Abstract
Objective
Mitochondrial depolarization aids platelet activation. Oxidized LDL (oxLDL) contains the medium length oxidatively truncated phospholipid hexadecyl azelaoyl-lysoPAF (HAz-LPAF) that disrupts mitochondrial function in nucleated cells, so oxLDL may augment platelet activation.
Methods and Results
Flow cytometry showed intact oxLDL particles synergized with sub-threshold amounts of soluble agonists to increase intracellular Ca++, and initiate platelet aggregation and surface expression of activated gpIIb/IIIa and P-selectin. oxLDL also induced aggregation and increased intracellular Ca++ in FURA2-labeled cells by itself at low, although not higher, concentrations. HAz-LPAF, alone and in combination with sub-stimulatory amounts of thrombin, rapidly increased cytoplasmic Ca++ and initiated aggregation. HAz-LPAF depolarized mitochondria in intact platelets, but this required concentrations beyond those that directly activated platelets. An unexpectedly large series of chemically pure truncated phospholipids generated by oxidative fragmentation of arachidonoyl-, docosahexaneoyl-, or linoleoyl alkyl phospholipids were platelet agonists. The PAF receptor, thought to effectively recognize only phospholipids with very short sn-2 residues, was essential for platelet activation because PAF receptor agonists blocked signaling by all these medium length phospholipids and oxLDL.
Conclusions
Intact oxLDL particles activate platelets through the PAF receptor, and the PAF receptor responds to a far wider range of oxidized phospholipids in oxLDL than anticipated.
Keywords: oxidized LDL, PAF, platelet, phospholipid, PAF acetylhydrolase
Introduction
Platelet mitochondria maintain cellular energetics and viability1, but these organelles also affect platelet activation. Thrombin produces a rapid decrease in mitochondrial transmembrane potential, thereby increasing reactive oxygen specie formation and caspase 3 activation needed for maximal aggregation 2. Combining maximal amounts of thrombin with collagen3 or with activators of Bax4, a pro-apoptotic protein that physically targets mitochondria, generates a subpopulation of highly activated platelets, and blockade of the mitochondrial permeability transition pore suppresses this form of platelet activation 5, 6.
Oxidized low density lipoprotein (oxLDL) has a fundamental role in thrombotic disease and atherogenesis through activation of inflammatory cells that includes platelets7 8, although the identity of the agonist(s) is unclear because oxLDL contains numerous bioactive compounds. 9 Oxidation of lipoprotein particles, either chemically or enzymatically10, 11, fragments phospholipids that contain polyunsaturated fatty acyl residues. One such oxidatively-truncated phospholipid, hexadecyl azelaoyl lysoPAF (HAz-LPAF), derived from oxidation of common linoleoyl residues, rapidly enters nucleated cells, traffics to mitochondria and initiates the mitochondrial-dependent pathway to apoptotic cell death. 12
We determined whether oxidized LDL contains agents that disrupt platelet mitochondrial function, thereby increasing cellular responsiveness. We prepared pure samples of a series of fragmented alkyl glycerophosphocholines—acylated lysoPAFs—found in oxidized LDL, and discovered that many of these stimulated platelets. However these lipids, and intact oxLDL particles, were direct platelet agonists that did not require mitochondrial involvement. Instead, we find the range of PAF receptor ligands is larger than previously predicted and includes medium length oxidatively truncated alkyl phospholipids found in oxLDL. We show low concentrations of intact oxLDL particles activate platelets only through their PAF receptor.
Materials and Methods
HAz-LPAF and PAF were from Avanti Polar Lipids (Birmingham, AL); human LDL was oxidized with Cu2SO4 as described13; fractions from oxidized arachidonoyl-, linoleoyl-, and docosahexaneoyl-glycerophosphocholine and those prepared synthetically were as described14; other materials are described in the Supplement.
Cell isolation
Protocols using human blood were approved by the Cleveland Clinic IRB committee. Platelets were isolated as described in the online Supplement.
Flow cytometry
Surface proteins were quantified as described (online Supplement). Platelet mitochondrial transmembrane potential was examined in washed platelets (107/ml) incubated (1h) with HAz-LPAF in serum-free RPMI, then with JC-1 (10 µg/ml, 15 min) before they were washed twice with PBS and analyzed by 2 color flow cytometry.
Microscopy
Electron microscopy was performed as described in the Supplement. For fluorescent microscopy, washed platelets (500µl) were mixed with calcein-AM, treated with the specified reagents with mild shaking for 10 min at room temperature in a 4-well cover slip chamber before unattached cells were removed by washing.
Intracellular Ca++ measurement
Platelets (108/ml) were incubated for 30 min with 1 µM FURA2-AM in PSG containing 1 µg/ml prostaglandin E1, collected by centrifugation and suspended in Hank’s buffered salt solution. Fluorescence was continuously recorded at 25 °C by alternating the excitation wavelength between 340 and 380 nm, and detecting the fluorescent emission at 510 nm with the bandwidth set at 2.5 nm for both emission and excitation.
Data Expression and statistics
Experiments were performed at least three times with cells from different donors with representative experiments or mean ± SE from all experiments shown. Supplementary Tables 1,2 provide quantitative Ca++ data.
Results
Intact oxidized LDL particles activate platelets
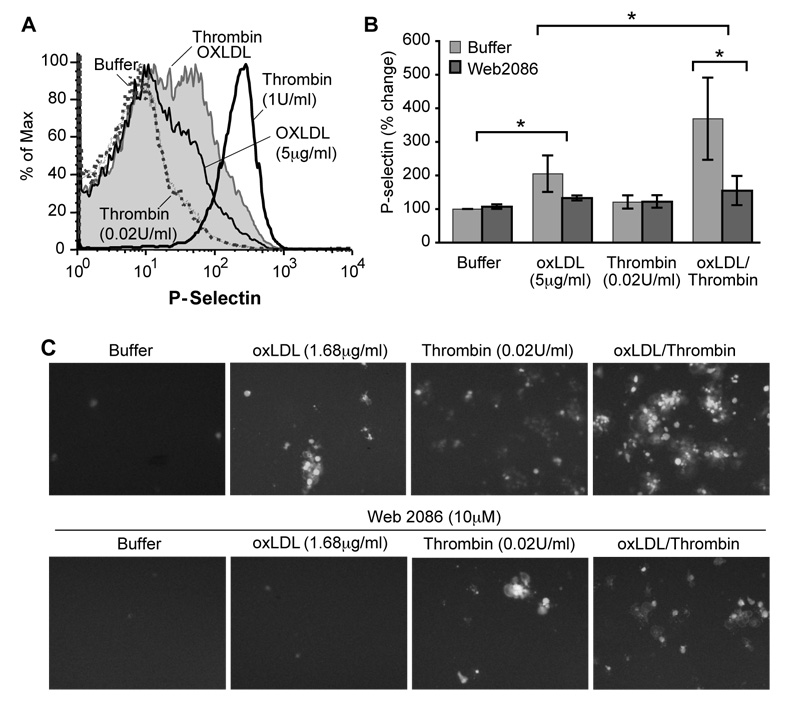
Low amounts of intact oxLDL particles (5 µg/ml) stimulated mobilization of platelet alpha granules as shown by surface expression of P-selectin (Fig. 1A). Platelets failed to respond to a sub-threshold concentration of thrombin (0.02 U/ml), but did so when a low concentration of oxLDL was included in the incubation (Fig. 1A, B). This combined response was greater than that induced by oxLDL alone, so oxLDL and thrombin signaling interact to promote platelet activation. Intact oxLDL particles alone also stimulated the adhesive response of platelets, and in combination with thrombin again evoked a response that was more robust than by either single agonist (Fig. 1C) OxLDL particles, like thrombin, enhanced spreading and scanning electron microscopy showed the combination of agonists produced highly spread clusters or aggregates of platelets (Supplementary Fig. 1).
Figure 1. Intact oxidized LDL particles activate platelets.
A. P-selectin surface expression. Washed human platelets were treated with 5 µg/ml Cu+-oxidized LDL particles (oxLDL), 0.02 U/ml thrombin or a combination of the two agonists for 10 min and fixed before P-selectin expression was quantified by flow cytometry (n=3). The positive control was 1 U/ml thrombin. B. Quantitation of surface P-selectin expression. * = P< 0.05 C. Adhesion. Calcein-AM loaded platelets were treated with the stated agonist(s) in the presence of 10 µM WEB 2086, or not, and imaged by fluorescent microscopy (n=3).
oxLDL priming and activation requires the PAF receptor
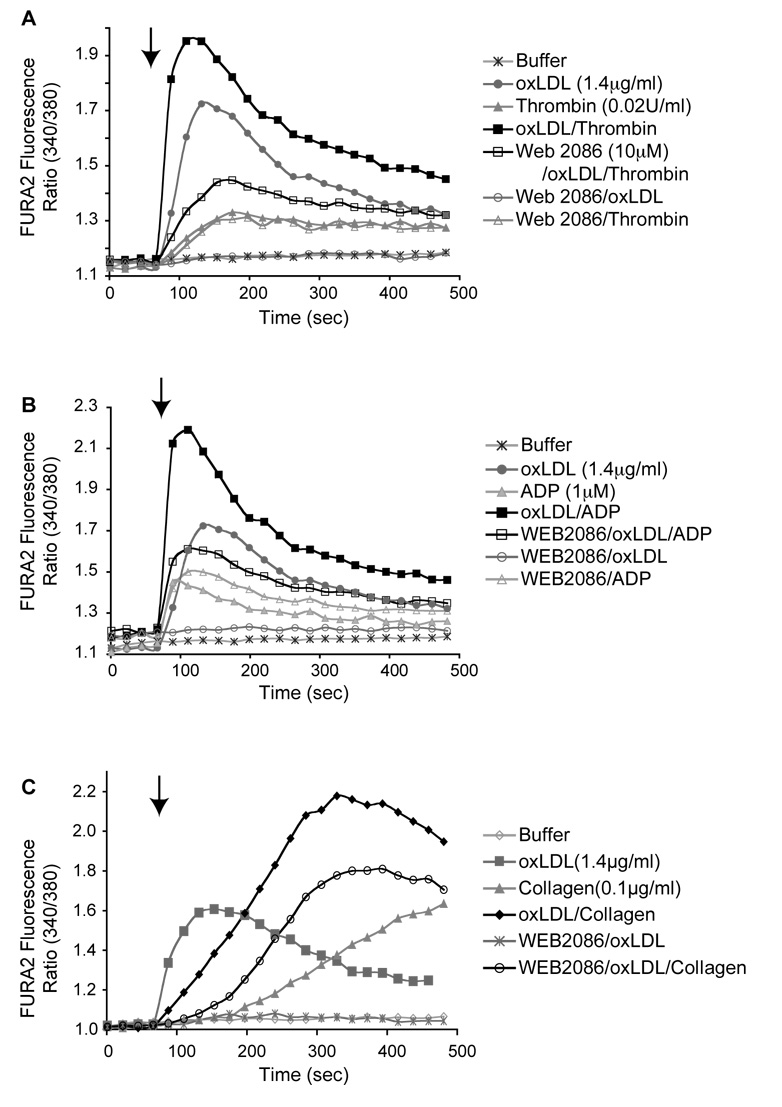
Platelet activation by oxLDL was concentration dependent with concentrations from just under 1.5 µg/ml up to 5 µg/ml stimulating intracellular Ca++ flux, but then activation was significantly reduced as the concentration of oxLDL increased above 7 µg/ml (Supplementary Fig. 2). Thrombin at 0.02 U/ml induced a modest increase in intracellular free Ca++ (Fig. 2A). oxLDL at 1.4 µg/ml was a better agonist than this very low level of thrombin, and the combination of oxLDL and thrombin greatly increased the rapidity and extent of platelet activation compared to either agonist alone. Similarly, oxidized LDL augmented the Ca++ flux induced by ADP (Fig. 2B) and collagen (Fig. 2C). The inhibitory PAF receptor inverse agonist15 WEB2086 abolished signaling by oxLDL, without altering the response to thrombin, ADP, or collagen (Fig. 2). WEB2086 also completely suppressed the cooperative effect of oxLDL on thrombin stimulated Ca++ flux (Fig. 2), and largely suppressed the oxLDL enhancement of the response to ADP or collagen (Fig. 2B,C).
Figure 2. Oxidized LDL acts through the PAF receptor to enhance thrombin stimulation.
Platelets loaded with the Ca++-sensitive dye FURA2 were treated with a threshold amount oxidized LDL particles (1.4 µg/ml) and (A) thrombin (0.02U/ml), (B) 1 µM ADP, (C) 0.1 µg/ml µg collagen or their combination with or without inclusion of the inverse PAF receptor agonist WEB 2086 (10 µM). Quantitative values are shown in Supplementary Table I.
Platelets are activated by the truncated phospholipid HAz-LPAF
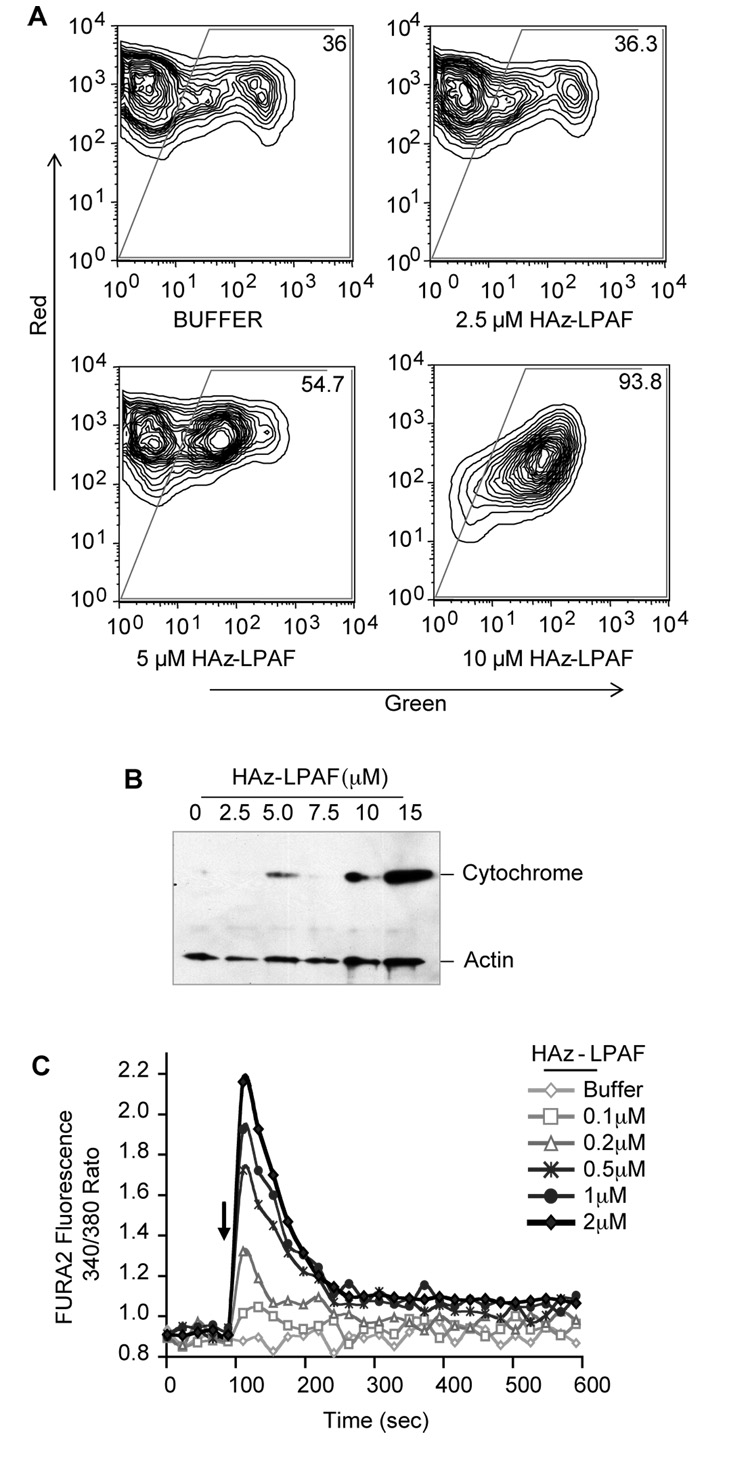
Agonist-induced mitochondrial depolarization enhances platelet reactivity3, 6. The synthetic oxidatively truncated alkyl phospholipid HAz-LPAF depolarizes mitochondria of nucleated cells12, and it depolarized mitochondria in intact washed platelets (Fig. 3A): Platelets exposed to 2.5 µM HAz-LPAF were not different from control cells, but—as with nucleated cells—5 and 10 µM HAz-LPAF increased the population with depolarized mitochondria. Physical isolation showed that cytochrome c escaped from its mitochondrial compartment in platelets exposed to the higher concentrations of HAz-LPAF (Fig. 3B). Pure HAz-LPAF increased platelet cytoplasmic Ca++ levels in a concentration dependent way (Fig. 3C), so this oxidatively truncated phospholipid is a direct platelet agonist. However, this Ca++ response was unrelated to the mitochondrial dysfunction because HAz-LPAF was ~25-times more potent at stimulating platelets than it was in depolarizing their mitochondria (compare Fig. 3A with Fig. 3C).
Figure 3. A pure oxidatively truncated phospholipid perturbs platelet homeostasis.
A. Mitochondrial potential. Platelets were treated (n=3) with the stated concentration of HAz-LPAF and changes in mitochondrial potential were assessed with the potentiometric dye JC-1 that fluoresces red when concentrated in polarized mitochondria and green as a cytoplasmic monomer. B. Cytochrome C release. Platelets (n=2) were treated with the stated concentration of HAz-LPAF as above and lysed before cytoplasmic cytochrome c was recovered by centrifugation and visualized by western blotting. C. HAz-LPAF induced a concentration-dependent transient increase in intracellular free Ca++ levels in FURA2-loaded platelets (n=3).
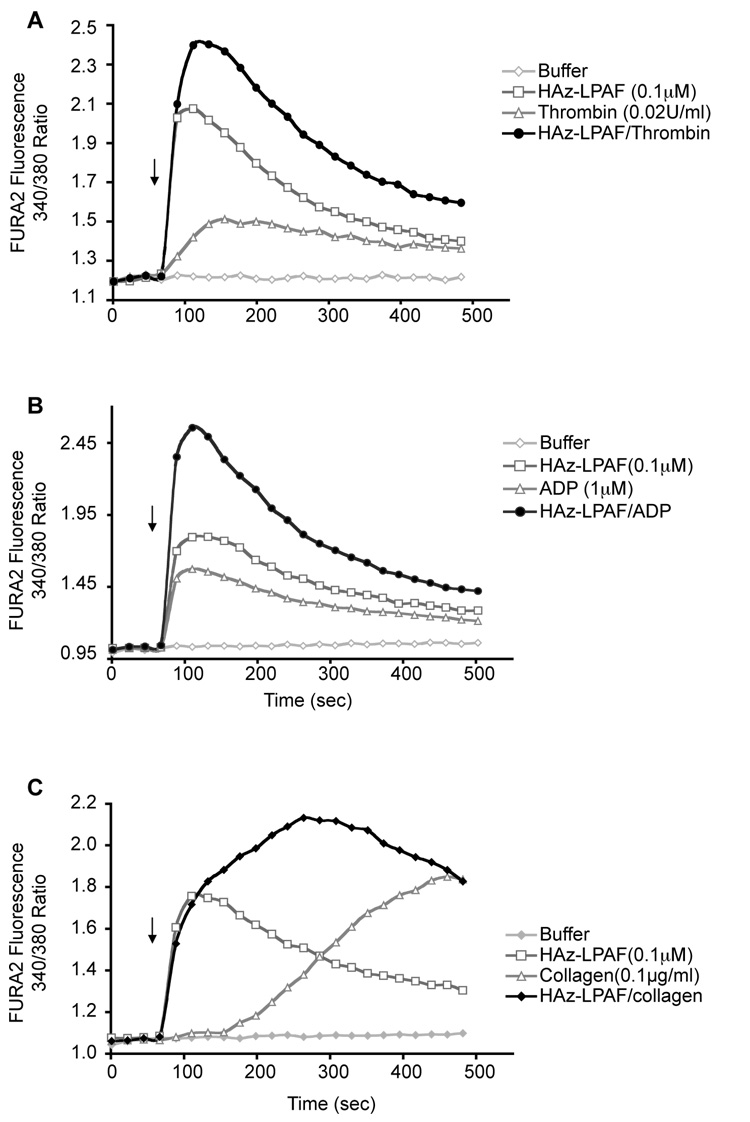
Sub-micromolar concentrations of HAz-LPAF, like oxLDL, synergistically enhanced thrombin-induced increases in intracellular free Ca++ (Fig. 4A). HAz-LPAF at 10−7 M also enhanced platelet activation by sub-optimal amounts of ADP (Fig. 4B) and collagen (Fig. 4C). HAz-LPAF induced an immediate increase in intracellular Ca++, in contrast to the delayed response to collagen, and it appears the response to the combination of HAz-LPAF and collagen is a direct summation of independent events.
Figure 4. HAz-LPAF primes platelet reactivity.
A. Intracellular Ca++. A low concentration of HAz-LPAF (0.1 µM) increased intercellular Ca++ levels in FURA2-loaded cells and aided the response to a low (A) 0.02 U/ml thrombin, (B) 1 µM ADP (C) 0.1 µg/ml collagen concentrations. Quantitative values are shown in Supplementary Table II.
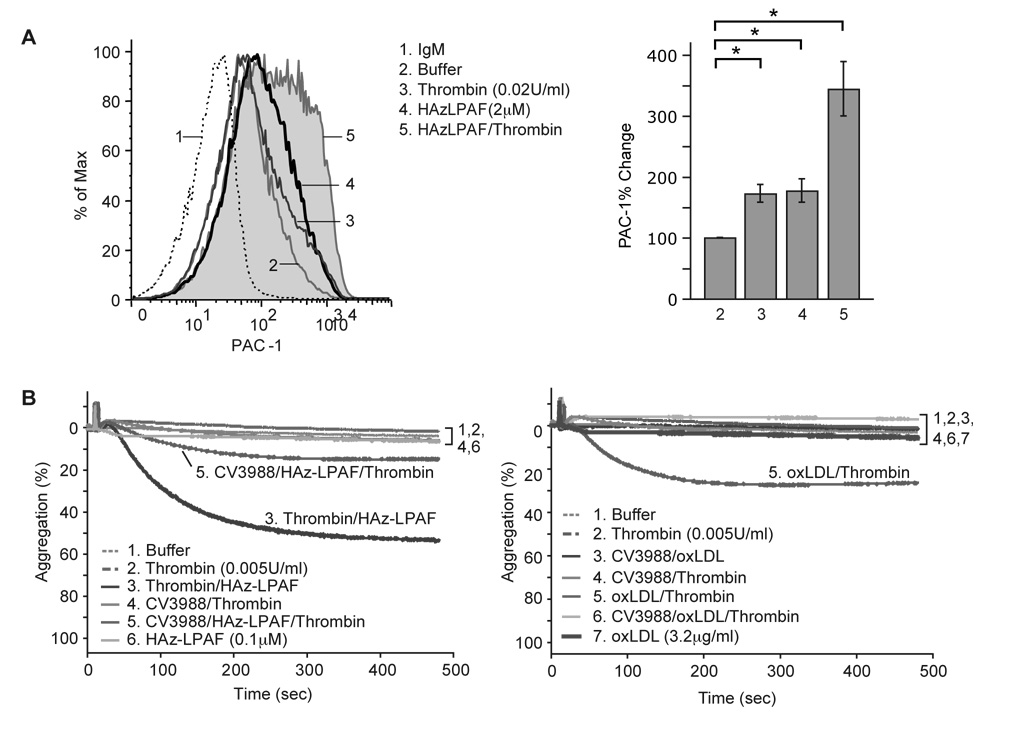
The response of platelets to HAz-LPAF included changes in the conformation of surface gpIIb/IIIa to its active state recognized by PAC-1 antibody (Fig. 5A). Again, the combination of HAz-LPAF and trace amounts of thrombin was more effective than either agonist alone. HAz-LPAF induced platelet aggregation and this truncated phospholipid potentiated the effect of thrombin present at a concentration too low to induce aggregation by itself (Fig. 5B). This synergistic effect of HAz-LPAF on sub-stimulatory amounts of thrombin comes from PAF receptor signaling because CV3988, a PAF receptor analog and receptor antagonist, suppressed this co-stimulus dependent aggregation. Suppression of the synergistic activation was not complete because CV3988 has partial agonistic activity 16 (Supplemental Fig. 3). Blockade of the PAF receptor by CV3988 also suppressed the ability of low concentrations of oxLDL to promote platelet aggregation by sub-stimulatory amounts of thrombin (Fig. 5B). Although CV3988 was only partially successful in reducing platelet activation and priming by HAz-LPAF and oxLDL, this stimulatory effect derived from PAF receptor activation because the more effective PAF receptor antagonists BN52021 and WEB2086 abolished HAz-GPC signaling and interaction with thrombin signaling (Supplemental Fig. 4).
Figure 5. HAz-LPAF activates platelet traditional functional responses.
A. HAz-LPAF alone and in combination with thrombin changed the conformation of IIb/IIIa to its activated form as measured by exposure of the PAC-1 epitope during flow cytometry. B. HAz-LPAF induced aggregation through the PAF receptor. Aggregometry of platelet rich plasma in responses to thrombin (0.005 u/ml) and/or oxLDL in the presence or absence of the PAF receptor antagonist CV3988 (1 µM).
Numerous phospholipid truncation products activate platelets
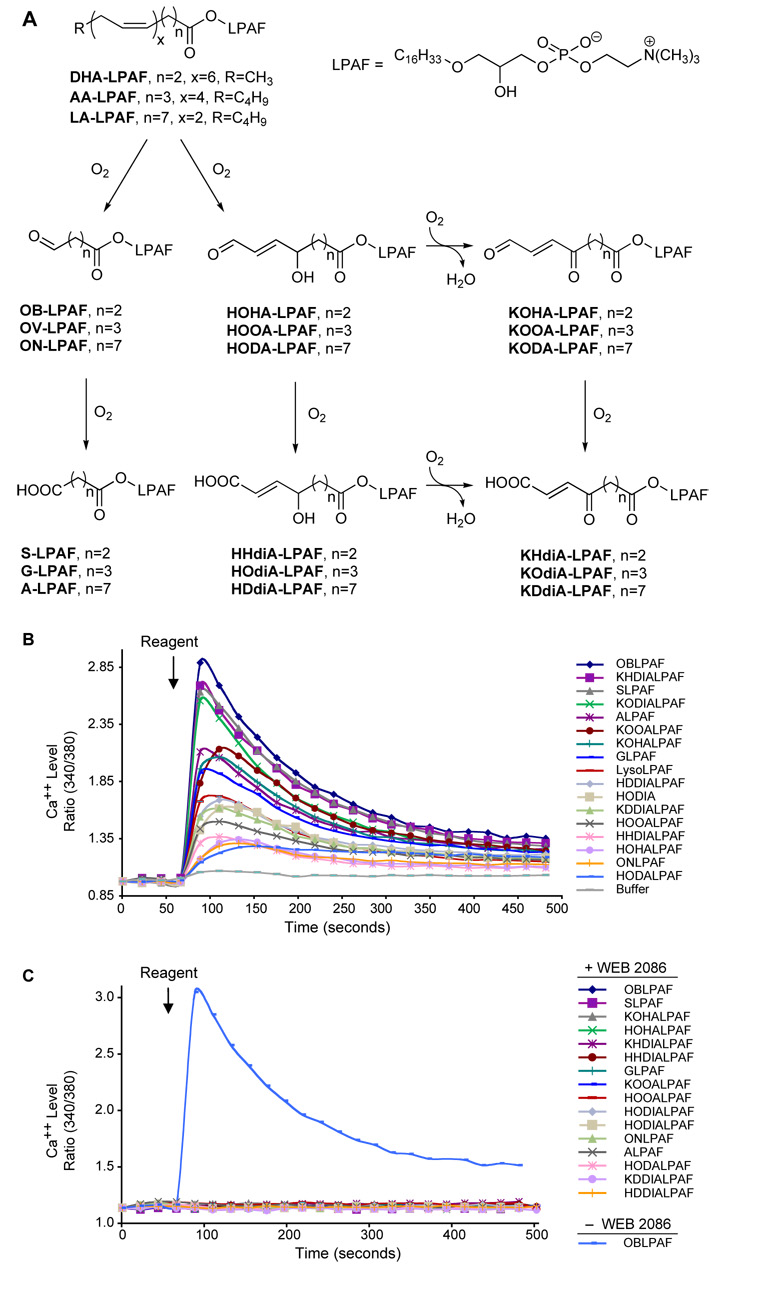
We determined whether HAz-LPAF was unique in its ability to stimulate platelets among the many oxidatively truncated phospholipids found in oxLDL, and so prepared pure samples of a series (Fig. 6A) of phospholipids that are generated by the oxidative fragmentation of alkyl phospholipids with sn-2 docosahexenoyl, arachidonoyl, and linoleoyl residues.14, 17 The backbone of this series of phospholipids is the lysoPAF (alkyl glycerophosphocholine) molecule that contains the sn-1 ether bond that is highly preferred by the PAF receptor. A large number of these structures at submicromolar concentrations induced a rapid increase in platelet cytoplasmic Ca++ level (Fig. 6B). The effectiveness of these oxidatively truncated alkyl phospholipids varied significantly, and did so in a largely non-obvious way. For instance, the most active group of truncated phospholipids included those with the shortest sn-2 residues tested—four carbon oxobutyryl and succinoyl residues—as expected, but also included seven and eight carbon long acidic α β unsaturated keto residues. While there was no distinct pattern to platelet activation by these phospholipids, HAz-LPAF was a member of the most active group of the oxidatively truncated phospholipids. The PAF receptor antagonist WEB 2086 was completely effective in suppressing the response to HAz-LPAF, and this blockade and inactivation of the PAF receptor also completely suppressed platelet activation by every other oxidatively truncated alkyl phospholipid we tested (Fig. 6C). The structurally unrelated PAF receptor antagonists BN52021 and WEB2086 were equally effective in suppressing platelet activation by HAz-LPAF (Supplementary Fig. 4), so the PAF receptor has a far wider range of agonist selectivity than previously determined using diacyl phospholipid homologs with unmodified chain fatty acyl residues.
Figure 6. Truncated phospholipids created by oxidation of common phospholipids activate platelets through the PAF receptor.
A. Reaction scheme depicting pathways forming specific phospholipid oxidation products starting from acyl lysoPAFs (alkyl glycerophosphocholines) containing the common arachidonoyl (AA), docosahexaneoyl (DHA), or linoleoyl (LA) polyunsaturated fatty acyl residues. B. Platelet stimulation by oxidized phospholipids. Intracellular Ca++ as a function of time in platelets treated with 0.5 µM of the stated phospholipid. C. Effect of PAF receptor inhibition. Platelets were pretreated with 10 µM WEB2086, except for one sample of OB-LPAF that served as the positive control, before Ca++ levels were determined.
Discussion
Thrombin is present at low concentrations during the initiation phase of thrombosis18 and low concentrations of oxLDL cooperated with sub-stimulatory concentrations of thrombin—or collagen or ADP—to activate platelets. However, oxLDL particles by themselves also activated platelets because oxidative fragmentation of its pool of alkyl phospholipids generated PAF receptor agonists. Oxidation of polyunsaturated fatty acids in diacyl and alkyl phospholipids proceeds by identical mechanisms, but all the newly formed agonists arise from oxidation of the less abundant alkyl phospholipids.13 There are two unusual aspects of platelet stimulation by the lipid agonists of oxidized LDL. First is that intact oxidized LDL particles were potent platelet agonists. We13, 19 and others20–23 have shown that oxidation of LDL generates a host of agonists for platelets, monocytes and polymorphonuclear leukocytes. Among these agonists are very low amounts of Platelet-activating Factor (PAF)24 and other very short-chained PAF analogs 19, 25 that are ligands for the PAF receptor that greatly prefers alkyl phosphatidylcholines. However to assay and identify these agents, the approach has been to extract, fractionate and concentrate the biologically active species to remove the bulk of the inactive lipids that sequester the active lipids in inactive insoluble complexes. Here we find that either the relevant biologically active species are sufficiently soluble to allow them to partition between the lipoprotein particle and the platelet surface, or the sn-2 residue and the sn-1 ether bond of the newly formed oxidized alkyl phospholipids is available to PAF receptors on the platelet surface as found in the “lipid whisker” model for oxidized phospholipid interaction with CD36. 26
The second—and surprising—aspect of oxidized LDL activation of platelets is that the relevant receptor recognizing the lipids of oxLDL was the PAF receptor. Lysophosphatidic acid in oxLDL is described as the agonist in oxLDL that stimulates platelets via their LPA receptors27, although this stimulation occurred at higher concentrations of oxLDL than required for PAF receptor-dependent signaling. Platelet activation by very low concentrations of oxLDL was abolished by PAF receptor antagonists, establishing the primacy of the PAF receptor in platelet responses to microgram quantities of intact oxidized LDL particles. Lysophosphatidic acid is some 10-fold less effective22 in stimulating platelets than oxidatively truncated phospholipids (Supplementary Fig. 2) that act through the PAF receptor, and so stimulation of platelets at higher concentrations of oxLDL22 may have a larger contribution by this lysolipid when PAF receptor signaling becomes ineffective at higher oxLDL concentrations.
The PAF receptor, which has critical roles in inflammation, thrombosis, and vascular disease, is expressed by all cells of the innate immune system. 28–30 This receptor displays marked ligand selectivity28, 31 , allowing it to detect infinitesimal amounts of PAF in a sea of membrane phospholipids and lysophosphatidylcholines. 32, 33 A central feature of this selectivity is recognition of the unique, short acetyl sn-2 residue of PAF, accompanied by a similarly strong recognition of the sn-1 ether bond and the choline headgroup. A model34 of PAF interaction with its receptor postulates a binding pocket that physically rejects longer sn-2 residues.
This modeling, however, is based on synthetic phospholipids where the sn-2 residue is a straight, short-chained fatty acyl molecule, and this understanding was established prior to a molecular definition of the plethora of oxidatively truncated phospholipids found in oxidized lipoprotein particles20–23, 35, apoptotic cells36 and platelet microparticles. 37 Oxidized alkyl phospholipids break the paradigm established with these short, straight-chained fatty phospholipids and introduce a host of new structures with a variety of oxy functions and carbon skeletons that can productively interact with the PAF receptor.
Examination of the PAF-like activity of a series of oxidatively truncated alkyl phospholipids shows that a short sn-2 residue is not an absolute criterion for an effective PAF receptor ligand. This means that oxidative fragmentation of any polyunsaturated fatty acyl residue, whether docosahexaenoyl or arachidonoyl residues that yield shorter 4 and 5 carbon fragments or linoleoyl residues forming 9 carbon long fragments, can generate effective PAF receptor agonists. Thus, PAF receptor agonists now also include longer sn-2 acidic residues such as the nine carbon long HAz-LPAF, and seven- and an eight-carbon long keto acids (KHdiA-GLPAF, and KOdiA-LPAF, respectively). Only a few of the oxidatively truncated phospholipids bearing an ω-terminal aldehyde function were agonists, excluding a role for protein adduction by Shiff base formation in platelet activation by aldehydic phospholipids.
The pattern of platelet activation through the PAF receptor by ether-containing phospholipids is distinct from the pattern of effective CD36 ligands. 38 This is epitomized by KODA-PC, the most efficient diacyl CD36 ligand20, 38, that failed to stimulate platelets (Fig. 6). Although CD36 stimulation by oxLDL requires several hundred micrograms of oxLDL39, CD36 could still bridge lower concentrations of oxidized LDL to platelets in a way that enhances platelet reactivity and promotes intravascular thrombosis. 20
Platelets in different environments are exposed to vastly different levels of oxLDL, from the low number of modified particles in the circulation40 to the high levels sequestered in atherosclerotic lesions. OxLDL itself was a potent platelet agonist, but it also was synergistic with amounts of thrombin (0.02 U/ml) too low to be stimulatory by itself, so the PAF receptor ligands in oxLDL are positioned to promote events early in thrombosis. Additionally, OxLDL contains, at a minimum, various oxidatively modified phospholipids and cholesterols, isoprostanes and oxidized arachidonoyl residues41, lysolipids generated by PAF acetylhydrolase hydrolysis of the newly minted oxidized phospholipids42, 43, and lysophosphatidic acid. 9 As might be expected from this, the effect of oxLDL on inflammatory cells is complex, dependent on the particle concentration, and extent and mode of oxidation. 9
We observed that intact oxLDL particles functioned as full, direct platelet agonists at a few µg/ml, and achieved a maximal response by ~7 µg/ml. This response then faded as the oxLDL concentration increased, suggesting the additional presence of a less potent antagonist(s). One lipid of oxLDL that can interfere with platelet function is lysophosphatidylcholine9, 44, but its role is enigmatic. Its actions are concentration dependent, either aiding or suppressing platelet function, and acts in receptor dependent and independent ways. Other anti-inflammatory agents present in oxLDL45 may also participate in forming the bell-shaped action profile, while the variety of agonists—particularly lysophosphatidic acid22 — could produce the stimulation found at oxLDL higher concentrations.
Controversy exists46 whether the circulating enzyme PAF acetylhydrolase / lipoprotein-associated phospholipase A2 that hydrolyzes PAF and oxidized phospholipids is anti-inflammatory29, 47, 48 or pro-inflammatory. 49 These opposing views depend on whether one regards the substrate PAF or its lysoPAF product as the primary bioactive entity of oxidized LDL. Here we find several oxidatively truncated phospholipids that were less effective agonists than the lysolipid product, so their hydrolysis would enhance platelet activation. Conversely for the larger number of oxidatively truncated phospholipids that were more effective than the corresponding lysolipid hydrolytic product, hydrolysis by PAF acetylhydrolase would decrease PAF receptor activity. Resolution of these disparate views based on substrate selectivity is problematic.
Supplementary Material
Acknowledgements
Sources of funding: This work was supported by P50 HL081011, R01 HL092747, P01 HL087018, R01 GM21249, and R01 HL53315.
Acknowledgements: The authors greatly appreciate the aid of Mark Calabro and Erin Brady in preparing platelets and Dr. Gopal K. Marathe for advice and LDL preparation. We appreciate the expertise and aid of Mei Yin and the Flow and Imaging core for SEM imaging. The helpful advice of Mitali Das regarding Pac1 staining is also greatly appreciated. Figure preparation by Diana Lim is greatly appreciated.
Footnotes
This is an un-copyedited author manuscript that was accepted for publication in Arteriosclerosis, Thrombosis, and Vascular Biology, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at Arteriosclerosis, Thrombosis, and Vascular Biology. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
Disclosure: The authors have no conflicts of interest.
References
- 1.Leaver HA, Schou AC, Rizzo MT, Prowse CV. Calcium-sensitive mitochondrial membrane potential in human platelets and intrinsic signals of cell death. Platelets. 2006;17:368–377. doi: 10.1080/09537100600757216. [DOI] [PubMed] [Google Scholar]
- 2.Rosado JA, Lopez JJ, Gomez-Arteta E, Redondo PC, Salido GM, Pariente JA. Early caspase-3 activation independent of apoptosis is required for cellular function. J Cell Physiol. 2006;209:142–152. doi: 10.1002/jcp.20715. [DOI] [PubMed] [Google Scholar]
- 3.Dale GL. Coated-platelets: an emerging component of the procoagulant response. J Thromb Haemost. 2005;3:2185–2192. doi: 10.1111/j.1538-7836.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- 4.Dale GL, Friese P. Bax activators potentiate coated-platelet formation. J Thromb Haemost. 2006;4:2664–2669. doi: 10.1111/j.1538-7836.2006.02211.x. [DOI] [PubMed] [Google Scholar]
- 5.Remenyi G, Szasz R, Friese P, Dale GL. Role of mitochondrial permeability transition pore in coated-platelet formation. Arterioscler Thromb Vasc Biol. 2005;25:467–471. doi: 10.1161/01.ATV.0000152726.49229.bf. [DOI] [PubMed] [Google Scholar]
- 6.Jobe SM, Wilson KM, Leo L, Raimondi A, Molkentin JD, Lentz SR, Di Paola J. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood. 2008;111:1257–1265. doi: 10.1182/blood-2007-05-092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witztum JL, Steinberg D. The oxidative modification hypothesis of atherosclerosis: does it hold for humans? Trends Cardiovasc Med. 2001;11:93–102. doi: 10.1016/s1050-1738(01)00111-6. [DOI] [PubMed] [Google Scholar]
- 8.Daub K, Lindemann S, Langer H, Seizer P, Stellos K, Siegel-Axel D, Gawaz M. The evil in atherosclerosis: adherent platelets induce foam cell formation. Semin Thromb Hemost. 2007;33:173–178. doi: 10.1055/s-2007-969031. [DOI] [PubMed] [Google Scholar]
- 9.Siess W. Platelet interaction with bioactive lipids formed by mild oxidation of low-density lipoprotein. Pathophysiol Haemost Thromb. 2006;35:292–304. doi: 10.1159/000093222. [DOI] [PubMed] [Google Scholar]
- 10.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28:1717–1725. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 11.Heinecke JW. Cellular mechanisms for the oxidative modification of lipoproteins: implications for atherogenesis. Coron Artery Dis. 1994;5:205–210. doi: 10.1097/00019501-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Chen R, Yang L, McIntyre TM. Cytotoxic phospholipid oxidation products: Cell death from mitochondrial damage and the intrinsic caspase cascade. J Biol Chem. 2007;282:24842–24850. doi: 10.1074/jbc.M702865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marathe GK, Davies SS, Harrison KA, Silva AR, Murphy RC, Castro-Faria Neto H, Prescott SM, Zimmerman GA, McIntyre TM. Inflammatory PAF-like lipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. 1999;274:28395–28405. doi: 10.1074/jbc.274.40.28395. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Zhang W, Laird J, Hazen SL, Salomon RG. Polyunsaturated phospholipids promote the oxidation and fragmentation of gamma -hydroxyalkenals: formation and reactions of oxidatively truncated ether phospholipids. J Lipid Res. 2008;49:832–846. doi: 10.1194/jlr.M700598-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupre DJ, Thompson C, Chen Z, Rollin S, Larrivee JF, Le Gouill C, Rola-Pleszczynski M, Stankova J. Inverse agonist-induced signaling and down-regulation of the platelet-activating factor receptor. Cell Signal. 2007;19:2068–2079. doi: 10.1016/j.cellsig.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Shen TY, Hwang S-B, Doebber TW, Robbins JC. The chemical and biological properties of PAF agonists, antagonists, and biosynthetic inhibitors. In: Snyder F, editor. Platelet-Activating Factor and Related Lipid Mediators. New York: Plenum Press; 1987. pp. 153–190. [Google Scholar]
- 17.Reis A, Domingues MR, Amado FM, Ferrer-Correia AJ, Domingues P. Separation of peroxidation products of diacyl-phosphatidylcholines by reversed-phase liquid chromatography-mass spectrometry. Biomed Chromatogr. 2005;19:129–137. doi: 10.1002/bmc.429. [DOI] [PubMed] [Google Scholar]
- 18.Chong AJ, Pohlman TH, Hampton CR, Shimamoto A, Mackman N, Verrier ED. Tissue factor and thrombin mediate myocardial ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:S649–S655. doi: 10.1016/s0003-4975(02)04691-x. [DOI] [PubMed] [Google Scholar]
- 19.Smiley PL, Stremler KE, Prescott SM, Zimmerman GA, McIntyre TM. Oxidatively fragmented phosphatidylcholines activate human neutrophils through the receptor for platelet-activating factor. J Biol Chem. 1991;266:11104–11110. [PubMed] [Google Scholar]
- 20.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pegorier S, Stengel D, Durand H, Croset M, Ninio E. Oxidized phospholipid: POVPC binds to platelet-activating-factor receptor on human macrophages Implications in atherosclerosis. Atherosclerosis. 2005;188:433–443. doi: 10.1016/j.atherosclerosis.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Maschberger P, Bauer M, Baumann-Siemons J, Zangl KJ, Negrescu EV, Reininger AJ, Siess W. Mildly oxidized low density lipoprotein rapidly stimulates via activation of the lysophosphatidic acid receptor Src family and Syk tyrosine kinases and Ca2+ influx in human platelets. J Biol Chem. 2000;275:19159–19166. doi: 10.1074/jbc.M910257199. [DOI] [PubMed] [Google Scholar]
- 23.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 24.Tsoukatos DC, Arborati M, Liapikos T, Clay KL, Murphy RC, Chapman MJ, Ninio E. Copper-catalyzed oxidation mediates PAF formation in human LDL subspecies. Protective role of PAF:acetylhydrolase in dense LDL. Arterioscler Thromb Vasc Biol. 1997;17:3505–3512. doi: 10.1161/01.atv.17.12.3505. [DOI] [PubMed] [Google Scholar]
- 25.Androulakis N, Durand H, Ninio E, Tsoukatos DC. Molecular and mechanistic characterization of platelet-activating factor-like bioactivity produced upon LDL oxidation. J Lipid Res. 2005;46:1923–1932. doi: 10.1194/jlr.M500074-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, Hazen SL. The lipid whisker model of the structure of oxidized cell membranes. J Biol Chem. 2008;283:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 27.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci USA. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braquet P, Touqui L, Shen TY, Vargaftig BB. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987;39:97–145. [PubMed] [Google Scholar]
- 29.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating Factor and Related Lipid Mediators. Ann Rev Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 30.Ishii S, Nagase T, Shimizu T. Platelet-activating factor receptor. Prostaglandins Other Lipid Mediat. 2002;68–69:599–609. doi: 10.1016/s0090-6980(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 31.O'Flaherty JT, Salzer WL, Cousart S, McCall CE, Piantadosi C, Surles JR, Hammett MJ, Wykle RL. Platelet-activating factor and analogues: Comparative studies with human neutrophils and rabbit platelets. Res Comm Chem Pathol Pharm. 1983;39:291–309. [PubMed] [Google Scholar]
- 32.Drobnik W, Liebisch G, Audebert FX, Frohlich D, Gluck T, Vogel P, Rothe G, Schmitz G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J Lipid Res. 2003;44:754–761. doi: 10.1194/jlr.M200401-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Subbaiah PV, Chen CH, Bagdade JD, Albers JJ. Substrate specificity of plasma lysolecithin acyltransferase and the molecular species of lecithin formed by the reaction. J Biol Chem. 1985;260:5308–5314. [PubMed] [Google Scholar]
- 34.Kajihara A, Komooka H, Kamiya K, Yoneda T, Yoneda S, Nakamura M, Shimizu T, Umeyama H. Three-dimensional model of the human PAF receptor. J Lipid Mediat Cell Signal. 1994;9:185–196. [PubMed] [Google Scholar]
- 35.Tuominen A, Miller YI, Hansen LF, Kesaniemi YA, Witztum JL, Horkko S. A natural antibody to oxidized cardiolipin binds to oxidized low-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2006;26:2096–2102. doi: 10.1161/01.ATV.0000233333.07991.4a. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber J, Vales A, Mitulovic G, Blumer M, Schmid R, Witztum JL, Binder BR, Leitinger N. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte- endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 38.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 39.Korporaal SJ, Van Eck M, Adelmeijer J, Ijsseldijk M, Out R, Lisman T, Lenting PJ, Van Berkel TJ, Akkerman JW. Platelet activation by oxidized low density lipoprotein is mediated by CD36 and scavenger receptor-A. Arterioscler Thromb Vasc Biol. 2007;27:2476–2483. doi: 10.1161/ATVBAHA.107.150698. [DOI] [PubMed] [Google Scholar]
- 40.Hodis HN, Kramsch DM, Avogaro P, Bittolo-Bon G, Cazzolato G, Hwang J, Peterson H, Sevanian A. Biochemical and cytotoxic characteristics of an in vivo circulating oxidized low density lipoprotein (LDL-) J Lipid Res. 1994;35:669–677. [PubMed] [Google Scholar]
- 41.Li R, Mouillesseaux KP, Montoya D, Cruz D, Gharavi N, Dun M, Koroniak L, Berliner JA. Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circ Res. 2006;98:642–650. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- 42.Stremler KE, Stafforini DM, Prescott SM, McIntyre TM. Human plasma platelet-activating factor acetylhydrolase: Oxidatively-fragmented phospholipids as substrates. J Biol Chem. 1991;266:11095–11103. [PubMed] [Google Scholar]
- 43.Davis B, Koster G, Douet LJ, Scigelova M, Woffendin G, Ward JM, Smith A, Humphries J, Burnand KG, Macphee CH, Postle AD. Electrospray ionization mass spectrometry identifies substrates and products of lipoprotein-associated phospholipase A2 in oxidized human low density lipoprotein. J Biol Chem. 2008;283:6428–6437. doi: 10.1074/jbc.M709970200. [DOI] [PubMed] [Google Scholar]
- 44.Mahfouz MM, Kummerow FA. Oxidized low-density lipoprotein (LDL) enhances thromboxane A(2) synthesis by platelets, but lysolecithin as a product of LDL oxidation has an inhibitory effect. Prostaglandins Other Lipid Mediat. 2000;62:183–200. doi: 10.1016/s0090-6980(00)00078-2. [DOI] [PubMed] [Google Scholar]
- 45.Bochkov VN, Leitinger N. Anti-inflammatory properties of lipid oxidation products. J Mol Med. 2003;81:613–626. doi: 10.1007/s00109-003-0467-2. [DOI] [PubMed] [Google Scholar]
- 46.Karabina SA, Ninio E. Plasma PAF-acetylhydrolase: an unfulfilled promise? Biochim Biophys Acta. 2006;1761:1351–1358. doi: 10.1016/j.bbalip.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Gomes RN, Bozza FA, Amancio RT, Japiassu AM, Vianna RC, Larangeira AP, Gouvea JM, Bastos MS, Zimmerman GA, Stafforini DM, Prescott SM, Bozza PT, Castro-Faria-Neto HC. Exogenous platelet-activating factor acetylhydrolase reduces mortality in mice with systemic inflammatory response syndrome and sepsis. Shock. 2006;26:41–49. doi: 10.1097/01.shk.0000209562.00070.1a. [DOI] [PubMed] [Google Scholar]
- 48.Ninio E. Phospholipid mediators in the vessel wall: involvement in atherosclerosis. Curr Opin Clin Nutr Metab Care. 2005;8:123–131. doi: 10.1097/00075197-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Macphee CH, Nelson J, Zalewski A. Role of lipoprotein-associated phospholipase A2 in atherosclerosis and its potential as a therapeutic target. Curr Opin Pharmacol. 2006;6:154–161. doi: 10.1016/j.coph.2005.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.