Abstract
Pericardiocentesis for therapeutic drainage of pericardial fluid may be associated with a variety of complications, including laceration of the right ventricle or coronary artery, arrhythmias, viscus perforation, hypotension, pneumothorax, adult respiratory distress syndrome and death. Hemodynamic derangements such as acute left ventricular failure, pulmonary edema and cardiogenic shock are infrequent and, hence, less well recognized. The present report describes a patient with pericardial effusion and tamponade who developed cardiogenic shock requiring inotropic support shortly following uncomplicated ultrasound-guided pericardial drainage.
Keywords: Left ventricular dysfunction, Pericardial effusion, Pericardiocentesis
Abstract
Une ponction péricardique en vue du drainage thérapeutique du liquide péricardique peut s’associer à diverses complications, y compris une lacération du ventricule droit ou de l’artère coronaire, des arythmies, une perforation des viscères, une hypotension, un pneumothorax, un syndrome de détresse respiratoire aiguë et un décès. Les perturbations hémodynamiques, telles qu’une insuffisance ventriculaire gauche aiguë, un œdème pulmonaire et un choc cardiogène sont rares, donc moins bien dépistées. Le présent rapport décrit le cas d’un patient souffrant d’effusion péricardique et de tamponnage, qui a développé un choc cardiogène exigeant un soutien inotrope peu après un drainage péricardique orienté par échographie sans complication.
A 45-year-old Caucasian woman with recently diagnosed acute myelogenous leukemia was admitted to hospital with complaints of right lower extremity swelling and progressively worsening dyspnea. Her past history included Hodgkin’s lymphoma (in remission). Doppler ultrasound of her lower extremities ruled out deep venous thrombosis. On day 3 of hospitalization, the patient complained of shortness of breath at rest and cough. On physical examination, she had engorged jugular veins, tachycardia and a pulsus paradoxus of 20 mmHg. An electrocardiogram (ECG) demonstrated sinus tachycardia, low voltage and electrical alter-nans. The chest x-ray was unremarkable except for small bilateral pleural effusions. Emergent transthoracic echocardiography (TTE) showed normal left ventricular (LV) wall motion and an ejection fraction of 60% to 65%. Moderate circumferential pericardial effusion with compression of the right heart chambers and echocardiographic evidence of tamponade was noted.
Echocardiography- and fluoroscopy-guided pericardiocentesis was performed for incipient tamponade, and approximately 500 mL of serosanguinous fluid was drained. A pigtail catheter was left in place. Cytological evaluation did not demonstrate any malignant cells.
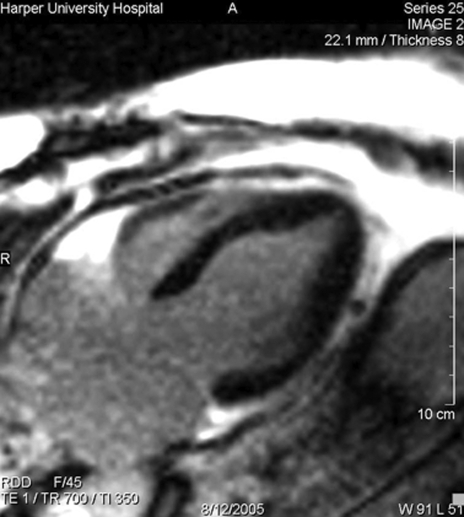
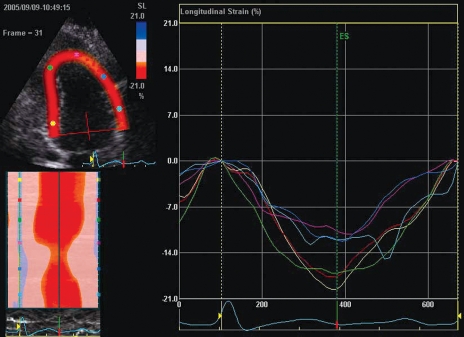
Six hours following the pericardiocentesis, the patient became hypotensive, tachypneic and tachycardic, requiring endotracheal intubation and inotropic support. A repeat TTE showed mid-anterior and anteroseptal akinesis, apical sparing and moderate LV dysfunction with an estimated ejection fraction of 30%. Minimal residual pericardial fluid was observed. A 12-lead ECG showed sinus tachycardia without ST-T changes. Chest x-ray showed pulmonary edema. Cardiac biomarkers, including troponin I, demonstrated minimally elevated values, with a peak value of 1.2 μg/mL (normal peak: 0.40 μg/mL). Cardiac magnetic resonance imaging (CMR) was performed to assess for myocarditis and myonecrosis. No evidence of delayed enhancement following gadolinium contrast was identified (Figure 1). The patient remained in cardiogenic shock for approximately 72 h, requiring ventilatory and inotropic support. Serial TTE showed progressive improvement in systolic function, and the patient was weaned off inotropic and ventilatory support approximately 96 h after the initial hypotensive event. A repeat outpatient echocardiogram with strain imaging to identify regional myocardial dysfunction showed normalization of global and segmental LV function, and no recurrence of effusion (Figure 2). A pharmacological nuclear perfusion stress test performed following discharge was normal.
Figure 1).
A long-axis magnetic resonance image showing no evidence of delayed enhancement following gadolinium administration
Figure 2).
Two-dimentional strain imaging (apical view) showing normalization of global and segmental left ventricular function. Note the homogeneity of segmental systolic strain curves representing the anterior and inferior walls
DISCUSSION
In 1983, Vandyke et al (1) first described pulmonary edema following pericardiocentesis for cardiac tamponade. They hypothesized that LV dysfunction following pericardiocentesis for chronic tamponade may be related to acute hemodynamic changes and interventricular volume mismatch in the setting of elevated systemic vascular resistance and tachycardia. The initial LV response to the release of pericardial constraint and restoration of right ventricular output accompanying pericardiocentesis is characterized by optimization of the Frank-Starling mechanism. Further increments in preload can cause increasing systolic wall stress, a reduction in stroke volume and pulmonary edema. These and other authors (2,3) have suggested that this response may be related to the magnitude and the velocity at which the load develops.
An imbalance between the sympathetic and parasympathetic limbs of the autonomic system, with an apparent attenuation of sympathetic outflow (following relief of tamponade) unmasking occult LV dysfunction previously compensated by high catecholamine levels, has also been postulated as an alternative mechanism. However, the complete recovery of LV function in our patient and in prior cases (4,5) makes this explanation less compelling.
Moreover, primary alterations in intramyocardial blood distribution, myocardial ischemia and subendocardial hemorrhage during tamponade, resulting in ‘stunned myocardium’, have also been proposed (6,7). Whether pericardiocentesis provides a milieu for the development of myocardial stunning or a variant of reperfusion injury remains unknown. The absence of delayed gadolinium enhancement on CMR imaging rules out myocardial necrosis as the predominant mechanism of systolic dysfunction in our patient. Delayed-enhancement CMR has been validated as a very sensitive tool for the diagnosis of myocardial infarction. Typically, gadolinium does not penetrate noninfarcted myocardium; nonetheless, its access into the infarcted myocardium is feasible due to disruption of the extracellular compartment, allowing delayed distribution to occur 10 min to 15 min after injection of gadolinium contrast. Imaging with an inversion recovery sequence set to null the uninfarcted myocardium highlights areas of infarct that appear ‘bright’ relative to normal, ‘dark’ myocardium. With this novel application, it is possible to visualize even subtle subendocardial infarcts hitherto impossible to detect using conventional techniques.
Furthermore, the absence of obstructive coronary disease in our patient and in previous cases (5,8) essentially eliminates epicardial coronary disease as a potential mechanism.
While the exact pathophysiological mechanism for LV dysfunction following pericardiocentesis remains speculative, we believe that the abrupt disproportionate increase in LV wall stress, coupled with the chronicity of tamponade, may be critical determining factors (4–6). Myocardial stunning may play a contributory role, considering the almost complete and uniform recovery of function seen in previously reported cases and in our patient, as verified by the homogeneity of two-dimensional strain measurements. Two-dimensional strain echocardiography, also termed speckle-tracking imaging, is akin to magnetic resonance tagging. Acoustic markers from the image are temporally tracked in two dimensions, providing information on the rate and extent of tissue deformation. A superior and quantitative assessment of regional function is possible with this technique compared with visual assessment. Importantly, ‘visual artifacts’, such as passive tethering and translational motion, are overcome, which is an important discriminatory feature in the assessment of patients with regional wall motion abnormalities.
Finally, in patients with chronic effusion and tamponade, it appears prudent to initially decompress the pericardium gradually until tamponade physiology resolves. There are no guidelines or recommendations in the literature either for the quantity of fluid that may be safely removed or for the rate of fluid drainage during pericardiocentesis. However, in acute tamponade, intrapericardial pressure typically declines rapidly, and symptomatic improvement ensues after aspiration of the first 50 mL to 200 mL of pericardial fluid. Thereafter, as proposed by Vandyke et al, the fluid should be drained slowly via an indwelling pericardial pigtail catheter, perhaps in steps of less than 1 L at a time, over 24 h. This may permit adaptive changes in coronary flow, myocardial mechanics and wall stress by minimizing abrupt fluctuations in loading conditions otherwise associated with a more rapid decompression of the pericardial space.
REFERENCES
- 1.Vandyke WH, Jr, Cure J, Chakko CS, Gheorghiade M. Pulmonary edema after pericardiocentesis for cardiac tamponade. N Engl J Med. 1983;309:595–6. doi: 10.1056/NEJM198309083091006. [DOI] [PubMed] [Google Scholar]
- 2.Spodick DH. The normal and diseased pericardium: Current concepts of pericardial physiology, diagnosis and treatment. J Am Coll Cardiol. 1983;1:240–51. doi: 10.1016/s0735-1097(83)80025-4. [DOI] [PubMed] [Google Scholar]
- 3.Glasser F, Fein AM, Feinsilver SH, Cotton E, Niederman MS. Non-cardiogenic pulmonary edema after pericardial drainage for cardiac tamponade. Chest. 1988;94:869–70. doi: 10.1378/chest.94.4.869. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe MW, Edelman ER. Transient systolic dysfunction after relief of cardiac tamponade. Ann Intern Med. 1993;119:42–4. doi: 10.7326/0003-4819-119-1-199307010-00007. [DOI] [PubMed] [Google Scholar]
- 5.Chamoun A, Cenz R, Mager A, et al. Acute left ventricular failure after large volume pericardiocentesis. Clin Cardiol. 2003;26:588–90. doi: 10.1002/clc.4960261209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamaya Y, Dohi S, Ueda N, Akamatsu S. Severe circulatory collapse immediately after pericardiocentesis in a patient with chronic cardiac tamponade. Anesth Analg. 1993;77:1278–81. doi: 10.1213/00000539-199312000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Wechsler AS, Auerbach BJ, Graham TC, Sabiston DC., Jr Distribution of intramyocardial blood flow during pericardial tamponade. Correlation with microscopic anatomy and intrinsic myocardial contractility. J Thorac Cardiovasc Surg. 1974;68:847–56. [PubMed] [Google Scholar]
- 8.Ligero C, Leta R, Bayes-Genis A. Transient biventricular dysfunction following pericardiocentesis. Eur J Heart Fail. 2006;8:102–4. doi: 10.1016/j.ejheart.2005.05.012. [DOI] [PubMed] [Google Scholar]