Abstract
BACKGROUND:
Heart rate recovery (HRR) within the first few minutes of graded exercise has been associated with impaired clinical outcomes in patients being evaluated for coronary artery disease. HRR is abnormal in patients with heart failure (HF), but has not been associated with clinical outcomes in these patients. The objective of the present study was to determine whether HRR following cardiopulmonary exercise testing (CPET) correlates with peak oxygen consumption (VO2), and whether it impacts clinical outcomes, including HF hospitalizations and total mortality, or the need for cardiac transplantation.
METHODS:
CPET was performed in 78 patients referred to the Montreal Heart Institute (Montreal, Quebec) with congestive HF between January 2000 and December 2002. All patients had New York Heart Association class II or III HF with a left ventricular ejection fraction of 45% or lower. Mean (± SD) age was 53±11 years and left ventricular ejection fraction was 27±9%. Forty-four per cent had ischemic cardiomyopathy, 88% received beta-blockers and 79% received angiotensin-converting enzyme inhibitors. HRR was defined as the difference from peak exercise HR to HR measured at specific time intervals. HRR was calculated 30 s, 60 s, 90 s and 120 s after exercise.
RESULTS:
Mean peak VO2 was 18.0±5.3 mL/kg/min, resting HR was 74±13 beats/min and peak HR was 119±22 beats/min. HRR measured was 10±9 beats/min after 30 s, 20±12 beats/min after 60 s, 25±15 beats/min after 90 s and 30±13 beats/min after 120 s. At 90 s, patients with an HRR below 24 beats/min were more likely to have an HF hospitalization at five-year follow-up (eight hospitalizations [22.2%] versus two hospitalizations [2.7%]; P=0.0134). There was a correlation between peak VO2 and HRR 90 s and 120 s after completion of the exercise test (r=0.40 after 90 s, P=0.001, and r=0.41 after 120 s, P=0.008).
CONCLUSIONS:
In patients with HF, blunted HRR 90 s and 120 s after CPET correlate with peak VO2 and are associated with increased risk of worsening HF. HRR is easily measured and a useful marker for morbidity in patients with HF.
Keywords: Beta-blockers, Exercise testing, Heart failure, Heart rate recovery
Abstract
HISTORIQUE :
Le rétablissement de la fréquence cardiaque (RFC) au cours des quelques premières minutes suivant un exercice croissant a été associé à une issue clinique anormale chez les patients évalués pour coronaropathie. Le RFC est anormal chez les insuffisants cardiaques, mais n’a pas été associé à des complications cliniques chez ces patients. L’objectif de la présente étude était de déterminer si le RFC après une épreuve d’effort cardiopulmonaire est en corrélation avec la consommation d’oxygène (VO2) de pointe et s’il exerce un impact sur l’issue clinique, y compris sur l’hospitalisation pour insuffisance cardiaque et la mortalité totale, ou la nécessité de procéder à une transplantation cardiaque.
MÉTHODES :
L’épreuve d’effort a été réalisée chez 78 patients atteints d’insuffisance cardiaque congestive en consultation à l’Institut de cardiologie de Montréal (Montréal, Québec) entre janvier 2000 et décembre 2002. Tous les patients présentaient une insuffisance cardiaque de classe II ou III selon la New York Heart Association et une fraction d’éjection ventriculaire gauche de 45 % ou moins. L’âge moyen (± É.-T.) était de 53 ± 11 ans et la fraction d’éjection ventriculaire gauche était de 27 ± 9 %. Quarante-quatre pour cent souffraient de cardiomyopathie ischémique; 88 % prenaient des bêtabloquants et 79 %, des inhibiteurs de l’enzyme de conversion de l’angiotensine. Le RFC était défini par la différence entre la FC de pointe à l’effort et la FC mesurée à intervalles spécifiques. Le RFC a été calculé 30, 60, 90 et 120 secondes après l’épreuve d’effort.
RÉSULTATS :
La VO2 de pointe moyenne était de 18,0 ± 5,3 mL/kg/min; la FC au repos était de 74 ± 13 battements/min et la FC de pointe était de 119 ± 22 battements/min. Le RFC mesuré a été de 10 ± 9 battements/min après 30 s, de 20 ± 12 battements/min après 60 s, de 25 ± 15 battements/min après 90 s et de 30 ± 13 battements/min après 120 s. À 90 s, les patients dont le RFC était inférieur à 24 battements/min étaient plus susceptibles de devoir être hospitalisés pour insuffisance cardiaque lors du suivi après cinq ans (huit hospitalisations [22,2 %], contre deux hospitalisations [2,7 %], p = 0,0134). On a noté une corrélation entre la VO2 de pointe et le RFC à 90 s et à 120 s après la fin de l’épreuve d’effort (r = 0,40 après 90 s, p = 0,001 et r = 0,41 après 120 s, p = 0,008).
CONCLUSIONS :
Chez les patients qui souffrent d’IC, un RFC émoussé 90 s et 120 s après l’épreuve d’effort, est en corrélation avec la VO2 de pointe et est associé à un risque accru d’aggravation de l’IC. Le RFC est facile à mesurer et se révèle un marqueur utile de la morbidité chez les insuffisants cardiaques.
Heart failure (HF) is very common in Canada and the United States, with over 550,000 new cases per year and an estimated mortality of up to 50% after one to two years (1,2). Functional capacity, as measured by peak oxygen consumption (VO2) using cardiopulmonary exercise testing (CPET), is commonly used, and is an important measure of prognosis and eligibility for cardiac transplantation in patients with HF (3). CPET, however, requires special equipment that measures parameters of gas exchange. Heart rate recovery (HRR) after exercise, however, is an important measure of autonomic function. Patients with congestive HF exhibit an increase in sympathetic activation associated with cardiac B1 adrenoreceptor downregulation, a decrease in parasympathetic tone and abnormal regulation of cardiopulmonary baroreflexes. These multiple abnormalities in autonomic regulation may play a significant role in HRR after exercise. This marker can easily be assessed without measuring gas exchange and is potentially useful as a marker of prognosis (4). HRR after exercise has been well studied among patients with and without cardiovascular disease (4,5). The impact of HRR on prognosis in patients with HF, however, has not been thoroughly investigated. Previous studies (6,7) examining HRR in HF patients did not include substantial numbers of patients on beta-blockers and only included information on HRR up to 1 min after completion of CPET. The present study examines HRR as a prognostic marker in a cohort of patients with HF in which the majority of patients received beta-blockers and who had HRR measured up to 2 min after completion of CPET.
METHODS
Subjects
Patients were referred for CPET at the Montreal Heart Institute (Montreal, Quebec) between January 2001 and December 2002. Patients were included in this retrospective cohort if they were in normal sinus rhythm and did not have a permanent pacemaker or automated implantable defibrillator. All subjects had New York Heart Association class II or III HF with a left ventricular ejection fraction of 45% or lower. The CPET database and retrospective review of patient charts were used. The present study was approved by the Research Ethics Committee at the Montreal Heart Institute.
CPET and HRR
All patients underwent symptom-limited cardiopulmonary testing (Jaeger) using an individualized ramp protocol. All exercise tests were limited by dyspnea or fatigue. Any other reasons for termination of exercise excluded patients from the study. Blood pressure was recorded at baseline, every 2 min during exercise and at the end of the recovery period. HR was measured at rest, during each minute of exercise, at maximum exercise, as well as 30 s, 60 s, 90 s and 120 s into recovery. The HR reserve was defined by HR recorded at peak exercise minus resting HR. HRR was defined as the difference from peak exercise HR to HR measured at specific time intervals, and was calculated 30 s, 60 s, 90 s and 120 s after exercise.
Statistical analysis
Data are expressed as mean ± SD for continuous variables and as number (percentage) for categorical variables. The Pearson correlation coefficient was used for establishing the link between peak VO2 and HRR. Independent sample t tests were used to compare means. Survival analysis was performed using the Kaplan-Meier method, and the log-rank test was used to compare HF hospitalization between groups. Analyses were performed with SAS release 8.2 (SAS Institute Inc, USA) and SPSS version 13.0 (SPSS Inc, USA). P<0.05 was considered to be statistically significant.
RESULTS
Baseline clinical characteristics and CPET results
A total of 104 patients were assessed. Based on inclusion and exclusion criteria, there were a total of 78 patients included in the cohort. Table 1 outlines the baseline characteristics and Table 2 provides the results of CPET in the patient cohort. Almost all patients (89%) were receiving beta-blockers and nearly one-half of the cohort (42%) had ischemic cardiomyopathy.
TABLE 1.
Clinical characteristics of the study population (n=78)
| Characteristic | |
|---|---|
| Age, years (mean ± SD) | 53±11 |
| New York Heart Association class II/III, % | 56/44 |
| Left ventricular ejection fraction, % (mean ± SD) | 27±9 |
| Diabetes, % | 13 |
| Etiology, % | |
| Ischemic | 42 |
| Idiopathic/viral | 42 |
| Hypertension | 3 |
| Other | 13 |
| Medication use, % | |
| Angiotensin-converting enzyme inhibitor | 79 |
| Beta-blocker | 89 |
| Angiotensin receptor blocker | 21 |
| Loop diuretic | 79 |
| Digoxin | 72 |
| Amiodarone | 20 |
TABLE 2.
Hemodynamic and gas exchange analyses for the study population
| Parameter | |
|---|---|
| Peak oxygen consumption, mL/kg/min | 18.0±5.3 |
| Respiratory exchange ratio | 1.1±0.1 |
| Systolic blood pressure, mmHg | |
| Resting | 118±21 |
| Peak | 139±33 |
| Diastolic blood pressure, mmHg | |
| Resting | 75±12 |
| Peak | 80±12 |
| Heart rate, beats/min | |
| Resting | 73±13 |
| Peak | 116±21 |
| Heart rate recovery, beats/min | |
| 30 s | 10±9 |
| 60 s | 20±12 |
| 90 s | 26±15 |
| 120 s | 30±13 |
Values are presented as mean ± SD
Correlation of HRR and peak VO2
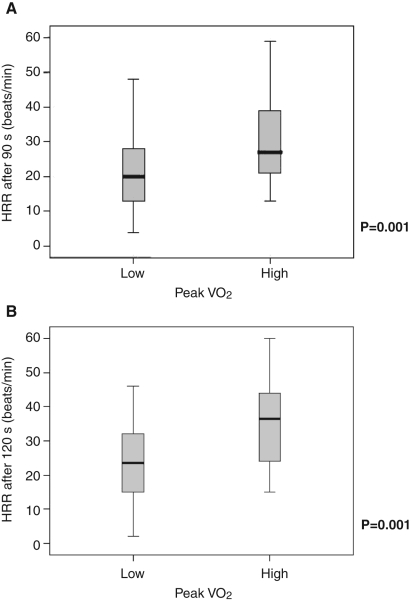
Patients with a peak VO2 that was below the median peak VO2 of the cohort (17.6 mL/kg/min) had a more blunted HRR after 90 s than patients with the median peak VO2 (mean HRR 18.2±3.1 beats/min versus 29.7±2.2 beats/min; P=0.003). Similar results were seen for HRR after 120 s (mean HRR 22.8±2.7 beats/min versus 33.1±2.7 beats/min) (Figures 1A and 1B). HRR after 30 s and 60 s was not associated with the degree of impairment in functional capacity as assessed by peak VO2.
Figure 1).
Heart rate recovery (HRR) after 90 s (A) and 120 s (B) based on whether patients were above or below the median peak oxygen consumption (VO2) (mean low VO2 13.9±2.3 mL/kg/min, mean high VO2 21.5±2.6 mL/kg/min)
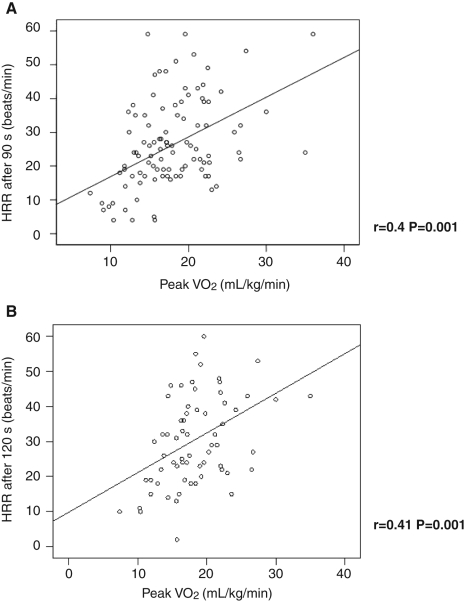
The relationship between HRR and peak VO2 was further investigated. The strongest correlations between HRR and peak VO2 existed 90 s and 120 s after completion of the exercise test (r=0.40 after 90 s, P=0.001, and r=0.41 after 120 s, P=0.008). The correlation between peak VO2 after 60 s was not as strong (r=0.29, P=0.02) and was not significant after 30 s following exercise (Figures 2A and 2B).
Figure 2).
Correlation of heart rate recovery (HRR) and peak oxygen consumption (VO2) after 90 s (A) and 120 s (B)
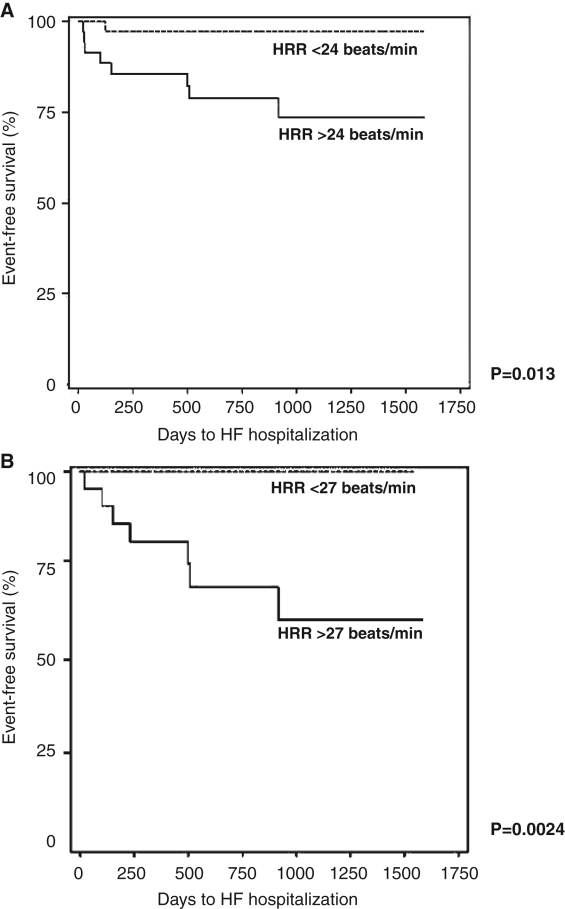
HRR and clinical outcomes (Figures 3A and 3B)
Figure 3).
Days free from hospitalization for heart failure (HF). Heart rate recovery (HRR) after 90 s (A) and 120 s (B)
At 90 s, patients with an HRR below 24 beats/min were more likely to have an HF hospitalization at five-year follow-up (eight hospitalizations [22.2%] versus two hospitalizations [2.7%]; P=0.0134). There was a similar difference seen after 120 s of HRR (seven years [30.4%] versus zero [0.0%]; P=0.0024). There was no significant relationship with HRR after 30 s or 60 s. There were no differences in overall death and/or cardiac transplant when comparing patients above or below the median HRR at 30 s, 60 s, 90 s or 120 s.
DISCUSSION
The present study has demonstrated that HRR correlates with peak VO2. In addition, poor HRR after 90 s and 120 s was associated with worse clinical outcomes, including more frequent HF hospitalizations. HRR has been established as a marker of prognosis in patients who do not have HF (4,5), but it has not been well studied in patients with HF who are receiving modern-era beta-blocker therapy (6–10). It is an easy-to-use, potential real-world measure of prognosis in HF patients. In our study of 78 patients with HF, HRR correlated with peak VO2, which is a very strong measure of prognosis. In addition, there was a trend toward increased mortality in patients with a blunted HRR. Last, patients with HF and a blunted HRR on baseline CPET were more likely to be rehospitalized for HF. Our study differs from others (6,7) in that nearly 90% of patients were receiving beta-blockers, and HRR was examined up to 2 min after the completion of exercise. No studies have examined the prognostic significance of HRR 120 s after completion of exercise in a population of patients with HF.
HRR is a measure of autonomic function, and reflects the parasympathetic tone and, possibly, the sympathetic withdrawal that exists in the recovery phase after exercise. In two separate studies of patients referred for exercise testing (6,7), a low HRR was a significant independent predictor of death. Blunted HRR reflects an increase in sympathetic activation associated with cardiac B1 adrenoreceptor downregulation, a decrease in parasympathetic tone and abnormal regulation of cardiopulmonary baroreflexes.
There are a number of markers of prognosis that have been well studied in patients with HF, including peak VO2 (3), left ventricular ejection fraction (11) and neurohormones such as brain natriuretic peptide (12). Measuring peak VO2 requires equipment to measure gas exchange during exercise, and there are a number of cardiovascular conditions and other comorbid conditions, such as renal failure, that lead to an abnormal level of brain natriuretic peptide. HRR, however, is an easy-to-measure, readily available exercise parameter that requires only the most basic of exercise testing equipment.
Because there is dysregulation of the autonomic nervous system in patients with HF (13,14), and because changes in HRR may reflect this dysregulation, HRR may help to stratify risk in patients with HF. To date, however, HRR in patients with HF has not been well studied and has not been established as a marker of prognosis (6,7). Imai et al (10) demonstrated that HRR was accelerated in athletes but appeared blunted in patients with HF. Racine et al (15) demonstrated that HRR was attenuated in patients with HF compared with healthy controls, and that there was no impact of beta-blockers on HRR.
Our study demonstrated similar HRR in patients with HF, and found that it strongly correlated with peak VO2, an important measure of sympathetic drive and prognosis in patients with HF. Patients with a low peak VO2 had a blunted HRR, potentially reflecting increased sympathetic drive. This correlation may also reflect the fact that patients with HF and a more attenuated HRR have less parasympathetic tone.
Our study has several limitations. First, we did not have a healthy control group with which we could compare HRR values. Second, we did not have any objective measures of autonomic function, such as plasma noradrenaline levels or an indirect assessment of vagal tone. These might have provided a physiological explanation for attenuated HRR in our patient cohort. Last, many patients were receiving beta-blocker therapy, which can influence autonomic function. Of note is that the values for HRR in patients not receiving beta-blockers were similar to those in patients receiving beta-blockers. In addition, studies have demonstrated that beta-blockers do not impact HRR in HF (15) or non-HF patients (16).
CONCLUSIONS
HRR in patients with HF was attenuated and correlated with other important measures of prognosis. In addition, patients with a more attenuated HRR were more likely to have recurrent HF hospitalizations. Although this relationship may reflect the impact of peak VO2, HRR is a much easier measure to perform. Studies with larger cohorts of patients are required to determine the impact of medical therapy on HRR, the incremental prognostic value of HRR with peak VO2 and the impact of HRR on mortality in patients with HF.
REFERENCES
- 1.Thom T, Haase N, Rosamond W, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics – 2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. (Errata in 2006;113:e696, 2006;114:e630). [DOI] [PubMed] [Google Scholar]
- 2.Heart and Stroke Foundation of Canada. The growing burden of heart disease and stroke in Canada 2003. <www.cvdinfobase.ca/cvdbook/En/Index.htm> (Version current at October 17, 2007).
- 3.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–86. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 4.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–7. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 5.Lacasse M, Maltais F, Poirier P, et al. Post-exercise heart rate recovery and mortality in chronic obstructive pulmonary disease. Respir Med. 2005;99:877–86. doi: 10.1016/j.rmed.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Nanas S, Anastasiou-Nana M, Dimopoulos S, et al. Early heart rate recovery after exercise predicts mortality in patients with chronic heart failure. Int J Cardiol. 2005;110:393–400. doi: 10.1016/j.ijcard.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Arena RM, Guazzi M, Myers J, Peberdy MA. Prognostic value of heart rate recovery in patients with heart failure. Am Heart J. 2006;151:851.e7–13. doi: 10.1016/j.ahj.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: The case of stress echocardiography. Circulation. 2001;104:1911–6. [PubMed] [Google Scholar]
- 9.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2001;285:1392–8. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 10.Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–35. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN, Johnson GR, Shabetai R, et al. Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norepinephrine as determinants of prognosis in heart failure. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87(6 Suppl):VI5–16. [PubMed] [Google Scholar]
- 12.Gardner RS, Ozalp F, Murday AJ, Robb SD, McDonagh TA. N-terminal pro-brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003;24:1735–43. doi: 10.1016/j.ehj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Francis GS, Cohn JN. The autonomic nervous system in congestive heart failure. Annu Rev Med. 1986;37:235–47. doi: 10.1146/annurev.me.37.020186.001315. [DOI] [PubMed] [Google Scholar]
- 14.Nolan J, Flapan AD, Capewell S, MacDonald TM, Neilson JM, Ewing DJ. Decreased cardiac parasympathetic activity in chronic heart failure and its relation to left ventricular function. Br Heart J. 1992;67:482–5. doi: 10.1136/hrt.67.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Racine N, Blanchet M, Ducharme A, et al. Decreased heart rate recovery after exercise in patients with congestive heart failure: Effect of beta-blocker therapy. J Card Fail. 2003;9:296–302. doi: 10.1054/jcaf.2003.47. [DOI] [PubMed] [Google Scholar]
- 16.Shetler K, Marcus R, Froelicher VF, et al. Heart rate recovery: Validation and methodologic issues. J Am Coll Cardiol. 2001;38:1980–7. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]