attending to the global level when the global stimuli were composed of local targets. Such local interference was less with longer abstinence. Callosal microstructural integrity compromise predicted degree of interference from stimulus incongruency in the alcoholic group. This relationship was not observed for lateral or third ventricular volumes, which are measures of nonspecific cortical volume deficits.
Conclusion
Global–local feature perception was generally spared in abstinent chronic alcoholics, but impairments were observed when directing attention to global features and when global and local information interfered at stimulus or response levels. Furthermore, the interference-callosal integrity relationship in alcoholics indicates that compromised visuospatial functions include those requiring bilateral integration of information.
Keywords: Alcohol, Alcoholism, Visuospatial, Global, Local, Corpus Callosum, Diffusion Tensor Imaging
Visuospatial Processing is a multifaceted function, commonly impaired in alcoholism (Beatty et al., 1996; Fama et al., 2006; Fein et al., 2006; Munro et al., 2000; Oscar-Berman, 2000; Schulte et al., 2005a, 2006; Sullivan et al., 2000, 2002); for reviews: Oscar-Berman and Marinkovic, 2007; Parsons and Nixon, 1993. Intact visuospatial perception relies on multiple component processes, including an individual’s ability to attend, perceive, and recognize objects at different hierarchical levels (e.g., global level: forest; local level: tree) (Navon, 1977; Robertson and Lamb, 1991). Depending on task requirements, either local features (details or parts) or the global composition of objects needs to be given priority to attend selectively to one or another level of spatial processing. For example, face recognition typically requires perception of the whole face rather than its parts, whereas reading requires attention to individual letters to spell out a word. In support of this depiction of attentional processes, a recent electrophysiological study found that slower processing of complex stimuli, such as faces, in alcoholics (ALC) was associated with prolonged latencies and reduced amplitudes of early visual components (P100 and N170) (Maurage et al., 2007). This suggests that ineffective visuospatial processing starts on a perceptual level and may limit information for further attentional and decisional processing stages. Despite the extensive body of literature on visuospatial impairment in alcoholism (e.g., Beatty et al., 1996; Kim et al., 2003; Polo et al., 2003; Ramachandran et al., 1996; Schulte et al., 2004), which of these processes is affected in chronic alcoholism is not fully established. The few studies that have addressed global–local visuospatial processing (Kramer et al., 1989; Robertson et al., 1985; Wegner et al., 2001) typically report global rather than local processing deficits in chronic ALC (e.g., Kramer et al., 1989; Robertson et al., 1985), but there are exceptions (e.g., Wegner et al., 2001).
Complementing its multifaceted functional characteristic, processing of visuospatial information employs multiple brain systems (Andersen, 1995; Graziano and Gross, 1996). Visuoperception and attention functions are primarily subserved by frontal and parietal networks (Devinsky and D’Esposito, 2003). Global–local perception is marked by cerebral hemispheric specialization, with global information by the right hemisphere and local information by the left hemisphere (Kimchi and Merhav, 1991; Sergent, 1982; Van Kleeck, 1989; for a review). Studies of patients with temporo–parietal brain lesions (Robertson and Lamb, 1991) and electrophysiological studies (e.g., Yamaguchi et al., 2000; Yoshida et al., 2007) have confirmed this right-global and left-local hemispheric specialization. Normal perception requires the integration of these 2 aspects of information, achieved through transfer of information between the hemispheres via the corpus callosum (CC) (Engel et al., 1991; Gazzaniga, 1987, 2000; Gazzaniga et al., 1965; Stephan et al., 2007). White matter microstructure compromise observed with diffusion tensor imaging (DTI) occurs in alcoholism, notably in the CC (Arnone et al., 2006; Chanraud et al., 2007; Pfefferbaum and Sullivan, 2005; Pfefferbaum et al., 2000, 2006) and the extent of compromise was shown to correlate with impairment in visuomotor interhemispheric processing speed (Schulte et al., 2005b) and visuospatial attention (Tuch et al., 2005). Results from studies of split-brain patients have provided further evidence of the importance of the CC in global–local processing (Robertson et al., 1993). Thus, it is reasonable to hypothesize that callosal compromise contributes to the impairment of component visuospatial global–local processes in alcoholism (e.g., Kramer et al., 1989; Robertson et al., 1985; Wegner et al., 2001).
To examine lateralization of perceptual function, Han et al. (2002), using functional magnetic resonance imaging (MRI), compared visual hemifield and central visual field presentation of local and global stimuli and found larger hemispheric asymmetries with central visual field stimulation than with hemifield stimulation. According to their competition hypothesis (Han et al., 2002; Volberg and Hubner, 2004), local and global feature processes compete with each other when both hemispheres have simultaneous access to the same visual information, as in the case of central visual field stimulation. Each hemisphere assigns resources to a given global or local target level, which in turn invokes lateralized activation patterns and hemispheric processing differences (Han et al., 2002, 2003; Heinze and Munte, 1993; Malinowski et al., 2002; Yamaguchi et al., 2000). Further, global–local perception is also mediated by attentional control functions (Banich et al., 2000; Devinsky and D’Esposito, 2003; Schulte et al., 2004), including attentional allocation, interference processing, and response control (Han and He, 2003; Han and Jiang, 2006; Muller-Oehring et al., 2007; Qin and Han, 2007; Yoshida et al., 2007). Perceptual and attentional processes are also disrupted in alcoholism as are frontal and parietal cortical areas subserving these functions (Hommer et al., 2001; Jernigan et al., 1991; Pfefferbaum et al., 1992).
We devised a modification of the global–local task based on the original paradigms of Navon (1977) and Delis et al. (1986) to examine visuoperceptual functions in sober ALC and age-matched controls (CTL). Contemporaneously, the microstructure integrity of the CC was assessed with DTI. We tested the hypotheses that chronic alcoholism would affect component global and local perception and attentional processes involving these 2 levels of perception, and that visuospatial processing would be related to CC integrity in ALC. Because the paradigm permitted examination of directed attention, conflict, and interference based on global versus local information, we could determine the pattern of sparing and impairment of these visuospatial processes in chronic alcoholism and test which processes were affected by regional compromise of callosal fiber structure.
METHODS
Subjects
The study sample comprised 18 detoxified ALC (13 men, 5 women) and 22 normal healthy CTL (12 men, 10 women) (Table 1). The sample was drawn from a larger cohort of subjects from our lab (Pfefferbaum et al., 2006). Only the participants described herein had taken the global–local hierarchical letter test. All subjects received a structured clinical interview for DSM-IV diagnosis (American-Psychiatric-Association, 1994) by trained clinicians to rule out nontarget psychiatric disease. Written informed consent was obtained from all participants and the Institutional Review Boards of Stanford University and SRI International approved the study in accordance with the ethical standards established in the 1964 Declaration of Helsinki. All participants had normal or corrected to normal visual acuity. The ALC and CTL groups did not differ significantly in sex distribution (χ2 = 1.32, p = 0.25), age [t(38) = 0.59, p = 0.56], or handedness [Crovitz score, t(37) = 0.09, p = 0.93;Crovitz and Zener, 1962] 1 (Table 1). ALC had fewer years of education than CTL [t(38) = 3.11, p < 0.004]; on average, however, both groups had an education beyond high school. Groups did not differ in their socioeconomic status (SES) measured with a 2-factor (education and lifetime occupation) scale (Hollingshead and Redlich, 1958) [CTL: 29.3 ± 15; ALC: 33.1 ± 13; t(34) = 0.81, p = 0.43]. Although ALC had lower estimated verbal intelligence [t(31) = 2.08, p = 0.046], both groups had average National Adult Reading Test (NART) IQs. (Nelson, 1982) 2 over 110. As expected, the ALC group consumed significantly more alcohol than CTL in their lifetime [t(38) = 5.79, p < 0.0001]. Alcoholic participants were, on average, abstinent for 427 days (±366 days, range = 28 to 1247 days) before testing. Five ALC and 4 control subjects were smokers (χ2=0.52, p = 0.47). Two ALC met the criteria for cannabis and amphetamine dependence in their past. One had last used amphetamines 3 years and cannabis 8 years prior to study participation; the other person had last used amphetamines a year ago and cannabis more than 3 months prior to study participation.
Table 1.
Demographic Characteristics (mean ± SD, range) and Group Differences for Alcoholics (ALC) and Controls (CTL)
| Age | Handednessa | Education (years) | SESa | NART IQ | Lifetime alcohol consumption (kg) | |
|---|---|---|---|---|---|---|
| ALC (n = 13M, 5F) | ||||||
| Mean | 42.3 | 21.6 | 13.6 | 31.5 | 110.7 | 628.2 |
| SD | 8.6 | 10.6 | 2.3 | 13.4 | 7.0 | 461.6 |
| Range | 29–58 | 14–54 | 9–18 | 11–51 | 101–121 | 40–1547 |
| CTL (n = 12M, 10F) | ||||||
| Mean | 40.7 | 22.0 | 15.8 | 29.3 | 115.4 | 51.6 |
| SD | 8.6 | 11.5 | 2.2 | 15.1 | 5.8 | 71.6 |
| Range | 26–55 | 14–66 | 12–20 | 11–65 | 103–124 | 1–301 |
| p | ns | ns | 0.002CTL > ALC | ns | 0.046CTL > ALC | 0.0001ALC > CTL |
NART, National Adult Reading Test (Nelson and Willison, 1991); SES, socioeconomic status. Higher values correspond to lower SES (Hollingshead and Redlich, 1958).
Handedness was determined by the Crovitz and Zener (1962) scale: right-handedness = 14 to 32, nonright = 33 to 49, and left-handedness = 50 to 70.
Global–Local Paradigm
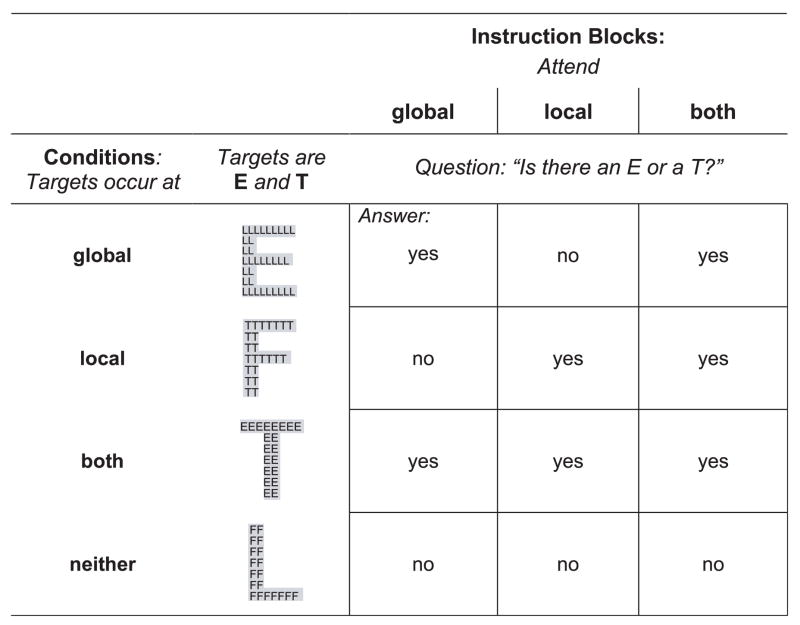
Hierarchical letters were presented in 2 selective attention blocks and 1 divided attention block. In the selective attention blocks, subjects attended either to the global or to the local spatial scale. In the divided attention block, subjects simultaneously attended to both spatial scales. Stimuli were the same for each block; only the attention instruction differed. Stimuli were the letters E, F, L, and T (e.g., a global F made out of local Es) (Fig. 1). Local letters were black; global letters had a light gray background to enhance their salience. Local and global letters were presented superimposed on a white background. The letters E and T were targets, F and L nontargets. There were 4 target conditions: a target letter appeared on the global, local, both levels, or not at all; and 3 attention conditions: attend to the global, local, or both levels (Fig. 1). Subjects answered the question, “Is there an E or T?” by pressing a YES button with the index finger of their dominant hand when a target letter appeared at the attended level, and a NO button with the middle finger of the same hand when a nontarget appeared at the attended level. Stimuli remained on the screen until the subject pressed the button initiating the onset of the next trial. Reaction times (RTs) and errors were collected for each trial. A total of 288 stimuli were presented. Each of the 3 attention blocks comprised 32 stimuli with 8 stimuli in each of the 4 target conditions, and each block was presented 3 times. Total task duration was approximately 9 minutes (3 minutes per block). All subjects performed a practice trial for each attention block before testing. Four global–local processing effects measured were as follows: (1) precedence of spatial level, (2) congruency of information at 2 spatial levels, (3) response conflict from nontarget information at the unattended level, and (4) response facilitation from target information at the unattended level.
Fig. 1. Design of the global–local paradigm.
3 attention blocks (1) attend to the global level, (2) attend to the local level, and (3) attend to both levels with 4 randomly intermixed conditions (1) global targets, (2) local targets, (3) both global and local targets, and (4) neither global nor local targets were repeatedly presented. Target letters were E and T, nontarget letters F and L. Subjects answered the question: Is there an E or a T? by pressing a Yes key for these target letters and a No key for the presence of nontarget letters F and L at the requested attended level (global, local, or both levels). Four specific component process effects were calculated from response times (Difference RTs): (1) Precedence: selective attention (SA) or divided attention (DA) blocks with targets at one level, (a) precedence (SA) = (global targets/attend global) − (local targets/attend local); (b) precedence (DA) = (global targets/attend global + local) − (local targets/attend global + local); (2) Congruency: SA and DA block with targets at both levels: congruency = incongruent trials (e.g., global T, local E) − congruent trials (global E, local E); (3) Response conflict for SA: (a) attention to global: inhibition = (local targets) − (no targets at either level), (b) attention to local: inhibition = (global targets) – (no targets at either level); 4) Response facilitation for SA: (a) attention to global: facilitation = (global targets) − (targets at both level), (b) attention to local: facilitation = (local targets) − (targets at both level).
The precedence effect indicates which level––global or local––was processed faster and calculated for selective and divided attention conditions. For selective attention conditions, mean RTs to local targets in the local attention block were compared with mean RTs to global targets in the global attention block. For divided attention conditions, mean RTs to local targets were compared with mean RTs to global targets in divided attention blocks requiring attention at both target levels.
The congruency effect is indicated by shorter RTs to congruent than incongruent stimuli. When target letters (E, T) appeared at local and global levels, they were either congruent (local E–global E, local T–global T) or incongruent (local E–global T, local T–global E). Moreover, both letters (E and T) required a YES response, i.e., the response to the question “Is there an E or T?” remained the same independent of the congruent/incongruent level information. Congruency effects were calculated for selective (attend global, attend local) and divided attention (attend global + local) conditions.
Response conflict usually occurs for stimuli where information at each level is associated with a different response (see Hubner and Malinowski, 2002). We tested response conflict between information provided from attended and unattended levels. When nontarget letters (F or L) appeared at the attended level, the correct response was NO, even in the presence of a target letter (E or T) at the unattended level. Thus, processing of unattended targets would lead to response conflict. Such conflicting trials were compared with nonconflicting trials, i.e., with nontarget letter (F or L) at either level. Difference in RT between conflicting and nonconflicting trials indexes response conflict elicited by unattended targets and was calculated for selective attention (attend global, attend local) conditions.
Response facilitation indicates faster responses to trials with target letters (E or T) at the attended level and the simultaneous presence of the target letter at the unattended level compared with trials where the nonattended level consists of nontarget letters (F or L). Differences in RT between trials with 2 targets relative to those with only 1 target index the amount of response facilitation elicited by unattended targets. Facilitation effects were calculated for selective attention conditions––for the global attention block by comparing RTs to global targets with RTs to targets at both spatial scales and for the local attention block by comparing RTs to local targets with RTs to targets at both spatial scales.
Stimuli were presented in the center of a 21-inch computer screen. Global stimuli were 9.5 cm high and 7.0 cm wide; local stimuli were 1.0 cm by 0.5 cm. With a subject–monitor distance of approximately 55 cm, global stimuli were ±5.0° visual angle vertically and ±3.5° visual angle horizontally. Local stimuli measured 1.0° by 0.5° visual angle.
Image Acquisition
MRI data were acquired on a 1.5 T General Electric (Milwaukee, WI) Signa human scanner (gradient strength = 40 mT/m; slew rate = 150 T/m/s). Fractional anisotropy (FA) and mean diffusivity (MD) values were based on DTI data from our published report (Pfefferbaum et al., 2007) and were measured on the midsagittal CC Two coronal structural MRI sequences were acquired: (1) a dual-echo fast spin echo (FSE) sequence (47 contiguous, 4 mm thick slices; TR/TE1/TE2 = 7500/14/98 milliseconds; matrix = 256 × 192); and (2) a spoiled gradient recalled echo (SPGR) sequence (94 contiguous, 2-mm thick slices; TR/TE = 25/5 milliseconds, flip angle = 30°; matrix = 256 × 192). All images were 0-filled to 256 × 256 pixels in-plane by the scanner reconstruction software.
DTI data were acquired in the coronal plane with the same slice location parameters as the dual-echo FSE, using a single shot spin- echo echo-planar imaging technique with a 24-cm field of view (47 contiguous, 4-mm thick slices, TR/TE = 10,000/103 milliseconds, matrix = 128 × 128, in-plane resolution = 1.875 mm2). The amplitude of the diffusion-sensitizing gradients was 1.46 Gauss/cm with 32 milliseconds duration and 38 milliseconds separation, resulting in a b-value of 860 s/mm2. Diffusion was measured along 6 noncollinear directions with alternating signs to minimize the need to account for cross-terms between imaging and diffusion gradients (Neeman et al., 1991). For each gradient direction, 6 images were acquired and averaged as were 6 images with no diffusion weighting (b = 0 s/mm2). The coronal MRI and DTI acquisitions produced either 2-mm or 4-mm thick slices and were prescribed for consistent slice locations so that each 4-mm slice encompassed a pair of 2-mm thick slices.
Image Processing
The dual-echo FSE images were passed through the FSL 3 Brain Extraction Tool BET (Smith, 2002) to extract the brain and exclude dura, skull, scalp, and other nonbrain tissue, and the mask was also used to extract the brain from the SPGR data.
Eddy-current-induced image distortions due to the large diffusion-encoding gradients cause spatial distortions in the diffusion-weighted DTI images that vary from 1 diffusion direction to the next. These artifacts were minimized by alignment with an average made of all 12 diffusion-weighted images with a 2D 6-parameter affine correction on a slice-by-slice basis to unwarp the eddy-current distortions in the diffusion-weighted DTI images for each direction. After correcting eddy-current and averaging the multiple acquisitions, the positive and negative directions were combined, and the DTI data were aligned with the FSE data with a nonlinear 3D warp (third-order polynomial), which provided in-plane and through-plane alignment.
Using the averaged images with b = 0 and b = 860 s/mm2, 6 maps of the apparent diffusion coefficient were calculated, each being a sum of 3 elements of the diffusion tensor. Based on the eigen values from the tensor, FA and MD (the mean of the tensor matrix eigen values) were calculated on a voxel-by-voxel basis. Thus, each diffusion-weighted study was reduced to a set of 3 images for each slice (FA, MD, and b = 0) to be used for analysis in conjunction with the anatomical images.
Warping to Common Coordinates
To place the images for all subjects into a coordinate system with a common origin and a standardized anatomical orientation, the anterior commissure and posterior commissure were manually identified on the native 2-mm-thick SPGR images and rotated into a common orientation. The parameters required to accomplish this transformation for each scan session were applied to all of the structural, FA, and diffusion images. All of the datasets were resliced to isotropic 1-mm3 voxels and the field-of-view was set to 20 cm for each axis. Each subject’s SPGR data were then aligned to a grand average laboratory SPGR template with a 12-parameter affine model, followed by creation of a new grand average used as a template for a higher-order (third to fifth polynomial) nonlinear warp. The early echo, dual-echo FSE data for each subject were also aligned to the SPGR grand average with AIR5.2.5 (third to fifth polynomial) nonlinear warp (Woods et al., 1998a,b). The b = 0 images were warped to the native late echo, dual-echo FSE images in 3D, first with a 12-parameter affine, followed by stepwise second and third order polynomial functions. Finally, for each subject all the registration transformation matrices were then combined into one function so that they could be applied only once to native data. This process allowed for anatomical identification of the CC in a common space for each subject from structural or FA images.
Identification of the Corpus Callosum and its Sectors
The CC was identified on the midsagittal slice extracted from the aligned FA data with a semi-automated edge identification procedure with high interrater reliability. Regional callosal areas were defined geometrically, as follows: the CC silhouette was rotated to a plane parallel to the inferior extremes of the rostrum anteriorly and splenium posteriorly. The midpoint along this plane between the anterior extreme of the genu and posterior extreme of the splenium was used as the center of a circle, and radii were projected at +30° and +150° angles relative to the x-axis from the plane, thus dividing the CC into genu + rostrum, body, and splenium (Schulte et al., 2004; Sullivan et al., 2002). FA and MD were expressed as the average values for 5, 1 mm thick, mid and parasagittal slices for each of the 3 callosal sectors. FA was expressed as the fraction of orientational coherence at an intravoxel level (ranging from 0 to 1.0), and MD was expressed as area/time (e.g., 10−6 mm2/s); neither FA nor MD is affected by the intracranial volume of the supratentorium and thus, do not necessitate head size correction (e.g., (Greenberg et al., 2008). Because white matter FA decreases and MD increases with advancing age, we adjusted these DTI metrics in all callosal regions for age based on 120 CTL previously published (Pfefferbaum et al., 2007) and expressed values as age-adjusted Z-scores (mean of the 120 control subjects = 0 ± 1 SD).
Atlas-Based Parcellation of Lateral and Third Ventricle
The third and lateral ventricles and CC were manually identified on a high-resolution, low-noise template brain. The SPGR data from each subject were aligned with the template brain in a 2-step process: a 9-parameter affine transformation followed by nonrigid alignment [multilevel, third-order b-spline, with 5-mm final control point spacing (Rohlfing and Maurer, 2003; Rueckert et al., 1999)]. This registration was then applied to warp the manual parcellation of the template brain (lateral and third ventricles) onto each subject’s native brain, producing subject-specific labeled brain structures (Pfefferbaum et al., 2006). The ventricular data were expressed as age- and intracranial volume-adjusted Z-scores for bilateral volumes of lateral ventricles and the third ventricle.
Statistical Analysis
Analysis of variance (ANOVA) and chi-square tests were used for group (ALC, CTL) and sex (men vs. women) comparisons of demographic data. RT analysis of global and local information processing was based only on correct responses. First, a series of ANOVAs used group and sex as between-subject variables, and the repeated measures component tested for the following 4 specific effects of global–local processing: precedence, congruency, response conflict, and response facilitation. A series of ANCOVAs tested for the influence of education on group differences on global–local specific effects. Second, relationships of these effects with clinical variables (e.g., life-time alcohol consumption, abstinence) and callosal microstructure (FA and MD in genu, body, splenium) were tested with 2-tailed Pearson product moment correlations. Partial correlations explored the contribution of education on significant relationships between global–local effects and callosal microstructure. Third, for significant correlations, the predictive value and regional specificity of callosal microstructure on special effects of global–local processing were tested using linear regression analysis, 1-tailed (SPSS 15.0). Additionally, we tested for differences between correlations of the 2 groups (Walker and Lev, 1953). The alpha level was set to 0.05 for all hypotheses tested.
To estimate the increasing risk of type-1 errors with multiple comparisons, we used a bootstrapping method. Accordingly, we performed 1,000 simulations using our data set, where within each group global–local special effect data were randomized and then used to calculate correlations with callosal FA andMD measures. We randomized the global–local RT measurements as blocks among subjects, thus maintaining the internal correlation structure of the global–local RT measurements (precedence, congruency, response conflict, and facilitation) and leaving the FA and MD measurements with the original subjects, thereby maintaining the correlation structure of FA andMD callosal area measurements. Thus, in these simulations we negated the cross-correlational structure between RT and callosal measurements, so that any statistically significant correlations between global–local RT and FA or MD measurements would be chance occurrences.
RESULTS
Error Frequency
The groups did not differ significantly in the number of errors committed [ALC: 5.1 ± 3, CTL: 5.5 ± 3; t(38) = 0.43, p = 0.67] and showed an overall low error rate, less than 2%. Higher error rate was associated with longer RT in ALC (Rho = 0.46, p = 0.05) but not in CTL (Rho = −0.15, ns). This positive correlation in ALC suggests absence of a speed-accuracy-tradeoff, which would have been indicated with a negative correlation.
Analyses of Specific Global–Local Processing Effects
Of primary interest to this study were the ANOVA effects and interactions involving group. In no case were group-by-sex interactions significant and are thus not further noted.
Precedence: Global Versus Local Levels of Processing
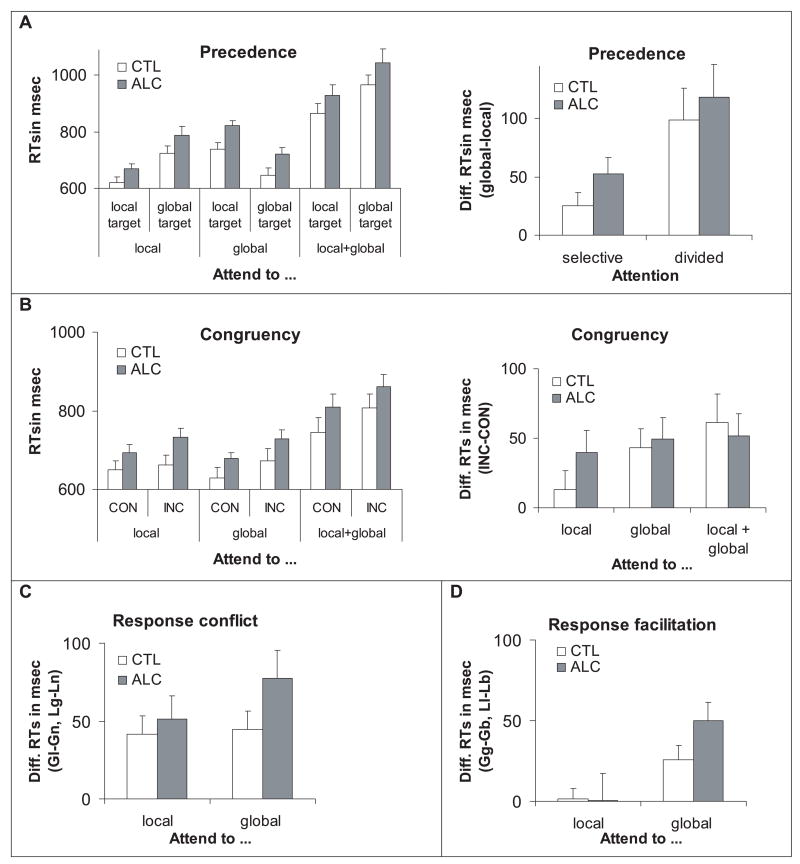
An ANOVA testing for group effects on precedence (global, local) and attention (selective, divided) processing revealed a local precedence effect with faster responses to local than to global targets for both groups [F(1,36) = 48.6, p < 0.001], a group effect with slower responses by ALC than CTL irrespective of attention condition [F(1,36) = 4.36, p = 0.044], and a trend for a group-by-precedence interaction [F(3,36) = 2.95, p = 0.095] (Fig. 2A). Relative to CTL, ALC had disproportionately slower responses to global than local level information [independent sample t-test for global level: t(38) = 2.53, p = 0.016; for local level: t(38) = 1.75, p = 0.09; selective attention]. Both groups showed slower RTs for divided than selective attention [F(1,36) = 250.55, p < 0.0001], which were even slower for global than local targets [F(1,36) = 13.93, p < 0.001] (Fig. 2A).
Fig. 2.
Mean reaction times and standard errors (SE) for (A) precedence, (B) congruency, (C) response conflict, and (D) response facilitation. CTL, controls; ALC, alcoholics. (A): Mean reaction times (RT) and SE to local and global targets under selective (attend to local or global) and divided (attend local + global) attention conditions (left). ALC and CTL show faster RT to local than global targets, indicative for a local precedence effect that was slightly more pronounced in ALC resulting from slower responses to global targets (right). (B): Mean RT and SE to congruent (CON) and incongruent (INC) stimuli under selective (attend to local or global) and divided (attend local + global) attention conditions (left). Both groups show similar congruency effects with longer RTs to INC than CON stimuli under selective and divided attention conditions (right). (C): Both groups show overall similar response conflict, i.e., longer RTs when targets are present at the nonattended level (attend global, local target: Gl, attend local, global target: Lg) than when no targets are present on either level (attend global, no target: Gn; attend local, no target: Ln). When attending to the global level, ALC showed slightly greater response inhibition from local targets than CTL. (D): Both groups showed response facilitation from local targets, i.e., shorter RTs to stimuli with 2 targets (attend global, both levels have targets: Gb) than 1 global target (attend global, global target: Gg) when attending the global level, whereas neither ALC nor CTL showed response facilitation from global targets (attend local, both levels have targets: Lb; attend local, local target: Ll).
Congruency: Targets at Global and Local Levels
Congruent trials (local E–global E, local T–global T) were compared with incongruent trials (local E–global T, local T–global E). Both groups (Fig. 2B) showed a congruency effect, with faster RTs to congruent than to incongruent target levels [F(1,36) = 31.25, p < 0.0001], an attention effect with longer RTs under divided than selective attention conditions [F(1,36) = 38.82, p < 0.0001], and a trend for a congruency-by-attention interaction with greater congruency effects under divided (local + global) than selective (local, global) attention conditions [F(1,36) = 3.12, p = 0.086] for both groups (Fig. 2B).
Response Conflict: Targets at the Unattended Level
Response conflict emerges when the correct response to nontarget letters (F or L) at the attended level is NO, but target letters (E or T) are present at the unattended level. Such conflicting trials were compared with nonconflicting trials, that is, when nontargets (F or L) were present at either level (Fig. 2C). ANOVA yielded a significant effect for response conflict, with faster RTs on trials with nonconflicting than with conflicting information [F(1,36) = 48.4, p < 0.0001] and longer RTs for selective attention at the global than the local level [F(1,36) = 4.54, p = 0.04] for both groups. In these conditions, ALC had longer RTs than CTL [F(1,36) = 4.45, p = 0.042]. ANOVAs with response conflict conducted for each selective attention condition revealed a trend for more conflict from local targets in ALC than CTL [F(1,36) = 3.06, p = 0.089; independent t-test for conflicting trials: t(38) = 2.17, p = 0.036; for nonconflicting trials: t(38) = 1.74, p = 0.09], but failed to reveal any significant group differences for conflict from global targets [F(1,36) = 0.003, p = 0.96].
Response Facilitation: Targets at the Unattended Level
Response facilitation emerges when responses to trials with targets (E or T) at the attended level are speeded by the concurrent presence of targets (E or T) at the unattended level. Such 2-target trials were compared with trials that had 1 target (E or T) at the attended level and nontargets (F or L) at the unattended level. Overall, ALC RTs were slower than those of CTL [F(1,36) = 4.77, p = 0.036] (Fig. 2D). Again, the ALC and CTL showed the same patterns: response facilitation indicated faster RTs on trials with targets at both attended and unattended levels than with 1 target presented at the attended level only [F(1,36) = 11.66, p < 0.002]; selective attention (global, local) effect was marked by slower responses when attention was directed to the global than local level; a facilitation-by-selective attention interaction [F(1,36) = 12.64, p < 0.001] indicated that slowing (i.e., when attention was directed to the global level) was attenuated with an additional target at the local level [F(1,36) = 7.68, p < 0.009]. ANOVAs with response facilitation conducted for each selective attention condition revealed that ALC showed greater facilitation from local targets (i.e., when attention was directed to the global level) than CTL [F(1,36) = 4.20, p = 0.048]. Group differences in response time were attenuated [independent t-test for 1 global target: t(38) = 2.53, p=0.016; for 2 global+local targets: t(38) = 1.75, p = 0.088], but no significant group differences were evident for facilitation from additional global targets (i.e., when attention was directed to the local level) [F(1,36) = 0.25, p = 0.62].
Education Level, Attention Span, Alcohol Consumption, and Abstinence
Groups differed significantly in education but not in SES (Table 1). Nonetheless, education was strongly related to verbal IQ and SES scores (education and NART IQ; ALC r = 0.71, p < 0.004; CTL r = 0.51, p = 0.025; education and SES: ALC r = −0.84, p < 0.0001; CTL r = −0.88, p < 0.0001). To explore the potential effect of education on group differences, we used education as a covariate in each analysis of global–local special effects. Although education was not a significant contributor in any ANCOVA [precedence F(1,35) = 0.04, p = 0.84; congruency F(1,35) = 0.24, p = 0.63; response conflict F(1,35) = 1.45, p = 0.24; response facilitation F(1,35) = 0.39, p = 0.54], adding education as a covariate attenuated group differences in overall RT [precedence F(1,35) = 3.22, p = 0.082; response conflict F(1,35) = 2.06, p = 0.16; response facilitation F(1,35) = 2.95, p = 0.095]. The observed group-by-precedence [F(1,35) = 2.69, p = 0.11] and group-by-response conflict [F(1,35) = 2.91, p = 0.097] interactions were reduced to be nonsignificant statistically, but the group-by-response facilitation interaction endured [F(1,35) = 4.31, p = 0.045].
To test whether possible group differences in attention span affected global–local task performance, we compared mean RTs from the first-third with the last-third of the task. Repeated measures ANOVA with task duration (first vs. last third) as within subjects factor and group (ALC, CTL) as between-subjects factor revealed no group-by-task duration interaction [F(1,38) = 1.09, p = 0.30]. Thus, no evidence was forthcoming to support the possibility that ALC exhibit more attention span difficulties than CTL over the global–local test period of about 9 minutes.
Finally, we tested for effects of alcohol abstinence and amount of lifetime alcohol consumption on global–local performance in ALC. Days of abstinence correlated with response conflict from local targets (global attention) (r = 0.52, p = 0.034) indicating less interference from conflicting local targets with longer abstinence. Multiple regression analysis with days abstinent, lifetime alcohol consumption, and education entered as predictors revealed that these factors explained 40% of the variance of response conflict from local targets [F(3,14) = 3.17, p = 0.058] with days of abstinence contributing independently (t = 2.43, p = 0.029) over the contribution of lifetime alcohol consumption (t = 1.77, p = 0.099) and education (t = 0.79, p = 0.44).
Callosal Microstructure and Global–Local Processing
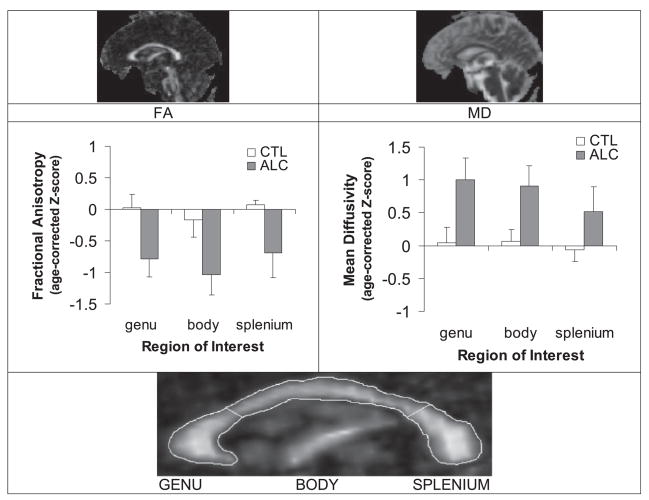
Relative to values adjusted for normal aging, ALC showed total callosal microstructure abnormalities in FA and MD of about 2/3 to 1 SD [group effect: FA F(1,33) = 4.68, p = 0.038; MD F(1,33) = 5.25, p = 0.028] (Fig. 3) that were not specific for any callosal region [no group-by-region interaction: FA F(1,33) = 0.02, p = 0.89; MD F(1,33) = 1.65, p = 0.21]. Also lateral and third ventricle Z-scores were larger in ALC (lateral ventricles: Z = 0.72 ± 2.49; third ventricle: Z = 0.33 ± 0. 92) than CTL (lateral ventricles: Z = −0.19 ± 0.69; third ventricle: Z = −0.05 ± 0.46) but the differences were not significant [lateral ventricles: t(33) = 1.44, p = 0.17; third ventricle: t(33) = 1.68, p = 0.11].
Fig. 3.
Midsagittal fractional anisotropy (FA) image (left), where white matter is the brightest and mean diffusivity (MD) image (right), where CSF is the brightest, of a 61-year-old healthy man. Mean ± SE of the regional FA (left) and MD (right), expressed as age-corrected Z-scores, for controls (CTL) and alcoholics (ALC). ALC showed callosal microstructure deficits evidenced in lower FA and higher MD than CTL. Bottom: Expanded midsagittal view of the FA image of the corpus callosum (CC). The CC (outlined) was identified with a semiautomated procedure. The genu and splenium were determined geometrically and defined the borders of the body.
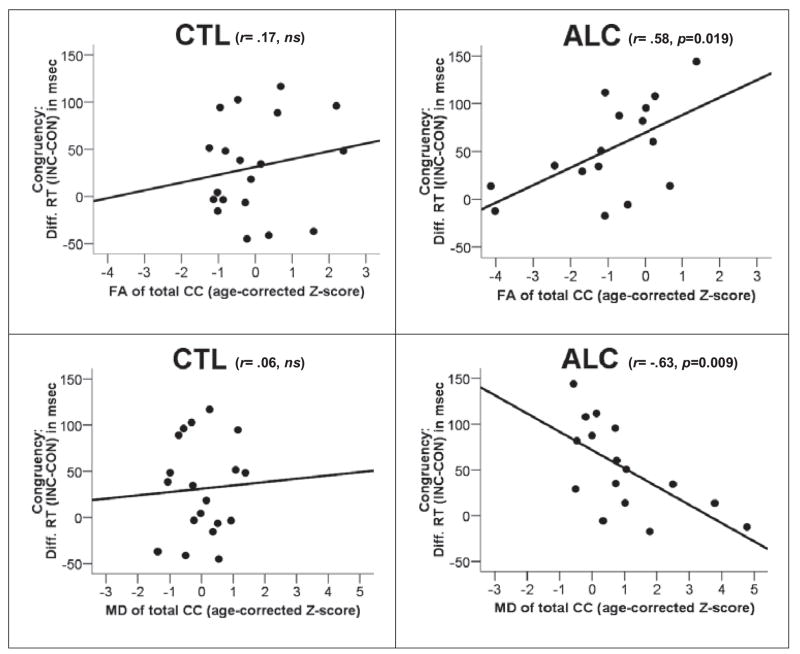
Lower FA and higher MD in the CC correlated with smaller congruency effects in the ALC (Table 2, Fig. 4) under selective, but not divided, attention conditions. Partial correlation controlling for education in each group revealed virtually identical results (Table 2). Bootstrapping analyses indicated that the likelihood of observing these significant correlations by chance was less than 0.3%, and less than 1.2% when the probability calculation also included divided attention trials.
Table 2.
Pearson Correlation Between Behavioral Congruency Effects (Selective Attention) and Regional Microstructural Integrity of the Corpus Callosum, Indexed as High Fractional Anisotropy (FA), and Low Mean Diffusivity (MD) in Alcoholics (ALC), and Controls (CTL)
| Total CC |
Genu |
Body |
Splenium |
|||||
|---|---|---|---|---|---|---|---|---|
| FA | MD | FA | MD | FA | MD | FA | MD | |
| ALC | 0.58* (0.58*) | −0.63** (−0.63**) | 0.61** (0.61*) | −0.67** (−0.65**) | 0.53* (0.52*) | −0.55* (−0.53*) | 0.56* (0.57*) | −0.70** (−0.71**) |
| CTL | 0.17 (0.24) | 0.06 (0.06) | 0.20 (0.28) | −0.06 (−0.06) | 0.16 (0.21) | 0.03 (0.02) | 0.13 (0.16) | 0.32 (0.36) |
| az | −2.14 | 1.38 | −2.0 | −1.77 | 1.37 | −3.24 | ||
| p | ns | 0.02 | 0.085 | 0.025 | ns | 0.04 | 0.085 | 0.001 |
Two-tailed;
p < 0.05;
p < 0.01.
Partial correlation controlling for education are presented in brackets.
We used r to zr transformation [zr = 0.5 loge (1 + r)/(1−r)] (Walker and Lev, 1953) to test whether correlations between congruency and callosal microstructure in ALC were statistically different from those observed in CTL.
Fig. 4.
Correlations between congruency (selective attention) and callosal microstructure indexed by fractional anisotropy (FA) and mean diffusivity (MD) of the total corpus callosum (CC) in controls (CTL) and alcoholics (ALC). An alcohol-specific relationship was observed between callosal microstructure and congruency effects evidenced by significantly higher MD-congruency correlations in ALC than CTL for the total CC and its subregions (genu, body, splenium).
The observed congruency by callosal microstructure correlations differed significantly between ALC and CTL for MD and showed trends for genu and splenium FA (Table 2, Fig. 4). The differences in correlations indicated an ALC-specific relationship between congruency effects (selective attention) and callosal microstructure (MD values).
To estimate the statistical contribution of regional FA and MD to the variance of global–local congruency effects in ALC, we first conducted a series of simple regression analyses with FA andMD for each region (genu, body, splenium) submitted as single predictors. Genu FA explained 38% [F(1,14) = 8.46, p = 0.011], genu MD 45% [F(1,14) = 11.33, p = 0.005], body FA 28% [F(1,14) = 5.55, p = 0.034], body MD 31% [F(1,14) = 6.16, p = 0.026], splenium FA 32%[F(1,14) = 6.54, p = 0.023], and splenium MD 50% [F(1,14) = 13.71, p = 0.002] of the total variance of congruency effects (selective attention). When FA and MD for genu, body, and splenium were entered as simultaneous predictors in a multiple regression analysis, FA for all 3 regions together accounted for 38% but was no longer a significant predictor [F(3,12) = 2.48, p = 0.11], whereas MD for all 3 regions together was the stronger predictor accounting for 53% [F(3,12) = 4.47, p = 0.025] of the total variance of congruency effects in ALC with no region contributing independently over the others.
Finally, we explored whether ventricle size is related to visuospatial global–local performance. In ALC, lateral and third ventricle sizes were not correlated to any global–local performance measure such as overall RT, errors, and global– local special effect (all p-values >0.10). In CTL, larger lateral ventricles correlated moderately with greater local precedence effects (selective attention) (r = 0.46, p = 0.050). However, the observed lateral ventricle by local precedence correlation in CTL was not significantly different from that observed in ALC (r = 0.20) (Z = 0.779, p = 0.22) indicating that ventricle size did not predict global–local precedence processing differently in CTL from ALC.
DISCUSSION
We examined component processes of visuospatial attention with a novel visuospatial paradigm, which assessed processing of global and local features and their competition under selective and divided attention demands in abstinent ALC and matched CTL. ALC were slower but as accurate as CTL in the global–local task, which may represent generalized slowing of processing speed (Davies et al., 2005; Parsons, 1998; Schulte et al., 2005a). These results also demonstrate sparing of general perceptual ability and identification of details (e.g., local features) and global structure in abstinent ALC. When selectively attending to global features under conditions of biasing towards local processing, ALC exhibited moderate compromise. This compromise in global processing reflects difficulty in inhibiting local information. Furthermore, degree of microstructural callosal compromise in ALC correlated with smaller interference effects from incongruent local and global letters at the respective unattended level (selective attention), a relationship that was not observed in healthy CTL.
Precedence Effects––What is Processed Faster, Global or Local Features?
ALC were differentiately affected depending on the attentional demands required for visuospatial processing. When local and global feature processing were directly compared, ALC showed slower global feature processing than CTL that resulted in greater local precedence effects (see Muller-Oehring et al., 2007). The difficulty ALC exhibited in processing global information is consistent with previous reports of a global processing deficit in visuospatial ability in alcoholism (Kramer et al., 1989, 1991; but see Wegner et al., 2001; for acute alcohol effects see: Lamb and Robertson, 1987). If ALC have difficulties perceiving global features, then global feature processing should interfere less with local feature processing, a pattern we did not observe.
Response inhibition, evidenced by longer response times to conflicting than nonconflicting global–local information, was observed in both groups. Our paradigm allowed testing for conflict elicited from global targets independently from conflict elicited from local targets. ALC did not differ from CTL when global features interfered with local feature processing at a response level but did show slightly greater response inhibition from conflicting local targets than CTL, i.e., when attention was directed to the global level. These difficulties in response inhibition are consistent with previous reports in ALC and have been associated with deficits in frontal executive control functions (Cohen et al., 1997; Dao-Castellana et al., 1998; Fallgatter et al., 1998; Lusher et al., 2004; Schulte et al., 2005a; Tedstone and Coyle, 2004). The present results specified the impairment to conflict information at the local level and normal processing of conflicting information at the global level. Thus, local unattended targets were a greater source of interference than global information in ALC as they are in CTL, whereas global unattended targets were processed normally in ALC. We observed the same pattern for response facilitation, evidenced by shorter response times to 2 than 1 target in both groups. ALC did not differ from CTL when additional global targets speeded responses to local targets but did show greater response facilitation from additional local targets than CTL, i.e., when attention was directed to the global level. The observed greater response conflict and facilitation from local targets together with slower global target processing (when attending the global level) in ALC than CTL (which resulted in an enhanced local precedence effect) indicate that ALC exhibit difficulties in focusing attention to the global level. Furthermore, ALC showed normal stimulus-related global–local interference from incongruent targets presented at the nonattended level. That ALC showed normal global interference and response conflict from unattended global targets argues against an alcoholism-specific deficit in global feature perception and for a modification of attentional focus to the global level. Even though this compromise was subtle, it has the potential to affect behavior when attention to the global scene or pattern is important (e.g., focusing on the overall traffic pattern when driving on the freeway during the rush-hour) and inhibition of local information is essential (e.g., the pretty color of an adjacent car). Yet, the finding of less local interference (attention to global) with longer alcohol abstinence likely illustrates recovery of global attention function with continued abstinence.
Microstructural degradation of the corpus callosum was observed in ALC and correlated with compromised performance of lateralized visuospatial functions. Visuospatial– callosal relationships were selectively observed when processing incongruent global–local level information, with equal contribution from both interfering global and local information. In ALC, degree of callosal integrity compromise, as evidenced by low FA and high MD, predicted smaller congruency effects (that is, speeded RT when a target appeared both at the attended and unattended levels). Although congruency effects are generally attributed to early processing stages, such as stimulus perception and identification (posterior system function) (Han et al., 2003; Ridderinkhof and van der Molen, 1995), and to lateralized functions requiring a combination of information from both hemispheres (Robertson and Lamb, 1991; Zhang and Han, 2007), the congruency-CC integrity relationship in ALC was not specific to posterior callosal regions. Although healthy subjects generally recruit posterior brain systems, ALC may recruit additional brain reserves drawing on bilateral fronto-parietal attention systems to achieve normal performance. Such compensatory brain recruitment has been observed in functional MRI studies in ALC who showed the same levels of performance as CTL but invoked bilateral neural systems to achieve normal performance (see, for example, in alcoholism: Desmond et al., 2003; Pfefferbaum et al., 2001; normal age: Cabeza, 2002; Townsend et al., 2006). More demonstrative callosal compromise, however, may attenuate efficient recruitment of bilateral brain reserves and reduce normal interference. An alternative explanation for the congruency-CC integrity relationship is that alcoholic individuals may have reduced gray matter in parietal and frontal regions that contribute to selective attention impairment to perceptual information at a given level (e.g., global). In vivo CT and MRI studies have reported dilated sulci, interhemispheric fissure, or ventricles in chronic alcoholism, which provide indirect evidence for nonspecific tissue shrinkage or alcohol-related cortical volume reduction (Cala and Mastaglia, 1981; Jernigan et al., 1991; Pfefferbaum et al., 1992, 1997). In the current sample, analyses of lateral and third ventricular size as control measures for gross abnormalities demonstrated that the observed relationship between callosal microstructure and global–local congruency effects in ALC cannot be attributed to a gross alcohol-related impairment on brain structure, thereby strengthening the hypothesis that callosal microstructure integrity predicts––at least in part–– visuospatial global–local integration ability in ALC.
In conclusion, our global–local paradigm coupled with assessment of callosal microstructural integrity provides evidence that selective components of visuospatial processing are spared in abstinent chronic ALC, namely, global–local feature perception. Impaired visuospatial global–local processing, however, was observed in ALC when focusing attention to the global level and when irrelevant local information served as a distractor to response selection in conditions of selective, but not divided, attention. Given the cerebral processing bias of local (left hemisphere) and global (right hemisphere) information, efficient integration of hierarchically organized stimuli (local or global) and speeded response selection directed to one or the other level require a fully functional CC. When compromised, however, the CC can adversely affect visuospatial attentional functions requiring integration of information from both hemispheres.
Acknowledgments
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (AA10723, AA12999, AA05965). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health. The authors thank Carla Raassi, B.A., Anne O’Reilly, PhD, Stephanie Sassoon, PhD, Andrea Spadoni, M.A., and Marya Schulte, M.A. for help with recruiting and screening study participants and assistance in data collection; Margaret Rosenbloom, M.A., for comments on the manuscript; and Harold Javitz, PhD, for support in statistics and applying the bootstrapping method to our data.
References
- American-Psychiatric-Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Andersen RA. Coordinate transformations and motor planning in posterior parietal cortex. In: Gazzaniga MS, editor. The Cognitive Neuroscience. MIT Press; Cambridge: 1995. pp. 519–532. [Google Scholar]
- Arnone D, Abou-Saleh MT, Barrick TR. Diffusion tensor imaging of the corpus callosum in addiction. Neuropsychobiology. 2006;54:107–113. doi: 10.1159/000096992. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang ZP, Wright A, Shenker J, Magin R. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cogn Neurosci. 2000;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Hames KA, Blanco CR, Nixon SJ, Tivis LJ. Visuospatial perception, construction and memory in alcoholism. J Stud Alcohol. 1996;57:136– 143. doi: 10.15288/jsa.1996.57.136. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cala LA, Mastaglia FL. Computerized tomography in chronic alcoholics. Alcohol Clin Exp Res. 1981;5:283–294. doi: 10.1111/j.1530-0277.1981.tb04902.x. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological correlates of response production and inhibition in alcoholics. Alcohol Clin Exp Res. 1997;21:1398–1406. [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Feline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Pandit SA, Feeney A, Stevenson BJ, Kerwin RW, Nutt DJ, Marshall EJ, Boddington S, Lingford-Hughes A. Is there cognitive impairment in clinically ‘healthy’ abstinent alcohol dependence? Alcohol Alcohol. 2005;40:498–503. doi: 10.1093/alcalc/agh203. [DOI] [PubMed] [Google Scholar]
- Delis DC, Robertson LC, Efron R. Hemispheric specialization of memory for visual hierarchical stimuli. Neuropsychologia. 1986;24:205–214. doi: 10.1016/0028-3932(86)90053-9. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Devinsky O, D’Esposito M. Neurology of Cognitive and Behavioral Disorders. Oxford University Press; Oxford: 2003. [Google Scholar]
- Engel A, Konig P, Kreiter A, Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991;252:1177– 1179. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Wiesbeck GA, Weijers HG, Boening J, Strik WK. Event-related correlates of response suppression as indicators of novelty seeking in alcoholics. Alcohol Alcohol. 1998;33:475–481. doi: 10.1093/alcalc/33.5.475. [DOI] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Visuoperceptual learning in alcoholic Korsakoff syndrome. Alcohol Clin Exp Res. 2006;30:680–687. doi: 10.1111/j.1530-0277.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2006;30:1538– 1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga MS. Perceptual and attentional processes following callosal section in humans. Neuropsychologia. 1987;25(1A):119–133. doi: 10.1016/0028-3932(87)90048-0. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123(Pt 7):1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Bogen JE, Sperry RW. Observations on visual perception after disconnexion of the cerebral hemispheres in man. Brain. 1965;88:221– 236. doi: 10.1093/brain/88.2.221. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Gross CG. Multiple pathways for processing visual space. In: Inui T, McClelland JL, editors. Attention and Performance XVI: Information Integration in Perception and Communication. MIT Press; Cambridge: 1996. pp. 181–207. [Google Scholar]
- Greenberg DL, Messer DF, Payne ME, Macfall JR, Provenzale JM, Steffens DC, Krishnan RR. Aging, gender, and the elderly adult brain: an examination of analytical strategies. Neurobiol Aging. 2008;29:290–302. doi: 10.1016/j.neurobiolaging.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, He X. Modulation of neural activities by enhanced local selection in the processing of compound stimuli. Hum Brain Mapp. 2003;19:273–281. doi: 10.1002/hbm.10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Jiang Y. Neural correlates of within-level and across-level attention to multiple compound stimuli. Brain Res. 2006;1076:193–197. doi: 10.1016/j.brainres.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Han S, Weaver JA, Murray SO, Kang X, Yund EW, Woods DL. Hemispheric asymmetry in global/local processing: effects of stimulus position and spatial frequency. Neuroimage. 2002;17:1290–1299. doi: 10.1006/nimg.2002.1255. [DOI] [PubMed] [Google Scholar]
- Han S, Yund EW, Woods DL. An ERP study of the global precedence effect: the role of spatial frequency. Clin Neurophysiol. 2003;114:1850–1865. doi: 10.1016/s1388-2457(03)00196-2. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Munte TF. Electrophysiological correlates of hierarchical stimulus processing: dissociation between onset and later stages of global and local target processing. Neuropsychologia. 1993;31:841–852. doi: 10.1016/0028-3932(93)90132-j. [DOI] [PubMed] [Google Scholar]
- Hollingshead A, Redlich F. Social Class andMental Illness. John Wiley and Sons; New York: 1958. [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Hubner R, Malinowski P. The effect of response competition on functional hemispheric asymmetries for global/local processing. Percept Psychophys. 2002;64:1290–1300. doi: 10.3758/bf03194772. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak LS. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15:418– 427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Jeong J, Kim KS, Chae JH, Jin SH, Ahn KJ, Myrick H, Yoon SJ, Kim HR, Kim SY. Complexity changes of the EEG induced by alcohol cue exposure in alcoholics and social drinkers. Alcohol Clin Exp Res. 2003;27:1955–1961. doi: 10.1097/01.ALC.0000100943.83959.1F. [DOI] [PubMed] [Google Scholar]
- Kimchi R, Merhav I. Hemispheric processing of global form, local form, and texture. Acta Psychol (Amst) 1991;76:133–147. doi: 10.1016/0001-6918(91)90042-x. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Blusewicz MJ, Robertson LC, Preston K. Effects of chronic alcoholism on perception of hierarchical visual stimuli. Alcohol Clin Exp Res. 1989;13:240–245. doi: 10.1111/j.1530-0277.1989.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Kaplan E, Blusewicz MJ, Preston KA. Visual hierarchical analysis of Block Design configural errors. J Clin Exp Neuropsychol. 1991;13:455–465. doi: 10.1080/01688639108401063. [DOI] [PubMed] [Google Scholar]
- Lamb MR, Robertson LC. Effect of acute alcohol on attention and the processing of hierarchical patterns. Alcohol Clin Exp Res. 1987;11:243–248. doi: 10.1111/j.1530-0277.1987.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Lusher J, Chandler C, Ball D. Alcohol dependence and the alcohol Stroop paradigm: evidence and issues. Drug Alcohol Depend. 2004;75:225–231. doi: 10.1016/j.drugalcdep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Malinowski P, Hubner R, Keil A, Gruber T. The influence of response competition on cerebral asymmetries for processing hierarchical stimuli revealed by ERP recordings. Exp Brain Res. 2002;144:136–139. doi: 10.1007/s00221-002-1057-1. [DOI] [PubMed] [Google Scholar]
- Maurage P, Philippot P, Verbanck P, Noel X, Kornreich C, Hanak C, Campanella S. Is the P300 deficit in alcoholism associated with early visual impairments (P100, N170)? An oddbal paradigm. Clin Neurophysiol. 2007;118:633–644. doi: 10.1016/j.clinph.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Muller-Oehring EM, Schulte T, Raassi C, Pfefferbaum A, Sullivan EV. Global-local interference is modulated by age, sex and anterior corpus callosum size. Brain Res. 2007;1142:189–205. doi: 10.1016/j.brainres.2007.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro CA, Saxton J, Butters MA. The neuropsychological consequences of abstinence among older alcoholics: a cross-sectional study. Alcohol Clin Exp Res. 2000;24:1510–1516. [PubMed] [Google Scholar]
- Navon D. Forest before trees: the precedence of global features in visual perception. Cognit Psychol. 1977;9:353–383. [Google Scholar]
- Neeman M, Freyer JP, Sillerud LO. A simple method for obtaining cross-term-free images for diffusion anisotropy studies in NMR microimaging. Magn Reson Med. 1991;21:138–143. doi: 10.1002/mrm.1910210117. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Eckhard M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. Vol. 34. NIAAA Research Monograph; Bethesda, MD: 2000. pp. 437–472. [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA. Neurocognitive deficits in alcoholics and social drinkers: a continuum? Alcohol Clin Exp Res. 1998;22:954–961. [PubMed] [Google Scholar]
- Parsons OA, Nixon SJ. Neurobehavioral sequelae of alcoholism. Review Neurol Clin. 1993;11:205–218. [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14(1 Pt 1):7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130(Pt 1):48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, Sullivan EV. Contribution of alcoholism to brain dysmorphology in HIV infection: effects on the ventricles and corpus callosum. Neuroimage. 2006;33:239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24:1214– 1221. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Polo MD, Escera C, Yago E, Alho K, Gual A, Grau C. Electrophysiological evidence of abnormal activation of the cerebral network of involuntary attention in alcoholism. Clin Neurophysiol. 2003;114:134–146. doi: 10.1016/s1388-2457(02)00336-x. [DOI] [PubMed] [Google Scholar]
- Qin J, Han S. The role of parietal cortex in global/local processing of hierarchical stimuli: a transcranial magnetic stimulation study. Neuroreport. 2007;18:1921–1924. doi: 10.1097/WNR.0b013e3282f1c9d2. [DOI] [PubMed] [Google Scholar]
- Ramachandran G, Porjesz B, Begleiter H, Litke A. A simple auditory oddball task in young adult males at high risk for alcoholism. Alcohol Clin Exp Res. 1996;20:9–15. doi: 10.1111/j.1530-0277.1996.tb01035.x. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van der Molen MW. When global information and local information collide: a brain potential analysis of the locus of interference effects. Biol Psychol. 1995;41:29–53. doi: 10.1016/0301-0511(95)05125-t. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR. Neuropsychological contributions to theories of part/whole organization. Cognit Psychol. 1991;23:299–330. doi: 10.1016/0010-0285(91)90012-d. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR, Zaidel E. Interhemispheric relations in processing hierarchical patterns: evidence from normal and commisurotomized subjects. Neuropsychology. 1993;7:325–342. [Google Scholar]
- Robertson LC, Stillman R, Delis DC. The effect of alcohol abuse on perceptual reference frames. Neuropsychologia. 1985;23:69–76. doi: 10.1016/0028-3932(85)90045-4. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Maurer CR., Jr Nonrigid image registration in shared memory multiprocessor environments with application to brains, breasts, and bees. IEEE Trans Inf Technol Biomed. 2003;7:16–25. doi: 10.1109/titb.2003.808506. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE TransMed Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Schulte T, Mueller-Oehring EM, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Differential effect of HIV infection and alcoholism on conflict processing, attentional allocation, and perceptual load: evidence from a Stroop Match-to-Sample task. Biol Psychiatry. 2005a;57:67–75. doi: 10.1016/j.biopsych.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Salo R, Pfefferbaum A, Sullivan EV. Callosal involvement in a lateralized stroop task in alcoholic and healthy subjects. Neuropsychology. 2006;20:727–736. doi: 10.1037/0894-4105.20.6.727. [DOI] [PubMed] [Google Scholar]
- Schulte T, Pfefferbaum A, Sullivan EV. Parallel interhemispheric processing in aging and alcoholism: relation to corpus callosum size. Neuropsychologia. 2004;42:257–271. doi: 10.1016/s0028-3932(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Schulte T, Sullivan EV, Muller-Oehring EM, Adalsteinsson E, Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb Cortex. 2005b;15:1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- Sergent J. The cerebral balance of power: confrontation or cooperation? J Exp Psychol Hum Percept Perform. 1982;8:253–272. doi: 10.1037//0096-1523.8.2.253. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Marshall JC, Penny WD, Friston KJ, Fink GR. Interhemispheric integration of visual processing during task-driven lateralization. J Neurosci. 2007;27:3512–3522. doi: 10.1523/JNEUROSCI.4766-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24:611– 621. [PubMed] [Google Scholar]
- Tedstone D, Coyle K. Cognitive impairments in sober alcoholics: performance on selective and divided attention tasks. Drug Alcohol Depend. 2004;75:277–286. doi: 10.1016/j.drugalcdep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Townsend J, Adamo M, Haist F. Changing channels: an fMRI study of aging and cross-modal attention shifts. Neuroimage. 2006;31:1682–1692. doi: 10.1016/j.neuroimage.2006.01.045. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci U S A. 2005;102:12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kleeck MH. Hemispheric differences in global versus local processing of hierarchical visual stimuli by normal subjects: new data and a metaanalysis of previous studies. Neuropsychologia. 1989;27:1165–1178. doi: 10.1016/0028-3932(89)90099-7. [DOI] [PubMed] [Google Scholar]
- Volberg G, Hubner R. On the role of response conflicts and stimulus position for hemispheric differences in global/local processing: an ERP study. Neuropsychologia. 2004;42:1805–1813. doi: 10.1016/j.neuropsychologia.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Walker H, Lev J. Statistical Inference. Holt; New York: 1953. [Google Scholar]
- Wegner AJ, Gunthner A, Fahle M. Visual performance and recovery in recently detoxified alcoholics. Alcohol Alcohol. 2001;36:171–179. doi: 10.1093/alcalc/36.2.171. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998a;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998b;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Yamagata S, Kobayashi S. Cerebral asymmetry of the “top-down” allocation of attention to global and local features. J Neurosci. 2000;20:RC72. doi: 10.1523/JNEUROSCI.20-09-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Yoshino A, Takahashi Y, Nomura S. Comparison of hemispheric asymmetry in global and local information processing and interference in divided and selective attention using spatial frequency filters. Exp Brain Res. 2007;181:519–529. doi: 10.1007/s00221-007-0948-6. [DOI] [PubMed] [Google Scholar]
- Zhang X, Han S. Global perception depends on coherent work of bilateral visual cortices: transcranial magnetic stimulation (TMS) studies. Sci China C Life Sci. 2007;50:557–565. doi: 10.1007/s11427-007-0067-4. [DOI] [PubMed] [Google Scholar]