Summary
TWEAK is a relatively recently identified proinflammatory cytokine which functions through binding to Fn14 receptor in target cells. Although TWEAK has been shown to modulate several biological responses, the TWEAK-induced signaling pathways remain poorly understood. In this study, we tested the hypothesis that TAK1 is involved in TWEAK-induced activation of NF-κB and MAPK, and expression of proinflammatory protein. TWEAK increased the phosphorylation and kinase activity of TAK1 in cultured myoblast and fibroblast cells. The activation of NF-κB was significantly inhibited in TAK1-deficinet (TAK1−/−) mouse embryonic fibroblasts (MEF) compared to wild-type MEF. Deficiency of TAK1 also inhibited the TWEAK-induced activation of IκB kinase and phosphorylation and degradation of IκBα protein. However, there was no difference in the levels p100 protein in TWEAK-treated wild-type and TAK1−/− MEF. Furthermore, TWEAK-induced transcriptional activation of NF-κB was significantly reduced in TAK1−/− MEF and in C2C12 myoblasts transfected with a dominant-negative TAK1 or TAK1 siRNA. TAK1 was also required for the activation of AP-1 in response to TWEAK. Activation of JNK1 and p38 MAPK but not ERK1/2 or Akt kinase was significantly inhibited in TAK1−/− MEF compared to wild-type MEF upon treatment with TWEAK. TWEAK-induced expression of proinflammatory genes such as MMP-9, CCL-2 and VCAM-1 was also reduced in TAK1−/− MEF compared to wild-type MEF. Furthermore, the activation of NF-κB and the expression of MMP-9 in response to TWEAK involved the upstream activation of Akt kinase. Collectively, our study demonstrates that TAK1 and Akt are the important components of the TWEAK-induced proinflammatory signaling and gene expression.
Keywords: TAK1, TWEAK, NF-κB, AP-1, JNK1, p38 kinase
Introduction
Tumor necrosis factor (TNF) super family (TNFSF) consists of 19 ligands which mediate their effect through engagement of TNF receptor superfamily members (1). These ligands are involved in regulation of several important physiological functions such immune response, hematopoiesis, and differentiation (2, 3). Conversely, aberrant expression of TNFSF ligands has been found to be associated with a wide variety of disease states such as graft-versus-host reaction, septic shock, viral replication, tumorigenesis, bone resorption, and cachexia (1-3).
TNF-like weak inducer of apoptosis (TWEAK) is a member of the TNFSF ligands (4). TWEAK is initially synthesized as a type II transmembrane protein, cleaved to its soluble form, and signals as a trimerized molecule (4-6). TWEAK signaling occurs through binding to Fn14 (also known as TWEAKR), a type I transmembrane receptor belonging to the TNF receptor super family (5, 7). Like other TNFSF members, TWEAK/Fn14 signaling also mediates unique and context-dependent pleiotropic effects. For example, in contrast to TNF-α, TWEAK attenuates the transition from innate to adaptive immunity by suppressing the production of interferon-γ and IL-12 cytokines (8). TWEAK has been shown to regulate a number of biological processes including cell survival, proliferation, angiogenesis, migration, and apoptosis (reviewed in (5, 6, 9)). Furthermore, elevated levels of TWEAK and/or Fn14 have been found to be associated with the pathogenesis of rheumatoid arthritis (10), skeletal-muscle wasting (11, 12), systemic lupus erythematosus (13, 14), multiple sclerosis (5, 15), stroke (16), neuroinflammation and neurodegeneration (17, 18), and several types of cancer (6, 19, 20).
The pathological functions of TWEAK are primarily attributed to its ability to induce the expression of several proinflammatory cytokines, chemokines, cell adhesion molecules, and matrix-degrading enzymes mainly through the activation of nuclear factor-kappa B (NF-κB), a major proinflammatory transcription factor which regulates expression of more than 400 genes (1, 5, 21). In contrast to TNF-α which activates NF-κB in a rapid and transient manner through the canonical pathway, TWEAK causes sustained activation of both classical and alternative NF-κB signaling pathways (11, 22). TWEAK has also been shown to activate p44/p42 mitogen activated protein kinases (MAPK), c-jun-N-terminal kinase 1 (JNK1), and activator protein-1 (AP-1) transcription factor in some cell types (23, 24). Although the precise mechanisms by which TWEAK/Fn14 interaction leads to the activation of NF-κB, AP-1, and MAPK remain poorly understood, it has been reported that the cytoplasmic domain of Fn14 receptor contains a TNF-receptor associated factor (TRAF)-binding site, the mutation of which inhibits signaling through this receptor (25). Indeed, TRAF-1, 2, 3, and 5 have been found to interact with the cytoplasmic domain of Fn14 in yeast two-hybrid system (25) and they are required for TWEAK-induced activation of NF-κB in cultured mammalian cells (22, 25, 26). Recently, Vince et al (27) demonstrated that the binding of TWEAK leads to the recruitment of TRAF2/cIAP complex to Fn14 cytoplasmic domain.
Transforming growth factor beta activated kinase-1 (TAK1), a member of the mitogen-activated protein kinase kinase kinase (MAP3K) family, was originally identified as a key regulator of TGF-β-induced activation of MAPK (28). However, subsequent studies have shown that TAK1 is an important component of the cell signaling pathways culminating in the activation of NF-κB and AP-1 in response to several cytokines (e.g. IL-1β, IL-18, RANKL, and TNF-α) and microbial products (29-33). A recent report also suggests that biomedical stress inhibits the expression of proinflammatory genes by inhibiting the phosphorylation of TAK1 in NF-κB pathway (34). Activation of TAK1 complex, which also contains TAB1 and TAB2 proteins, leads to the phosphorylation of IKKβ in its activation domain leading to the activation of NF-κB (35-37). Activated TAK1 complex can also phosphorylate MKK6 leading to the activation of p38 MAPK (38, 39). Furthermore, the activation of JNK1 in response to certain stimuli is also mediated through the activation of TAK1 (40, 41). However, the role of TAK1 in TWEAK-induced cell signaling pathways leading to the activation of NF-κB, AP-1, and MAPK and the expression of proinflammatory genes remain unknown.
We hypothesize that TAK1 is an important component of the TWEAK-induced activation of proinflammatory signaling pathways. To test our hypothesis, we have studied the role of TAK1 in the activation of NF-κB, AP-1, and MAPK in response to TWEAK. Our results demonstrate that TWEAK activates TAK1 in cultured C2C12 myoblasts and mouse embryonic fibroblasts (MEF). The TWEAK-induced activation of NF-κB and AP-1 and the expression of proinflammatory genes (e.g. MMP-9, CCL-2, and VCAM-1) are significantly impaired in MEF lacking TAK1. Furthermore, our results demonstrate that the phosphatidylinositide 3-kinase (PI3K)/Akt pathway also contributes to the TWEAK-induced activation of NF-κB and the expression of MMP-9 gene.
Materials and Methods
Materials
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum were obtained from Invitrogen (Carlsbad, CA). Horse serum, lipopolysaccharide, and β-actin antibody were purchased from Sigma Chemical Company (Saint Louis, MO). Poly (dI·dC) was from GE Healthcare. Effectene transfection reagent was obtained from Qiagen (Valencia, CA). NF-κB and AP-1 consensus oligonucleotides and luciferase assay kits were purchased from Promega (Madison, WI). Antibody against phospho p38 (Thr180/Tyr182) was purchased from Millipore (Billerica, MA). Antibodies against phospho p44/42 (Thr202/Tyr204), phospho IκBα (Ser-32), phospho TAK1 (Ser412), phospho TAK1 (Thr184/187), total p100/p52, TAK1, p38, TAB1, and TAB2 and LY294002 were obtained from the Cell Signaling Technology (Beverly, MA). Antibodies against IκBα, IKKα/β, TRAF-6, and JNK1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse TAK1 SMARTpool siRNA and control non-targeting siRNA and DharmaFect-3 transfection reagent was obtained from Dharmacon RNA Technologies (Lafayette, CO). Recombinant mouse TWEAK and TNF-α protein and antibody against mouse MMP-9 protein were obtained from R&D Systems, Inc. (Minneapolis, MN). [γ-32P]ATP (specific activity, 3000 (111 TBq) Ci/mmol) was obtained from MP Biomedicals (Solon, OH).
Cell Culture
TAK1+/+ and TAK1−/− murine embryonic fibroblasts (MEF) were kindly provided by Prof. Shizuo Akira of Osaka University, Osaka, Japan. The cells were grown in DMEM containing 10% FBS. C2C12 myoblastic cell line was obtained from the American Type Culture Collection (Manassas, VA). These cells were grown in DMEM containing 10% fetal bovine serum. C2C12 myoblasts were differentiated into myotubes by incubation in differentiation medium (DM, 2% horse serum in DMEM) for 96h. Myotubes were maintained in DM, and medium was changed every 48 h.
Plasmids
Plasmid constructs encoding murine wild-type HA-TAK1 or a dominant negative mutant (HA-TAK1K63W) and His-MKK6 as described were kindly provided by Dr. Jun Ninomiya-Tsuji of North Carolina State University. Dominant negative mutant of Akt kinase (i.e. HA-AktK179M) was from Dr. Mien-Chie Hung and obtained through Addgene (Cambridge, MA). Mouse MMP-9 gene promoter (1 to −1174 bp)-luciferase reporter plasmid was from Dr. S.V. Reddy of Children's Research Institute, Charleston, SC. pNF-κB-SEAP, pNF-κB-Luc, pAP1-Luc, and pTAL-SEAP were purchased from Clontech (Mountain View, CA).
Western Blotting
Cells were washed with phosphate buffered saline (PBS) and homogenized in lysis buffer A (50 mM Tris-Cl [pH 8.0], 200 mM NaCl, 50mM NaF, 0.3% NP-40, 2 μg/mL leupeptin, 2 μg/mL aprotinin, 1 mM phenylmethylsulfonyl fluoride, 0.5 μg/mL benzamidine, 1 mM dithiothreitol, 1 mM sodium orthovanadate, 50 mM NaF, and 10 mM β-glycerophosphate). The protein concentration of the samples was measured using BioRad protein assay reagent (BioRad Laboratories, Hercules, CA) and 60−80 μg of protein was resolved on 10% SDS-PAGE gel. The proteins were then electrotransferred to a nitrocellulose membrane blocked with 3% nonfat milk and probed with primary antibodies (dilution, 1:1000) for 4−5 h. The blot was washed, exposed to horseradish peroxidase-conjugated secondary antibodies for 1 h, and detected by chemiluminescence. The bands obtained were quantitated with Personal Densitometer Scan version 1.30 using ImageQuantTL (GE Healthcare, Piscataway, NJ) software.
Immunoprecipitation and in vitro kinase assays
The activity of JNK1, IKK, and TAK1 was determined by immunoprecipitation followed by in vitro kinase assay using a method as previously described (11, 42, 43). In brief, cells were treated with TWEAK, cell extracts were made in lysis buffer, and the concentration of protein was measured. 500−700 μg of protein was immunoprecipitated with JNK1, IKK-γ or TAK1 antibody and the immune complex was collected using protein A-Sepharose beads. After washing two times with lysis buffer and two times with kinase buffer (50 mM HEPES (pH 7.4), 10 mM MgCl2, and 1 mM dithiothreitol), the beads were suspended in 20 μl of kinase assay mixture containing 50 mM HEPES (pH 7.4), 20 mM MgCl2, 2 mM dithiothreitol, 10 μCi of [γ-32P]ATP, 1 μm unlabeled ATP, and 2 μg of either GST-cJun (1−79) (for JNK1), GST-IκBα (1-36) (for IKK), or His-MKK6 (for TAK1) as substrate. After incubation at 37 °C for 15 min, the reaction was terminated by boiling with 20 μl of 2× Laemmli sample buffer for 3 min. Finally, the protein was resolved on 10% polyacrylamide gel, the gel was dried, and the radioactive bands were visualized by exposing to a PhosphorImager screen and quantified using ImageQuantTL (GE Healthcare, Piscataway, NJ) software.
Electrophoretic Mobility Shift Assay (EMSA)
The activation of NF-κB and AP-1 transcription factors was measured by EMSA as described (11, 44). Briefly, after treatment with soluble TWEAK protein approximately 2 × 106 cells were washed with cold PBS, scraped, and suspended in 100 μl of hypotonic lysis buffer (10 mM HEPES (pH 7.9), 10 mM KCl, 1.5 mM MgCl2, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 2.0 μg/ml leupeptin, 2.0 μg/ml aprotinin, 0.5 mg/ml benzamidine) for 10 min. The cells were then lysed with 3.25 μl of 10% IPEGAL, the homogenates were centrifuged, and the supernatants containing the cytoplasmic extracts were stored frozen at −80 °C. The nuclear pellets were resuspended in 40 μl of ice-cold high salt nuclear extraction buffer (20 mM HEPES [pH 7.9], 420 mM NaCl, 1 mM EDTA, 1 mM EGTA, 25% glycerol, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 2.0 μg/ml leupeptin, 2.0 μg/ml aprotinin, 0.5 mg/ml benzamidine). After 30 min of intermittent vortexing the extracts were centrifuged, and the supernatants containing the nuclear extracts were collected. The protein content was measured and 8μg nuclear extracts were incubated with 16 fmol of 32P end-labeled NF-κB or AP-1 consensus oligonucleotides (Promega) at 37 °C for 20 min. The DNA-protein complex formed was resolved on a 7.5% native polyacrylamide gel. The gel was dried, and the radioactive bands were visualized and quantitated by Storm 820 PhosphorImager (GE Healthcare, Piscataway, NJ) and using ImageQuantTL (GE Healthcare, Piscataway, NJ) software.
Gelatin Zymography
The MMP-9 activity in conditioned medium was determined by gelatin zymography as described (42). In brief, conditioned media samples were separated on 8% SDS-polyacrylamide gels containing 1 mg/ml gelatin B (Fisher Scientific) under nonreducing conditions. Gels were washed in 2.5% Triton X-100 for 1 h at room temperature followed by incubation in reaction buffer (50 mm Tris-HCl (pH 7.5), 50 mm NaCl, 5 mm CaCl2, and 0.02% sodium azide) for 36h at 37 °C. To visualize gelatinolytic bands, gels were stained with Coomassie Blue at room temperature followed by extensive washing in destaining buffer (10% methanol and 10% acetic acid in distilled water). The gels were dried and scanned under a densitometer for determination of gelatinolytic activity.
Semiquantitative and Quantitative Reverse Transcriptase (RT)-PCR
Isolation of total RNA and semi-quantitative RT-PCR was done as previously described (11, 23, 42) with minor modification. Briefly, a RNeasy Mini Kit (Qiagen, Valencia, CA, USA) was used to extract RNA from cells. Any contaminating DNA was removed using DNA-free™ kit from Ambion (Ambion, Austin, TX, USA). RNA was quantified using NanoDrop instrumentation (NanoDrop Technologies, Wilmington, DE). Total RNA samples (2 μg) was reverse-transcribed in a 20-μl reaction volume using Ambion's oligo(dT) primer and Qiagen's Omniscript reverse transcriptase according to the manufacturer's instructions. Ten percent of the RT reaction volume (2 μl, corresponding to 200 ng of original input RNA) was used for each subsequent 50-μl PCR reaction. The primers were designed using Vector NTI software and were purchased from Integrated DNA Technologies. The sequences of primers used were as follows: MMP-9, 5′-GCG TGT CTG GAG ATT CGA CTT G-3′ (forward) and CAT GGT CCA CCT TGT TCA CCT C (reverse); CCL2: 5’-GCC AGC TCT CTC TTC CTC CAC-3’ (forward) and 5’-GAG TAG CAG CAG GTG AGT GGG -3’ (reverse); VCAM-1: 5’-TCT ATT TCA CTC ACA CCA GCC CG-3’ (forward) and 5’-ATC CAA AGT ACC GTT GAG GCT CC-3’ (reverse); GAPDH: 5′-ATG ACA ATG AAT ACG GCT ACA GCA A-3′ (forward) and 5′-GCA GCG AAC TTT ATT GAT GGT ATT-3′ (reverse). Hot start PCR reactions were conducted as 94 °C × 3′; (94 °C × 1′, 56°C × 30′, 72°C × 1′) × 30 cycles; 72 °C × 10′. RT-PCR was also performed for GADPH as a control to check the amount and integrity of total RNA samples. The specificity of the amplified products was confirmed by the size of the templates and also by performing PCR reactions in the absence of RT products.
Quantitative real-time PCR was carried out using the same primers as described above and following a method as previously detailed (11, 45). Briefly, approximately 25 μl of reaction volume was used for the real-time PCR assay that consisted of 2× (12.5 μl) Brilliant SYBR Green QPCR Master Mix (Applied Biosystem), 400 nM of primers (0.5 μl each from the stock), 11 μl of water, and 0.5 μl of template. The thermal conditions consisted of an initial denaturation at 95°C for 10 min followed by 40 cycles of denaturation at 95°C for 15 s, annealing and extension at 60 °C for 1 min, and, for a final step, a melting curve of 95°C for 15 s, 60°C for 15 s, and 95°C for 15 s. All reactions were carried out in triplicate to reduce variation. The data were analyzed using SDS software version 2.0, and the results were exported to Microsoft Excel for further analysis. Data normalization was accomplished using the endogenous control (GAPDH, glyceraldehyde-3-phosphate dehydrogenase) and the normalized values were subjected to a 2−ΔΔCt formula to calculate the fold change between the control and experiment groups.
Transient Transfection and Reporter Gene Activity
Cells plated in 24-well tissue culture plates were transfected with different plasmids using Effectene transfection reagent according to the protocol suggested by the manufacturer (Qiagen). Transfection efficiency was controlled by cotransfection of myoblasts with Renilla luciferase encoding plasmid pRL-TK (Promega). For transfection of C2C12 with siRNA, we used DharmaFect-3 transfection reagent (Dharmacon RNA Technologies). After appropriate treatments, specimens were processed for luciferase expression using Dual luciferase assay systems with reporter lysis buffer per the manufacturer's instructions (Promega, Madison, WI). Luciferase measurements were made using a luminometer (Berthold Detection Systems, USA). Secreted alkaline phosphatase activity (SEAP) in culture supernatants was measured by a standard assay using para-nitrophenyl phosphate as a substrate (42).
Statistical Analysis
Results are expressed as mean ± standard deviation (SD). The Student's t test or ANOVA was used to compare quantitative data populations with normal distributions and equal variance. A value of P < 0.05 was considered statistically significant unless otherwise specified.
RESULTS
Using TAK1+/+ and TAK1−/− MEF and the molecular and pharmacological agents, here we have investigate the role of TAK1 in the TWEAK-induced activation of NF-κB, AP-1, p44/42, JNK1, p38 MAPK, and Akt kinase and the expression of proinflammatory genes MMP-9, CCL-2, and VCAM-1.
TWEAK activates TAK1 in C2C12 myoblasts and mouse embryonic fibroblasts (MEF)
We first studied the effect of TWEAK on the phosphorylation and activation of TAK1. C2C12 myoblasts were treated with recombinant TWEAK protein for different time intervals ranging from 0 to 60 minutes. The phosphorylation of TAK1 was measured using antibody that recognizes TAK1 protein phosphorylated at Se412 or Thr184/187 residues. Treatment of C2C12 myoblasts with recombinant TWEAK protein increased the phosphorylation of TAK1 at Ser412 (Fig1A, top panel) and Thr184/187 (Fig. 1A, second panel) residues. We also measured kinase activity of TAK1 by immunoprecipitation with TAK1 antibody followed by in vitro kinase assay using His-MKK6 as substrate. Treatment with TWEAK increased the kinase activity of TAK1 in C2C12 myoblasts (Fig. 1A, third panel from top). TWEAK did not affect the cellular level of either TAK1 or other proteins such as TRAF6, TAB1, and TAB2 which form complex with TAK1 and involved in its activation (Fig. 1A). Furthermore, TWEAK was also found to increase the phosphorylation of TAK1 in mouse embryonic fibroblasts (Fig. 1B) suggesting that the effect of TWEAK on TAK1 was not limited to C2C12 myoblasts and other cells respond similarly with respect to TAK1 activation on treatment with TWEAK.
Figure 1. Effect of TWEAK on the activation of TAK.

A). C2C12 myoblasts were treated with TWEAK (100 ng/ml) for indicated time interval, cell extracts made were used for western blotting or immunoprecipitated with TAK1 antibody for in vitro kinase assay. Representative gel pictures presented here show that TWEAK augments the phosphorylation of TAK1 at Ser412 (top panel) and Thr184/187 (second from top) residues and increased its enzymatic activity (third from top). Treatment of cells with TWEAK did not affect the cellular level of TAK1, TRAF-6, TAB1, and TAB2 protein. B). TWEAK treatment also increased the phosphorylation of TAK1 in murine embryonic fibroblasts (MEF) without affecting the total level of TAK1 protein. KA, kinase assay.
TAK1 is required for TWEAK-induced activation of NF-κB
To investigate the role of TAK1 in TWEAK-induced activation of NF-κB, we employed wild-type (TAK1+/+) and TAK1-deficient (TAK1−/−) murine embryonic fibroblasts (MEF). TAK1+/+ and TAK1−/− MEF were treated with 100 ng/ml of soluble TWEAK protein for different time intervals ranging from 0 to 90 min. The activation of NF-κB was measured by electrophoretic mobility shift assay (EMSA). As shown in Fig. 2A, TWEAK increased the DNA-binding activity of NF-κB in TAK1+/+ MEF. However, the activation of NF-κB was significantly impaired in TAK1−/− MEF providing initial evidence that TAK1 is required for TWEAK-induced activation of NF-κB.
Figure 2. Role of TAK1 in TWEAK-induced activation of NF-κB pathways.


A). TAK1+/+ and TAK1−/− MEF were treated with recombinant TWEAK protein (100 ng/ml) for indicated time intervals and the activation of NF-κB was measured by EMSA. A representative EMSA gel presented here show that compared to TAK1+/+ MEF, the activation of NF-κB was significantly inhibited in TAK1−/− MEF in response to TWEAK-treatment. B). Cell extracts prepared from TWEAK-treated TAK1+/+ and TAK1−/− MEF were subjected to western blotting to measure the levels of phosphorylated and total IκBα protein. Representative immunoblots presented here show that TWEAK-induced phosphorylation (upper panel) and degradation (middle panel) of IκBα protein was significantly inhibited in TAK1−/− cells compared to TAK1+/+ MEF. The level of an unrelated protein β-actin did not change due to TWEAK treatment. C). TAK1+/+ and TAK1−/− MEF were treated with 100 ng/ml TWEAK protein for indicated time intervals and the activation of IKK was measured. Data presented here show that the activation of IKK was significantly inhibited in TAK1−/− MEF as compared to TAK1+/+ MEF (top panel). There was no difference in the total level of IKKα/β proteins in TWEAK-treated TAK1+/+ and TAK1−/− cells (middle panel). Western blotting confirmed the deficiency of full length TAK1 protein in TAK1−/− MEF (lower panel). D). TAK1+/+ and TAK1−/− MEF were treated with 100 ng/ml TWEAK protein for indicated time intervals and the level of p100 and p52 protein was measured by western blotting. Data presented here show that TWEAK induces the degradation of p100 in MEF but there was no significant difference in the amount of p100 between TAK1+/+ and TAK1−/− MEF at indicated time points.
TAK1 is required for TWEAK-induced activation of canonical NF-κB pathway
In the canonical signaling pathway, the activation and translocation of NF-κB to nucleus is preceded by the phosphorylation, ubiquitination, and proteolytic degradation of IκBα protein (21). We examined whether TAK1 was required for TWEAK-induced phosphorylation and degradation of IκBα protein. As shown in Fig. 2B, TWEAK rapidly increased the phosphorylation of IκBα protein in TAK1+/+ MEF. However, the TWEAK-induced phosphorylation of IκBα protein was significantly lower in TAK1−/− MEF compared to TAK1+/+ MEF. Similarly, the TWEAK-induced degradation of IκBα protein (evident at 30 and 60 min) in TAK1+/+ MEF was blunted in TAK1−/− MEF (Fig. 2B).
We also measured the activation of IKK in response to TWEAK treatment. The activation of IKK was studied by immunoprecipitation with IKKγ followed by in vitro kinase assay using GST-IκBα (1-36) as substrate. TWEAK drastically increased the activity of IKK complex in TAK1+/+ MEF. However, the TWEAK-induced activation of IKK was prevented in TAK1−/−MEF (Fig. 2C, top panel). TWEAK treatment had no effect on the total level of IKKα/β protein (Fig. 2C, middle panel). By performing western blotting with TAK1 antibody, we also confirmed that TAK1−/− MEF lack wild-type TAK1 protein (Fig. 2C, bottom panel).
TWEAK-induced activation of alternative NF-κB pathway does not involve TAK1
The activation of alternative (non-canonical) NF-κB pathway involves the upstream activation of IKKα and proteolytic processing of p100 into p52 protein (21). A recent report suggests that along with canonical pathway, TWEAK can also activate non-canonical NF-κB signaling pathway (22). To investigate the role of TAK1 in activation of alternative NF-κB signaling pathway, we measured the degradation of p100 protein in TWEAK-treated TAK1+/+ and TAK1−/− MEF. TWEAK treatment reduced the cellular level of p100 protein (Fig. 2D) confirming that TWEAK activates alternative NF-κB signaling pathway in MEF. However, there was no significant difference in the degradation of p100 protein between TAK1+/+ and TAK1−/− MEF treated with TWEAK at different time points. These data suggest that although TWEAK activates alternative NF-κB signaling pathway, TAK1 is not involved in the TWEAK-induced activation of alternative pathway.
TAK1 is required for TWEAK-induced transactivation of NF-κB
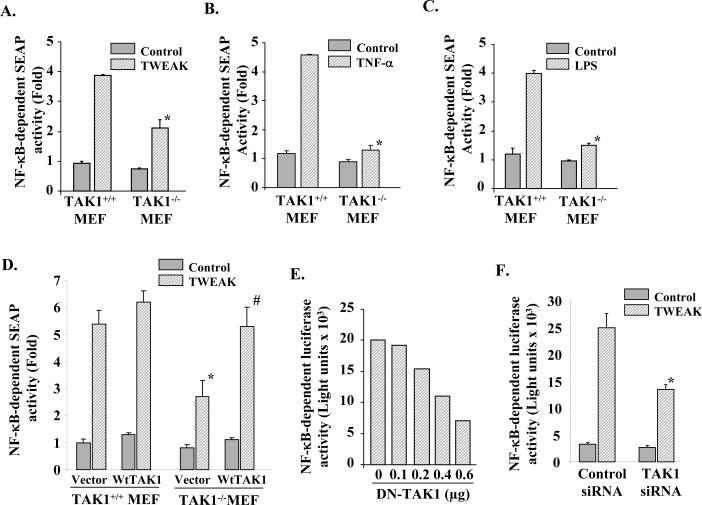
Because increased DNA-binding activity of NF-κB does not always result in its transcriptional activation (21, 46), we investigated whether TWEAK augments the transcriptional activity of NF-κB and whether the inhibition of TAK1 can block the TWEAK-induced transcriptional activation of NF-κB. TAK1+/+ and TAK1−/− cells were transiently transfected with pNF-κB-SEAP plasmid (Clontech). After 36h of transfection, the cells were treated with TWEAK for 18h and the amount of secreted alkaline phosphatase (SEAP) in culture supernatants was measured. As shown in Fig. 3A, the production of SEAP in culture supernatants was significantly reduced in TAK1−/− MEF as compared to TAK1+/+ MEF. Interestingly, we observed that while the transactivation of NF-κB was significantly inhibited (∼ 50%) in TAK1−/− MEF compared to wild-type MEF, the inhibition was not complete. To address this issue and to determine the extent of TAK1 involvement in NF-κB activation, we compared TWEAK-induced NF-κB activation with TNF-α and LPS which also activates NF-κB through the recruitment of TAK1 (37). Interestingly, the inhibition of NF-κB in TAK1−/−MEF in response to TNF-α (Fig. 3B) or LPS (Fig. 3C) was more drastically compared to TWEAK (Fig. 3A).
Figure 3. Role of TAK1 in transcriptional activation NF-κB.
A). TAK1+/+ and TAK1−/− MEF were transiently transfected with pNF-κB-SEAP plasmid for 36h followed by treatment with A) TWEAK (100 ng/ml), B) TNF-α (10 ng/ml), or C) LPS (1 μg/ml) for 18h and measurement of SEAP activity in culture supernatants. Data presented here show the deficiency of TAK1 (i.e. TAK1−/−) in MEF significantly reduced the transcriptional activation of NF-κB in response to TWEAK, TNF-α, or LPS. *p<0.01, values significantly different from TAK1+/+ MEF treated with TWEAK, TNF-α or LPS. D). TAK1+/+ and TAK1−/− MEF were transiently transfected with vector alone or wild-type (Wt)-TAK1 along with pNF-κB-SEAP plasmid (in 1:10 ratio). After 36h of transfection, the cells were treated with TWEAK (100 ng/ml) for 18h and the production of SEAP in culture supernatants was measured. Data presented here demonstrate that overexpression wild-type TAK1 protein restores the TWEAK-induced activation of NF-κB in TAK1−/−MEF. *p <0.05, values significantly different from vector alone transfected and TWEAK-treated TAK1+/+ MEF. #p <0.05, values significantly different from vector alone transfected and TWEAK-treated TAK1−/− MEF. E). C2C12 myoblasts were transiently transfected with indicated amounts DN-TAK1 plasmid or control vector along with pNF-κB-Luc (1:10) for 36h. The cells were then treated with TWEAK for 18h and the expression of luciferase in the cell extracts was measured. Data presented here show that overexpression of DN-TAK1 inhibits the TWEAK-induced transcriptional activation of NF-κB in C2C12 myoblasts. F). C2C12 myoblasts were first transfected with control or mouse TAK1 SMARTpool siRNA duplexes for 24h. The cells were then transfected with pNF-κB-Luc plasmid. After 24h the cells were treated with TWEAK (100ng/ml) and expression of luciferase in myoblasts was assayed. Data presented here show that siRNA-mediated knockdown of TAK1 also inhibits the TWEAK-induced activation of NF-κB in C2C12 myoblasts. *p <0.05, values significantly different from control siRNA-transfected and TWEAK-treated C2C12 myoblasts.
To further confirm the role of TAK1 in TWEAK-induced NF-κB activation, we investigated whether the expression of wild-type TAK1 protein can restore the TWEAK-induced activation of NF-κB in TAK1−/−MEF. As shown in Fig. 3D, overexpression of wild-type TAK1 protein restored the TWEAK-induced activation of NF-κB in TAK1−/− MEF. We next studied the role of TAK1 in TWEAK-induced activation of NF-κB using a dominant negative mutant of TAK1 (i.e. TAK1 K63W). C2C12 myoblasts were transiently transfected with increasing amount of DN-TAK1 expressing plasmid along with pNF-κB-Luc plasmid. The cells were then treated with TWEAK for 18h and the cell extracts made were analyzed for luciferase activity. Overexpression of DN-TAK1 dose-dependently inhibited the TWEAK-induced transactivation of NF-κB in C2C12 myoblasts (Fig. 3E). Finally, we investigated the effect of siRNA-mediated knockdown of TAK1 in TWEAK-induced transactivation of NF-κB in C2C12 myoblasts. C2C12 myoblasts were first transfected with mouse TAK1 SMARTpool siRNA or non-targeting control siRNA along with pNF-κB-Luc plasmid. After 36h of transfection, the cells were treated with TWEAK for 18h and the amount of TAK1 and luciferase in cell lysates was measured. Transfection of C2C12 myoblasts with TAK1 siRNA considerably (by almost 70%) reduced the level of TAK1 protein in C2C12 myoblasts (data not shown). Furthermore, siRNA-mediated knockdown significantly inhibited the TWEAK-induced NF-κB activation in C2C12 myoblasts (Fig. 3F). Taken together, these data suggest that TAK1 is required for the TWEAK-induced NF-κB activation.
TAK1 is required for TWEAK-induced activation of AP-1
We also measured the DNA-binding and transcriptional activation of AP-1, a proinflammatory transcription factor which generally cooperates with NF-κB to augment the expression of various proinflammatory genes (46). Similar to NF-κB, the TWEAK-induced increase in DNA-binding activity of AP-1 was significantly reduced in TAK1−/− MEF compared to TAK1+/+MEF (Fig. 4A). To study the role of TAK1 in transcriptional activation of AP-1, C2C12 myoblasts were transiently transfected with increasing concentration of DN-TAK1 along with pAP1-Luc plasmid (Clontech). The cells were then treated with TWEAK and luciferase activity was measured in cell extracts. As shown in Fig. 4B, the overexpression of DN-TAK1 reduced the TWEAK-induced transactivation of AP-1 in C2C12 myoblasts. These data suggest that TAK1 is involved in the activation of AP-1 in response to TWEAK.
Figure 4. Role of TAK1 in TWEAK-induced activation of AP-1 transcription factor.

A). TAK1+/+ and TAK1−/− MEF were treated with recombinant TWEAK protein (100 ng/ml) for indicated time intervals and the activation of AP-1 was measured by EMSA. A representative EMSA gel presented here show that compared to TAK1+/+ MEF, TWEAK-induced activation of AP-1 was inhibited in TAK1−/− MEF. B). C2C12 myoblasts were transiently transfected with indicated amounts DN-TAK1 plasmid or control vector along with AP1-Luc (1:10) plasmid for 36h. The cells were then treated with TWEAK for 18h and the expression of luciferase in cell extracts was measured. Data presented here show that the overexpression of DN-TAK1 blocked the TWEAK-induced transcriptional activation of AP-1 in a dose-dependent manner.
TAK1 is involved in TWEAK-induced activation of JNK1 and p38MAPK but not p44/p42 MAPK and Akt kinase
We next investigated the role of TAK1 in the activation of MAPK in response to TWEAK treatment. The activation of p44/p42 and p38MAPK was measured by immunoblotting with antibody that recognize phosphorylated form of p44/p42 or p38 MAPK. The activation of JNK1 was measured by immunoprecipitation and in vitro kinase assay. As depicted in Fig. 5A, there was no significant difference in the level of activation of p44/42 between TWEAK-treated TAK1+/+ and TAK1−/− MEF. However, the TWEAK-induced activation of JNK1 (Fig. 5B) and p38 MAPK (Fig. 5C) was significantly inhibited in TAK1−/−MEF compared to TAK1+/+ MEF. We also measured the activation of Akt by immunoblotting with antibody that recognize phosphorylated Akt protein. There was no significant difference in the level of phosphorylation of Akt between TAK1+/+ and TAK1−/− MEF upon treatment with TWEAK. Furthermore, during this time of TWEAK treatment, the total levels of p44/p42, JNK1, p38 MAPK or Akt did not change in either TAK1+/+ or TAK1−/− MEF (Fig. 5).
Figure 5. Role of TAK1 in TWEAK-induced activation of ERK1/2, JNK1, p38 MAPK and Akt.
TAK1+/+ and TAK1−/− MEF were treated with TWEAK (100 ng/ml) for indicated time intervals and the activation of A) ERK1/2, B) JNK1 C) p38MAPK, and D) Akt kinase was measured. Representative gel pictures presented here show that the TWEAK-induced activation of JNK1 and p38 MAPK but not ERK1/2 or Akt kinase was significantly inhibited in TAK1−/− MEF compared to TAK1+/+ MEF. There was no significant difference in total level of ERK1/2, JNK1, p38MAPK, or Akt kinase between TAK1+/+ and TAK1−/− MEF or after treatment with TWEAK.
TAK1 is required for the TWEAK-induced expression of MMP-9, CCL-2, and VCAM-1 gene
Since TWEAK can activate the expression of a number of proinflammatory proteins including MMP-9, CCL-2, and VCAM-1 (10, 47, 48), we investigate whether TAK1 is involved in TWEAK-induced expression of MMP-9, CCL-2, and VCAM-1. TAK1+/+ and TAK1−/− MEF were treated with soluble TWEAK protein for 12h and the expression of MMP-9, CCL-2, and VCAM-1 was studied by semi-quantitative RT-PCR method. As shown in Fig. 6A, TWEAK-induced the expression of MMP-9, CCL-2, and VCAM-1 in TAK1+/+ MEF. However, the mRNA levels of these genes was significantly lower in TAK1−/− cells as compared to TAK1+/+ cells.
Figure 6. Involvement of TAK1 in TWEAK-induced expression of MMP-9, CCL-2, and VCAM-1.

TAK1+/+ and TAK1−/− MEF were treated with TWEAK (100 ng/ml) for 12h, the total RNA was extracted and used for measuring the transcript level of MMP-9, CCL-2, and VCAM-1. A). Representative agarose gels pictures of semi-quantitative RT-PCR assay show that TWEAK-induced expression of MMP-9, CCL-2, and VCAM-1 protein was significantly inhibited in TAK1−/− MEF compared to TAK1+/+ MEF. B). Reduced level of MMP-9, CCL-2, or VCAM-1 mRNA in TAK1−/− MEF compared to TAK1+/+ MEF was also confirmed by quantitative real-time PCR. *p < 0.05 values significantly different from corresponding TWEAK-treated TAK1+/+ MEF.
We also quantified the fold difference in the mRNA levels of MMP-9, CCL-2, and VCAM-1 by quantitative real time-PCR. Consistent with semiquantitative reverse transcriptase PCR data, the transcript levels of MMP-9, CCL-2, and VCAM-1 were found to be significantly lower in TAK1−/− MEF compared to TAK1+/+ MEF (Fig. 6B). These results suggest that TWEAK induces the expression of MMP-9, CCL-2, and VCAM-1 through the activation of TAK1.
Akt is involved in TWEAK-induced activation of NF-κB and the expression of MMP-9
Published reports suggest an important role for Akt kinase in the activation of NF-κB in response to specific stimuli (49-51). We investigated whether TWEAK-induced activation of NF-κB involved the activation of Akt kinase. Similar to MEF, TWEAK increased the phosphorylation of Akt kinase without affecting its cellular level in C2C12 myotubes (Fig. 7A). To investigate the role of Akt in TWEAK-induced activation of NF-κB, C2C12 myoblasts were transiently transfected with a dominant negative mutant of Akt (DN-Akt) along with pNF-κB-SEAP plasmid (in 1:10 ratio). The cells were then treated with TWEAK and 18h later, the amount of SEAP in culture supernatants was measured. Overexpression of DN-Akt in C2C12 myoblasts significantly blocked the TWEAK-induced transactivation of NF-κB (Fig. 7B) suggesting that Akt kinase is involved in NF-κB activation in response to TWEAK. In another experiment, we measured the effect of inhibition of Akt on TWEAK-induced expression of MMP-9. C2C12 myotubes were preincubated with LY294002 (a pharmacological inhibitor of Akt kinase) for 2h followed by treatment with TWEAK for 24h and the production of MMP-9 in culture supernatants was measured by gelatin zymography and Western blotting techniques. TWEAK-induced MMP-9 production in culture supernatants was significantly inhibited by pretreatment of myotubes with LY294002 (Fig. 7C). Furthermore, the transactivation of MMP-9 promoter in response to TWEAK was significantly inhibited by overexpression of DN-Akt in myotubes (Fig. 7D).
Figure 7. Role of Akt kinase in TWEAK-induced activation of NF-κB and expression of MMP-9.
A). C2C12 myotubes were treated with 100 ng/ml TWEAK protein for indicated time intervals and the phosphorylation of Akt was measured by western blotting using antibody that recognizes phosphorylated Akt protein. Data presented here show that TWEAK augments the phosphorylation of Akt without affecting its total cellular levels. B). C2C12 myoblasts were transiently transfected with vector alone or expressing DN-Akt protein along with pNF-κB-SEAP plasmid in 1:10 ratio. The myoblasts were differentiated into myotubes, treated with TWEAK for 18h and the production of SEAP in culture supernatants was measured. Data presented here show that overexpression of DN-Akt inhibits the TWEAK-induced production of SEAP in culture supernatants. *p<0.01, values significantly different from vector alone transfected TWEAK-treated myotubes. C). C2C12 myotubes were preincubated with 50μM LY294002 for 2h followed by treatment with TWEAK (100 ng/ml) for 24h. The production of MMP-9 in culture supernatants was measured by gelatin zymography (upper panel) or western blotting (lower panel). Data presented here demonstrate that LY294002 significantly inhibited the TWEAK-induced MMP-9 production in myotubes. D). C2C12 myoblasts were transiently transfected with vector alone or plasmid expressing DN-Akt protein along with mouse MMP-9 promoter luciferase plasmid in 1:10 ratio. The myoblasts were then differentiated into myotubes, treated with TWEAK for 24h, and the expression of luciferase in cell extracts was measured. Data presented here show that the overexpression of DN-Akt significantly inhibits the TWEAK-induced activation of MMP-9 promoter in myotubes. #p<0.01, values significantly different from vector alone transfected TWEAK-treated myotubes. E). TAK1+/+ and TAK1−/− MEF were transfected with vector alone or DN-Akt plasmid along with pNF-κB-SEAP plasmid in 1:10 ratio for 36h. The cells were then treated with TWEAK (100ng/ml) for additional 18h and the production of SEAP in culture supernatants was measured. Data presented here demonstrate that overexpression of DN-Akt more drastically reduced the TWEAK-induced transcriptional activation of NF-κB in TAK1−/− MEF. p<0.01, values significantly different from vector alone transfected and TWEAK-treated TAK1+/+ MEF. #p<0.01, values significantly different from vector alone transfected and TWEAK-treated TAK1+/+ MEF. @p<0.05, values significantly different from vector alone transfected and TWEAK-treated TAK1−/− MEF.
Because we found that both TAK1 and Akt were involved in TWEAK-induced activation of NF-κB, we investigated whether the overexpression of DN-Akt will completely block the TWEAK-induced activation of NF-κB in TAK1−/− MEF. TAK1+/+ and TAK1−/− MEF were transiently transfected with vector alone or DN-TAK1 plasmid for 36h followed by treatment with TWEAK for 18h. As shown in Fig. 7D, overexpression of DN-TAK1 further reduced the TWEAK-induced activation of NF-κB in TAK1−/− MEF suggesting that both TAK1 and Akt-mediated signaling pathways contribute to the activation of NF-κB in response to TWEAK.
DISCUSSION
TWEAK is an important member of the TNFSF which regulates several physiological and pathological processes (5). However, the signaling mechanisms by which TWEAK modulates various cellular responses remain largely unknown. In this report, we demonstrate that TWEAK activates TAK1 and the activation of TAK1 is required for the TWEAK-induced activation of NF-κB. We also report that TAK1 is required for the activation of JNK1, p38 MAPK, and AP-1 transcription factor in response to TWEAK. Consistent with the role of TAK1 in TWEAK-induced activation of proinflammatory transcription factors NF-κB and AP-1, the expression of proinflammatory genes MMP-9, CCL-2, and VCAM-1 was reduced in TAK1-deficient cells upon treatment with TWEAK. Finally, our results demonstrate that TWEAK-induced activation of NF-κB and the expression of MMP-9 also involve the activation of Akt kinase. A schematic representation of the biochemical signaling mechanisms that might be involved in the TWEAK-induced activation of NF-κB and MAPK is depicted in Fig. 8.
Figure 8. Proposed signaling pathway involving TAK1 and Akt in TWEAK-induced activation of NF-κB.
Binding of TWEAK to Fn14 receptors leads to the activation of TAK1 and Akt through recruitment of TRAF-1, 2, 3, and/or 5 proteins. Activated TAK1 and Akt then phosphorylate and activate IKKβ in IKK complex leading to the activation NF-κB through canonical pathway. Activated TAK1 can also activate JNK1 and p38 MAPK which in their turn activates AP-1. Increased activation of NF-κB and AP-1 leads to the increased expression of proinflammatory genes.
In the recent years, significant progress has been made towards understanding the intracellular signaling pathways activated in response to various growth factors, microbial products, and cytokines. Although TWEAK has been shown to activate NF-κB and MAPK in different cell types (11, 12, 22, 24), the upstream signaling events culminating in the activation of NF-κB or MAPK are not yet fully understood. TAK1 is a well-characterized MAPK kinase kinase family member that is activated in response to a number of stimuli such as TNF-α, IL-1β, IL-18, RANKL, osmotic stress, toll-like receptors and B cell receptors, and upon antigen stimulation (30, 31, 37). TAK1 has been shown to participate in diverse cellular responses, including innate and adaptive immune responses (52, 53), survival of hematopoietic cells and heptocytes (54), and growth and differentiation of epidermis (55). Furthermore, TAK1 appears to be an important regulator of inflammatory response in both physiological and pathological conditions (42, 56, 57). Accumulating evidence suggests that the activation of TAK1 in response to different stimuli leads to the activation of IKK complex resulting in the phosphorylation and degradation of IκB proteins and activation of NF-κB (35-37). Interestingly, we found that recombinant TWEAK protein rapidly activates TAK1 in cultured myoblasts and MEF (Fig. 1). Furthermore, our data demonstrate that genetic ablation of TAK1 (i.e. TAK1−/− MEF) inhibits the TWEAK-induced activation of IKK and NF-κB (Fig. 2 A, B and C). These results suggest that TAK1 is involved in the TWEAK-induced signaling pathway leading to the activation of NF-κB. Our results also suggest that TAK1 is not involved in the activation of alternative NF-κB signaling pathway (21) which involves upstream activation of IKKα and proteolytic degradation of p100 protein (Fig. 2D). The role of TAK1 in TWEAK-induced canonical pathway is consistent with published reports suggesting that activated TAK1 causes the phosphorylation and activation of IKKβ but not IKKα (30).
Several published reports suggest that TAK1 is indispensable for the activation of NF-κB in response to many proinflammatory stimuli such as TNF-α, IL-1β, and LPS (29, 30, 37). To further understand the contribution of TAK1 in TWEAK-induced NF-κB activation, we compared the transactivation of NF-κB in response to TWEAK, TNF-α and LPS in wild-type and TAK1-deficinet MEF. Interestingly, we found that although the transactivation of NF-κB in response to TWEAK was significantly inhibited in TAK1−/− cells compared to TAK1+/+ MEF, the inhibition was not complete. Loss of TAK1 reduced NF-κB transactivation by almost 50% (Fig 4A). On the other hand, TNF-α and to some extent LPS-induced activation of NF-κB was completely suppressed in TAK1−/− cells (Fig. 4B, and C). The lack of total inhibition of NF-κB transcriptional activity in TAK1−/− cells suggests that in addition to TAK1, there might be some other kinases/pathways which mediate TWEAK-induced activation of IKKβ and NF-κB. Although the identity of such kinases remains unknown, NF-κB-inducing kinase (NIK) and Akt kinase (Fig. 7) represents two potential upstream activators of IKKβ and NF-κB in response to TWEAK. In a recent report, Saitoh et al showed that functional NIK was required for the TWEAK-induced activation of NF-κB (22). Furthermore, recent studies in our laboratory have shown that the inhibition of NIK or IKKβ strongly inhibits the TWEAK-induced activation of NF-κB and the expression of NF-κB regulated genes in skeletal muscle indicating that NIK mediates the activation NF-κB in response to TWEAK (Li et al., Submitted for publication). Indeed, there are other reports which suggest that in addition to IKKα, NIK can also activate IKKβ in response to specific stimuli (58, 59).
Another possibility why the inhibition of NF-κB was partial in response to TWEAK but complete on treatment with TNF-α or LPS could be the pathways, which TNF-α and TWEAK employ to activate NF-κB. While TNF-α activates NF-κB mainly through canonical pathway, TWEAK can activate both canonical and non-canonical (or alternative) NF-κB pathways (22). Because TAK1 is involved only in the activation of NF-κB via canonical (i.e. IKKβ-dependent) pathway (30, 37), the residual NF-κB activity in TWEAK-treated TAK1−/− cells might be attributed to the activation of NF-κB through non-canonical pathway which TWEAK activates in both TAK1+/+ and TAK1−/−MEF (Fig. 2D). Although more studies are required to understand how interaction of various ligands to their specific cell surface receptors culminates in TAK1 activation, the involvement of TAK1 in the activation of NF-κB suggests that TAK1 acts in a signaling nexus that respond to a variety of stimuli including TWEAK.
We also found that the TWEAK-induced activation of AP-1, JNK1, and p38 but not p44/42 MAPK was significantly inhibited in the TAK1−/− MEF (Fig. 3 and 4). Our results are consistent with the published reports suggesting that TAK1 is an upstream activator of JNK1 and p38 MAPK but not p44/p42 (30, 38, 39, 41). Recent reports also suggest that TWEAK can augment the expression of several proinflammatory molecules including CCL-2 (a chemokine), MMP-9 (a matrix-degrading enzyme), and VCAM-1 (a cell adhesion molecule) (10, 47, 48). The increased production of these and several other inflammatory molecules has been suggested as mechanistic in TWEAK-induced pathological responses (5, 6, 9, 15). Interestingly, the expression of several proinflammatory cytokines, chemokines, cell adhesion molecules, and extracellular matrix degrading enzymes is regulated through the activation NF-κB and AP-1 transcription factors (46). Our results demonstrating that the expression of CCL-2, MMP-9 and VCAM-1 is inhibited in TAK1-deficient cells suggest that TAK1 constitute the molecular pathway that leads to the TWEAK-induced production of CCL-2, MMP-9 and VCAM-1 possibly through the activation of NF-κB and AP-1.
The PI3K/Akt pathway is one of the well studied cell-signaling pathways involved in regulation of multiple biological processes such as apoptosis, metabolism, cell proliferation, and cell growth (60-62). Published reports suggest that in response to specific stimuli, Akt can activate NF-κB via the activation of IKK complex (49-51). However, the role of Akt in TWEAK-mediated signaling is not very clear. Our data in this study (Fig. 7A) and in a recently published report from our group (23) suggest that TWEAK transiently activates (maximum after 15 min) Akt kinase. However, prolonged (> 2h) treatment of cells with TWEAK results in the inhibition of Akt kinase (12, 23, 63). Our results in this study suggest that the activation of Akt kinase contributes to the TWEAK-induced activation of NF-κB and the expression of NF-κB regulated genes (e.g. MMP-9). Overexpression of a kinase-dead (i.e. dominant negative) mutant of Akt drastically inhibited the TWEAK-induced activation of NF-κB in myotubes (Fig. 7B). Furthermore, pharmacological inhibition of Akt using LY294002 or overexpression of a dominant negative Akt mutant inhibited the TWEAK-induced production of MMP-9 and activation of MMP-9 promoter, respectively (Fig. 7 C and D). In addition, overexpression of DN-Akt further attenuated the TWEAK-induced activation of NF-κB in TAK1−/− MEF (Fig. 7E). Although our results clearly indicate that both TAK1 and Akt are involved in TWEAK-induced activation of NF-κB, they seem to work in parallel pathways to activate NF-κB. This contention is supported by our data demonstrating that there was no difference in the TWEAK-induced phosphorylation of Akt kinase between TAK1+/+ and TAK1−/− MEF (Fig. 5D). Furthermore, the pharmacological inhibition of Akt kinase using LY294002 did not affect the TWEAK-induced activation of TAK1 in C2C12 myoblasts (data not shown).
In summary, we provide the first evidence that TAK1 and Akt constitute the molecular pathway that leads to the activation of NF-κB and AP-1 in response to TWEAK. Furthermore, our study suggests that TWEAK-induced expression of proinflammatory molecules is mediated through the activation of TAK1 indicating that TAK1 can serve as an important molecular target to regulate inflammation in TWEAK-related diseases.
Acknowledgments
We thank Prof. Shizuo Akira and Dr. Osamu Takeuchi of Osaka University, Osaka, Japan for providing wild-type and TAK1-deficient mouse embryonic fibroblasts. We also thank Dr. Jun Ninomiya-Tsuji for providing DN-TAK1, WT-TAK1, and His-MKK6 plasmid constructs and Prof. S.V. Reddy for mouse MMP-9 promoter-luciferase construct. This work was supported by a National Institute of Health Grant (RO1 AG129623) to A K.
Abbreviations
- AP-1
activator protein-1
- CCL2
chemokine (C-C motif) ligand 2
- DN
dominant negative
- EMSA
electrophoretic mobility shift assay
- ERK1/2
extracellular signal-related kinase
- IKK
IκB kinase
- JNK1
c-Jun N-terminal kinase-1
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MEF
mouse embryonic fibroblasts
- MMP-9
matrix metalloprotease-9
- NF-κB
nuclear factor-B
- PI3K
phosphoinositide 3-kinase
- SEAP
secreted alkaline phosphatase
- siRNA
short interfering RNA
- TGF-β
transforming growth factor-beta
- TAK1
TGF-β activated kinase 1
- TNF
tumor necrosis factor
- TNFSF
TNF super family
- TRAF
TNF-receptor associated factor
- TWEAK
TNF-related weak inducer of apoptosis
- VCAM-1
vascular cell adhesion molecule-1
REFERENCES
- 1.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 2.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB. Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Ann Rheum Dis. 2000;59(Suppl 1):i6–16. doi: 10.1136/ard.59.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 5.Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov. 2008;7:411–425. doi: 10.1038/nrd2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkles JA, Tran NL, Brown SA, Stains N, Cunliffe HE, Berens ME. Role of TWEAK and Fn14 in tumor biology. Front Biosci. 2007;12:2761–2771. doi: 10.2741/2270. [DOI] [PubMed] [Google Scholar]
- 7.Wiley SR, Cassiano L, Lofton T, Davis-Smith T, Winkles JA, Lindner V, Liu H, Daniel TO, Smith CA, Fanslow WC. A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity. 2001;15:837–846. doi: 10.1016/s1074-7613(01)00232-1. [DOI] [PubMed] [Google Scholar]
- 8.Maecker H, Varfolomeev E, Kischkel F, Lawrence D, LeBlanc H, Lee W, Hurst S, Danilenko D, Li J, Filvaroff E, Yang B, Daniel D, Ashkenazi A. TWEAK attenuates the transition from innate to adaptive immunity. Cell. 2005;123:931–944. doi: 10.1016/j.cell.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Wiley SR, Winkles JA. TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev. 2003;14:241–249. doi: 10.1016/s1359-6101(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 10.Perper SJ, Browning B, Burkly LC, Weng S, Gao C, Giza K, Su L, Tarilonte L, Crowell T, Rajman L, Runkel L, Scott M, Atkins GJ, Findlay DM, Zheng TS, Hess H. TWEAK is a novel arthritogenic mediator. J Immunol. 2006;177:2610–2620. doi: 10.4049/jimmunol.177.4.2610. [DOI] [PubMed] [Google Scholar]
- 11.Dogra C, Changotra H, Mohan S, Kumar A. Tumor necrosis factor-like weak inducer of apoptosis inhibits skeletal myogenesis through sustained activation of nuclear factor-kappaB and degradation of MyoD protein. J Biol Chem. 2006;281:10327–10336. doi: 10.1074/jbc.M511131200. [DOI] [PubMed] [Google Scholar]
- 12.Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J. 2007;21:1857–1869. doi: 10.1096/fj.06-7537com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan MJ, Lewis EE, Shelden EA, Somers E, Pavlic R, McCune WJ, Richardson BC. The apoptotic ligands TRAIL, TWEAK, and Fas ligand mediate monocyte death induced by autologous lupus T cells. J Immunol. 2002;169:6020–6029. doi: 10.4049/jimmunol.169.10.6020. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Z, Burkly LC, Campbell S, Schwartz N, Molano A, Choudhury A, Eisenberg RA, Michaelson JS, Putterman C. TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J Immunol. 2007;179:7949–7958. doi: 10.4049/jimmunol.179.11.7949. [DOI] [PubMed] [Google Scholar]
- 15.Aktas O, Prozorovski T, Zipp F. Death ligands and autoimmune demyelination. Neuroscientist. 2006;12:305–316. doi: 10.1177/1073858405285208. [DOI] [PubMed] [Google Scholar]
- 16.Yepes M, Brown SA, Moore EG, Smith EP, Lawrence DA, Winkles JA. A soluble Fn14-Fc decoy receptor reduces infarct volume in a murine model of cerebral ischemia. Am J Pathol. 2005;166:511–520. doi: 10.1016/S0002-9440(10)62273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iocca HA, Plant SR, Wang Y, Runkel L, O'Connor BP, Lundsmith ET, Hahm K, van Deventer HW, Burkly LC, Ting JP. TNF superfamily member TWEAK exacerbates inflammation and demyelination in the cuprizone-induced model. J Neuroimmunol. 2008;194:97–106. doi: 10.1016/j.jneuroim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Potrovita I, Zhang W, Burkly L, Hahm K, Lincecum J, Wang MZ, Maurer MH, Rossner M, Schneider A, Schwaninger M. Tumor necrosis factor-like weak inducer of apoptosis-induced neurodegeneration. J Neurosci. 2004;24:8237–8244. doi: 10.1523/JNEUROSCI.1089-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran NL, McDonough WS, Donohue PJ, Winkles JA, Berens TJ, Ross KR, Hoelzinger DB, Beaudry C, Coons SW, Berens ME. The human Fn14 receptor gene is up-regulated in migrating glioma cells in vitro and overexpressed in advanced glial tumors. Am J Pathol. 2003;162:1313–1321. doi: 10.1016/S0002-9440(10)63927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran NL, McDonough WS, Savitch BA, Sawyer TF, Winkles JA, Berens ME. The tumor necrosis factor-like weak inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14 (Fn14) signaling system regulates glioma cell survival via NFkappaB pathway activation and BCLXL/BCL-W expression. J Biol Chem. 2005;280:3483–3492. doi: 10.1074/jbc.M409906200. [DOI] [PubMed] [Google Scholar]
- 21.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 22.Saitoh T, Nakayama M, Nakano H, Yagita H, Yamamoto N, Yamaoka S. TWEAK induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation. J Biol Chem. 2003;278:36005–36012. doi: 10.1074/jbc.M304266200. [DOI] [PubMed] [Google Scholar]
- 23.Dogra C, Hall SL, Wedhas N, Linkhart TA, Kumar A. Fibroblast growth factor inducible 14 (Fn14) is required for the expression of myogenic regulatory factors and differentiation of myoblasts into myotubes. Evidence for TWEAK-independent functions of Fn14 during myogenesis. J Biol Chem. 2007;282:15000–15010. doi: 10.1074/jbc.M608668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polek TC, Talpaz M, Darnay BG, Spivak-Kroizman T. TWEAK mediates signal transduction and differentiation of RAW264.7 cells in the absence of Fn14/TweakR. Evidence for a second TWEAK receptor. J Biol Chem. 2003;278:32317–32323. doi: 10.1074/jbc.M302518200. [DOI] [PubMed] [Google Scholar]
- 25.Brown SA, Richards CM, Hanscom HN, Feng SL, Winkles JA. The Fn14 cytoplasmic tail binds tumour-necrosis-factor-receptor-associated factors 1, 2, 3 and 5 and mediates nuclear factor-kappaB activation. Biochem J. 2003;371:395–403. doi: 10.1042/BJ20021730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han S, Yoon K, Lee K, Kim K, Jang H, Lee NK, Hwang K, Young Lee S. TNF-related weak inducer of apoptosis receptor, a TNF receptor superfamily member, activates NF-kappa B through TNF receptor-associated factors. Biochem Biophys Res Commun. 2003;305:789–796. doi: 10.1016/s0006-291x(03)00852-0. [DOI] [PubMed] [Google Scholar]
- 27.Vince JE, Chau D, Callus B, Wong WW, Hawkins CJ, Schneider P, McKinlay M, Benetatos CA, Condon SM, Chunduru SK, Yeoh G, Brink R, Vaux DL, Silke J. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J Cell Biol. 2008;182:171–184. doi: 10.1083/jcb.200801010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 29.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 30.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wald D, Commane M, Stark GR, Li X. IRAK and TAK1 are required for IL-18-mediated signaling. Eur J Immunol. 2001;31:3747–3754. doi: 10.1002/1521-4141(200112)31:12<3747::aid-immu3747>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Huangfu WC, Omori E, Akira S, Matsumoto K, Ninomiya-Tsuji J. Osmotic stress activates the TAK1-JNK pathway while blocking TAK1-mediated NF-kappaB activation: TAO2 regulates TAK1 pathways. J Biol Chem. 2006;281:28802–28810. doi: 10.1074/jbc.M603627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizukami J, Takaesu G, Akatsuka H, Sakurai H, Ninomiya-Tsuji J, Matsumoto K, Sakurai N. Receptor activator of NF-kappaB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol Cell Biol. 2002;22:992–1000. doi: 10.1128/MCB.22.4.992-1000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madhavan S, Anghelina M, Sjostrom D, Dossumbekova A, Guttridge DC, Agarwal S. Biomechanical signals suppress TAK1 activation to inhibit NF-kappaB transcriptional activation in fibrochondrocytes. J Immunol. 2007;179:6246–6254. doi: 10.4049/jimmunol.179.9.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 36.Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, Matsumoto K. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell. 2000;5:649–658. doi: 10.1016/s1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 38.Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- 39.Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, Nishida E, Hagiwara M. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem. 1996;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- 40.Shirakabe K, Yamaguchi K, Shibuya H, Irie K, Matsuda S, Moriguchi T, Gotoh Y, Matsumoto K, Nishida E. TAK1 mediates the ceramide signaling to stress-activated protein kinase/c-Jun N-terminal kinase. J Biol Chem. 1997;272:8141–8144. doi: 10.1074/jbc.272.13.8141. [DOI] [PubMed] [Google Scholar]
- 41.Silverman N, Zhou R, Erlich RL, Hunter M, Bernstein E, Schneider D, Maniatis T. Immune activation of NF-kappaB and JNK requires Drosophila TAK1. J Biol Chem. 2003;278:48928–48934. doi: 10.1074/jbc.M304802200. [DOI] [PubMed] [Google Scholar]
- 42.Srivastava AK, Qin X, Wedhas N, Arnush M, Linkhart TA, Chadwick RB, Kumar A. Tumor necrosis factor-alpha augments matrix metalloproteinase-9 production in skeletal muscle cells through the activation of transforming growth factor-beta-activated kinase 1 (TAK1)-dependent signaling pathway. J Biol Chem. 2007;282:35113–35124. doi: 10.1074/jbc.M705329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar A, Manna SK, Dhawan S, Aggarwal BB. HIV-Tat protein activates c-Jun N-terminal kinase and activator protein-1. J Immunol. 1998;161:776–781. [PubMed] [Google Scholar]
- 44.Kumar A, Boriek AM. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J. 2003;17:386–396. doi: 10.1096/fj.02-0542com. [DOI] [PubMed] [Google Scholar]
- 45.Wedhas N, Klamut HJ, Dogra C, Srivastava AK, Mohan S, Kumar A. Inhibition of mechanosensitive cation channels inhibits myogenic differentiation by suppressing the expression of myogenic regulatory factors and caspase-3 activity. FASEB J. 2005;19:1986–1997. doi: 10.1096/fj.05-4198com. [DOI] [PubMed] [Google Scholar]
- 46.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 47.Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Matsuo T. Proinflammatory effects of tumour necrosis factor-like weak inducer of apoptosis (TWEAK) on human gingival fibroblasts. Clin Exp Immunol. 2006;146:540–549. doi: 10.1111/j.1365-2249.2006.03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SH, Kang YJ, Kim WJ, Woo DK, Lee Y, Kim DI, Park YB, Kwon BS, Park JE, Lee WH. TWEAK can induce pro-inflammatory cytokines and matrix metalloproteinase-9 in macrophages. Circ J. 2004;68:396–399. doi: 10.1253/circj.68.396. [DOI] [PubMed] [Google Scholar]
- 49.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 50.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 51.Dogra C, Changotra H, Wergedal JE, Kumar A. Regulation of phosphatidylinositol 3-kinase (PI3K)/Akt and nuclear factor-kappa B signaling pathways in dystrophin-deficient skeletal muscle in response to mechanical stretch. J Cell Physiol. 2006;208:575–585. doi: 10.1002/jcp.20696. [DOI] [PubMed] [Google Scholar]
- 52.Liu HH, Xie M, Schneider MD, Chen ZJ. Essential role of TAK1 in thymocyte development and activation. Proc Natl Acad Sci U S A. 2006;103:11677–11682. doi: 10.1073/pnas.0603089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 54.Tang M, Wei X, Guo Y, Breslin P, Zhang S, Wei W, Xia Z, Diaz M, Akira S, Zhang J. TAK1 is required for the survival of hematopoietic cells and hepatocytes in mice. J Exp Med. 2008;205:1611–1619. doi: 10.1084/jem.20080297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sayama K, Hanakawa Y, Nagai H, Shirakata Y, Dai X, Hirakawa S, Tokumaru S, Tohyama M, Yang L, Sato S, Shizuo A, Hashimoto K. Transforming growth factor-beta-activated kinase 1 is essential for differentiation and the prevention of apoptosis in epidermis. J Biol Chem. 2006;281:22013–22020. doi: 10.1074/jbc.M601065200. [DOI] [PubMed] [Google Scholar]
- 56.Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC, Ninomiya-Tsuji J. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem. 2006;281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, Mihara M, Tsuchiya M, Matsumoto K. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem. 2003;278:18485–18490. doi: 10.1074/jbc.M207453200. [DOI] [PubMed] [Google Scholar]
- 58.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 59.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 60.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest. 2005;115:2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 62.Merlot S, Firtel RA. Leading the way: Directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J Cell Sci. 2003;116:3471–3478. doi: 10.1242/jcs.00703. [DOI] [PubMed] [Google Scholar]
- 63.Feng F, Wang L, Albanese N, Holmes A, Xia P. Tumor necrosis factor-like weak inducer of apoptosis attenuates the action of insulin in hepatocytes. Endocrinology. 2008;149:1505–1513. doi: 10.1210/en.2007-1119. [DOI] [PubMed] [Google Scholar]