Abstract
BACKGROUND:
Vein arterialization following bypass surgery often leads to graft occlusion, but the underlying cellular mechanisms have been poorly studied.
OBJECTIVES:
Cell cycle progression and the activation of proliferation signalling were compared in arterialized grafts prepared either according to the conventional procedure or using pharmacological relaxation with the native vein.
METHODS:
Using the porcine carotid-jugular bilateral interposition graft model on one side, a segment of porcine jugular vein was prepared for grafting using the conventional procedure, with pressure distention at 300 mmHg; the segment grafted on the other side was treated with a combination of pharmacological vasodilators. Both veins were grafted into the carotid artery for two weeks.
RESULTS:
On the immunolabelling of proliferation cell nuclear antigen, a greater number of proliferating cells was found in the conventionally prepared grafts compared with pharmacologically prepared grafts. Cyclin D1 expression and phosphorylation of retinoblastoma increased after implantation, coinciding with nuclear accumulation of beta-catenin, activation of the Akt and mitogen-activated protein kinase cascades, and upregulated phosphatase and tensin homologue phosphorylation. Replacement of distention with pharmacological relaxation reduced the increase in cyclin D1 expression, phosphorylation of retinoblastoma, Akt-Thr308, glycogen synthase kinase 3 beta and p38, but not extracellular signal-regulated kinases. This technique preserved the active phosphatase and tensin homologue, as well as the expression of cyclin-dependent kinase inhibitor p21Cip1, while elevating the expression of p27Kip1.
CONCLUSIONS:
It was concluded that two-week arterial implantation stimulates proliferation signalling and promotes the cell cycle in vein grafts. Replacement of the conventional preparation procedures with pharmacological vasorelaxation restricts the activation of proliferation and cell cycle progression, and can be beneficial for improving vein graft patency.
Keywords: Bypass, Grafting, Signal transduction, Smooth muscle, Veins
Abstract
HISTORIQUE :
L’artérialisation d’une veine après un pontage aortocoronarien provoque souvent une occlusion de la greffe, mais les mécanismes cellulaires sous-jacents sont peu étudiés.
OBJECTIFS :
Les auteurs ont comparé la progression du cycle cellulaire et l’activation des signaux proliférants des greffes artérialisées préparées d’après l’intervention classique ou à l’aide d’une dépression pharmacologique de la veine native.
MÉTHODOLOGIE :
À l’aide du modèle de greffe porcine avec interposition bilatérale carotide-jugulaire d’un côté, les auteurs ont préparé à la greffe un segment de veine jugulaire porcine au moyen de l’intervention classique, avec une distension de pression de 300 mmHg. Le segment greffé de l’autre côté était traité avec une association de vasodilatateurs pharmacologiques. Les deux veines ont été greffées dans l’artère carotide pendant deux semaines.
RÉSULTATS :
À l’immunomarquage de l’antigène nucléaire des cellules proliférantes, on a trouvé plus de cellules proliférantes dans les greffes classiques que dans les greffes avec préparation pharmacologique. L’expression de la cycline D1 et la phosphorylation du rétinoblastome augmentent après l’implantation, en même temps que l’accumulation de la bêta-carénine, l’activation des cascades de protéine-kinase Akt et activée par le mitogène et la phosphorylation par régulation positive de la phosphatase et de l’homologue de la tensine. Le remplacement de la distension par des dépresseurs pharmacologiques réduit l’augmentation de l’expression de la cycline D1, la phosphorylation du rétinoblastome, l’Akt-Thr308, le glycogène synthase-kinase 3 bêta et p38, mais pas les kinases reliées aux signaux extracellulaires. Cette technique préservait la phosphatase active et l’homologue de tensine de même que l’expression de l’inhibiteur de la kinase cycline-dépendante p21Cip1, tout en accroissant l’expression du p27Kip1.
CONCLUSIONS :
Les auteurs ont conclu que l’implantation artérielle pendant deux semaines stimule les signaux proliférants et favorise le cycle cellulaire dans les greffes veineuses. Le remplacement des interventions préparatoires classiques par des vasodépresseurs pharmacologiques limite l’activation de la prolifération et la progression du cycle cellulaire et peut être bénéfique pour améliorer la capacité de la greffe veineuse.
The saphenous vein is a convenient conduit for revascularization, especially in patients with multivessel coronary artery disease; however, its patency is limited in comparison with arterial grafts (1). Vein graft occlusion is the result of intimal hyperplasia and the accelerated development of atherosclerosis, a cellular response that is triggered by hypoxia and surgical trauma, and increased wall stress due to the implantation of the vein in the arterial bed (1). Nevertheless, the cellular signalling mechanisms and the impact of the preparation procedures in vein bypass graft remodelling have not been studied nearly as well as arterial damage due to balloon angioplasty. There are a few interesting exceptions: the recently published study on the activation of mitogen-activated protein kinase (MAPK) in vein grafts (2) and the antiproliferative and anti-inflammatory effects of the topically applied MAPK inhibitor to the vein before grafting (3), and the inhibitory effect of transfection of nuclear factor-kappa beta decoy on neointimal hyperplasia (4).
Smooth muscle cell (SMC) proliferation and migration are the critical events in vein graft remodelling (1). The cell proliferation cycle is regulated by cyclin-dependant kinases (Cdks), which are members of a family of serine and threonine kinases. Cdks are activated by binding cyclins to their regulatory subunits. Cyclin D1 is one of the first cyclins synthesized in response to growth factors, and, in a complex with Cdk4, it controls the G1/S phase transition. The cyclin D1-Cdk4 complex inactivates the growth-suppressive function of retinoblastoma (Rb) through its phosphorylation. This releases the transcription factor E2F and induces DNA synthesis, thus initiating the cell cycle (5,6).
The phosphoinositide 3 kinase (PI3K)/Akt, MAPK and beta-catenin cascades are the major proliferation signalling pathways that can contribute to vascular remodelling (7–12). Glycogen synthase kinase 3 beta (GSK3β) is an intermediate in PI3K/Akt-dependent proliferation (8,9) and the regulator of beta-catenin levels (12). The conventional preparation procedure, including pressure distention routinely used for overcoming vasospasm, is likely to enhance remodelling of the vein graft (13–15). This can be realized through Akt, MAPK and beta-catenin cascades, which are known to be activated by mechanical stimuli (9,16). In previous studies (14,15), we have shown that the replacement of pressure distention with effective pharmacological vasodilators (alpha-adrenergic antagonist, Rho kinase inhibitor and calcium blocker) prevents vasospasm, preserves endothelial function, and decreases matrix metalloproteinase activation and neointima formation in the vein graft, all of which should be beneficial for graft patency.
In the present study, we determined the underlying cellular mechanisms of accelerated remodelling of the vein graft and studied how the modification of the vein graft preparation procedure affects these events. In the porcine interposition jugular-carotid vein graft model, we compared the activation of cell proliferation signalling in grafts prepared using the conventional procedure (CP), including pressure distention of the vein, and those topically treated with a combination of effective pharmacological vasodilators instead of pressure distension exceeding physiological levels. We hypothesized that vein arterialization upregulates cell proliferation signalling pathways and activates cell cycle progression, both of which can be attenuated by a modification of the preparation procedure.
MATERIALS AND METHODS
Materials
Primary antibodies used in the present study include mouse mono-clonal anti-cyclin D1, anti-phospho-extracellular signal-regulated kinase (ERK1/2) (Cell Signaling Technology Inc, USA); anti-beta-catenin antibodies (BD Biosciences, USA); rabbit polyclonal anti-Rb, anti-phospho-Rb, anti-GSK3β, anti-phospho-GSK3β (Ser9), anti-PTEN phosphatase and tensin homologue, anti-phospho-PTEN (Ser380), anti-phospho-p38 MAPK (Thr180/Tyr182), anti-Akt, anti-phospho-Akt (Thr308), anti-phospho-Akt (Ser473) (Cell Signaling Technology Inc); and anti-Cdk4, anti-p21, anti-p27 and anti-proliferating cell nuclear antigen (PCNA) antibodies (Santa Cruz Biotechnology, USA). Biotinylated goat anti-mouse immunoglobulin (Ig) G (Vector Laboratories, USA); avidin-biotin complex (Dako, USA); alkaline phosphatase (Vector Red; Vector Laboratories, USA). TRIZOL Reagent (Invitrogen Life Technologies, USA) was also used. Gene-specific primers were synthesized by the Nucleic Acid-Protein Service Unit at the Oligonucleotide Synthesis Laboratory (Biotechnology Laboratory, University of British Columbia, Vancouver, British Columbia). All other reagents were of highest molecular grade and purchased from Sigma-Aldrich (USA), unless mentioned otherwise in the text.
Animal surgical procedures
Surgical procedures have previously been explained in detail (15). In brief, grafting of the jugular vein in the carotid artery was performed in eight female white pigs (weight 60 kg to 65 kg). All animal procedures were approved by the University of British Columbia Animal Care Committee. Pigs were given a general anesthetic (a single dose of ketamine 800 mg to 1400 mg), and under sterile conditions, a midline neck incision was used to expose both common carotid arteries and internal jugular veins. A 6 cm segment of the dissected vein was grafted into the carotid artery as an interposition vein graft using an end-to-end anastomotic technique (Figure 1). Before grafting, a piece of the vein from the side randomly assigned to pharmacological relaxation (pharmacological preparation [PP]) was taken as the ‘undistended’ sample, and the rest was placed in a bath containing a combination of vasodilatatory drugs (alpha-adrenergic antagonist, phenoxybenzamine, at 10 μmol/L; Rho kinase inhibitor, HA-1077 [fasudil], at 50 μmol/L, and a calcium blocker, nicardipine, at 1 μmol/L) for 30 min (14,15). The vein from the other side was first exposed to distention with heparinized saline at a distention pressure of 300 mmHg for 2 min. Following this, a piece of the distended vein was taken as the ‘distended’ sample, and the rest of the vein was grafted. After two weeks, the interpositioned vein grafts were harvested, and all animals were euthanized. Flash-frozen and formalin-fixed samples were prepared from the veins before grafting and from the grafts.
Figure 1).
Diagram showing the experimental procedures in the porcine model
Morphology and immunostaining
Movat’s staining was used for graft cross-section visualization and counting SMCs, as previously reported (15). For PCNA staining, the graft cross-sections were deparaffinized and rehydrated. Antigen retrieval was achieved by steaming for 30 min in a citrate buffer (pH 6.0). Nonspecific antibody-antigen interactions were blocked by incubation in 10% normal goat serum for 30 min at room temperature. Anti-PCNA antibody was applied at a 1:350 dilution (an equal concentration of IgG2A antibody was used as a negative control) and incubated overnight at 4°C. Indirect detection was performed by incubating biotinylated goat anti-mouse IgG, followed by avidin-biotin complex and alkaline phosphatase. Nuclei were counterstained with hematoxylin. All the stained sections were imaged using a Nikon inverted microscope (Nikon, USA) and SPOT Digital Camera (Diagnostic Instruments, USA). The images were processed with Image-Pro Plus 5 software (Media Cybernetics, USA). The number of proliferating cells was counted separately in the clusters and in the areas free of clusters. For the latter, eight spots through each cross-section were quantified.
Extraction of messenger RNA
Total RNA was extracted from flash-frozen segments using TRIZOL Reagent according to the manufacturer’s instructions; the detailed experimental procedures were previously described (14,15). RNA concentration was quantified by measuring absorbance at 260 nm (Ultrospec 3000 UV/visible spectrophotometer; Pharmacia Biotech, USA).
Reverse transcription-polymerase chain reaction
Total RNA (1 μg) was reverse-transcribed into complementary DNA in a final volume of 25 μL containing 50 mM Trishydrochloride (pH 8.3), 75 mM potassium chloride, 3 mM magnesium chloride, 1 μg random primer oligonucleotides, 2 mM deoxyribonucleotide triphosphate, 16 mM dichlorodiphenyltrichloroethane, 10 units ribonuclease inhibitor and 300 units Moloney murine leukemia virus reverse transcriptase (Invitrogen Life Technologies). Reverse transcription was initiated in a GeneAmp thermocycle (GeneAmp PCR System 9700; Applied Biosystems, USA) for 60 min at 37°C, then 15 min at 70°C. The reverse trasnscriptase products were cooled and stored at −20°C until use.
Polymerase chain reaction was carried out in a volume of 25 μL containing 5 μL first-strand complementary DNA, 10 mM Trishydrochloride (pH 8.3), 50 mM potassium chloride, 1.5 mM magnesium chloride, 0.2 μM forward and reverse primer, 0.2 mM deoxyribonucleotide triphosphate mix and two units Taq DNA polymerase (Applied Biosystems) (14,15). The primer sequence of cyclin D1 was as follows: forward – TGC-TGA-ATT-CAA-GCC-TGC-GCC-AG; reverse – TGA-AGC-TTT-CCC-TTC-TGG-TAT-CAA (352 base pairs). The reaction profile for amplification consisted of an initial denaturation at 94°C for 8 min, followed by 33 cycles denaturing at 94°C for 1 min, annealing at 57°C for 1 min and extension at 72°C for 1 min, with a final elongation at 72°C for 10 min. Aliquots of polymerase chain reaction products (10 μL) were electrophoresed in 1.25% agarose gel in Tris-acetate-EDTA buffer containing 1 μg/mL ethidium bromide. Gels were illuminated with ultraviolet light and photographed using Digi Doc-It software (Bio-Rad Laboratories Inc, USA). Densitometry was analyzed by Quantity One imaging software (Bio-Rad Laboratories Inc). Gene transcription of beta-actin was used as the loading control.
Western immunoblotting
Procedures of protein extraction and Western immunoblotting have been described previously (14,15). In brief, equal amounts of protein (10 μg) were electrophoresed in sodium dodecyl sulfate-polyacrylamide gel and then transferred to polyvinyldifluo-ride membranes (Bio-Rad Laboratories Inc). The membranes were incubated with primary antibodies for 2 h and then with peroxidase-conjugated secondary antibodies for 1 h at room temperature. The immunoreactive protein was detected by enhanced chemiluminescence (Amersham Life Sciences, United Kingdom). As a control of equal loading and transfer, membranes were stripped and reprobed with anti-beta-actin antibody. Densitometric analysis was performed.
Separation of cell fractions
Four-millimetre vessel segments were ground into powder by mortar and pestle in liquid nitrogen. Cell fractionation was achieved by suspension of the tissue powder in nine volumes of ice-cold lysis buffer (1% sodium dodecyl sulfate, 10 μg/mL leupeptin, 100 mM sodium chloride, 43.5 mM Tris, pH 7.6, 1 mM phenylmethylsul-fonyl), as previously described (17). After 20 min of incubation on ice, samples were homogenized by a glass homogenizer. The portion containing nuclei was precipitated by centrifugation at 1600 g for 5 min at 4°C. The supernatant was centrifuged at 16,000 g for 1 h at 4°C to separate the membrane and cytoplasmic fractions. Each fraction was subjected to Western blotting to quantify the cellular distribution of beta-catenin.
Statistics
Data were reported as mean with SEM from six to eight independent experiments.
A paired t test was performed to compare the differences between the two groups (GraphPad Prism; Hearne Scientific Software, USA). P<0.05 was considered to be significant.
RESULTS
Development of neointima, and quantification and proliferation of SMCs
Before grafting, the intima was composed of a single layer of endothelial cells (Figures 2A and 2B). The tunica media of the vein included a few layers of SMCs of elongated shape, which comprised 17% of the jugular vein body, and a large area was stained as collagen. PCNA staining did not detect proliferating cells in the native vein (Figure 3A). Following two weeks of grafting, pronounced remodelling of the vein grafts occurred (Figures 2C and 2D). The medial layer was drastically thickened and included a large amount of small, nonelongated SMCs, some of which were proliferating (Figures 3C and 3D). SMCs are often organized as clusters when they are proliferating (Figure 3E). Grafts developed profuse neointima, consisting of small, nonelon-gated SMCs and proteoglycans (Figures 2C and 2D). Interestingly, no proliferating cells were found in the neointima (Figure 3B).
Figure 2).
Representative Movat-stained micrographs from six to eight independent experiments showing native jugular vein (A), distended vein (B), conventionally prepared graft after two weeks of implantation (C) and pharmacologically prepared graft after two weeks of implantation (D). *Neointima. A and B scale bars = 0.1 mm; C and D scale bars = 0.5 mm
Figure 3).
Representative micrographs from five independent experiments showing proliferating cell nuclear antigen-stained cross-sections of native jugular vein (A), neointima (B), conventionally prepared graft after two weeks of implantation (C), pharmacologically prepared graft after two weeks of implantation (D), and smooth muscle cell clusters in the conventionally prepared graft (E) and the pharmacologically prepared graft (F). Scale bars = 0.05 mm
However, these changes were more pronounced in the CP grafts than in the grafts where the distention was replaced with PP. From the Movat’s stained histological slides, the percentage of SMCs, which were labelled as purple and pink areas (Figure 2), was upregulated by 110±8% in CP grafts (P<0.001) after implantation, while in the PP grafts there was no significant difference from the pregrafted level (22.6±3.1% versus 17.3±4.5% of total area). Thus, the SMC area was 56.2% higher in the CP than in the PP grafts.
From the PCNA-stained cross-sections, the average number of proliferative SMC clusters was 5.33 per vessel in the CP grafts and 1.67 per vessel in the PP grafts. The percentage of proliferating cells was also lower in PP grafts than in CP grafts: 4.86±0.66% in the PP versus 9.05±1.37% in the CP grafts in the nonclustered areas (Figures 3C and 3D) and 32.7±8.1% versus 55.6±5.7% in the cluster areas (Figures 3E and 3F) (P<0.05).
Cell cycle control
The two-week arterial implantation increased cyclin D1 messenger RNA expression in the CP grafts by 78.3±4.3%, but had no notable effect in the PP ones (Figure 4A). The changing trend of cyclin D1 in gene transcription was similar to that in protein expression, which was increased in the CP grafts by 164±11%, but did not differ significantly in the PP grafts (Figure 4B).
Figure 4).
Representative images from reverse-transcriptase polymerase chain reaction (A) and Western blotting (B) showing the gene transcription and protein levels of cyclin D1 in the veins and conventionally prepared (CP) or pharmacologically prepared (PP) grafts. C Representative immunoblots indicating protein expression of retinoblastoma (Rb) (dilution of primary antibody 1:2000), phosphorylated Rb (p-Rb) (1:1000), cyclin-dependent kinase 4 (Cdk4) (1:1000), p21Cip1 (1:200), p27Kip1 (1:200) and the subcellular distribution of beta-catenin (β-catenin) (1:500) in the veins and grafts. A summary of the densitometric measurements is given in Table 1. bp Base pair; C Cytoplasm; M Membrane; MW Molecular weight; N Nuclear; T Total
A summary of densitometric analyses for the levels of gene transcription and protein expression is provided in Table 1. Cdk4 protein expression was equally upregulated in both types of grafts (Figure 4C). The levels of Cdk inhibitor p21Cip1 and p27Kip1, which inactivate Cdk-cyclin complexes in SMCs and counteract cell cycle progression (7,18,19), were increased only in the PP grafts (Figure 4C). As a result, the levels of phosphorylated Rb in the CP grafts were significantly higher than those in the PP grafts by 41.7±4.5% (Figure 4C), while the total Rb level was practically unchanged in all four vessels.
TABLE 1.
Summary of densitometric analyses
| Pregrafting
|
Postgrafting
|
|||
|---|---|---|---|---|
| Parameters | Native | Distended | CP | PP |
| Cyclin D1 mRNA | 1601±142 | 1533±153 | 2733±248* | 1910±112 |
| Cyclin D1 protein | 1420±203 | 1292±163 | 3412±230* | 1668±177 |
| Total Rb | 2578±204 | 2742±287 | 2747±210 | 2925±301 |
| p-Rb | 2009±189 | 2234±316 | 4325±323* | 3143±199** |
| Beta-catenin | 2218±204 | 2102±173 | 2147±214 | 1925±241 |
| Total | 100% | 100% | 100% | 100% |
| Cytoplasmic | 93±5% | 90.2±7.5% | 16.4±2.3%* | 102±8% |
| Nuclear | 12±2.5% | 9.7±1.0% | 95±12%* | 10±2.4% |
| Cdk-4 | 1718±154 | 1609±141 | 2347±195 | 2225±189** |
| p21 | 2110±204 | 1989±183 | 1307±114* | 2200±207 |
| p27 | 1718±191 | 1909±146 | 1787±101* | 2925±219** |
| Total Akt | 3589±277 | 3936±182 | 3737±202 | 3683±247 |
| p-AktThr308 | 1089±178 | 936.2±82.5 | 4037±227* | 2983±284** |
| p-AktSer473 | 2141±206 | 1841±457 | 3839±407 | 3738±203* |
| Total GSK3β | 3009±177 | 2956±272 | 3237±142 | 3383±317 |
| p-GSK3β | 1299±285 | 1425±310 | 2509±349* | 1544±205 |
| p-ERK1/2 | 1717±110 | 1564±186 | 2874±142 | 2702±176** |
| p-p38 MAPK | 1197±105 | 1225±110 | 2409±209* | 1649±155** |
| Total PTEN | 1877±164 | 1927±214 | 2174±200 | 2002±187 |
| p-PTEN | 1217±184 | 1124±184 | 2264±186* | 1202±117 |
Data are mean ± SEM, expressed as arbitrary unit of intensity.
*Significantly (P<0.05) different from pharmacologically prepared (PP) (postgrafting) grafts, (n=6 to n=8);
**Significantly (P<0.05) different from native (pregrafting) veins, (n=6 to n=8). Cdk Cyclin-dependent kinase; CP Conventionally prepared; ERK1/2 Extracellular signal-regulated kinase; GSK3β Glycogen synthase kinase 3 beta; MAPK Mitogen-activated protein kinase; mRNA Messenger RNA; p-Phosphorylated; PTEN Phosphatase and tensin homologue; Rb Retinoblastoma
Proliferation signalling pathways
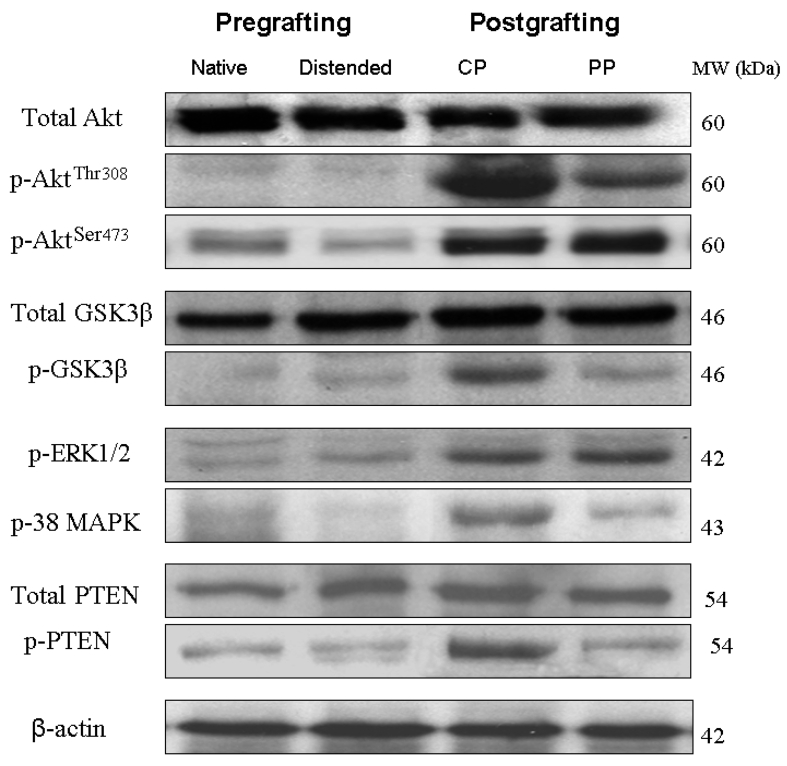
Upstream mechanisms that likely mediate the effects of arterial implantation and distention on the graft remodelling were further investigated. The expression and phosphorylation levels of key intermediates in the proliferation pathways were examined, and it was found that arterialization of the veins caused the activation of signalling cascades known to induce proliferation (Figure 5, Table 1). In the PI3K/Akt signalling cascade, arterial grafting resulted in a prominent increase in phosphorylation of Akt at Thr308 and Ser473, thus activating this enzyme in both types of grafts. However, protein expression levels of p-AktThr308 were significantly higher in the CP grafts than in the PP grafts, without differences in the total Akt or p-AktSer473 levels (Figure 5). The phosphorylation levels of the PI3K phosphatase PTEN were highly enhanced in the CP grafts, but remained at the pregrafted level in the PP grafts (Figure 5). Phosphorylation inactivates PTEN and enhances activity of the PI3K/Akt cascade. The phosphorylation levels of the target of Akt, GSK3β (9,20), were significantly enhanced in the CP grafts after arterial grafting, but not in the PP grafts (Figure 5). Active GSK3β phosphorylates cyclin D1, which promotes its degradation in proteosomes. Thus, the increased cyclin D1 protein level can be attributed to the inactivation of GSK3β. Another protein that is phosphorylated by GSK3β, and is thus exposed to degradation, is beta-catenin. Although changes in the total expression of beta-catenin (Figure 4C, Table 1) were not found, a higher accumulation of beta-catenin in the nuclei in the CP grafts (Figure 4C) was discovered, which might have been a result of its stabilization due to the inactivation of GSK3β.
Figure 5).
Representative immunoblots (n=6 to n=8) reveal the protein levels of total Akt, phosphorylated (p-) AktThr308, p-AktSer473, total glycogen synthase kinase 3 beta (GSK3β), p-GSK3β, phosphorylated extracellular signal-regulated kinase (p-ERK1/2), p38 mitogen-activated protein kinase (MAPK), total phosphatase and tensin homologue (PTEN) or p-PTEN (dilution of antibodies 1:1000) in the veins and the conventionally prepared (CP) or pharmacologically prepared (PP) grafts. Beta-actin (β-actin) (1:2500) is the loading control. Densitometric measurements are summarized in Table 1. MW Molecular weight
In the MAPK signalling cascades, phosphorylation levels of both ERK1/2 and p38 were increased after implantation, but the CP grafts had a substantially higher level of p38 MAPK than the PP grafts (Figure 5, Table 1). Therefore, both MAPK pathways contributed to the activation of proliferation during vein grafting; however, additional activation of p38 may further contribute to cell cycle stimulation in the distended grafts.
DISCUSSION
The present study demonstrated that the grafting of the jugular vein in the arterial bed after the conventional procedure of surgical preparation causes pronounced activation of MAPK, Akt and beta-catenin signalling pathway, which results in cell cycle progression. The replacement of the distention of the PP vein graft decreases the activation of the proliferative signalling pathways and downregulates cell cycle proteins. Therefore, we suggest that PP may be a beneficial alternative technique for preventing vasospasm and attenuating graft remodelling.
During arterialization, the vein graft is exposed to high and cyclic blood pressures, which cause overstretching of the vessel wall. The graft also undergoes hypoxia during surgery and the postsurgical period as a result of the dissection of the vasa vasorum. These conditions, together with the surgical trauma, trigger inflammatory responses that activate proliferation signalling. Distention with pressure exceeding physiological levels applied during preparation for grafting can be expected to cause additional graft remodelling, because it adversely affects endothelium structure and function (13,21,22), decreases production of antiatherogenic nitric oxide (14,22) and prostacyclin (21), and traumatizes the smooth muscle layer, causing a loss of the contractile ability of the vein (23,24). Distension also activates matrix metalloproteinases (15), which stimulates SMC proliferation and migration.
In the present study, we showed that grafting of the CP vein for two weeks resulted in drastic thickening of the medial layer and development of the profound neointima. In the neointima, there was a large amount of small nonelongated SMCs (Figures 2C and 3B), which is characteristic of the low-differentiated cells of proliferative phenotype (25). PCNA immunolabelling revealed the presence of the proliferating cells in the media (Figure 3C), but practically not at all in the neointima (Figure 3B). In contrast to some studies related to the origin of SMCs composing the neointima of injured arteries (20,25), we found a low level of intimal SMC replication (Figure 3B) in vein grafts.
We showed that two-week arterial grafting modulated cell cycle-regulating proteins in the CP grafts through increasing protein expression of cyclin D1, accompanied by considerably a decreased level of the Cdk inhibitor p21Cip1 compared with the native vein (Figure 4). The active cyclin D1-Cdk4 complex phos-phorylates Rb, thus promoting the cell cycle.
During exploration of upstream mechanisms causing these orchestrated alterations in the cell cycle regulatory proteins, we found that arterial grafting activates signalling cascades known to stimulate proliferation (Figure 6). Phosphorylation of ERK1/2, p38 MAPK, AktThr308 and AktSer473 was strongly increased in the grafts. ERK activation plays a crucial role in growth factor-induced vascular SMC proliferation (26), is well documented in SMC proliferation and neointimal formation following balloon injury (10,27), and is required for the induction of cyclin D1 (28). p38 can inactivate Rb through a mechanism complementary to cyclin D1 by phosphorylation at a distinct site, leading to increased transcriptional activity (11). Phasic activation of both p38 and ERK1/2 has been observed previously in a similar jugular vein graft model in dogs (2,3,26). Activated Akt signalling in SMC induces migration, proliferation and hypertrophy, leading to vascular remodelling, intimal formation and restenosis (8). Constitutive activation of Akt features the highly proliferate phenotype of neointimal SMCs (20). In vivo transfection of the balloon-injured vessel with dominant-negative Akt reduced SMC proliferation and neointima formation (7). The Akt/GSK3β pathway has been shown to increase cyclin D1 synthesis and to downregulate expression of cell cycle inhibitors p21Cip1 and p27Kip1 (7,18,19).
Figure 6).
Scheme of signalling pathways stimulating cell cycle progression. These pathways could be activated by mechanical stimuli. Arrows indicate activation of the signalling. Bars indicate inactivation/inhibition. Details of the mechanisms and abbreviations are explained in text. Cdk Cyclin-dependent kinase; ERK1/2 Extracellular signal-regulated kinase; GSK3β Glycogen synthase kinase 3 beta; Rb Retinoblastoma; PTEN Phosphatase and tensin homologue
Active GSK3β also phosphorylates cyclin D1 and promotes its proteasomal degradation (6,18,19).
Beta-catenin is another target of GSK3β that controls cyclin D1 expression (6,17). Phosphorylation of beta-catenin by GSK3β leads to its degradation in proteasomes, thus decreasing the cytosol and nuclear localization of beta-catenin. The inactivation of GSK3β due to Akt signalling allows beta-catenin to be accumulated in nuclei, where it enhances cyclin D1 transcription (17). We showed a higher accumulation of beta-catenin in nuclei in the CP grafts (Figure 4C, Table 1), which might have been a result of its stabilization due to inactivation of GSK3β.
We found that phosphorylated PTEN was increased in the grafts. The protein phosphatase PTEN dephosphorylates PI3K and, thus, functions as a negative regulator of the Akt cascade and cell proliferation (20,29,30). PTEN induces cell cycle arrest by decreasing the nuclear localization of cyclin D1 and increasing expression of cell cycle inhibitors p21Cip1 and p27Kip1 (30). The adenovirus-mediated intra-arterial delivery of PTEN inhibits neointimal hyperplasia after balloon injury (29). In addition to their roles in the regulation of cell cycle proteins, Akt and MAPK pathways are also important for SMC migration (16,31) and matrix metalloproteinase activation (15,32). Therefore, all the orchestrated changes we found in the vein grafts led to activation of proliferative signalling and cell cycle progression in the implanted grafts.
However, we found that the replacement of distension during the preparation of the vein for grafting with PP attenuated activation of the proliferation signalling and impeded cell cycle progression. In the PP grafts, lower levels of cyclin D1 messenger RNA and protein expression (Figures 4A and 4B), supported by higher levels of expression of cell cycle inhibitors p21Cip1 and p27Kip1, reduced Rb phosphorylation (Figure 4C). This appeared to result from reduced activation of signalling pathways in the PP grafts. Indeed, while ERK1/2 is equally phosphorylated in both CP and PP grafts, phosphorylation levels of p38 and Akt at Thr308 were significantly higher in CP grafts than in the veins before grafting and in the grafts where distention was avoided. Nuclear accumulation of beta-catenin was also much higher in the CP grafts than in the native veins or PP grafts. Moreover, cell cycle inhibitor p27Kip1 expression was increased only in the PP grafts, which provides additional control of the cyclin D1-Cdk4 complex activity and decreases the SMC growth.
The avoidance of distension and the beneficial effects of the drugs might have participated in these favourable results. Distention with pressure considerably exceeding physiological levels can be expected to cause additional graft remodelling, because-mechanical stimuli are well known to stimulate SMC proliferation, migration and hypertrophy (Figure 6). ERK1/2 and p38 MAPK can be activated by a mechanical stretch in vascular SMCs in a time- and strength-dependent manner (33–35). Akt signalling can be stimulated by mechanical stimuli through the cadherin-beta-catenin complex (12) as a sensor. Cadherins act as signalling receptors by regulating the intracellular localization of beta-catenin, which forms a complex with cadherin. The increase in nuclear beta-catenin, which we found in the CP grafts, was probably the composite result of the release of beta-catenin from its complex with cadherin and GSK3β inactivation.
It should be kept in mind that the drugs used for pharmacological treatment might also have contributed to the beneficial outcomes of the grafts. Pharmacological treatment for inhibition of vein graft neointimal hyperplasia has been recognized (35). The antiproliferative and anti-inflammatory effects of the topical application of MAPK inhibitor to the vein during surgery before grafting have recently been shown in a similar dog model (3). These experiments also suggest that the application of the antiproliferative drugs during the preparation procedure may be of critical importance for improving vein graft patency. However, the MAPK inhibition itself cannot replace the distension of the vein during surgery, because there is no evidence for the acute antispasmodic effects of MAPK inhibitors. The pharmacological treatment tested herein achieved both goals – prevention of vasospasm and attenuation of further remodelling. The rapid and prolonged vasodilatory effect of the suggested combination of drugs has been shown in our previous study of the human saphenous vein (23). The antiproliferative and anti-inflammatory effects of these drugs have been described. Fasudil attenuates migration and proliferation of SMCs and enhances cell apoptosis, and thus suppresses vascular remodelling and intimal hyperplasia (36). Roles for alpha-adrenergic stimulation in DNA synthesis and SMC proliferation (37) suggesting the inhibition of alpha-adrenergic receptors can restrict vascular remodelling. Nicardipine also resulted in the reduction of intimal and medial thickness in a rabbit venous graft model (38).
Limitations
One limitation of the present study is the relatively small number of animals used. However, our results were of sufficient statistical power to delineate differences in CP and PP vein grafts. Furthermore, long-term study is required to confirm the improvement in vein graft patency with PP during vein graft preparation.
CONCLUSIONS
The pronounced proliferation and development of intimal hyperplasia with the eventual occlusion of the CP vein graft may be attributed to both the arterialization of the graft and distention during the surgical procedure. The implantation of the vein graft in the arterial circulation causes pronounced activation of Akt, MAPK and beta-catenin signalling pathways, which is likely to be responsible for graft remodelling. The conventional procedure, which includes pressure distention of the vein graft, appears to cause additional stimulation of Akt and p38 signalling pathways, as well as the nuclear accumulation of beta-catenin in grafts, leading to orchestrated alterations in cell cycle regulatory proteins, which promotes the cell cycle. Pharmacological vasodilation during vein graft preparation appears to confer distinct benefits with regard to the maintenance of graft patency. The signalling cascades discussed herein provide a number of promising pharmacological targets for the prevention of vessel remodelling.
Acknowledgments
This study was supported by a Grant-in-Aid from the Heart and Stroke Foundation of British Columbia and Yukon. Ada WY Chung is the recipient of Heart and Stroke Foundation of Canada/AstraZeneca Canada Research Fellowship Award. Honglin Luo is a New Investigator of the CIHR/St Paul’s Hospital Foundation Award and a Scholar of the Michael Smith Foundation for Health Research.
REFERENCES
- 1.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: Pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 2.Saunders PC, Pintucci G, Bizekis CS, et al. Vein graft arterialization causes differential activation of mitogen-activated protein kinases. J Thorac Cardiovasc Surg. 2004;127:1276–84. doi: 10.1016/j.jtcvs.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Pintucci G, Saunders PC, Gulkarov I, et al. Anti-proliferative and anti-inflammatory effects of topical MAPK inhibition in arterialized vein grafts. FASEB J. 2006;20:398–400. doi: 10.1096/fj.05-4114fje. [DOI] [PubMed] [Google Scholar]
- 4.Miyake T, Aoki M, Shiraya S, et al. Inhibitory effects of NF B decoy oligodeoxynucleotides on neointimal hyperplasia in a rabbit vein graft model. J Mol Cell Cardiol. 2006;41:431–40. doi: 10.1016/j.yjmcc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–9. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Boehm M, Nabel EG. The cell cycle and cardiovascular diseases. Prog Cell Cycle Res. 2003;5:19–30. [PubMed] [Google Scholar]
- 7.Stabile E, Zhou YF, Saji M, et al. Akt controls vascular smooth muscle cell proliferation in vitro and in vivo by delaying G1/S exit. Circ Res. 2003;93:1059–65. doi: 10.1161/01.RES.0000105086.31909.1B. [DOI] [PubMed] [Google Scholar]
- 8.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–71. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Zhou RH, Lee TS, Tsou TC, et al. Stent implantation activates Akt in the vessel wall: Role of mechanical stretch in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:2015–20. doi: 10.1161/01.ATV.0000095161.06906.ED. [DOI] [PubMed] [Google Scholar]
- 10.Izumi Y, Kim S, Namba M, et al. Gene transfer of dominant-negative mutants of extracellular signal-regulated kinase and c-Jun NH2-terminal kinase prevents neointimal formation in balloon-injured rat artery. Circ Res. 2001;88:1120–6. doi: 10.1161/hh1101.091267. [DOI] [PubMed] [Google Scholar]
- 11.Nath N, Wang S, Betts V, Knudsen E, Chellappan S. Apoptotic and mitogenic stimuli inactivate Rb by differential utilization of p38 and cyclin-dependent kinases. Oncogene. 2003;22:5986–94. doi: 10.1038/sj.onc.1206843. [DOI] [PubMed] [Google Scholar]
- 12.Slater SC, Koutsouki E, Jackson CL, et al. R-cadherin:beta-catenin complex and its association with vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 2004;24:1204–10. doi: 10.1161/01.ATV.0000130464.24599.e0. [DOI] [PubMed] [Google Scholar]
- 13.Angelini GD, Bryan AJ, Williams HM, Morgan R, Newby AC. Distention promotes platelet and leukocyte adhesion and reduces short-term patency in pig arteriovenous bypass grafts. J Thorac Cardiovasc Surg. 1990;99:433–9. [PubMed] [Google Scholar]
- 14.Chung AW, Rauniyar P, Luo H, Hsiang YN, van Breemen C, Okon EB. Pharmacologic relaxation of vein grafts is beneficial compared with pressure distention caused by upregulation of endothelial nitric oxide synthase and nitric oxide production. J Thorac Cardiovasc Surg. 2006;132:925–32. doi: 10.1016/j.jtcvs.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Chung AW, Rauniyar P, Luo H, Hsiang YN, van Breemen C, Okon EB. Pressure distention compared with pharmacologic relaxation in vein grafting upregulates matrix metalloproteinase-2 and -9. J Vasc Surg. 2005;42:747–56. doi: 10.1016/j.jvs.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Hu Y, Mayr M, Xu Q. Cyclic strain stress-induced mitogen-activated protein kinase (MAPK) phosphatase 1 expression in vascular smooth muscle cells is regulated by Ras/Rac-MAPK pathways. J Biol Chem. 1999;274:25273–80. doi: 10.1074/jbc.274.36.25273. [DOI] [PubMed] [Google Scholar]
- 17.Uglow EB, Slater S, Sala-Newby GB, et al. Dismantling of cadherin-mediated cell-cell contacts modulates smooth muscle cell proliferation. Circ Res. 2003;92:1314–21. doi: 10.1161/01.RES.0000079027.44309.53. [DOI] [PubMed] [Google Scholar]
- 18.Sedding DG, Seay U, Fink L, et al. Mechanosensitive p27Kip1 regulation and cell cycle entry in vascular smooth muscle cells. Circulation. 2003;108:616–22. doi: 10.1161/01.CIR.0000079102.08464.E2. [DOI] [PubMed] [Google Scholar]
- 19.Rössig L, Badorff C, Holzmann Y, Zeiher AM, Dimmeler S. Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation. J Biol Chem. 2002;227:9684–9. doi: 10.1074/jbc.M106157200. [DOI] [PubMed] [Google Scholar]
- 20.Mourani PM, Garl PJ, Wenzlau JM, Carpenter TC, Stenmark KR, Weiser-Evans MC. Unique, highly proliferative growth phenotype expressed by embryonic and neointimal smooth muscle cells is driven by constitutive Akt, mTOR, and p70S6K signaling and is actively repressed by PTEN. Circulation. 2004;109:1299–306. doi: 10.1161/01.CIR.0000118462.22970.BE. [DOI] [PubMed] [Google Scholar]
- 21.Angelini GD, Breckenridge IM, Psaila JV, Williams HM, Henderson AH, Newby AC. Preparation of human saphenous vein for coronary artery bypass grafting impairs its capacity to produce prostacyclin. Cardiovasc Res. 1987;21:28–33. doi: 10.1093/cvr/21.1.28. [DOI] [PubMed] [Google Scholar]
- 22.Liu ZG, Liu XC, Yim AP, He GW. Direct measurement of nitric oxide release from saphenous vein: Abolishment by surgical preparation. Ann Thorac Surg. 2001;71:133–7. doi: 10.1016/s0003-4975(00)02231-1. [DOI] [PubMed] [Google Scholar]
- 23.Crowley CM, Lee CH, Gin SA, Keep AM, Cook RC, van Breemen C. The mechanism of excitation-contraction coupling in phenylephrine-stimulated human saphenous vein. Am J Physiol Heart Circ Physiol. 2002;283:H1271–81. doi: 10.1152/ajpheart.01129.2001. [DOI] [PubMed] [Google Scholar]
- 24.Okon EB, Millar MJ, Crowley CM, et al. Effect of moderate pressure distention on the human saphenous vein vasomotor function. Ann Thorac Surg. 2004;77:108–14. doi: 10.1016/j.athoracsur.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Hao H, Gabbiani G, Bochaton-Piallat ML. Arterial smooth muscle cell heterogeneity: Implications for atherosclerosis and restenosis development. Arterioscler Thromb Vasc Biol. 2003;23:1510–20. doi: 10.1161/01.ATV.0000090130.85752.ED. [DOI] [PubMed] [Google Scholar]
- 26.Zhan Y, Kim S, Izumi Y, et al. Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression. Arterioscler Thromb Vasc Biol. 2003;23:795–801. doi: 10.1161/01.ATV.0000066132.32063.F2. [DOI] [PubMed] [Google Scholar]
- 27.Pyles JM, March KL, Franklin M, Mehdi K, Wilensky RL, Adam LP. Activation of MAP kinase in vivo follows balloon overstretch injury of porcine coronary and carotid arteries. Circ Res. 1997;81:904–10. doi: 10.1161/01.res.81.6.904. [DOI] [PubMed] [Google Scholar]
- 28.Nofer JR, Junker R, Pulawski E, et al. High density lipoproteins induce cell cycle entry in vascular smooth muscle cells via mitogen activated protein kinase-dependent pathway. Thromb Haemost. 2001;85:730–5. [PubMed] [Google Scholar]
- 29.Huang J, Niu XL, Pippen AM, Annex BH, Kontos CD. Adenovirus-mediated intraarterial delivery of PTEN inhibits neointimal hyperplasia. Arterioscler Thromb Vasc Biol. 2005;25:354–8. doi: 10.1161/01.ATV.0000151619.54108.a5. [DOI] [PubMed] [Google Scholar]
- 30.Moon SK, Kim HM, Kim CH. PTEN induces G1 cell cycle arrest and inhibits MMP-9 expression via the regulation of NF-kappaB and AP-1 in vascular smooth muscle cells. Arch Biochem Biophys. 2004;421:267–76. doi: 10.1016/j.abb.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Birukov KG, Lehoux S, Birukova AA, Merval R, Tkachuk VA, Tedgui A. Increased pressure induces sustained protein kinase C-independent herbimycin A-sensitive activation of extracellular signal-related kinase 1/2 in the rabbit aorta in organ culture. Circ Res. 1997;81:895–903. doi: 10.1161/01.res.81.6.895. [DOI] [PubMed] [Google Scholar]
- 32.Aikawa R, Nagai T, Kudoh S, et al. Integrins play a critical role in mechanical stress-induced p38 MAPK activation. Hypertension. 2002;39:233–8. doi: 10.1161/hy0202.102699. [DOI] [PubMed] [Google Scholar]
- 33.Kawabe J, Okumura S, Lee MC, Sadoshima J, Ishikawa Y. Translocation of caveolin regulates stretch-induced ERK activity in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H1845–52. doi: 10.1152/ajpheart.00593.2003. [DOI] [PubMed] [Google Scholar]
- 34.Zampetaki A, Zhang Z, Hu Y, Xu Q. Biomechanical stress induces IL-6 expression in smooth muscle cells via Ras/Rac1-p38 MAPK-NF B signaling pathways. Am J Physiol Heart Circ Physiol. 2005;288:H2946–54. doi: 10.1152/ajpheart.00919.2004. [DOI] [PubMed] [Google Scholar]
- 35.Schachner T. Pharmacologic inhibition of vein graft neointimal hyperplasia. J Thorac Cardiovasc Surg. 2006;131:1065–72. doi: 10.1016/j.jtcvs.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 36.Shibata R, Kai H, Seki Y, et al. Role of Rho-associated kinase in neointima formation after vascular injury. Circulation. 2001;103:284–9. doi: 10.1161/01.cir.103.2.284. [DOI] [PubMed] [Google Scholar]
- 37.Nakaki T, Nakayama M, Yamamoto S, Kato R. Alpha 1-adrenergic stimulation and beta 2-adrenergic inhibition of DNA synthesis in vascular smooth muscle cells. Mol Pharmacol. 1990;37:30–6. [PubMed] [Google Scholar]
- 38.Gökçe O, Gökçe C, Günel S, et al. Preventive effect of nicardipine on hyperplastic changes in venous bypass grafts. World J Surg. 1993;17:94–9. doi: 10.1007/BF01655716. [DOI] [PubMed] [Google Scholar]