Abstract
BACKGROUND:
To compare the demographics, inpatient mortality and short-term survival following hospital discharge between cocaine-using and non-cocaine-using patients presenting with acute aortic dissection.
METHODS:
Retrospective analysis of 46 consecutive patients admitted with the diagnosis of acute aortic dissection at the Mount Sinai Hospital (Chicago, USA) between 1996 and 2005. Among these 46 patients, cocaine use was temporally related to the presenting symptom in 13 patients (28%, group 1). Patients who were not cocaine users were grouped into group 2 (33 patients [72%]).
RESULTS:
Patients in group 1 were younger than those in group 2 (mean age 38±9 years versus 63±17 years, P=0.001), more likely to be smokers (13 of 13 patients [100%] versus 15 of 33 patients [45%], P=0.001) and had a higher prevalence of accelerated hypertension (mean blood pressure 210/130 mmHg) compared with group 2 (10 of 13 patients [77%] versus 11 of 33 patients [33%]) (P=0.01). Group 1 patients had a higher prevalence of type B dissection than group 2 (nine of 13 patients [69%] versus one of 33 patients [3%]). After hospital discharge, eight of 13 patients (62%) in the cocaine group continued to use cocaine. Mortality following hospital discharge was significantly higher in cocaine users (nine of 13 patients [69%]) compared with the non-cocaine users (four of 33 patients [12%], P=0.01). Recurrent dissection was the cause of death in five of the 13 deaths (42%) in the cocaine group.
CONCLUSIONS:
Patients presenting with acute aortic dissection temporally related to cocaine use are more likely to be younger, smokers, have higher prevalence of hypertensive crises, more likely to have type B aortic dissection and may have a higher mortality following hospital discharge, possibly due to continued cocaine use and recurrent aortic dissection.
Keywords: Aortic dissection, Clinical outcome, Cocaine, Mortality
Abstract
HISTORIQUE :
Comparer la démographie, la mortalité en milieu hospitalier et la survie à court terme après un congé de l’hôpital de patients utilisateurs et non utilisateurs de cocaïne présentant une dissection aortique aiguë.
MÉTHODOLOGIE :
L’analyse rétrospective de 46 patients consécutifs hospitalisés à cause d’une dissection aortique aiguë au Mount Sinai Hospital (de Chicago, aux États-Unis) entre 1996 et 2005. Chez ces 46 patients, l’utilisation de cocaïne était temporellement reliée aux symptômes à la consultation chez 13 patients (28 %, groupe 1). Les patients qui n’étaient pas utilisateurs de cocaïne étaient placés dans le groupe 2 (33 patients [72 %]).
RÉSULTATS :
Les patients du groupe 1 étaient plus jeunes que ceux du groupe 2 (âge moyen de 38±9 ans par rapport à 63±17 ans, p = 0,001), étaient plus susceptibles d’être fumeurs (13 patients sur 13 [100 %] par rapport à 15 sur 33 [45 %], p = 0,001) et avaient une plus forte prévalence d’hypertension accélérée (tension artérielle de 210/130 mmHg) par rapport au groupe 2 (10 patients sur 13 [77 %] par rapport à 11 sur 33 [33 %]) (p = 0,01). Les patients du groupe 1 avaient une plus forte prévalence de dissection de type B que ceux du groupe 2 (neuf patients sur 13 [69 %] par rapport à un sur 33 [3 %]). Après leur congé de l’hôpital, huit des 13 patients (62 %) du groupe prenant de la cocaïne ont continué à en prendre. La mortalité après le congé était considérablement plus élevée chez les utilisateurs de cocaïne (neuf patients sur 13 [69 %]) par rapport aux non-utilisateurs (quatre patients sur 33 [12 %], p = 0,01). Une dissection récurrente était la cause du décès dans cinq des 13 cas (42 %) du groupe utilisateur de cocaïne.
CONCLUSIONS :
Les patients consultant à cause d’une dissection aortique aiguë reliée temporellement à l’utilisation de cocaïne étaient plus susceptibles d’être plus jeunes, d’être fumeurs, d’avoir une plus forte prévalence de crises d’hypertension, de souffrir d’une dissection aortique de type B et de présenter un plus haut taux de mortalité après leur congé de l’hôpital, peut-être imputable à la poursuite de l’utilisation de cocaïne et à une dissection aortique récurrente.
In 2002 and 2003, almost six million (2.5%) Americans 12 years of age or older had used cocaine within the previous year, and more than 2.1 million (0.9%) had used cocaine within the previous month (1). The Substance Abuse and Mental Health Service Administration estimates that the rates of emergency department visits per 100,000 population were higher for cocaine (n=125,921) than for any other illicit drug (2). The Drug Abuse Warning Network and local or state mortality data for 2003 and 2004, respectively, show that cocaine-related deaths tend to exceed those for other drugs in 13 community epidemiology work group areas (3).
Cocaine use is associated with a variety of cardiovascular manifestations, including hypertension, myocardial infarction and coronary ischemia, coronary vasospasm, arrhythmia and cardiomyopathy (4). Approximately five million people annually undergo evaluation in emergency departments in the United States for acute chest pain (5,6). The proportion of these patients who present with chest pain related to cocaine use is difficult to ascertain (7). A patient presenting with a chest pain syndrome following cocaine use poses a diagnostic challenge because of the unreliability of the electrocardiogram in diagnosing myocardial infarction (8–10), a low index of clinical suspicion of aortic dissection due to younger age, imprecise clinical history and patient noncompliance (11).
Acute aortic dissection is an uncommon but important differential diagnosis of a chest pain syndrome, because if undiagnosed and untreated, the mortality rate is 25% within 24 h. More than 50% of patients die in the first week, 75% die by the first month and 90% die within the first year (12). The purpose of the present study was to determine the demographics, inpatient mortality and short-term survival following hospital discharge of cocaine users presenting with acute aortic dissection, and to compare them with patients with aortic dissection who do not use cocaine.
METHODS
Study population
Retrospective analysis of 46 consecutive patients hospitalized with the ICD-9 code diagnosis of aortic dissection (441.0 – dissection of aorta; 441.00 – aortic dissection, unspecified site; 441.01 – aortic dissection, thoracic aorta; 441.02 – aortic dissection, abdominal aorta; and 441.03 – aortic dissection, thoracic-abdominal aorta) between 1996 and 2005 at an inner-city teaching hospital Mount Sinai Hospital (Chicago, USA) was completed. Patients with aortic dissection secondary to trauma were excluded. The data were collected by trained chart abstractors and independently verified by two physicians using the Stanford criteria for aortic dissection (12). Admission and inpatient data were collected by medical record review, while follow-up data were collected by ambulatory chart review and telephone survey. Information was recorded on patient age, sex, ethnicity, cocaine use (route), cardiovascular risk factors (including history of hypertension, cigarette smoking and diabetes), presentation (symptoms and blood pressure at presentation), type of dissection, in-hospital mortality, continued cocaine use and all-cause mortality after hospital discharge. Among 46 patients identified, cocaine use was temporally related to symptom onset in 13 of 46 patients (28%) (group 1; ‘crack’ cocaine use in 11 of 13 patients and powder cocaine use in two of 13 patients). Patients in whom the aortic dissection was not associated with cocaine use were placed into group 2 (33 of 46 patients [72%]). Table 1 lists the patient demographics of groups 1 and 2. Patients who continued to use cocaine in group 1 were labelled group A (eight of 13 patients [62%]), and those who did not were labelled group B. The demographics and clinical outcomes of patients in the cocaine group who did and did not continue to use cocaine after hospital discharge (subgroups A and B) are described in Table 2.
TABLE 1.
Demographics of the study population
| Cocaine users (group 1, n=13) | Non-cocaine users (group 2, n=33) | P | |
|---|---|---|---|
| Age, years (mean ± SD) | 37.8±96 | 62.9±17 | 0.001 |
| Male sex, n (%) | 9 (69.2) | 16 (48.5) | NS |
| African-American, n (%) | 12 (92) | 32 (97) | 0.49 |
| Prior hypertension, n (%) | 9 (69.2) | 22 (66.7) | NS |
| Cigarette smoking, n (%) | 13 (100) | 15 (45.5) | 0.001 |
| Diabetes mellitus, n (%) | 0 | 8 (24.2) | NS |
| Marfan syndrome | 0 | 0 | NA |
| Bicuspid aortic valve | 0 | 0 | NA |
| Prior aortic surgery, n (%) | 0 | 2 (6.1) | NS |
| Type A dissection, n (%) | 4 (30.8) | 32 (97) | 0.001 |
| Type B dissection, n (%) | 9 (69.2) | 1 (3) | 0.001 |
| Accelerated hypertension, n (%) | 10 (76.9) | 11 (33.3) | 0.01 |
| Myocardial ischemia, n (%) | 6 (46.2) | 7 (21.2) | NS |
| Myocardial infarction, n (%) | 0 | 5 (15.2) | NS |
| Inpatient mortality, n (%) | 1 (7.7) | 4 (12.1) | NS |
| Outpatient mortality, n (%) | 9 (75) | 4 (13.7) | 0.01 |
P<0.05 was considered to be significant. NA Not applicable; NS Not significant
TABLE 2.
Demographics of patients who reported past cocaine use
| Continued cocaine use after discharge (group A, n=8) | No cocaine use after discharge (group B, n=4) | |
|---|---|---|
| Age ≤40 years | 4 (50) | 4 (100) |
| Male sex | 7 (87.5) | 2 (50) |
| African-American | 8 (100) | 3* (75) |
| History of hypertension | 6 (75) | 2 (50) |
| History of smoking | 8 (100) | 4 (100) |
| Accelerated hypertension | 6 (75) | 3 (75) |
| Recurrent aortic dissection | 5 | 0 |
| All-cause mortality | 6 (75) | 3 (75) |
Values shown are number of patients (%).
One patient in group B was Hispanic
Diagnoses were confirmed using transesophageal echocardiography, magnetic resonance imaging or computed tomography at the discretion of the treating emergency room physician in consultation with a cardiovascular specialist. History on cocaine use was obtained from each patient. In group 1, a urine drug screen was positive for seven of 13 cocaine-related patients and was not measured in the remaining six. In the non-cocaine group, three patients were tested, and all were negative. In subgroup A, a urine drug screen was positive for two of eight patients and was not measured in the remaining six patients. In subgroup B, none of the patients were tested for cocaine use and exclusion was based on history alone. The local institutional review board approved the present study.
Statistical analysis
Data analysis was performed using SPSS statistical analysis software (version 10; SPSS Inc, USA). Differences in continuous variables were analyzed by independent sample t tests. Categorical variables were analyzed by χ2 cross tabulations. A logistic regression analysis was performed using a set of variables to assess each as an independent predictor of mortality. Mortality data were assessed using Kaplan-Meier survival analysis. All P values were two-sided, and P<0.05 was considered to be significant.
RESULTS
All patients in the cocaine group reported a history of cocaine use within 48 h, whereas none of the patients in the non-cocaine group reported any use in the past. The mean interval between cocaine use and the onset of chest pain or other symptoms could not be precisely determined due to a lack of documentation in hospital records. Nine of 13 patients in the cocaine group (69%) had hypertension, and six of nine patients (67%) were not compliant with their antihypertensive medication. Patients in group 2 diagnosed with myocardial infarction (five of 33 patients [15%]) had native coronary thrombosis as documented by coronary angiography. Following hospital discharge, eight of 13 patients (62%) in the cocaine group continued to use cocaine.
All-cause mortality following hospital discharge was higher in group 1 (nine of 13 patients [70%]) compared with group 2 (four of 33 patients [12%]) (P=0.01). Patient demographics are listed in Table 1. The demographics and clinical outcomes of patients in the cocaine group who did (group A) and did not continue (group B) to use cocaine are described in Table 2. Recurrent dissection (defined as recurring aortic dissection within six months of the first episode) (13) was the cause of death in five of the 13 deaths (38%) in the cocaine group. All five who died of the recurrent dissection were those patients who continued to use cocaine (Table 2). Accelerated hypertension was defined as a systolic blood pressure of 210 mmHg or higher and a diastolic blood pressure of 130 mmHg or higher accompanied by headache, blurred vision or focal neurological symptoms (13).
A logistic regression analysis was performed using a set of variables to assess each as an independent predictor of mortality among cocaine users. It was observed that cocaine use was the strongest predictor (P<0.001), followed by male sex (P<0.011) and history of a hypertensive crisis (P<0.02). Age was not a predictor of mortality in cocaine users (P not significant). A trend was observed, in that smoking, history of prior hypertension and a prior aortic dissection were predictors of mortality, although P was not significant.
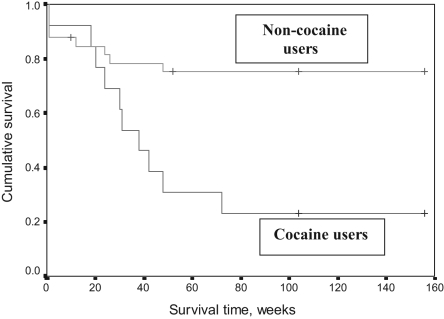
The difference between groups in survival times was analyzed by the Kaplan-Meier method; the result is graphically presented in Figure 1. Patients were followed up for three years, and the mean follow-up time was 25 months (median 24 months, range two to 36 months). Figure 1 shows patient survival as a function of time. Most of the deaths occurred during the first year of follow-up. In the non-cocaine group, eight of the 33 patients died during the first year, resulting in a 75% cumulative survival. No deaths occurred beyond one year in this group. The mortality rate was much higher among cocaine users, resulting in a much smaller proportion of patients who survived for one year (31%). By the end of the 72nd week, the cumulative survival was 23% in the cocaine group, with no further fatalities beyond 72 weeks. The Kaplan-Meier estimate of the mean survival time among cocaine users of 61±15 weeks (95% CI 31 to 91 weeks) was significantly shorter than the mean survival time of 121±11 weeks in non-cocaine users (95% CI 100 to 142 weeks) (P<0.005).
Figure 1).
Survival following aortic dissection
DISCUSSION
Our study demonstrates that in an inner-city environment, patients presenting with acute aortic dissection following cocaine use are younger, more likely to be smokers and have a higher prevalence of hypertensive crises. They are also more likely to have type B aortic dissection and a higher risk of mortality following hospital discharge, probably due to continued cocaine use and recurrent aortic dissection.
Previous studies
Cocaine use has been known to be associated with cardiovascular complications, including myocardial ischemia and coronary thrombosis, as well as acceleration of the development of atherosclerosis, hypertension, cardiac arrhythmia, cardiomyopathy and endocarditis. The association of cocaine use with acute aortic dissection and the causal relationship of cocaine use with acute aortic dissection are not well defined (4,10,14). Several case reports of aortic dissection have been reported (15–19), but the article by Hsue et al (20) was the only study that clearly linked cocaine use and aortic dissection (20). Hsue et al noted that cocaine, particularly crack cocaine, had a definitive role in precipitating aortic dissection among a cohort of young, predominantly African-American and hypertensive individuals. In their study, and in the previously reported case reports, the pattern of dissection (Stanford type A versus type B) was similar among cocaine users and non-cocaine users (15–20).
Our findings suggest a clear distinction between the two groups. We noted that presenting cocaine users had a statistically significantly higher likelihood of suffering from type B dissection than non-cocaine users.
The present study also suggests that more than two-thirds of patients with cocaine-related aortic dissection continue to use cocaine following hospital discharge. This was likely the cause of repeated admissions for recurrent aortic dissection and, eventually, death in this cohort of patients. This finding is consistent with those of previous case reports (10,16).
Pathophysiology
The aortic arch joins the descending part of aorta at the aortic isthmus. This part of aorta is particularly vulnerable to trauma, because at this junction, the ascending aorta and the aortic arch become relatively fixed to the thoracic cage by the pleural reflections, the paired intercostal vessels and the left subclavian artery (12). Thus, cocaine may induce acute hemodynamic stress particularly at this junction.
Cocaine acts as a powerful sympathomimetic agent (14). It blocks the presynaptic reuptake of noradrenaline and dopamine, producing high levels of these neurotransmitters at the postsynaptic receptors (4).
In the present study, nine of 13 patients in the cocaine group (69%) had hypertension, and six of nine patients (67%) were not compliant with their antihypertensive medication. Cocaine produces a dose-dependent increase in blood pressure and heart rate, and may therefore increase the risk of aortic dissection, especially if baseline arterial pressure is elevated (4,21,22).
Cocaine also induces vasoconstriction through synaptic stimulation and endothelin release (21). Cigarette smoking has been reported to increase vasoconstriction associated with cocaine use (21). All 13 patients in the cocaine group were smokers.
The pathophysiology of acute aortic dissection in patients following cocaine use is therefore likely to be multifactorial, involving the effects of systemic hypertension and cigarette smoking, as well as cocaine-induced progression of atherosclerosis, medial necrosis and acute hemodynamic shear stress.
Pharmacology of cocaine
Cocaine (benzoylmethylecgonine, C17H21NO4) is an alkaloid extract from the leaf of the Erythroxylon coca plant, which is usually grown in South America (14). It is available in hydrochloride salt and freebase (‘crack’ cocaine) forms. Due to its rapidity of onset and potency, crack cocaine and its hemodynamic effects are well suited to precipitate acute aortic dissection. Indicator data reported by National Forensic Laboratory Information System for 2004 showed that crack cocaine accounted for one-half or more of primary cocaine treatment admissions in 17 community epidemiology work group areas (3). In our study, crack cocaine was the form used by 11 of 13 patients, and powder cocaine was used by two of 13 patients at the time of admission.
Thus, in the present study, the possible pathophysiological mechanism for type B aortic dissection being more commonly observed in young cocaine users may be explained by the anatomical vulnerability of the aortic isthmus region, as mentioned above, as well as the superimposed acute hemodynamic stress induced by crack cocaine.
Susceptible subgroups
The most common predisposing factor for acute aortic dissection is systemic hypertension (12). African-American patients tend to have more prevalent and severe hypertension, which can be compounded by the hemodynamic effects of cocaine and the resultant precipitation of acute aortic dissection. The majority of patients in the present study were African-Americans, who presented with significantly more hypertensive crises in the cocaine group than in the than in the non-cocaine group.
In our study group, the average age of cocaine users was 38 years, and 52% of crack cocaine users were women. These results are consistent with a survey performed in 2002, which showed that the average age at admission among crack cocaine users was 37 years, 41% of whom were female (23). Also, we found no significant difference in inpatient mortality between the two groups. In a study by Hsue et al (20), the conclusion was similar, suggesting that the two groups of patients behaved similarly in a hospitalized setting.
Although these data are not statistically significant because of the small number of patients in the cocaine group, the data clearly indicate that there is a higher mortality rate among patients who continue to use cocaine after discharge (six of eight patients in group A; Table 2). A review of literature suggests higher long-term mortality rates among those with continued exposure to cocaine (10,16).
We recommend that patients with acute aortic dissection who use cocaine should be routinely referred for substance use treatment, because there is an increased likelihood of aortic dissection and other cardiovascular complications associated with continued cocaine use.
Study limitations
The retrospective design and the lack of systematic and focused data collection relating to cocaine use is a limitation of the present study. As mentioned before, a substantial percentage of subjects who had recently used cocaine denied doing so when asked about it in the emergency department. It seems reasonable, then, that some cocaine users during this 10-year observation period were undetected. In one study of patients with chest pain who were examined in emergency departments, only 72% of those with positive urine tests for cocaine admitted to cocaine use within the preceding week (7). The current study was conducted in an inner city hospital that serves a predominantly African-American population. Therefore, these data may not be applicable to patients of other racial and ethnic backgrounds. While the outcome data are striking, inferences should be made with caution.
CONCLUSIONS
Our findings suggest that crack cocaine is a common precipitating factor of acute aortic dissection in an inner-city population and should be considered as a diagnosis in cocaine users who have severe chest pain. The typical patient with cocaine-related dissection tends to be younger and is more likely to be African-American.
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration. 2003 National Survey on Drug Use & Health: Results. <www.oas.samhsa.gov/nhsda/2k3nsduh/2k3Results.htm> (Version current at August 29, 2007).
- 2.Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2003: Interim national estimates of drug-related emergency department visits. <www.drugabusestatistics.samhsa.gov/DAWN/2k3interimED.pdf> (Version current at August 29, 2007).
- 3.National Institute on Drug Abuse. Epidemiologic trends in drug abuse – advance report. 2005. <www.drugabuse.gov/PDF/CEWG/AdvReport605.pdf> (Version current at August 29, 2007).
- 4.Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345:351–8. doi: 10.1056/NEJM200108023450507. (Erratum in 2001;345:1432). [DOI] [PubMed] [Google Scholar]
- 5.National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey: 1995 emergency department summary. Advanced data from vital and health statistics. <www.cdc.gov/nchs/products/pubs/pubd/ad/290-281/ad285.htm> (Version current at August 29, 2007).
- 6.Haberer J, Waters D. “Chest pain” in patients who use cocaine. In: Hurst JW, Morris DC, editors. Chest Pain. Armonk: Futura Publishing Co; 2001. pp. 307–16. [Google Scholar]
- 7.Hollander JE, Todd KH, Green G, et al. Chest pain associated with cocaine: An assessment of prevalence in suburban and urban emergency departments. Ann Emerg Med. 1995;26:671–6. doi: 10.1016/s0196-0644(95)70035-8. [DOI] [PubMed] [Google Scholar]
- 8.Gitter MJ, Goldsmith SR, Dunbar DN, Sharkey SW. Cocaine and chest pain: Clinical features and outcome of patients hospitalized to rule out myocardial infarction. Ann Intern Med. 1991;115:277–82. doi: 10.7326/0003-4819-115-4-277. [DOI] [PubMed] [Google Scholar]
- 9.Hollander JE, Hoffman RS, Gennis P, et al. Prospective multicenter evaluation of cocaine-associated chest pain. Cocaine Associated Chest Pain (COCHPA) Study Group. Acad Emerg Med. 1994;1:330–9. doi: 10.1111/j.1553-2712.1994.tb02639.x. [DOI] [PubMed] [Google Scholar]
- 10.Weber JE, Shofer FS, Larkin GL, Kalaria AS, Hollander JE. Validation of a brief observation period for patients with cocaine-associated chest pain. N Engl J Med. 2003;348:510–7. doi: 10.1056/NEJMoa022206. [DOI] [PubMed] [Google Scholar]
- 11.Januzzi JL, Isselbacher EM, Fattori R, et al. International Registry of Aortic Dissection (IRAD) Characterizing the young patient with aortic dissection: Results from the International Registry of Aortic Dissection (IRAD) J Am Coll Cardiol. 2004;43:665–9. doi: 10.1016/j.jacc.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 12.Isselbacher EM. Diseases of the aorta. In: Zipes DP, Libby P, Bonow RO, Braunwald E, editors. Braunwald’s Heart Disease: A Text Book of Cardiovascular Medicine. 7th edn. Philadelphia: Elsevier Saunders; 2005. pp. 1403–35. [Google Scholar]
- 13.Morrison AR. Hypertension. In: Green GB, Harris IS, Lin GA, Moylan KC, editors. The Washington Manual of Medical Therapeutics. 31st edn. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 72–91. [Google Scholar]
- 14.Egred M, Davis GK. Cocaine and the heart. Postgrad Med J. 2005;81:568–71. doi: 10.1136/pgmj.2004.028571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rashid J, Eisenberg MJ, Topol EJ. Cocaine-induced aortic dissection. Am Heart J. 1996;132:1301–4. doi: 10.1016/s0002-8703(96)90486-x. [DOI] [PubMed] [Google Scholar]
- 16.Chang RA, Rossi NF. Intermittent cocaine use associated with recurrent dissection of the thoracic and abdominal aorta. Chest. 1995;108:1758–62. doi: 10.1378/chest.108.6.1758. [DOI] [PubMed] [Google Scholar]
- 17.Perron AD, Gibbs M. Thoracic aortic dissection secondary to crack cocaine ingestion. Am J Emerg Med. 1997;15:507–9. doi: 10.1016/s0735-6757(97)90196-0. [DOI] [PubMed] [Google Scholar]
- 18.Madu EC, Shala B, Baugh D. Crack-cocaine-associated aortic dissection in early pregnancy – a case report. Angiology. 1999;50:163–8. doi: 10.1177/000331979905000212. [DOI] [PubMed] [Google Scholar]
- 19.Famularo G, Polchi S, Di Bona G, Manzara C. Acute aortic dissection after cocaine and sildenafil abuse. J Emerg Med. 2001;21:78–9. doi: 10.1016/s0736-4679(01)00345-6. [DOI] [PubMed] [Google Scholar]
- 20.Hsue PY, Salinas CL, Bolger AF, Benowitz NL, Waters DD. Acute aortic dissection related to crack cocaine. Circulation. 2002;105:1592–5. doi: 10.1161/01.cir.0000012524.44897.3a. [DOI] [PubMed] [Google Scholar]
- 21.Moliterno DJ, Willard JE, Lange RA, et al. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. N Engl J Med. 1994;330:454–9. doi: 10.1056/NEJM199402173300702. [DOI] [PubMed] [Google Scholar]
- 22.Goldfrank LR, Hoffman RS. The cardiovascular effects of cocaine. Ann Emerg Med. 1991;20:165–75. doi: 10.1016/s0196-0644(05)81217-x. [DOI] [PubMed] [Google Scholar]
- 23.Substance Abuse and Mental Health Services Administration. Smoked Cocaine vs. Non-Smoked Cocaine Admissions: 2002. The DASIS Report, February 2005. <oas.samhsa.gov/2k5/crackTx/crackTx.Pdf> (Version current at August 29, 2007).