Abstract
Very little is known about the human pulvinar; suggestions for its function include relaying input from cortical areas, allocating visual attention, supporting feature binding, and other integrative processes. The diversity of hypotheses about pulvinar function highlights our lack of understanding of its basic role. A conspicuously missing piece of information is whether the human pulvinar encodes visual information topographically. The answer to this question is crucial, as it dramatically constrains the sorts of computational and cognitive processes that the pulvinar might carry out. Here we used fMRI to test for position‐sensitive encoding in the human pulvinar. Subjects passively viewed flickering Gabor stimuli, and as the spatial separation between Gabors increased, the correlation between patterns of activity across voxels within the right pulvinar decreased significantly. The results demonstrate the existence of precise topographic coding in the human pulvinar lateralized to the right hemisphere, and provide a means of functionally localizing this topographic region. Hum Brain Mapp 2009. © 2007 Wiley‐Liss, Inc.
Keywords: vision, perception, localization, retinotopy, topography, V1, V2, fMRI, LGN
INTRODUCTION
Nearly all of what is known about the organization of the pulvinar comes from research on cats and monkeys. In both of these species, the pulvinar has widespread connectivity with visual cortex, establishing cortico‐thalamo‐cortical loops with nearly every visual area [Casanova, 2004]. The pulvinar receives its driving afferents from layer 5 of several cortical areas including V1, V2, and V3; its projections to the cortex are modulatory and arrive primarily in layer 3 [Sherman and Guillery, 2002]. Since the visual areas of the pulvinar are driven by descending pathways from the cortex, it is considered a higher‐order visual nucleus.
The cat pulvinar is referred to as the LP‐pulvinar complex and consists of three main subdivisions: the lateral and medial portions of the LP nucleus (LPl and LPm) and the pulvinar. There is good agreement on the boundaries of these subdivisions established independently by anatomical studies and retinotopic parcellation. Each subdivision contains a retinotopic map of the contralateral visual field which extends slightly over the vertical meridian. Progressing ventrally, receptive fields generally advance downward through the visual field across the horizontal meridian [Raczkowski and Rosenquist, 1981]. Receptive field characteristics differ among LPl, LPm, and the pulvinar. Receptive fields in LPl are mostly binocular and are orientation‐ and direction‐sensitive [Casanova et al., 1989; Chalupa and Abramson, 1989; Mason, 1981]. These cells are also highly sensitive to textured patterns and visual noise [Casanova and Savard, 1996]. Whereas LPl receives cortical projections from areas 17 to 18, LPm receives subcortical projections from the superior colliculus. Receptive fields in LPm are about four times larger than those in LPl; some may cover whole hemifield. Fewer LPm cells are orientation‐selective than in LPl, and their tuning is broader [Chalupa and Abramson, 1989]. Less is known about the receptive fields in the pulvinar subdivision; they are perhaps similar to those in LPl.
In monkeys, there is less agreement on how the pulvinar should be subdivided, but traditionally four nuclei are identified on cytoarchitectonic grounds: inferior (PI), lateral (PL), medial (PM), and anterior (PA). There is not a precise correspondence between these subdivisions and the response properties of cells therein, but inferior and lateral pulvinar are generally considered the main visual nuclei, while anterior pulvinar has connections with somatosensory cortex and medial pulvinar connects with multisensory areas [Kaas and Lyon, 2007]. Both inferior and lateral subdivisions contain a complete retinotopic map of the contralateral visual field, with the smallest receptive fields corresponding to the fovea [Bender, 1981, 1982; Petersen et al., 1985]. Accordingly, the central visual field is overrepresented [Bender, 1981]. These spatiotopic maps are organized such that the inferior field is represented dorsally, while the superior field is represented ventrally [Bender, 1981]. There is comparatively little data on receptive fields in the monkey pulvinar, but they are generally smaller than in cats, averaging 1°–5° in diameter in inferior pulvinar [Bender, 1982]. Most are binocular and orientation selective, and about half are selective for the direction of movement [Bender, 1982; Felsten et al., 1983]. Interestingly, most cells in the inferior pulvinar show fluctuations in responsiveness correlated with the general level of arousal [Bender, 1982].
Very little is known about the organization of the human pulvinar outside of what is inferred from work on cats and monkeys. On the basis of the retinotopy that is observed in the cat and monkey pulvinar, it seems likely that similar spatiotopic organization exists in the human pulvinar. There is some evidence from a single subject to support this: Ward et al. [ 2002] found that a patient with damage to the anterior and dorsal boundary of the pulvinar showed performance deficits in her inferior contralesional visual quadrant where one would expect to see a deficit if the inferior visual field is represented dorsally in humans as it is in monkeys. This finding hints at spatiotopic coding in the human pulvinar, but only in the limited context of the affected quadrant in a single patient, and it cannot speak to what the resolution of such a mapping would be. Another recent study that explored the organization of the human pulvinar with fMRI was less conclusive [Kastner et al., 2004].
The role of the pulvinar in vision is poorly understood. However, in contrast with the LGN which acts as a first‐order relay of retinal signals to striate cortex [Guillery and Sherman, 2002; Sherman and Guillery, 2002], the pulvinar's widespread bidirectional connectivity, forming cortico‐thalamo‐cortical loops with nearly all visual areas, indicates that it is not simply a passive relay [Casanova, 2004].
The pulvinar has long been implicated in visual attentional processing. Specifically, several studies have suggested that the pulvinar is involved in filtering unwanted information in the visual scene [Desimone et al., 1990; LaBerge and Buchsbaum, 1990]. Desimone et al. [ 1990] found that when the pulvinar was chemically deactivated, responses to targets in the visual field contralateral to deactivation were unaffected when the target was presented alone but were significantly impaired when a distractor was present. An analogous PET study on human subjects showed an elevated level of glucose uptake in the pulvinar when distractors were present in a target identification task (though it could not be determined whether left, right, or both hemispheres contributed to this effect) [LaBerge and Buchsbaum, 1990]. Recent fMRI studies have confirmed that a portion of the pulvinar is active during attentionally demanding tasks. Yantis et al. [ 2002] found that during attentional shifts across hemifield (left to right or vice versa), an area in inferior left pulvinar was significantly activated. Also, Cotton and Smith [ 2007] have shown that attention to a lateralized visual stimulus produces activity in inferior pulvinar contralateral to the stimulus and suppression in the inferior pulvinar ipsilateral to the stimulus. Other studies on humans and monkeys have found deficits in spatially directed attention accompanying specific lesions of the pulvinar [Karnath et al., 2002; Michael and Desmedt, 2004; Petersen et al., 1987]. Together, these studies reveal that the pulvinar likely has a role in spatially directed attention; however, the nature of this role in humans depends on the extent and precision of spatial coding in the pulvinar, which has not yet been established.
A more recent hypothesis is that the pulvinar might play a key role in feature binding. Ward et al. [ 2002] found that a subject with pulvinar damage because of a stroke made proportionally many more feature conjunction errors in the contralesional visual field than in the ipsilesional field. Since spatial coding is requisite to establish feature binding [Cohen and Ivry, 1989; Wolford and Shum, 1980], and the precision of spatial coding affects the accuracy of feature binding [Prinzmetal et al., 1995; Treisman and Schmidt, 1982], one possible role of topographic maps in the pulvinar might be to carry feature position information for feature binding. In pursuing this possibility, however, it is important to determine whether and to what degree the human pulvinar carries information about object position.
The aim of this study is to test for the existence and the precision of topographic encoding of visual stimuli in the human pulvinar, using fMRI. Establishing the precision of topographic encoding in the pulvinar is crucial to address its function, whether that be in the allocation or control of attention [Karnath et al., 2002; Petersen et al., 1987], the binding of features [Ward et al., 2002], or even passive visual processing.
MATERIALS AND METHODS
Stimuli
Stimuli consisted of one fixation baseline condition and five stimulus conditions. In each stimulus condition, four Gabors (sine wave luminance modulations within a Gaussian contrast envelope) were presented simultaneously, one in each of the four quadrants of the visual field. These conditions differed from each other with respect to the eccentricities of the Gabors: the centers of the Gabors, defined by the points of peak contrast, fell at 8.73°, 8.92°, 9.05°, 9.32°, and 9.68° eccentricity in the five conditions, respectively (Fig. 1). These fine position increments between conditions were chosen to ensure that BOLD activity from any two adjacent conditions was highly correlated. As with any parametric manipulation, shifting the Gabors by too large an increment might have resulted in having precise position sensitivity hidden by a floor effect in our analysis. All Gabors had an identical contrast envelope with a standard deviation of 1.70°. The spatial frequency of the luminance sine wave was 0.38 cycles per degree. The Gabors were flickered in counterphase at 7.5 Hz, and the phase of each Gabor was randomized on each trial. Stimuli were always presented bilaterally in order to maximize signal‐to‐noise (Kastner et al. [ 2004], only observed activations in the pulvinar with bilaterally presented checkerboard stimuli; no functional activations were found when stimuli were presented unilaterally).
Figure 1.
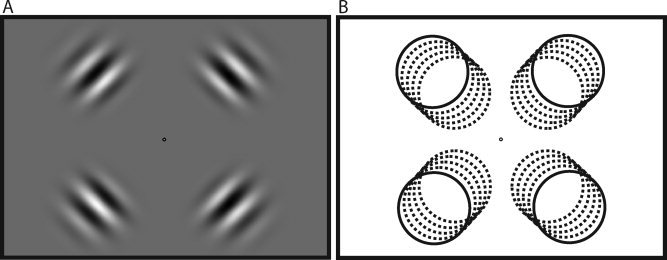
Gabor stimuli. (A) A sample stimulus condition. Each condition consisted of four Gabors (1.70° standard deviation contrast envelope, 0.38 cycle/degree sine wave) flickered in counterphase at 7.5 Hz. (B) Across the five conditions, Gabor centers (defined by peak contrast) were shifted to 8.73°, 8.92°, 9.05°, 9.32°, and 9.68° eccentricity from the fixation point.
In all six conditions, there was a fixation task. During each 10 s trial, a small textured annulus (either circular or radial grating, chosen randomly; 0.98° diameter) was flashed for 500 ms surrounding the fixation point at least seven times but no more than 11 times, and one texture was always presented more often than the other (determined randomly). Subjects were instructed to count the number of times that each type of grating (circular and radial) appeared in each 10 s block and report which had occurred more often. In this way, subjects always attended at the fixation point and passively viewed the surrounding Gabors. During the fixation baseline condition, only this fixation task was visible. Effects related to the fixation task were therefore factored out of the analysis.
fMRI Data Collection
In each functional imaging run, the six conditions were randomly interleaved in 36 ten second blocks (360 s runs). Each subject participated in a minimum of five functional runs. Subjects maintained fixation and performed the counting task at a central point (0.39° diameter) throughout the entire experiment.
Seven subjects participated in the experiment. Scanning protocols were approved by the University of California, Davis, Human Subject Review Board, and informed consent was obtained from all subjects. Imaging was conducted on a 3‐Tesla Siemens TRIO scanner located at the UC Davis Imaging Research Center. Stimuli were back‐projected with a Digital Projection Mercury 5000HD projector (75 Hz) onto a semitransparent screen from outside the bore. A mirror angled at 45°, located 9.5 cm directly above the subject, provided a reflected view of the stimuli. Functional images were acquired with a gradient‐recalled echo EPI sequence. Whole‐brain structural images were collected with a high resolution (1 mm3) Turbo Spin Echo scan that was used to align functional images. The acquisition parameters were: TR = 2,000 ms, TE = 26 ms, FA = 90°, FOV = 22 × 22 cm2, voxel size = 1.528 × 1.528 × 2.5 mm3, 20 slices/volume. The imaging volume was centered on the calcarine sulcus, covering the occipital lobe and the pulvinar.
All preprocessing, including linear trend removal and three‐dimensional (3D) motion correction, as well as GLM analyses were conducted with Brain Voyager QX (Brain Innovation B.V., Maastricht, The Netherlands). Motion correction was performed separately for each run using trilinear interpolation, and with the exception of the first run for each subject, motion corrected data from the previous run was used for intrasession alignment. The images were not spatially smoothed. A correction for serial correlations (removal of first‐order autocorrelation) was used prior to all GLM analyses. Each subject's high resolution anatomical image was transformed to Talaraich coordinates, and the data for each functional run was individually aligned to the subject's Talairach‐transformed anatomical image. Performing individual alignments for each functional run, within each subject, mitigated any effect of subject movement between functional runs.
For each functional run, a GLM was fit to the data with five predictors (corresponding to the five Gabor eccentricities). These five Gabor eccentricities were separately contrasted against a sixth predictor (the fixation baseline) to discover the pattern of activity produced by each Gabor eccentricity. Separate activation maps based on the GLM were created for each of the five Gabor eccentricities. In these 3D maps, every voxel had a statistical value associated with it (there was no threshold—each voxel had a t value, though many were very close to zero and many were negative). The reason for including all voxel responses—not thresholding—is because spatial patterns of negative and near‐zero voxel responses can carry meaningful and precise information about object position in the visual cortex [Bressler et al., 2007].
Correlational Method
To obtain regions of interest (ROIs) for right and left pulvinar, we queried the Talairach Atlas [Talairach and Tournoux, 1988] using Talairach Daemon Client [Lancaster et al., 1997] for all points that fell within the pulvinar. The corresponding coordinates were divided by hemisphere and then used to define the voxels in the right and left pulvinar ROIs (Fig. 2). This provided the advantage of using nonarbitrary, anatomically defined ROIs. However, these ROIs encompassed the entire pulvinar and thus contained both visual and nonvisual areas. This was a conservative but necessary tradeoff, since the visual subdivisions of the human pulvinar have not been well defined.
Figure 2.
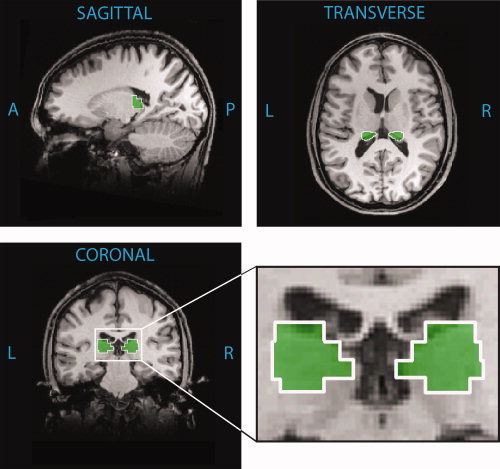
Anatomically defined regions of interest. ROIs for right and left hemisphere pulvinar are outlined in white, shown in a coronal plane (y = −24), a sagittal plane (x = 18), and a transverse plane (z = 13). These ROIs were obtained by querying the Talairach Atlas [Talairach and Tournoux, 1988] using Talairach Daemon Client [Lancaster et al., 1997] for all points that fell within the pulvinar. Resulting coordinates were used to define the voxels in the right and left hemisphere ROIs separately. An enlarged view of the coronal plane shows the ROIs adjacent to the lateral ventricles. ROIs are depicted on an anatomical image from a single subject for clarity.
The GLM analyses, described earlier, produced five volumetric statistical maps. Within each subject, we computed the correlation between each possible pair of maps (10 such pairs were possible) for the voxels within the right and left pulvinar ROIs. That is to say, we took two activity maps (corresponding to two eccentricity conditions), paired the voxels from corresponding points in the two maps, and computed a Pearson r value for the pairs (Fig. 3A). This was computed independently for voxels in the left pulvinar ROI and the right pulvinar ROI, and repeated for all 10 possible pairings of statistical maps.
Figure 3.
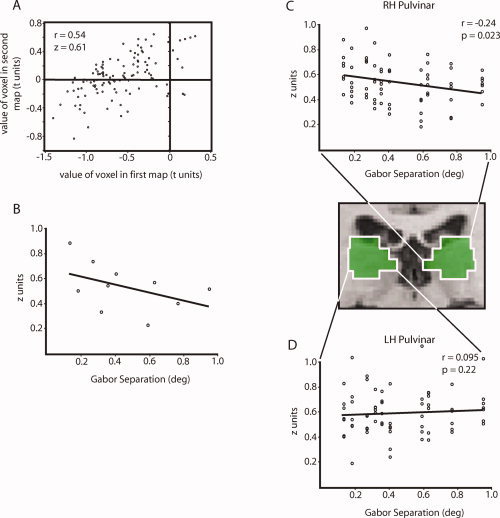
Computation of position discrimination slopes. (A) A sample correlation between two volumetric activity maps, performed on voxels within a 5 mm3 ROI in the right pulvinar. Each voxel's value in the second map was plotted against its value in the first map, and a Pearson r was computed on this plot to provide a measure of the similarity of the two maps. The resulting r value from this correlation was converted to a Fisher z score by
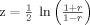 . Note that many voxels have negative values in one or both activity maps. Although activity within these voxels is downwardly modulated by the stimuli, this activity still has the potential to carry precise information about the positions of the stimuli [Bressler et al., 2007]. (B) Each z score computed as in (A) was plotted with nine other z scores computed in the same way, representing all possible pairs taken from the five volumetric activity maps associated with each subject. Each z score was plotted against the corresponding increment in Gabor position. The slope of a regression line fit to this plot provides an index of stimulus discrimination within the given ROI. This plot shows the z scores for the right hemisphere pulvinar ROI in a representative subject. (C) Fisher z scores from the right pulvinar ROI in all seven subjects; z scores for each subject were normalized to that subject's mean z score. A linear regression fit to this plot revealed a significant correlation (r = 0.24, P = 0.023, slope = 0.155), indicating significant position discrimination in the right hemisphere pulvinar. (D) Fisher z scores from the left pulvinar ROI in all seven subjects, normalized as in the previous plot. A linear regression fit to this plot was not significant (r = 0.073, P = 0.27, slope = 0.049). The regression coefficients for the left and right pulvinar plots were also significantly different from each other (Z = 1.84, P = 0.033).
. Note that many voxels have negative values in one or both activity maps. Although activity within these voxels is downwardly modulated by the stimuli, this activity still has the potential to carry precise information about the positions of the stimuli [Bressler et al., 2007]. (B) Each z score computed as in (A) was plotted with nine other z scores computed in the same way, representing all possible pairs taken from the five volumetric activity maps associated with each subject. Each z score was plotted against the corresponding increment in Gabor position. The slope of a regression line fit to this plot provides an index of stimulus discrimination within the given ROI. This plot shows the z scores for the right hemisphere pulvinar ROI in a representative subject. (C) Fisher z scores from the right pulvinar ROI in all seven subjects; z scores for each subject were normalized to that subject's mean z score. A linear regression fit to this plot revealed a significant correlation (r = 0.24, P = 0.023, slope = 0.155), indicating significant position discrimination in the right hemisphere pulvinar. (D) Fisher z scores from the left pulvinar ROI in all seven subjects, normalized as in the previous plot. A linear regression fit to this plot was not significant (r = 0.073, P = 0.27, slope = 0.049). The regression coefficients for the left and right pulvinar plots were also significantly different from each other (Z = 1.84, P = 0.033).
The resulting r values were converted to Fisher z scores because equal distances between these scores are equally probable [Cohen, 1988], and Fisher z scores can be directly compared, unlike r values. We then plotted each of the 10 z scores as a function of the eccentricity separation between the two conditions that produced the z score, such that the horizontal axis representing separation had values at 0.136°, 0.184°, 0.271°, 0.320°, 0.359°, 0.407°, 0.591°, 0.630°, 0.766°, and 0.950° visual angle. A linear regression was fit to this plot (Fig. 3C).
The slope of the linear regression is an index of position discrimination within the selected ROI. Note that if the pattern of activity in the ROI showed no selectivity for object position, the slope of the linear regression should be zero. However, if the ROI can discriminate object position, then the spatial correlation of activity produced by two Gabor stimuli should be higher when the two Gabors are nearer to each other. Therefore, the linear regression slope within a given ROI is an indicator of that region's ability to discriminate changes in Gabor position.
As a control, in a separate analysis, we defined every possible 5 × 5 × 5 mm3 of voxels in the entire brain, creating thousands of ROIs. Because each ROI overlapped many others, each voxel in the brain was covered by 125 ROIs. We performed the above correlational analysis to obtain a position discrimination slope value for each ROI. Because any particular voxel was covered by 125 ROIs, we assigned the average slope of those 125 ROIs to that particular voxel. Each voxel therefore represented the average position discrimination ability of the overlapping ROIs that surrounded it. With these average position discrimination values, we created a new map revealing clusters of voxels that are best able to discriminate the Gabor positions (Fig. 5).
Figure 5.
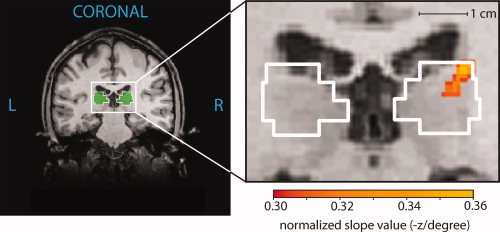
Location of the strongest position discrimination within the right pulvinar. The enlarged coronal view (y = −24) shows colored voxels where the position discrimination slope value exceeded a threshold of 0.30. The color gradation shows the variation in slope values obtained for the voxels within this region of strong position discrimination. The voxels with the largest slopes in the pulvinar had a value of 0.36 and were centered on (x, y, z = 19, −24, 14). A map of slope values for all voxels in the brain was obtained by performing the correlational analysis in all possible 5 mm3 ROIs across the entire brain. The slope assigned to a particular voxel was the average of the slopes associated with the 125 ROIs containing that voxel. Resulting position discrimination maps were averaged across the seven subjects.
All correlational analyses were conducted in Matlab 7.1 (The Mathworks, Natick, MA).
RESULTS
Figure 3B shows a sample cross‐correlation plot and slope fit for a representative subject. For voxels within the right‐hemisphere pulvinar ROI (Fig. 2), Fisher z scores from all subjects were plotted together revealing a significant linear regression (Fig. 3C; r = 0.24, P = 0.023, slope = 0.155; Bonferroni‐corrected αβ = 0.025 to account for testing right‐ and left‐hemisphere pulvinar independently). This indicates that shifting the Gabor stimuli by one degree of visual angle resulted in a decrease in the spatial correlation across voxels in the right pulvinar of 0.155 Fisher z units. It is worth keeping in mind that because functional subdivisions of the human pulvinar have not been established, this analysis was conducted on an anatomically defined pulvinar ROI (see Materials and methods section). As this ROI probably included nonvisual regions of the pulvinar, the position discrimination slope of 0.155 above is likely a conservative estimate. Nonetheless, this significant decorrelation in the pattern of BOLD responses with slight changes in stimulus position shows that the population of neurons in the right pulvinar ROI is quite sensitive to stimulus location.
The data show a surprising lateralization. In contrast to the strong position discrimination found in the right‐hemisphere pulvinar, when we performed the same analysis using an anatomically defined left‐hemisphere pulvinar ROI, we found no indication of position sensitivity (Fig. 3D, r = 0.095, P = 0.22). The right pulvinar was significantly better at discriminating Gabor positions (Z = 1.97, P = 0.025). This laterality effect is evident in individual subject data as well. Figure 4A shows cross‐correlation plots and slope fits for all subjects, divided by hemisphere. While slope fits in the right pulvinar are consistently negative, slopes in left pulvinar are variable but distributed around zero. This is easily visualized in Figure 4B, where slope fits from all subjects are plotted together. This lateralization is statistically significant within subjects (there was a significant negative slope in RH, but not in LH Pulvinar: RH, t = 4.11, P < 0.01; LH, t = −0.50, P = 0.64).
Figure 4.
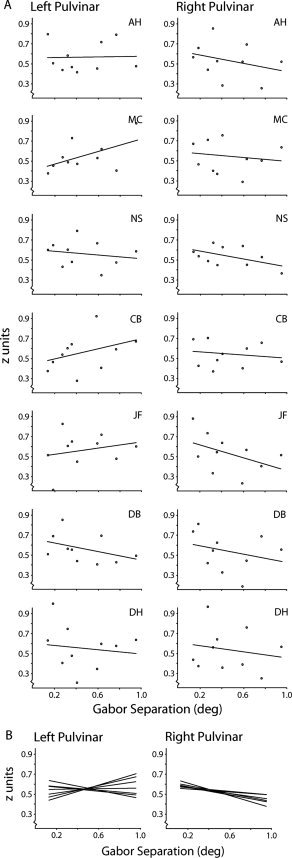
Position discrimination plots for individual subjects. (A) Cross‐correlation plots for left‐ and right‐hemisphere pulvinar are shown for each subject, along with regression lines. While slope fits in the right pulvinar are consistently negative, slopes in left pulvinar are variable and distributed around zero. (B) Regression lines from all subjects are plotted together. This lateralization is statistically significant within subjects (there was a significant negative slope in RH, but not in LH Pulvinar: RH, t = 4.11, P < 0.01; LH, t = −0.50, P = 0.64).
A control analysis was performed on voxels in left‐ and right‐hemisphere V1 independently to ensure that the lateralization was unique to the pulvinar. The boundaries of V1 for each subject were delineated using a standard flickering bowtie stimulus that localized the horizontal and vertical meridians [Sereno et al., 1995]. Using the analysis described earlier, slopes in both right and left V1 were significant (r = 0.77 and r = 0.72 for left and right V1, respectively, P < 0.0001 for both). This indicates that the lateralization of position discrimination found in the pulvinar is not an artifact of scanning conditions or of the statistical methods. The lateralization is also not because of inconsistency or lateralized variability in the pulvinar location across subjects: the pulvinar ROIs conservatively encompassed the whole pulvinar of each subject, and, more importantly, the analyses in Figures 3 and 4 were conducted on the individual within‐subject data.
Precision of topographic encoding can be defined as the smallest stimulus shift to which the pattern of BOLD activity significantly changes. To estimate precision in the right pulvinar, pairwise t tests were performed on the data in Figure 3C to compare the mean of z scores at the smallest (0.136°) separation with the means at the nine other separations. The mean at 0.136° differed significantly from the mean at a separation of 0.591 (t = 4.20, P = 0.0028, αβ = 0.0056, using an incremental application of Bonferroni correction as suggested by [Benjamini and Hochberg, 1995]). Since a position shift of 0.455° visual angle produced a significant decrease in the mean correlation (z score) across subjects, this gives a lower bound on the precision of topographic encoding in the pulvinar.
As a control, we calculated the position discrimination slope (as in Fig. 3C) for all 5 mm3 of voxels across the entire brain, computing an average slope value for each voxel (see Experimental Procedures). Figure 5 shows these computed slope values overlaid on an anatomical image (volumetric maps of slope values were averaged across subjects). Within the anatomically defined right pulvinar ROI, there was a cluster of voxels that displayed strong position discrimination (colored voxels in Fig. 5). This area of peak discrimination was centered on the Talairach coordinates (x, y, z = 19, −24, 14), corresponding to the dorsal‐lateral region of the pulvinar ROI. Table I gives the locations of peak position discrimination within the right pulvinar for individual subjects. There was some variability from subject to subject. However, in five of the seven subjects the location of peak discrimination fell in the lateral portion of the right pulvinar ROI, near the highly precise region reported for the grouped data.
Table I.
Location of peak position discrimination for all seven subjects
| Subject | x | y | z |
|---|---|---|---|
| AH | 20 | −24 | 16 |
| MC | 18 | −25 | 11 |
| NS | 18 | −29 | 4 |
| CB | 20 | −24 | 16 |
| JF | 18 | −25 | 12 |
| DB | 22 | −32 | 6 |
| DH | 9 | −27 | 11 |
| Average | 17.9 | −26.6 | 10.9 |
| Standard error | 4.2 | 3.0 | 4.6 |
| Group analysis | 19 | −24 | 14 |
Volumetric position discrimination maps were created for each subject as in Figure 5, and the voxel within the right pulvinar with the largest slope value was taken as the location of peak position discrimination. Although there was some variability from subject to subject, in five of the seven subjects the location of peak discrimination fell in the lateral portion of the right pulvinar ROI, within the region corresponding to the PLvl subdivision in macaques. This matches the region of peak position discrimination that we found in Figure 5.
Figure 6 shows the peak position discrimination slope in the dorsal‐lateral right pulvinar in relation to the distribution of slopes across the voxels in the whole brain (Experimental Procedures); the pulvinar clearly falls in the right‐hand tail of the distribution, and was able to discriminate Gabor position better than 98.6% of the voxels in the rest of the brain. Also indicated are the peak slopes for select visual cortical areas. Early visual areas, including V1, V2, and V3, had very steep position discrimination slopes [Bressler et al., 2007]; the position discrimination slope in the pulvinar was close behind. This shows that position discrimination in the pulvinar is comparable to that in extrastriate visual cortex.
Figure 6.
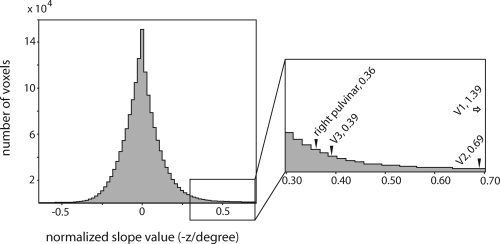
Distribution of slope values for all voxels in the brain. Position discrimination maps were averaged across the seven subjects as in Figure 5, and the 1,762,396 voxels in the resulting position discrimination map that fell inside the brain were plotted in a histogram with bin size 0.02. Right hemisphere pulvinar, along with the early visual cortical areas, falls in the extreme right tail of this histogram. The voxels in the right pulvinar showing the strongest position discrimination had slope values of 0.36, placing them among the top 1.43% of voxels in the brain. Peak slope values for V1, V2, and V3 are shown for comparison.
DISCUSSION
In working toward an understanding of the function of the pulvinar, a natural first step is to characterize its limits. To this end, establishing whether the human pulvinar encodes position information is critical. Finding retinotopic coding in the human pulvinar on a precise scale would provide strong evidence that the pulvinar's role is tied to position information. Here we demonstrated that precise topographic encoding exists in the human pulvinar. Our results indicated that the right hemisphere pulvinar contains a topographic map of the visual environment, discriminating objects that are separated by about one half of a degree. Position coding in the right pulvinar is less precise than that observed in early visual cortical areas, but still remarkably precise, discriminating object position better than 98.6% of the voxels in the entire brain. By contrast, we found no evidence of such position coding in the left hemisphere pulvinar.
The human pulvinar is notoriously difficult to image. A handful of studies have observed activation in the pulvinar during attentional tasks [Corbetta et al., 1990; Cotton and Smith, 2007; Yantis et al., 2002]. In an elegant study, Kastner et al. [ 2004] activated the pulvinar using bilateral attended stimuli; lateralized or passively viewed stimuli produced no detectable differential BOLD response in the pulvinar. Among the reasons for the difficulty in detecting activation in the pulvinar is the fact that neurons in the pulvinar are modulated bidirectionally by certain stimuli and tasks [Bender and Youakim, 2001], potentially resulting in little net change in the BOLD signal at the population level [Logothetis and Wandell, 2004]. Thus a major strength of the correlational method that we employ here is that it provides a reliable means of functionally localizing the unique topographic portions of the human pulvinar by making use of the information inherent in the distributed spatial pattern of the BOLD signal, rather than the net response.
The lateralization of position coding to the right pulvinar is surprising. One possibility is that left pulvinar has some position sensitivity on a dramatically coarser scale than right pulvinar, outside of the range of our stimuli. However, our data do not show any hint of this. There is not even a slight mean reduction in z scores between the smallest and largest separations in left pulvinar (t 12 = 1.10, P = 0.29). While bilateral retinotopy has been reported in cats and monkeys, the pulvinar has undergone considerable changes in morphology throughout evolution [Browne and Simmons, 1984; Casanova, 2004]. Hence, it is reasonable that organizational differences might exist between the human pulvinar and the phylogenetically older cat and monkey pulvinars. Consistent with this possibility, there is evidence for lateralization in the function of the human pulvinar. Karnath et al. [ 2002] identified the right pulvinar and putamen as the particular subcortical structures whose damage often leads to spatial neglect. Neglect itself is a lateralized phenomenon, occurring more severely and roughly four times as often in patients with right brain damage than in patients with left brain damage when assessed with a cancellation task [Kleinman et al., 2007; Weintraub and Mesulam, 1987]. This has been taken to indicate a specialization of the right hemisphere [Mapstone et al., 2003; Weintraub and Mesulam, 1987], and the right pulvinar [Karnath et al., 2002], for spatial attention.
One advantage of our spatial correlation technique is that it can detect any systematic position coding scheme, retinotopic or otherwise. Our analysis shows that the pattern of activity in the right pulvinar varies systematically as stimulus location varies, which demonstrates position sensitive encoding and is consistent with a retinotopic mapping in human pulvinar. However, this does not preclude the possibility that the mapping is a nonretinotopic one in which activity still varies systematically with stimulus position. One such example would be head or body‐centered position coding. Indeed, Ward and Danziger [ 2005] report that a retinotopic map would not account for results from their lesion experiments, and have instead suggested that spatial coding may be relative to the center of attention. Our results would be consistent with such a coordinate system, since subjects attended to the fixation point in all conditions. A preferential representation of the central visual field (i.e., a signal gain for objects presented more foveally) could produce the systematic changes in correlation that we report, but we would expect to see a trend in beta values indicating that there is a greater BOLD response for the more foveal stimuli (or even vice versa). No such trend is present in the individual subject data, and there are no main effects of stimulus position on signal strength in the grouped data (F = 1.23, P = 0.33). Thus the position discrimination in the right pulvinar is not driven by a larger BOLD response for more foveal stimuli.
The voxels in the pulvinar showing the strongest position discrimination fall in the lateral and superior portion of the right pulvinar ROI (Fig. 5). Given a good correspondence between the morphology of monkey and human pulvinars, this area of highly precise position coding falls squarely within the ventrolateral nucleus of the lateral pulvinar (PLvl) as demarcated in macaque monkeys by Kaas and Lyon [ 2007]. This subdivision is retinotopically organized in monkeys and projects in a topographic manner to areas V1, V2, and V4. Since the bulk of V1 and V2 output goes to ventral stream area V4, Kaas and Lyon [ 2007] conclude that the predominant influence of PLvl is on processing in the ventral stream. However, the homology between monkey and human pulvinar is less than clear. While the inferior subdivision of the monkey pulvinar is retinotopically organized, the position discrimination in voxels in the inferior portion of the right pulvinar ROI was only marginally significant (r = 0.196, P = 0.052, based on analysis using the inferior half of the right pulvinar ROI). While there is some degree of homology between the monkey and human pulvinars, it appears that there may be specialization in the function of the subdivisions of the human pulvinar beyond that which exists in monkeys.
The correlational analysis employed in this study provides a novel and powerful method for characterizing stimulus discrimination within a given region of interest. It also provides a straightforward means of estimating the precision of stimulus discrimination by measuring the degree of change in a stimulus required to produce a significant decorrelation of BOLD response patterns. It is important to note, however, that such a precision estimate is not a direct indication of receptive field size. Very large receptive fields (even bilateral ones) can give rise to fine position discrimination if many of the receptive fields overlap [Eurich and Schwegler, 1997]. Thus, recovering RF size from our data is not possible. Conversely, the precision of position coding cannot be revealed by measuring receptive field size alone. The only way to accurately measure the resolution of position coding is to measure populations of neurons.
Whereas typical analyses often simply identify the areas of peak activation, this correlational method utilizes the information available in the distributed spatial pattern of the BOLD response. Consequently, it is sensitive to changes on a subvoxel scale. In the case of the pulvinar, some cells increase their firing rates while others decrease their firing rates under attentional control [Bender and Youakim, 2001]. This bidirectional modulation of activity within a population of cells will not necessarily produce any reliable net change in BOLD response [Logothetis and Wandell, 2004], which might account for the inconsistent results of several fMRI studies on attentional modulation of the pulvinar [Ward and Danziger, 2005]. However, evidence of these small modulations may be preserved in the distributed spatial pattern of the BOLD response such that a correlational analysis is successful in detecting the changes despite little or no net change in the BOLD response on a larger scale. Thus, while fMRI has the strength of measuring activity at the population level rather than just the single‐unit level, our technique utilizes information on a finer scale that most fMRI analyses.
The precise position coding in right hemisphere pulvinar demonstrated by this study provides strong evidence that the role of the pulvinar is tied to position information. Allocating spatial attention has been hypothesized as a possible function of the pulvinar [Bender and Youakim, 2001; Petersen et al., 1987], and with the spatial resolution reported here of 0.05E or better (where E is the eccentricity from fixation), highly precise spatial attention could be supported by the right hemisphere pulvinar. Neri and Levi [ 2006] report the resolution of feature binding at about 0.11E for a dense stimulus array and about 0.06E for a sparse array, so the precision of spatial coding in the pulvinar is also fine enough to account for these data. If the pulvinar is involved in feature binding, the coarser resolution for feature binding could be due to downstream noise, or to wiring scatter as proposed by Neri and Levi [ 2006]. The lateralization that we report is consistent with several previous findings, including the implication of the pulvinar in spatial neglect [Karnath et al., 2002] and the prospect that spatial attention is dominated by the right hemisphere [Mapstone et al., 2003; Mesulam, 1999]. In the stimuli and methods employed here, we have also provided a means of functionally localizing the specific portions of the human pulvinar that are sensitive to object position.
Acknowledgements
The authors thank David Bressler, Elizabeth Louie, Nicole Spotswood, Dr. Michael Buonocore, and two anonymous reviewers.
REFERENCES
- Bender DB ( 1981): Retinotopic organization of macaque pulvinar. J Neurophysiol 46: 672–693. [DOI] [PubMed] [Google Scholar]
- Bender DB ( 1982): Receptive‐field properties of neurons in the macaque inferior pulvinar. J Neurophysiol 48: 1–17. [DOI] [PubMed] [Google Scholar]
- Bender DB,Youakim M ( 2001): Effect of attentive fixation in macaque thalamus and cortex. J Neurophysiol 85: 219–234. [DOI] [PubMed] [Google Scholar]
- Benjamini Y,Hochberg Y ( 1995): Controlling the false discovery rate—A practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodological 57: 289–300. [Google Scholar]
- Bressler D,Spotswood N,Whitney D ( 2007): Negative BOLD fMRI response in the visual cortex carries precise stimulus‐specific information. PLoS ONE 2: e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne B,Simmons RM ( 1984): Quantitative studies of the evolution of the thalamus in primates. J Hirnforsch 25: 261–274. [PubMed] [Google Scholar]
- Casanova C ( 2004): The visual functions of the pulvinar In: Werner JS,Chalupa LM, editors. The Visual Neurosciences. Cambridge, MA: MIT Press; pp 592–608. [Google Scholar]
- Casanova C,Savard T ( 1996): Responses to moving texture patterns of cells in the striate‐recipient zone of the cat's lateral posterior‐pulvinar complex. Neuroscience 70: 439–447. [DOI] [PubMed] [Google Scholar]
- Casanova C,Freeman RD,Nordmann JP ( 1989): Monocular and binocular response properties of cells in the striate‐recipient zone of the cat's lateral posterior‐pulvinar complex. J Neurophysiol 62: 544–557. [DOI] [PubMed] [Google Scholar]
- Chalupa LM,Abramson BP ( 1989): Visual receptive fields in the striate‐recipient zone of the lateral posterior‐pulvinar complex. J Neurosci 9: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A,Ivry R ( 1989): Illusory conjunctions inside and outside the focus of attention. J Exp Psychol Hum Percept Perform 15: 650–663. [DOI] [PubMed] [Google Scholar]
- Cohen J ( 1988): Statistical Power Analysis for the Behavioral Sciences. Hillsdale,NJ: L. Erlbaum Associates; xxi, 567 p. [Google Scholar]
- Corbetta M,Miezin FM,Dobmeyer S,Shulman GL,Petersen SE ( 1990): Attentional modulation of neural processing of shape, color, and velocity in humans. Science 248: 1556–1559. [DOI] [PubMed] [Google Scholar]
- Cotton PL,Smith AT ( 2007): Contralateral visual hemifield representations in the human pulvinar nucleus. J Neurophysiol 98: 1600–1609. [DOI] [PubMed] [Google Scholar]
- Desimone R,Wessinger M,Thomas L,Schneider W ( 1990): Attentional control of visual perception: Cortical and subcortical mechanisms. Cold Spring Harb Symp Quant Biol 55: 963–971. [DOI] [PubMed] [Google Scholar]
- Eurich CW,Schwegler H ( 1997): Coarse coding: Calculation of the resolution achieved by a population of large receptive field neurons. Biol Cybern 76: 357–363. [DOI] [PubMed] [Google Scholar]
- Felsten G,Benevento LA,Burman D ( 1983): Opponent‐color responses in macaque extrageniculate visual pathways: The lateral pulvinar. Brain Res 288: 363–367. [DOI] [PubMed] [Google Scholar]
- Guillery RW,Sherman SM ( 2002): Thalamic relay functions and their role in corticocortical communication: Generalizations from the visual system. Neuron 33: 163–175. [DOI] [PubMed] [Google Scholar]
- Kaas JH,Lyon DC ( 2007): Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res Rev (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO,Himmelbach M,Rorden C ( 2002): The subcortical anatomy of human spatial neglect: Putamen, caudate nucleus and pulvinar. Brain 125 (Part 2): 350–360. [DOI] [PubMed] [Google Scholar]
- Kastner S,O'Connor DH,Fukui MM,Fehd HM,Herwig U,Pinsk MA ( 2004): Functional imaging of the human lateral geniculate nucleus and pulvinar. J Neurophysiol 91: 438–448. [DOI] [PubMed] [Google Scholar]
- Kleinman JT,Newhart M,Davis C,Heidler‐Gary J,Gottesman RF,Hillis AE ( 2007): Right hemispatial neglect: Frequency and characterization following acute left hemisphere stroke. Brain Cogn 64: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBerge D,Buchsbaum MS ( 1990): Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci 10: 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J,Summerln J,Rainey L,Freitas C,Fox P ( 1997): The Talairach Daemon, a database server for Talairach Atlas Labels. Neuroimage 5: S633. [Google Scholar]
- Logothetis NK,Wandell BA ( 2004): Interpreting the BOLD signal. Annu Rev Physiol 66: 735–769. [DOI] [PubMed] [Google Scholar]
- Mapstone M,Weintraub S,Nowinski C,Kaptanoglu G,Gitelman DR,Mesulam MM ( 2003): Cerebral hemispheric specialization for spatial attention: Spatial distribution of search‐related eye fixations in the absence of neglect. Neuropsychologia 41: 1396–1409. [DOI] [PubMed] [Google Scholar]
- Mason R ( 1981): Differential responsiveness of cells in the visual zones of the cat's LP‐pulvinar complex to visual stimuli. Exp Brain Res 43: 25–33. [DOI] [PubMed] [Google Scholar]
- Mesulam MM ( 1999): Spatial attention and neglect: Parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci 354: 1325–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GA,Desmedt S ( 2004): The human pulvinar and attentional processing of visual distractors. Neurosci Lett 362: 176–181. [DOI] [PubMed] [Google Scholar]
- Neri P,Levi DM ( 2006): Spatial resolution for feature binding is impaired in peripheral and amblyopic vision. J Neurophysiol 96: 142–153. [DOI] [PubMed] [Google Scholar]
- Petersen SE,Robinson DL,Keys W ( 1985): Pulvinar nuclei of the behaving rhesus monkey: Visual responses and their modulation. J Neurophysiol 54: 867–886. [DOI] [PubMed] [Google Scholar]
- Petersen SE,Robinson DL,Morris JD ( 1987): Contributions of the pulvinar to visual spatial attention. Neuropsychologia 25: 97–105. [DOI] [PubMed] [Google Scholar]
- Prinzmetal W,Henderson D,Ivry R ( 1995): Loosening the constraints on illusory conjunctions: Assessing the roles of exposure duration and attention. J Exp Psychol Hum Percept Perform 21: 1362–1375. [DOI] [PubMed] [Google Scholar]
- Raczkowski D,Rosenquist AC ( 1981): Retinotopic organization in the cat lateral posterior‐pulvinar complex. Brain Res 221: 185–191. [DOI] [PubMed] [Google Scholar]
- Sereno MI,Dale AM,Reppas JB,Kwong KK,Belliveau JW,Brady TJ,Rosen BR,Tootell RB ( 1995): Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268: 889–893. [DOI] [PubMed] [Google Scholar]
- Sherman SM,Guillery RW ( 2002): The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci 357: 1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐planar stereotaxic atlas of the human brain: 3‐dimensional proportional system: an approach to cerebral imaging. Stuttgart,New York: G. Thieme, Thieme Medical Publishers; viii, 122 p. [Google Scholar]
- Treisman A,Schmidt H ( 1982): Illusory conjunctions in the perception of objects. Cognit Psychol 14: 107–141. [DOI] [PubMed] [Google Scholar]
- Ward R,Danziger S ( 2005): Selective attention and response control following damage to the human pulvinar In: Humphreys GW,Riddoch MJ, editors. Attention in Action: Advances from Cognitive Neuroscience. New York: Psychology Press; pp 325–350. [Google Scholar]
- Ward R,Danziger S,Owen V,Rafal R ( 2002): Deficits in spatial coding and feature binding following damage to spatiotopic maps in the human pulvinar. Nat Neurosci 5: 99–100. [DOI] [PubMed] [Google Scholar]
- Weintraub S,Mesulam MM ( 1987): Right cerebral dominance in spatial attention. Further evidence based on ipsilateral neglect. Arch Neurol 44: 621–625. [DOI] [PubMed] [Google Scholar]
- Wolford G,Shum KH ( 1980): Evidence for feature perturbations. Percept Psychophys 27: 409–420. [DOI] [PubMed] [Google Scholar]
- Yantis S,Schwarzbach J,Serences JT,Carlson RL,Steinmetz MA,Pekar JJ,Courtney SM ( 2002): Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci 5: 995–1002. [DOI] [PubMed] [Google Scholar]


