Abstract
Background
Chronic alcohol consumption reduces the percentage and number of peripheral natural killer (NK) cells in mice and in humans. The underlying mechanism for these changes is only partly known. We recently found that chronic alcohol consumption inhibits NK cell release from the bone marrow (BM) and that this is associated with a decrease in splenic NK cells. The number of peripheral NK cells is tightly controlled by homeostatic proliferation. It is not known whether this mechanism is initiated in response to the reduction in splenic NK cells, or if so, why the steady state levels of NK cells are not restored.
Methods
To examine this mechanism, female C57BL/6 mice were given 20% w/v alcohol in the drinking water for 3 months. NK cell proliferation and apoptosis were determined before and after treatment with IL-15 alone or combined with its alpha receptor.
Results
Chronic alcohol consumption invoked homeostatic proliferation of splenic NK cells in an attempt to return NK cells to normal levels; however, this did not happen due to enhanced apoptosis of NK cells relative to proliferation. Chronic alcohol consumption decreased IL-15 producing cells in the spleen but not in the BM. The numbers of NK cells in the alcohol-consuming mice returned to normal levels in the spleen and were higher than normal in the BM after 2 daily injections of IL-15; however, the enhanced rate of apoptosis due to alcohol consumption was not decreased in the spleen or BM. Combined IL-15 and IL-15Rα treatment decreased apoptosis of NK cells from alcohol-consuming mice to levels similar to untreated water-drinking mice and greatly increased the percentage and number of NK cells in both the spleen and BM.
Conclusion
Chronic alcohol consumption causes a self-unrecoverable loss of NK cells in the spleen by compromising NK cell release from the BM and enhancing splenic NK cell apoptosis that can be reversed with IL-15/IL-15Rα treatment.
Keywords: IL-15, Alcohol, Natural Killer Cells, Homeostatic Proliferation, Apoptosis
NATURAL KILLER (NK) cells are a small population of lymphocytes that can kill virus-infected and tumor cells without prior immunization (Colucci et al., 2003; Yokoyama and Plougastel, 2003). By producing cytokines and chemokines and interacting with dendritic cells, NK cells also regulate T cell function (Zingoni et al., 2005). Thus, these cells play important roles in innate and adaptive immunity.
More than 90% of NK cells in the periphery originate from the bone marrow (BM); however, a small population of NK cells are derived from the thymus in mice (Di Santo and Vosshenrich, 2006; Kim et al., 2002; Vosshenrich et al., 2006) and the lymph nodes in humans (Farag and Caligiuri, 2006). Most of the peripheral NK cells are found in the spleen, blood, and liver. Like T and B cells, the numbers of peripheral NK cells are tightly controlled, and equilibrium is maintained by homeostatic proliferation. Although the precise mechanism of NK cell homeostatic proliferation is still not completely understood, there is convincing evidence that IL-15 plays an essential role in this process (Ranson et al., 2003b).
IL-15 is essential for NK cell commitment, development, differentiation, maturation, homeostatic proliferation, and survival (Prlic et al., 2003; Ranson et al., 2003b; Vosshenrich et al., 2005). This cytokine is expressed by multiple tissues (Waldmann and Tagaya, 1999), and it activates responding cells via its receptor. The IL-15 receptor (R) is composed of 3 subunits: IL-15Rα, β, and γ chains. IL-2 and IL-15 receptors share the same β and γ chains, and differences in the α chain make the 2 cytokines distinct in their activation of target cells (Waldmann et al., 2001). IL-2 binds the receptor with all 3 chains combined together on the target cell to activate downstream signaling. IL-15 first binds IL-15Rα, which is then trans-presented to the IL-2R β/γ chains on the target cell to induce activation (Dubois et al., 2002). Presence of the α receptor on target cells is not required for IL-15 induced target cell activation. IL-15Rα is synthesized by IL-15-producing cells and primes target cells by binding to IL-15 (Koka et al., 2003, 2004; Sandau et al., 2004). Because most of the IL-15 is bound to the α receptor on the IL-15 producing cell, it is difficult to detect this cytokine in serum or in tissue fluid via ELISA (Waldmann and Tagaya, 1999).
Experiments with IL-15Rα knockout mice showed that the NK cells from these mice survive and proliferate in IL-15Rα+/+ hosts; while, NK cells from IL-15Rα+/+ mice do not survive in IL-15Rα-/- hosts (Koka et al., 2003). This study indicates the importance of trans-presentation of IL-15 by IL-15Rα to NK cell survival and proliferation. This observation is underscored by the fact that exogenous administration of IL-15 has limited effects on NK cell proliferation, while administration of IL-15/IL-15Rα strongly boosts proliferation (Rubinstein et al., 2006; Stoklasek et al., 2006).
Chronic alcohol consumption decreases peripheral NK cells in humans (Perney et al., 2003) and in mice (Pan et al., 2006; Zhang and Meadows, 2005, 2008), and the underlying mechanism is still largely unknown. We recently found that chronic alcohol consumption compromises NK cell release from the BM (Zhang and Meadows, 2008), and this could contribute to decreased numbers of peripheral NK cells. Lymphopenia associated with decreased NK cells will invoke homeostatic proliferation to return NK cells to steady state levels (Jamieson et al., 2004; Prlic et al., 2003). It is not known whether the reduced availability of NK cells from the BM is the stimulus that invokes splenic NK cell homeostatic proliferation or if other events contribute to the loss of peripheral NK cells. Little is known regarding the effects of alcohol on IL-15. Acute alcohol intake inhibits IL-15 mRNA expression (Pruett etal., 2003). The effect of acute and chronic alcohol consumption on IL-15 protein expression, however, especially IL-15 expression on IL-15 presenting cells, is not known.
In this study, we found that chronic alcohol consumption results in a self-unrecoverable splenic NK cell depletion in mice. Although the reduction in NK cells induces homeostatic proliferation, chronic alcohol consumption also increases apoptosis of NK cells in the spleen and BM. This is associated with decreased numbers of IL-15+ cells in the spleen, but not in the BM. In vivo treatment with IL-15 enhances NK cell proliferation in the spleen, and the percentage and number of NK cells return to control levels in alcohol-consuming mice. IL-15 treatment increases the percentage and number of NK cells in the BM. This treatment does not abrogate enhanced NK cell apoptosis associated with chronic alcohol consumption in either the spleen or BM. Combined treatment with IL-15/IL-15Rα further increases NK cells in the spleen and BM and also rescues these cells from the enhanced apoptosis induced by chronic alcohol consumption.
MATERIALS AND METHODS
Animals and Alcohol Administration
Female C57BL/6 mice were obtained from NIH/Charles River laboratories at 6 to 7 weeks of age. Mice were single housed in sterile polycarbonate cages containing sterilized Carefresh paper pulp bedding, and the cages were covered with micro isolator air filter tops. All mice received sterilized Purina 5001 laboratory chow, and sterilized Milli-Q water. One week later, one-half of the mice were given 20% (w/v) alcohol prepared from 95% EverClear™ (Luxco, Inc., St. Louis, MO) as the sole fluid source for the indicated time period as described previously (Blank et al., 1992). Mice were housed under specific pathogen free conditions in the Wegner Hall vivarium, at Washington State University. The vivarium is accredited by AAALAC, and the animal protocol was approved by the Institutional Animal Care and Use Committee at Washington State University.
Antibodies and Reagents
The following antimouse monoclonal antibodies and reagents were used in this study: CD3-FITC (145-2C11), CD3-PE (145-2C11), NK1.1-PerCP-Cy.5.5 (PK136), NK1.1-PE (PK136), CD11c-FITC (HL3), and CD16 (2.4G2), all obtained from BD Pharmingen, San Diego, CA. Streptavidin-PE, Annexin-V-FITC, and Cytofix and CytoPerm reagents were from BD Pharmingen. BrdU was obtained from Sigma, St. Louis, MO. FITC anti-BrdU was from eBiosciences, San Diego, CA. Biotinylated antimouse IL-15 antibody, IL-15Rα-FITC, chimeric recombinant mouse-IL-15Rα/Fc, and recombinant mouse-IL-15 were from R & D Systems, Minneapolis, MN. Throughout this paper NK cells are defined as CD3-NK1.1+ cells.
In Vivo BrdU Administration for Measurement of Homeostatic Proliferation
The procedure for in vivo BrdU administration was described previously (Zhang and Meadows, 2005). Briefly, BrdU was added to sterilized Milli-Q water or 20% alcohol to form a concentration of 0.8 mg/ml. Freshly prepared BrdU-containing water and alcohol were presented ad libitum to mice in lightproof tubes, and the drinking fluid was changed every day. Mice were given the BrdU-containing fluids for 9 days. Then splenocytes were isolated using the methods described below. Antimouse CD16 antibodies were added to block nonspecific binding and then the splenocytes were stained with antimouse NK1.1-PerCP and antimouse CD3-PE for 20 minutes. Cells were washed and fixed with Cytofix and CytoPerm reagents following the manufacturer's instruction. Cells were treated with DNase I at 37°C for 30 minutes, washed with CytoPerm washing buffer, and then stained the cells with anti-BrdU antibody for 30 minutes. BrdU+ NK cells were analyzed by flow cytometry as described below.
Isolation of Splenocytes
The procedure for isolating splenocytes was described previously (Zhang and Meadows, 2005). Briefly, spleens were removed from individual mice and placed in a tissue culture dish containing phosphate buffered saline (PBS) plus 0.1% bovine serum albumin (BSA) (PBS + BSA) and forced through a stainless steel wire mesh screen. The cell suspension was transferred then filtered through a 100 μm cell strainer (BD Falcon™, Franklin Lakes, NJ). The collected cells were washed with ice-cold PBS + BSA, centrifuged at 800 × g and then resuspended in warm PBS + BSA. The cell suspension was layered onto warm Lympholyte-M (Cedarlane Laboratories Limited, Burlington, NC) and centrifuged at room temperature for 20 minutes. Splenocytes were collected from the interface of Lympholyte-M and buffer and then washed with ice-cold PBS + BSA. Total cell number and viability were determined with the Cell Viability Analyzer (Vi-Cell, Beckman Coulter, Fullerton, CA). Cells were resuspended in an appropriate volume of PBS + BSA for further use.
Flow Cytometric Analysis
The analysis followed our previous method (Zhang and Meadows, 2005). Briefly, 1 million cells were treated with an appropriate amount of anti-CD16 monoclonal antibody for 10 minutes to block nonspecific bending. Cells were washed and stained with various antibodies for 15 minutes, washed again, resuspended in FACS buffer (PBS + 0.1% BSA + 0.09% NaN3), and then analyzed with a FACscan cytometer (BD Immunocytometry Systems, San Jose, CA). Data acquisition and analysis were accomplished with CellQuest software (BD Biosciences, Mountain View, CA).
IL-15 and IL-15 Plus IL-15Rα Treatment
The administration of IL-15 alone and IL-15 combined with IL-15Rα followed an established method (Rubinstein et al., 2006). Briefly, recombinant mouse IL-15 was dissolved in sterilized PBS at 1.5 μg/100 μl. The combination of 7 μg recombinant mouse-IL-15Rα/Fc and 1.5 μg recombinant mouse IL-15 was dissolved in 100 μl sterilized PBS. One group of mice was injected intraperitoneally with 100 μl IL-15 daily for 2 days, and splenocytes were isolated 2 days later. Another group was injected once intraperitoneally with IL-15/IL-15Rα, and splenocytes were isolated 3 days later. A control group was injected daily for 2 days with 100 μl PBS. All mice were injected with 2 mg BrdU on day 1, and then given water or alcohol containing BrdU at the concentration of 0.8 mg/ml from day 1 through day 4. We did not include an IL-15Rα only control since it was previously reported that the receptor by itself did not enhance NK cell proliferation (Rubinstein et al., 2006).
Statistical Analysis
The results are expressed as mean ± SD. Data in which 2 groups are compared were analyzed with the statistical programs within Microsoft Excel. Significance between groups was analyzed by 2 tailed Student's t-test. Values were considered significantly different at p ≤ 0.05. The data involving multiple group comparisons in Figs. 5 and 6 were analyzed with the General Linear Models procedure of SAS followed by multiple pair-wise comparisons with the Least Squares method. Values were considered significant at p < 0.05.
RESULTS
Differential Effects of Chronic Alcohol Consumption on Splenic NK Cell Numbers
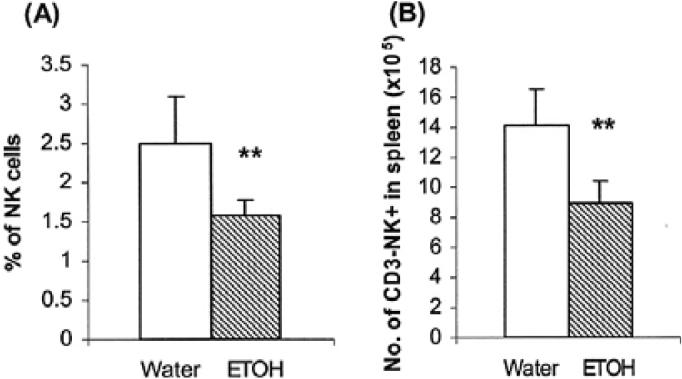
Previously, we found that short-term alcohol consumption (≤2 weeks) decreased the numbers of NK cells in the spleen (Blank et al., 1993). We also showed that mice consuming alcohol chronically for 2 and 6 months exhibited reduced numbers of splenic NK as well as other cell types (Zhang and Meadows, 2005, 2008). In the present study most of the mice used in the experiments consumed alcohol for 3 months. Since we did not previously examine the effect of alcohol consumption at 3 months, we conducted experiments to determine if NK cells were similarly affected. We found that the percentage and number of NK cells in the spleen also were lower at this time point (Fig. 1). These results suggest that the decrease in NK cells from the spleen of the chronic alcohol-consuming mice is persistent and cannot be recovered by homeostatic proliferation, which under normal conditions would correct the NK deficiency (Prlic et al., 2003).
Fig. 1.
Chronic alcohol consumption decreases the percentage and number of NK cells in the spleen. Mice were given alcohol for 3 months. Each group contained 10 mice. CD3-NK1.1+ NK cells were analyzed by flow cytometry. (A) Percentage of NK cells in the total splenic lymphocytes. (B) Number of NK cells in the spleen. **p < 0.001. Values are mean ± SD. The data are from a representative experiment that was repeated 3 times with similar results. ETOH, alcohol-consuming group.
Chronic Alcohol Consumption Induces NK Cells Homeostatic Proliferation
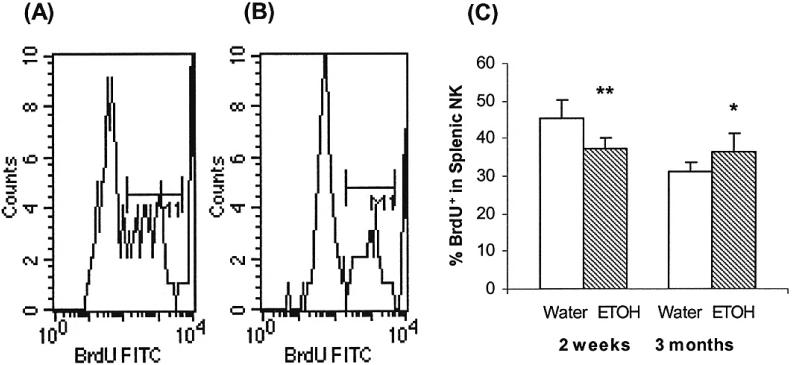
BrdU incorporation experiments were used to examine the effect of short-term and long-term alcohol consumption on NK cell homeostatic proliferation. The results in Fig. 2 indicate that the percentage of BrdU+ NK cells in the total splenic NK cell pool is significantly decreased in mice consuming alcohol for 2 weeks, while the percentage is significantly increased in mice consuming alcohol for 3 months. This indicates that short-term alcohol consumption inhibits NK cell proliferation and that chronic alcohol consumption stimulates NK cell homeostatic proliferation. The major focus of this study is chronic alcohol consumption. Therefore, no further experiments were conducted with short-term alcohol administration.
Fig. 2.
Effects of alcohol consumption on splenic NK cell proliferation. Mice were given alcohol for 2 weeks or 3 months. Each group contained 10 mice. NK cells proliferation was measured by in vivo BrdU incorporation as described in Material and Methods. BrdU+ cells were analyzed by flow cytometry. (A & B) Representative histograms of BrdU+ cells in the gated CD3-NK1.1+ cells in the spleen of mice given water or alcohol for 2 weeks. (C) Percentage of BrdU+ CD3-NK1.1+ cells in the NK cell population from mice consuming water or alcohol for 2 weeks and 3 months. Values are mean ± SD. *p < 0.05, **p < 0.001. The data are from a representative experiment that was repeated twice with similar results. ETOH, alcohol-consuming group.
Chronic Alcohol Consumption Induces NK Cell Apoptosis in the Spleen
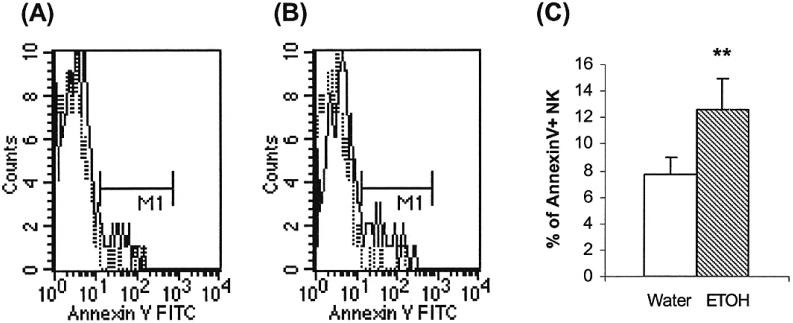
Chronic alcohol consumption stimulates NK cell homeostatic proliferation; however, NK cell numbers did not recover to the levels of water-drinking mice. We hypothesized that NK cells die at a faster rate than the renewal rate, and this was confirmed by the results in Fig. 3. This figure indicates that the percentage of apoptotic cells is 60% higher in NK cells from the alcohol-consuming mice than the water-consuming mice.
Fig. 3.
Chronic alcohol consumption increases NK cell apoptosis in the spleen. Mice were given alcohol for 3 months. Each group contained 10 mice. The apoptosis of CD3-NK1.1+ cells was measured by Annexin V staining and flow cytometry analysis. (A & B) Representative histograms of Annexin V+ cells in gated CD3-NK1.1+ cells isolated from water-drinking mice and alcohol-consuming mice, respectively. (C) Percentage of Annexin V+ cells in gated CD3-NK1.1+ cells. Values are mean ± SD. **p < 0.001. The data are from a representative experiment that was repeated 3 times with similar results. ETOH, alcohol-consuming group.
Chronic Alcohol Consumption Decreases IL-15 Producing Cells in the Spleen
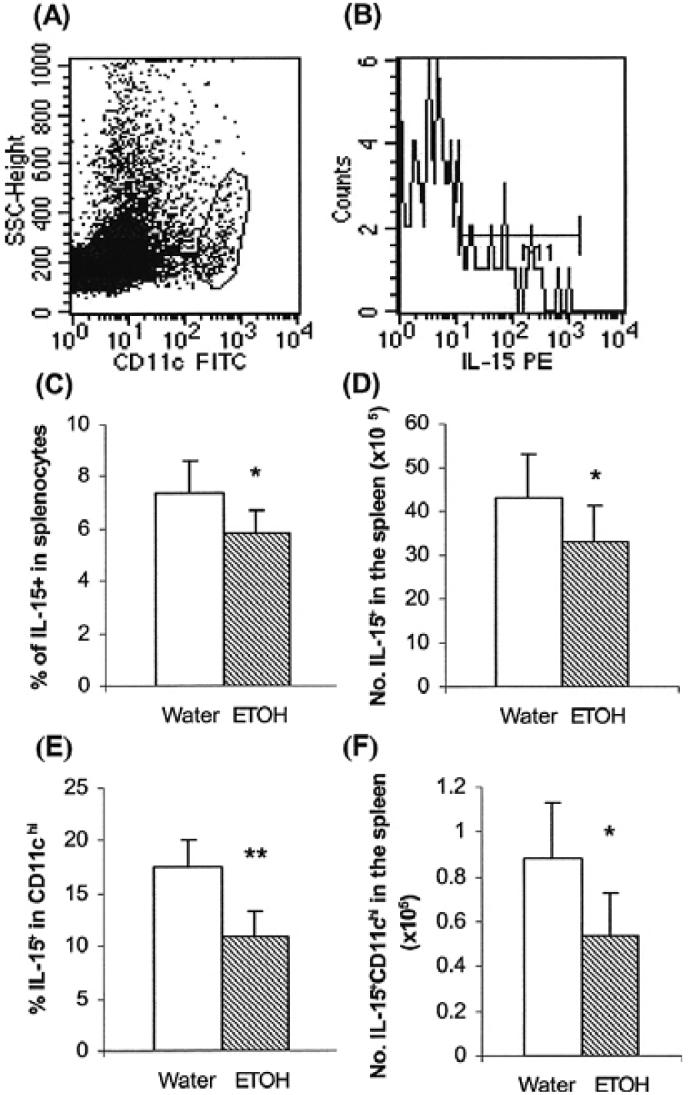
Many growth factors and signaling molecules affect NK cell survival. Among these, IL-15 and the IL-15R signaling system play an essential role in NK cell development and survival (Vosshenrich et al., 2005). We hypothesized that chronic alcohol consumption augmented NK cell apoptosis by inhibiting IL-15 expression or by decreasing IL-15 producing cells. To examine this, we measured the percentage of IL-15 producing cells in the spleen by flow cytometry since it is difficult to detect this cytokine in tissue fluid with conventional ELISA methodology (Waldmann and Tagaya, 1999). CD11chi IL-15-producing dendritic cells are essential for priming NK cells (Koka et al., 2004; Lucas et al., 2007). Thus, we also examined the percentage of IL-15+CD11chi cells in the total splenocyte population. We found that chronic alcohol consumption significantly decreased the percentage and number of IL-15 producing cells in the total splenocyte population and the IL-15+CD11chi dendritic cells in the spleen (Fig. 4). Consistent with the decrease in IL-15+CD11chi cells, we also found that the percentage of IL-15Rα+CD11chi cells was also lower in alcohol-consuming mice as compared to water-drinking mice (13.3 ± 3.1% vs. 18.4 ± 2.5%, p < 0.003).
Fig. 4.
Chronic alcohol consumption decreases IL-15 producing cells in the spleen. Mice were given alcohol for 3 months. Each group contained 10 mice. (A) Dot plot showing the gated CD11chi cells. (B) A representative histogram of IL-15+ CD11chi cells. (C) Percentage of IL-15+ cells. (D)Number of IL-15+ cells. (E) Percentage of IL-15+ CD11chi cells. (F) Number of IL-15+CD11chi cells. Values are mean ± SD. *p < 0.05, **p < 0.001. ETOH, alcohol-consuming group.
IL-15 and IL-15Rα Treatment Rescues NK Cells From Apoptosis Induced by Chronic Alcohol Consumption in the Spleen
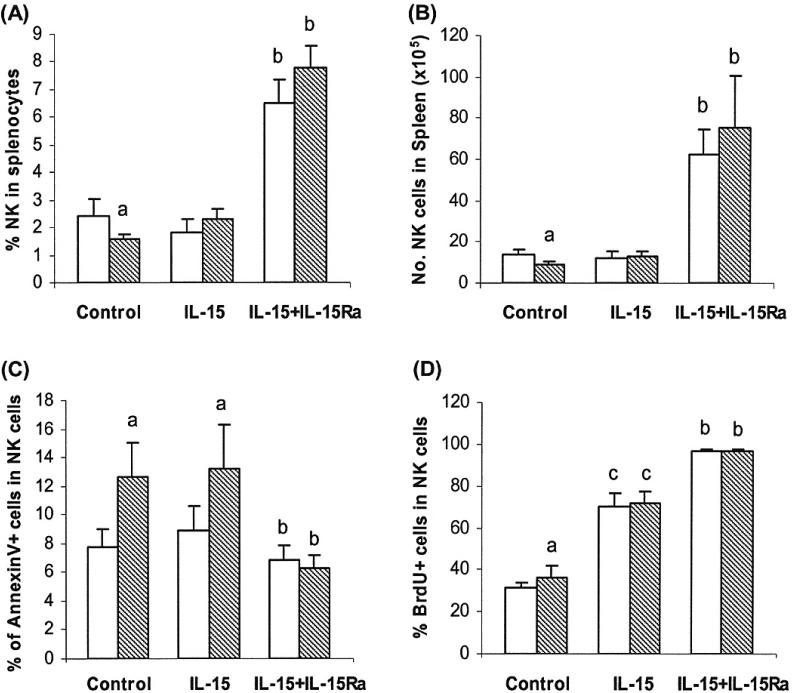
From the results above we hypothesized that the decrease in IL-15 producing cells was associated with the enhanced apoptosis of NK cells and that this related to the decrease in the numbers of NK cells in the spleen. We further hypothesized that exogenous IL-15 would halt the NK loss due to apoptosis. For these experiments we utilized the trans-presentation feature of IL-15 and IL-15Rα. Two groups report that IL-15 binding with IL-15Rα maximizes the effects of IL-15 in vivo (Rubinstein et al., 2006; Stoklasek et al., 2006). We found that IL-15 alone did not significantly alter the percentage and number of NK cells in the spleens from water-drinking mice (Fig. 5A and 5B) compared to the mice without IL-15 administration nor did it change the degree of NK cell apoptosis in the spleens of water-drinking or alcohol-consuming mice (Fig. 5C) compared to the mice without IL-15 treatment. In the alcohol-consuming mice, the percentage and number of NK cells increased to the levels of water-drinking mice after IL-15 treatment (Fig. 5A and 5B). The percentage of BrdU+ NK cells in the spleen increased greater than 2-fold in the water-drinking and alcohol-consuming mice treated with IL-15 (Fig. 5D) compared to the mice without IL-15 treatment.
Fig. 5.
Effects of exogenous IL-15 and combined IL-15 and IL-15Rα treatment on NK cell proliferation and apoptosis in water-drinking and alcohol-consuming mice. Groups containing 5 mice were given water or alcohol for 3 months and then treated with IL-15 alone or IL-15 combined with IL-15RαFc as described in Material and Methods. Values are mean ± SD. (A) Percentage of CD3-NK1.1+ cells in the spleens of treated mice. (B) Number of CD3-NK1.1+ cells in the spleen of treated mice. (C) Percentage of Annexin V+ CD3-NK1.1+ NK cells. (D) Percentage of BrdU+ CD3-NK1.1+ cells. IL-15Ra = IL-15Rα. White bars, water-drinking mice; hatched bars, alcohol-consuming mice. aDifferent from water group, p < 0.05. bDifferent from control and IL-15 treated groups but not from each other, p < 0.05. cDifferent from control and IL-15/IL-15Rα treated groups but not from each other, p < 0.05.
The response of NK cells to combined IL-15 and IL-15Rα treatment was more pronounced than to IL-15 treatment alone. The percentage of NK cells in the spleen increased about 3-fold and the number of NK cells increased 6- to 7-fold for the water-drinking mice and alcohol-consuming mice, respectively. Combined IL-15 and IL-15Rα treatment stimulated almost all the splenic NK cells to proliferate since 98% of the NK cells were BrdU+ (Fig. 5D). The combined treatment decreased the percentage of Annexin V+ apoptotic NK cells in the alcohol-consuming mice to levels not significantly different from the treated water-drinking mice (Fig. 5C).
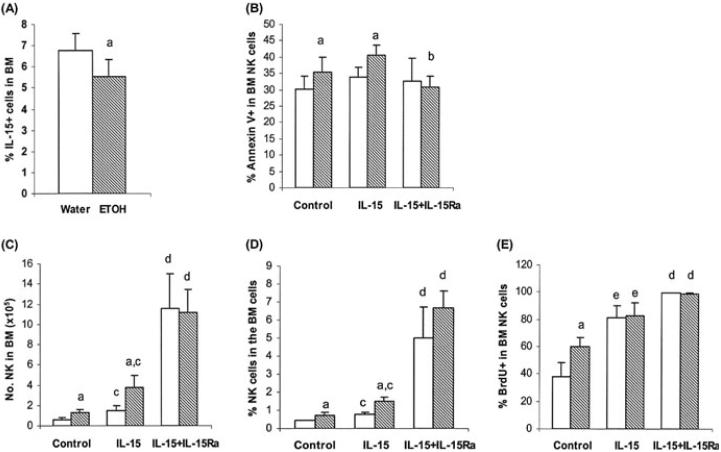
Chronic Alcohol Consumption Does Not Affect the Number of IL-15+ Cells or the Survival of NK Cells in the BM
We found previously that chronic alcohol consumption did not alter the proliferation rate, but compromised the release of NK cells from the BM (Zhang and Meadows, 2008). This suggested that the effects of alcohol consumption on NK cell proliferation in the spleen and BM are different. This is likely due to the availability of “space” in the spleen but not in the BM, which stimulates NK cell proliferation in the spleen. We further examined if chronic alcohol consumption affected IL-15+ cells and the survival of NK cells in the BM. We found that alcohol consumption decreased the percentage of IL-15+ cells in the total BM cell population (Fig. 6A); however, neither the total number of IL-15+ cells nor the percentage and number of IL-15+CD11chi cells were different from water-consuming mice (data not shown). Also, the percentage of IL-15Rα+CD11chi cells was not different between alcohol-consuming mice as compared to water-drinking mice (8.2 ± 2.7% vs. 7.6 ± 1.45%, p = 0.57).
Fig. 6.
Effects IL-15 and combined IL-15 and IL-15Rα on CD3-NK1.1+ cells in the BM. Mice consumed alcohol for 2 to 3 months. Control groups contained 8 to 10 mice, and each treatment group contained 5 mice. (A) Percentage of IL-15+ cells. (B) Percentage of Annexin V+ NK cells. (C) Number of NK cells. (D) Percentage of NK cells. (E) Percentage of BrdU+ NK cells. Values are mean ± SD. IL-15Ra = IL-15Rα. White bars, water-drinking mice; hatched bars, alcohol-consuming mice. aDifferent from water group, p < 0.05. bDifferent from IL-15 treated alcohol group, p < 0.05. cDifferent from respective control and alcohol group, p < 0.05. dDifferent from all control and IL-15 treated groups but not from each other, p < 0.05. eDifferent from all control and IL-15/IL-15Rα treated groups but not from each other, p < 0.05.
Chronic alcohol consumption increased NK cell apoptosis in the BM, but not to the same degree as in the spleen (~15% increase in the BM vs. >50% increase in the spleen). The modest increase in apoptosis of NK cells in the BM was not reflected in depletion of NK cells, and this was most likely due to the compromised release of these cells to the periphery (Zhang and Meadows, 2008). IL-15 treatment alone did not rescue NK cells from enhanced apoptosis related to alcohol consumption (Fig. 6B), and this is similar to the results observed in the spleen (Fig. 5C).
Unlike the spleen, IL-15 treatment increased the percentage and number of NK cells in the BM of the alcohol-consuming and water-drinking mice as compared to respective untreated controls (Fig. 6C and 6D). The percentage and number of NK cells in IL-15 treated alcohol-consuming mice remained higher than similarly treated water-drinking mice (Fig. 6C and 6D). Although combined treatment with IL-15 and IL-15Rα did not alter the basal level of apoptosis in the BM, it rescued NK cells from enhanced apoptosis related to alcohol consumption (Fig. 6B) and it greatly increased the percentage and number of NK cells in the BM to similar levels in both water-and alcohol-consuming mice as compared to control and IL-15 treatment alone (Fig. 6C and 6D). This is likely due to the overwhelming effect associated with enhanced proliferation resulting from the combined treatment.
DISCUSSION
Continuous feeding of alcohol in the drinking water results in loss of NK and other immune cells from the spleen (Blank et al., 1993; Cook et al., 2007; Zhang and Meadows, 2005, 2008; and Fig. 1). The mechanism underlying this sustained decrease in NK cells is not well understood. Generally, the numbers of NK cells in the spleen are tightly regulated by influx of cells from the BM (Kim et al., 2002), to a lesser extent from the thymus (Vosshenrich et al., 2006), and during lymphopenia through induction of homeostatic proliferation (Jamieson et al., 2004; Prlic et al., 2003). Previously, we found that chronic alcohol consumption compromised the release of NK cells from the BM, and that this contributed to the reduction in peripheral NK cells (Zhang and Meadows, 2008). Ordinarily, the decrease in splenic NK cells should be recovered by other homeostatic mechanisms to correct the deficit. Thus, the initial objective of this study was to determine if alcohol consumption impaired NK cell homeostatic proliferation.
We found that alcohol consumption had a biphasic effect on NK cell proliferation. Short-term alcohol administration (2 weeks) inhibited NK cell proliferation and long-term consumption administration (3 months) increased NK cell proliferation (Fig. 2C). The inhibition of proliferation is consistent with previous reports indicating that the proliferative response of NK cells to in vitro mitogenic stimuli is inhibited after 2 weeks of alcohol consumption (Boyadjieva et al., 2002; Gallucci and Meadows, 1996), and also that NK cells are reduced in the spleen (Blank et al., 1993).
The microenvironment of the immune system changes as a result of continuous alcohol administration, and at some point between 2 weeks and 3 months it is likely that the initial depletion of NK cells after 2 weeks of alcohol administration along with the persistent decrease in output of NK cells from the BM (measured from 2 to 6 months) creates a “space” for stimulation of NK cell homeostatic proliferation. The “space” effect is the most important factor (Jamieson et al., 2004) in regulation of homeostatic proliferation. Herein, we found evidence to support that homeostatic proliferation is invoked during chronic alcohol consumption; however, we also found that apoptosis of NK cells was also increased in the spleen (Fig. 3). It is likely that enhanced apoptosis of NK contributes to the sustained depletion of NK cells.
In addition to the “space” effect, homeostatic proliferation is also supported by cytokines. The homeostatic proliferation of NK cells, CD8+ memory T cells, γ/δT cells, and NKT cells is IL-15 dependent (Baccala et al., 2005; Koka et al., 2003; Lodolce et al., 1998), and this cytokine maintains NK cell homeostasis by supporting NK cell survival (Burkett et al., 2004; Jamieson et al., 2004). Moreover, T cells along with NK cells compete for available IL-15 during homeostatic proliferation (French et al., 2005; Ranson et al., 2003a). IL-15 is produced by multiple tissues and cell types. Different types of IL-15 responsive cells could selectively respond to a specific type of IL-15 producing cell (Schluns et al., 2004). For example, nonhematopoietic lung IL-15Rα+ cells are critical to homing of antigen specific CD8+ T cells to the lung during the contraction phase of the immune response, while hematopoietic IL-15Rα+ cells (CD11c+IL-15Rα+) in the lymph nodes and spleen are essential for the homeostatic proliferation of CD44hiCD8+ memory T cells (Sato et al., 2007). It is not known which type of IL-15 producing cell supports NK cell proliferation and survival. However, it appears that both stromal cells and CD11c+ hematopoietic cells are important (Ma et al., 2006). IL-15 expressed by CD11chi cells also is essential to prime NK cells (Koka et al., 2004; Lucas et al., 2007). We found that chronic alcohol consumption decreased splenic IL-15 producing cells, especially the CD11chi IL-15 producing cells (Fig. 4F). These cells also support the homeostatic proliferation and survival of CD8+ T cells and NKT cells. We previously found that chronic alcohol consumption also results in general depletion of lymphocytes in the spleen and that this induces T cell homeostatic proliferation (Zhang and Meadows, 2005). We also determined that CD44hi CD8+ memory T cells and NKT cells, which are highly dependent on IL-15 (Lodolce et al., 1998) also remain depleted after 3 months of alcohol consumption (data not shown). The competition among NK, CD8+T, and NKT cells for available IL-15 could limit the proliferation of NK cells in the spleen of alcohol-consuming mice. Thus, the amount of IL-15 could be insufficient to support proliferation and survival of NK cells. This is consistent with our observation that administration of exogenous IL-15 normalizes the numbers of NK cells in the spleen of alcohol-consuming mice.
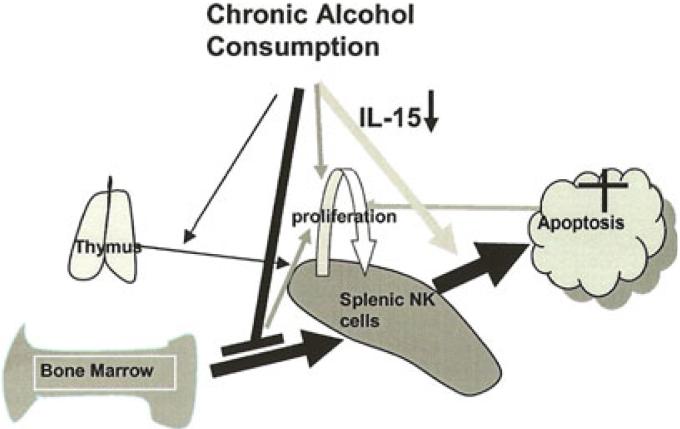
Given all the above interacting factors, we outline a scheme in Fig. 7 of a potential mechanism to explain how chronic alcohol consumption results in a persistent loss in splenic NK cells. Chronic alcohol consumption creates a positive feedback loop for NK cell depletion and homeostatic proliferation in the spleen. Compromised NK cell supply from the BM and enhanced apoptosis of NK cells due to limited availability of IL-15 in the spleen generate a space to stimulate NK cell homeostatic proliferation in the periphery. The decrease in NK cells cannot be recovered by homeostatic proliferation because of continuously enhanced apoptosis and this further drives splenic NK cell homeostatic proliferation.
Fig. 7.
Scheme outlining a mechanism for how chronic alcohol consumption decreases splenic NK cells. At steady state greater than 90% of the splenic NK cells are supplied by the BM. About 5% of the cells in the spleen originate from the thymus (Vosshenrich et al., 2006). Chronic alcohol consumption compromises NK cell release from the BM. Chronic alcohol consumption also enhances NK cell apoptosis in the spleen, which is associated with the decrease of splenic IL-15+ cells, especially IL-15+CD11chi cells. The decrease in splenic NK cells is related to the compromised release of NK cells from the BM and enhanced apoptosis of splenic NK cells. Thymic NK cells do not fill the void (Zhang and Meadows, 2008). The loss of NK cells in the spleen produces a “space,” which drives splenic NK cell homeostatic proliferation. Homeostatic proliferation cannot completely recover the deficiency in NK cells in the spleen due to enhanced apoptosis of these cells. Administration of exogenous IL-15 transiently restores the normal levels of NK cells, but does not alter the enhanced apoptosis induced by alcohol consumption. However, IL-15 in combination with IL15Rα reduces apoptosis of NK cells and also dramatically and equivalently increases the percentage and numbers of NK cells in the spleen.
The limited availability of IL-15 appeared to be a key factor that governs the inability of NK cells to return to their normal levels. In order to further examine this hypothesis, we exogenously administered IL-15 alone or IL-15 combined with its receptor and determined the effect on NK cell proliferation and survival. IL-15 normalized the percentage and number of NK cells in the spleen of alcohol-consuming mice. Chronic alcohol consumption increased the percentage and number of NK cells in the BM. After IL-15 treatment the percentage and number of NK cells in alcohol-consuming mice remained higher than in water-consuming mice. This result is consistent with our previous finding that alcohol consumption inhibits NK cell release from the BM (Zhang and Meadows, 2008). More pronounced effects were observed when IL-15 and Il-15Rα were administered together. The binding of IL-15 to IL-15Rα not only makes IL-15 producing cells a reservoir for IL-15, but also significantly extends the half-life of IL-15 in vivo (Sato et al., 2007; Stoklasek et al., 2006). The half-life of exogenous IL-15 is around 1 hour; whereas the half-life for IL-15 combined with IL-15Rα is about 20 hours (Stoklasek et al., 2006). This could relate to why IL-15 alone is not able to rescue NK cells from the apoptosis induced by chronic alcohol consumption.
In summary, the present study demonstrates that chronic alcohol consumption causes a self-unrecoverable decrease in the NK cells of the spleen. Although the loss of NK cells produces a “space” to stimulate NK cell homeostatic proliferation, intensified apoptosis of NK cells in the spleen during chronic alcohol consumption makes the “space” unfillable. In addition, apoptosis of NK cells could result from reduced availability of IL-15. The fact that chronic alcohol consumption decreases IL-15 producing cells in the spleen, especially CD11chi cells, and that administration of IL-15 combined with IL-15Rα dramatically increases the numbers of NK cells and reduces apoptotic cell death in alcohol-consuming mice in both the spleen and BM, supports these findings. Additionally, the combined treatment also decreased the baseline NK cell apoptosis in the spleen of water-consuming mice. Correcting the IL-15 deficiency induced by alcohol could be a promising new therapeutic intervention to correct in part immune dysfunction in the chronic alcoholic.
ACKNOWLEDGMENTS
This study was supported by NIH grants AA07293 and AA014200.
REFERENCES
- Baccala R, Witherden D, Gonzalez-Quintial R, Dummer W, Surh CD, Havran WL, Theofilopoulos AN. Gamma delta T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J Immunol. 2005;174:4606–4612. doi: 10.4049/jimmunol.174.8.4606. [DOI] [PubMed] [Google Scholar]
- Blank SE, Johansson J-O, Origines MM, Meadows GG. Modulation of NK cell activity by moderate intensity endurance training and chronic ethanol consumption. J Appl Physiol. 1992;72:8–14. doi: 10.1152/jappl.1992.72.1.8. [DOI] [PubMed] [Google Scholar]
- Blank SE, Pfister LJ, Gallucci RM, Meadows GG. Ethanol-induced changes in peripheral blood and splenic NK cells. Alcohol Clin Exp Res. 1993;17:561–565. doi: 10.1111/j.1530-0277.1993.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Boyadjieva NI, Dokur M, Advis JP, Meadows GG, Sarkar DK. Beta-endorphin modulation of lymphocyte proliferation: effects of ethanol. Alcohol Clin Exp Res. 2002;26:1719–1727. doi: 10.1097/01.ALC.0000036925.42090.9D. [DOI] [PubMed] [Google Scholar]
- Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt TJ. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion-absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin Exp Res. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo JP, Vosshenrich CA. Bone marrow versus thymic pathways of natural killer cell development. Immunol Rev. 2006;214:35–46. doi: 10.1111/j.1600-065X.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- French JD, Roark CL, Born WK, O'brien RL. γδ T cell homeostasis is established in competition with αβ T cells and NK cells. Proc Natl Acad Sci U S A. 2005;102:14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci RM, Meadows GG. Ethanol consumption suppresses the IL2-induced proliferation of NK cells. Toxicol Appl Pharmacol. 1996;138:90–97. doi: 10.1006/taap.1996.0102. [DOI] [PubMed] [Google Scholar]
- Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–870. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- Kim S, Iizuka K, Kang H-SP, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- Koka R, Burkett P, Chien M, Chai S, Boone DL, Ma A. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J Immunol. 2004;173:3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, Boone DL, Ma A. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- Pan HN, Sun R, Jaruga B, Hong F, Kim WH, Gao B. Chronic ethanol consumption inhibits hepatic natural killer cell activity and accelerates murine cytomegalovirus-induced hepatitis. Alcohol Clin Exp Res. 2006;30:1615–1623. doi: 10.1111/j.1530-0277.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- Perney P, Portales P, Corbeau P, Roques V, Blanc F, Clot J. Specific alteration of peripheral cytotoxic cell perforin expression in alcoholic patients: a possible role in alcohol-related diseases. Alcohol Clin Exp Res. 2003;27:1825–1830. doi: 10.1097/01.ALC.0000093742.22787.30. [DOI] [PubMed] [Google Scholar]
- Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q. Acute ethanol administration profoundly alters poly I:C-induced cytokine expression in mice by a mechanism that is not dependent on corticosterone. Life Sci. 2003;72:1825–1839. doi: 10.1016/s0024-3205(02)02507-9. [DOI] [PubMed] [Google Scholar]
- Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, Di Santo JP. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci U S A. 2003a;100:2663–2668. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003b;101:4887–4893. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J Immunol. 2004;173:6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci U S A. 2007;104:588–593. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc Natl Acad Sci U S A. 2004;101:5616–5621. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15 Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, Rogge L, Ezine S, Di Santo JP. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, Di Santo JP. Roles for common cytokine receptor gammachain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304–316. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- Zhang H, Meadows GG. Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation. J Leukoc Biol. 2005;78:1070–1080. doi: 10.1189/jlb.0605317. [DOI] [PubMed] [Google Scholar]
- Zhang H, Meadows GG. Chronic alcohol consumption perturbs the balance between thymus-derived and bone marrow-derived natural killer cells in the spleen. J Leukoc Biol. 2008;83:41–47. doi: 10.1189/jlb.0707472. [DOI] [PubMed] [Google Scholar]
- Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. NK cell regulation of T cell-mediated responses. Mol Immunol. 2005;42:451–454. doi: 10.1016/j.molimm.2004.07.025. [DOI] [PubMed] [Google Scholar]