Abstract
The first synthesis of a novel 5:7:5-fused heterocyclic ring system, a diimidazodiazepine, is reported. The propensity of the ring system to undergo facile, acid-catalyzed nucleophilic addition reactions by neutral carbon and nitrogen nucleophiles has been explored. The ring system has potential future applications in mechanistic studies of formation and repair of DNA interstrand crosslinks.
Mechanistic studies of formation and repair of DNA interstrand cross-links have elicited considerable interest of chemists, biochemists, and biophysicists alike for decades.1-5 Many bifunctional organic reagents like nitrogen mustards,6 organometallic compounds such as cis-platin,7 and natural products such as psoralen8 and mitomycin C9 are long known to cause interstrand cross-links in DNA, and this property has been extensively explored in cancer chemotherapy.10 A major problem in using these molecules for mechanistic studies at the molecular level is the formation of a host of products that are often unstable, making the isolation and purification of a specific cross-linked product difficult and cumbersome.2 Therefore, the challenge is to chemically synthesize a DNA duplex containing single cross-link at a defined site.2 Most of the known work in this area involves the synthesis of a single-stranded oligonucleotide covalently linked to one side of a cross-link, which would then be hybridized with a complementary DNA strand, and further subjected to chemical or phtochemical reaction to produce interstrand cross-links.4 In this regard, another novel strategy would be to start from a tricyclic heterocycle such as 1, serving as a single interstrand cross-link, from which the two DNA strands could be extended via (deoxy)ribosylation of each imidazole ring to produce 2, followed by conversion to phosphoramidite derivatives and oligomerization with a DNA synthesizer. The presence of 4 nitrogen atoms in two imidazole rings offers an additional scope for synthesis of a variety duplexes extending from different nitrogen atoms. These duplexes have potential applications for biochemical and biophysical explorations of DNA formation, structure, stability, and conformation in addition to DNA repair of interstrand cross-links.
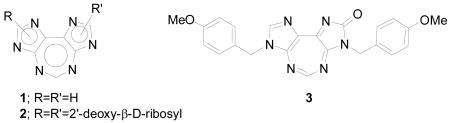
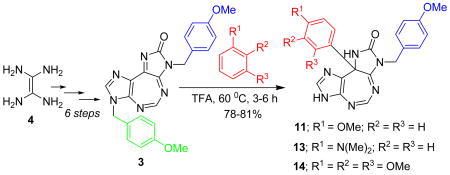
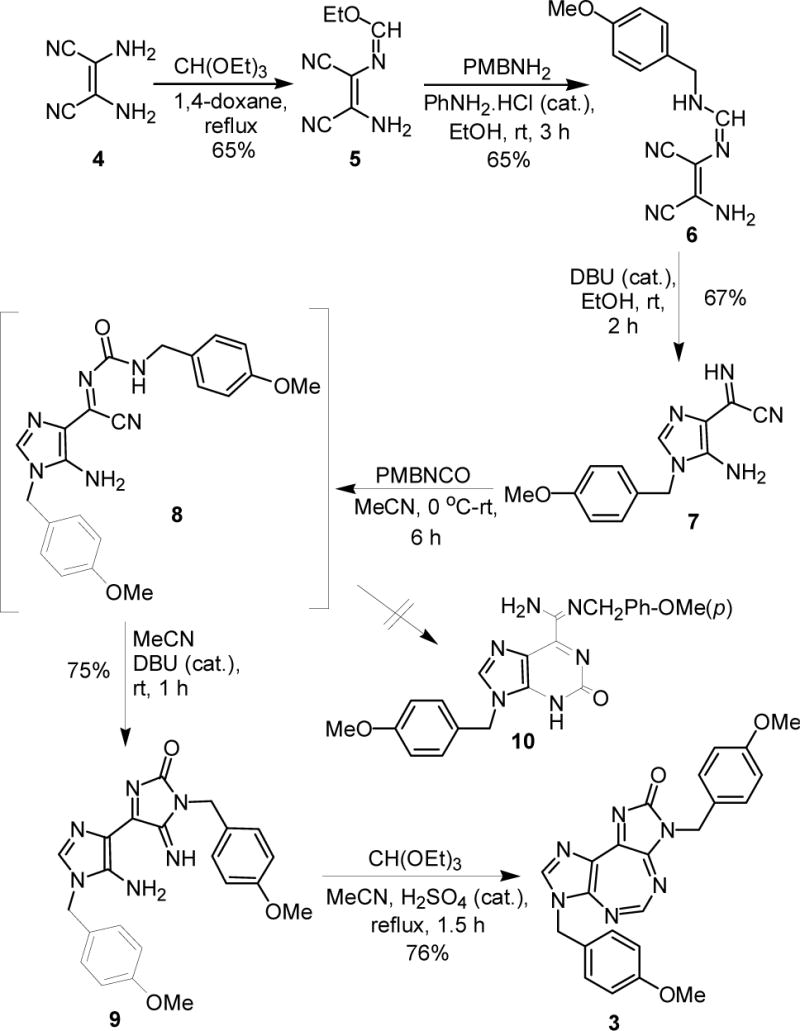
An examination of the literature revealed that the target 5:7:5 heterocyclic ring system is yet unknown. We report here our first entry into such a ring system employing a representative example 3, containing a removable p-methoxybenzyl (PMB) group attached to each of the two imidazole rings. Heating diaminomaleonitrile 4 (Scheme 1) with triethyl orthoformate in dioxane yielded formimidate 5.11 The reaction of 5 with p-methoxybenzylamine catalyzed by aniline hydrochloride formed formimidine 6,12 which underwent intramolecular cyclization in the presence of DBU to form imidazole derivative 7.13 The treatment of latter with p-methoxybenzyl isocyanate resulted into a mixture of 8 and 9.14 The complete coversion of 8 into 9 was achieved by treating the mixture with DBU in acetonitrile.14 The reaction of isolated 9 with triethyl orthoformate yielded the target heterocycle 3. We did not observe the reported rearrangement of compound such as 8 into an oxopurine such as 10.14 Such a rearrangement, however, has been limited to the use of N-tosylisocyanate, but not other isocyanates.14 This was further corroborated by the facile ring-closure of 9 to form 3. All intermediates and final product were fully characterized by spectroscopic and analytical data.15
Scheme 1.
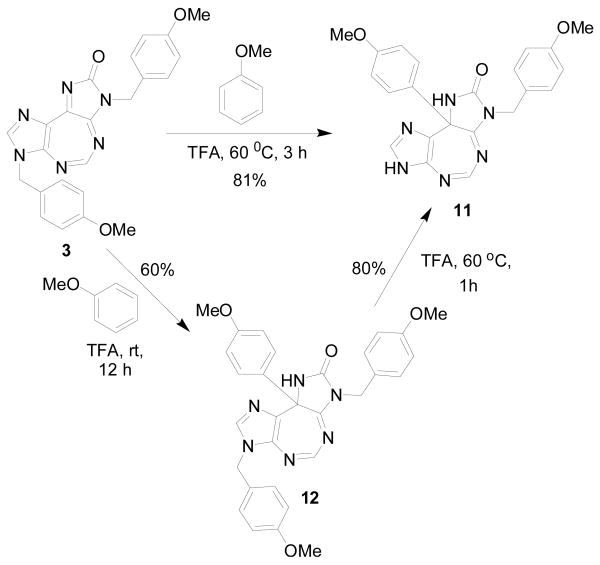
The core tricyclic structure of 3 containing 14 π electrons is aromatic by the Hückel rule. Nevertheless, with six nitrogen atoms and a conjugated carbonyl group present in the heterocyclic ring system, compound 3 is anticipated to be considerably electrophilic. In order to explore this aspect a little further, we set out to treat 3 with a few carbon and nitrogen nucleophiles. The carbon nucleophiles attempted include anisole, N,N-dimethylaniline, and 1,2,3-trimethoxybenzene, all of which contain electron-donating substituent(s) on their aromatic rings. Thus, the reaction of a mixture of 3 (1 mmol), anisole (5 mL), and TFA (10 mL) at 60 °C for 3 h (Scheme 2) formed a novel product 11 which was isolated, purified (81%) and characterized.
Scheme 2.
In order to throw more light on the pathway of formation of 11 from 3, the latter (1 mmol) was treated with a mixture of TFA (10 mL) and anisole (5 mL)16 at rt for 12 h (Scheme 2), which yielded a mixture of 11 (10%) and 12 (60%) which was found to be an adduct of anisole by spectroscopic and analytical data. Interestingly 12 was converted into 11 when heated with TFA at 60 °C for 1 h. This suggests that the reaction proceeds by first addition of anisole, followed by selective cleavage of the N-3 PMB group. As TFA is often used to remove the PMB group from heterocyclic rings,17 the observed deprotection of one of the imidazole rings under these conditions is not totally surprising.
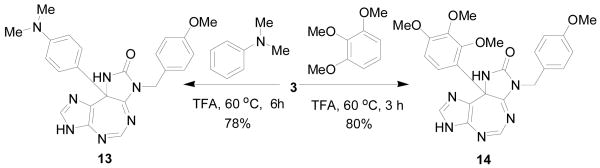
The generality of the above reaction was studied using two other electron-rich carbon nucleophiles, including N,N-dimethylaniline and 1,2,3-trimethoxybenzene. Thus, when 3 (1 mmol) was heated separately (Scheme 3) at 60°C for 6 h with N,N-dimethylaniline (5 mL) or 1,2,3-trimethoxy benzene (5 mL) in TFA (10 mL), compound 13 (78%) or 14 (80%), was formed, respectively.
Scheme 3.
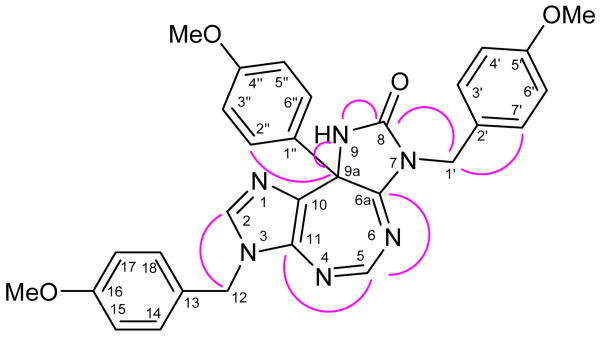
The structures of 3 and 11-14 were determined by 1D and 2D NMR experiments (some important peaks and correlations are presented in Figure 1 with an example of 12; and Tables 1-3), including HMQC, HMBC and DEPT experiments. In the HMBC spectra, H-5 showed the correlations with C-6a and C-11; H-1′ showed correlation with C-8, C-3′ and C-7′; C-8 showed correlations with H-1′. The addition of anisole/1,2,3-trimethoxybenzene/N,N-dimethylaniline at position 9a was determined by the correlation of H-2″(or/and) and H-6″ with C-9a; two-bond coupling enhancement between C-9a and H-9 (N) and H-9 (N) and C-8.
Figure 1.
Selected HMBCs of 12.
Table 1.
1H NMR (δ, DMSO-d6,J in Hz in parentheses) data of compounds 3 and 11-14a
| compd | H-2 | H-5 | H-12 | H-14 & H-18 | H-1′ | H-3′ & H-7′ | H-9 (N) | H-2″& H-6″ |
|---|---|---|---|---|---|---|---|---|
| 3 | 8.92 s | 8.75 s | 5.51 s | 7.33 d (8.7) | 5.08 s | 7.29 d (8.7) | -- | -- |
| 11 | 7.87 s | 7.53 s | -- | -- | 4.63 dd (15.1, 18.3) | 7.19 d (8.6) | 9.82 s | 6.68 d (8.6) |
| 12 (CDCl3) | 7.69 s | 7.65 s | 5.13 q (14.2) | 7.08 d (8.6) | 4.72 s | 7.33 d (8.6) | 9.47 s | 6.72 d (8.6) |
| 13 | 7.83 s | 7.50 s | -- | -- | 4.62 dd (15.1, 18.3) | 7.20 d (8.72) | 9.68 s | 6.56 d (9.2) |
| 14 | 7.85 s | 7.50 s | -- | -- | 4.60 dd (15.1, 18.3) | 7.22 d (8.72) | 9.4 s | 5.80 d (H-2″) (8.72) |
Assignments are based on DEPT, HMQC and HMBC experiments.
Table 3.
Three-Bond 1H-C13 Coupling (HMBC) in compounds 3 and 11-14
| H | 3 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|
| H-2 | C-12 | C-10 & C-11 | C-10 & C-11 | C-10 & C-11 | C-10 & C-11 |
| H-5 | C-6a | C-6a & C-11 | C-6a & C-11 | C-6a & C-11 | C-6a & C-11 |
| H-12 | C-2, C-14 & C-18 | -- | C-2, C-14 & C-18 | -- | -- |
| H-14 & C-18 | C-12 | -- | C-12 | -- | -- |
| H-1′ | C-6a, C-8, C-3′ & C-7′ | C-8, C-3′ & C-7′ | C-8, C-3′ & C-7′ | C-8, C-3′ & C-7′ | C-8, C-3′ & C-7′H- |
| H-3′ & H-7′ | C-1′ | C-1′ | C-1′ | C-1′ | C-1′ |
| H-9(N) | -- | C-8*, C-9a* | C-8*, C-9a* | C-8*, C-9a* | C-8*, C-9a* |
| H-2″ & H-6″ | -- | C-9a | C-9a | C-9a | C-9a (H-2″) |
Two-bond coupling enhancement obaserved.
The above reactions failed to proceed at room temperature as well as at 60 °C in the absence of acid catalysis by TFA. The attempted reactions with nitrogen nucleophiles, including benzylamine and n-butylamine, failed to produce any products at room temperature with or without TFA even after 6 hours. However, intractable multiple products were formed upon heating the reaction mixture at 60 °C in the presence or absence of TFA. The results were no different when stoichiometric amounts of nucleophiles were employed for the reactions.
The attempts to induce reaction of 3 (1 mmol) with a mixture of phenol (5 mL) and TFA (10 mL) resulted in the formation of o- and p-phenol adducts (see Supporting Information) and were not seperated, whereas reaction of 3 (1 mmol) with aniline (5 mL)-TFA (10 mL) mixture led to multiple products (> 8) as detected by TLC.
No reaction occurred when 3 (1 mmol) was treated with nitrobenzene (5 mL)-TFA (10 mL) mixtue proving that the reaction is feasible only with nucleophiles bearing electron donating substiuents with partcipation in resonance.
In conclusion, we have synthesised a novel 5:7:5-fused heterocycle containing a diimidazodiazepine system that has potential applications in mechanistic studies of DNA interstrand crosslinks and repair in addition to explorations of DNA structure, function, stability, and conformation. We have explored the electrophilicity of the ring system employing carbon and amine nucleophiles. The system is reasonably stable toward both carbon and nitrogen nucleophiles under physiological temperature and pH, but undergoes facile acid-catalyzed Michael-type conjugate addition reactions. While neutral, electron-rich carbon nucleophiles produce relatively clean products, those with nitrogen nucleophiles were found intractable.
Supplementary Material
Experimental details, spectra, and spectral data of all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Table 2.
13C NMR (δ, DMSO-d6) data of compounds 3 and 11-14a
| C | 3 | 11 | 12 (CDCl3) | 13 | 14 |
|---|---|---|---|---|---|
| C-2 | 148.9 | 137.5 | 138.3 | 137.2 | 137.2 |
| C-5 | 150.3 | 145.9 | 146.6 | 145.9 | 146.0 |
| C-6a | 160.2 | 154.3 | 154.6 | 154.7 | 154.2 |
| C-8 | 166.3 | 155.8 | 156.2 | 155.8 | 156.6 |
| C-9a | -- | 64.6 | 64.2 | 64.7 | 63.4 |
| C-10 | -- | 121.0 | 121.0 | 121.3 | 122.0 |
| C-11 | -- | 134.8 | 134.4 | 134.7 | 134.9 |
| C-12 | 47.0 | -- | 46.9 | -- | -- |
| C-14 & C-18 | 129.8 | -- | 129.0 | -- | -- |
| C-1′ | 43.4 | 40.6 | 43.4 | 42.8 | 42.9 |
| C-3′ & C-7′ | 129.7 | 129.4 | 129.9 | 129.4 | 129.9 |
| C-2″ & C-6″ | -- | 127.7 | 127.4 | 127.1 | 122.8 (C-2″) |
Assignments are based on DEPT, HMQC and HMBC experiments.
Acknowledgments
This research was supported by grants (#1 R01 GM087738-01A1 & #1 R21 AI071802) from the National Institutes of Health.
References
- 1.Swenson MC, Paranawithana SR, Miller PS, Kielkopf CL. Biochemistry. 2007;46:4545. doi: 10.1021/bi700109r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noll DM, McGregor Mason T, Miller PS. Chem Rev. Vol. 106. Washington, DC, U S: 2006. p. 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilds CJ, Noronha AM, Robidoux S, Miller PS. J Am Chem Soc. 2004;126:9257. doi: 10.1021/ja0498540. [DOI] [PubMed] [Google Scholar]
- 4.Noll DM, Noronha AM, Wilds CJ, Miller PS. Front Biosci. 2004;9:421. doi: 10.2741/1246. [DOI] [PubMed] [Google Scholar]
- 5.Noronha AM, Noll DM, Wilds CJ, Miller PS. Biochemistry. 2002;41:760. doi: 10.1021/bi011610u. [DOI] [PubMed] [Google Scholar]
- 6.Ojwang JO, Grueneberg DA, Loechler EL. Cancer Res. 1989;49:6529. [PubMed] [Google Scholar]
- 7.Kerpel-Fronius S. Analogue-Based Drug Discov. 2006;385 [Google Scholar]
- 8.Morison WL, Honigsmann H. Basic Clin Dermatol. 2007;38:347. [Google Scholar]
- 9.Remers WA. In: Anticancer Agents from Natural Products. Cragg GMK, David GI, Newman David J, editors. CRC Press LLC; Boca Raton, Fla: 2005. p. 475. [Google Scholar]
- 10.McHugh PJ, Spanswick VJ, Hartley JA. Lancet Oncology. 2001;2:483. doi: 10.1016/S1470-2045(01)00454-5. [DOI] [PubMed] [Google Scholar]
- 11.Sun Z, Hosmane RS. Synth Commun. 2001;31:549. [Google Scholar]
- 12.Yahya-Zadeh A, Booth BL. Synth Commun. 2001;31:3225. [Google Scholar]
- 13.Yahyazadeh A, Sharifi Z. Phosphorus, Sulfur, Silicon, Relat Elem. 2006;181:1339. [Google Scholar]
- 14.Dias AM, Cabral I, Proenca MF, Booth BL. J Org Chem. 2002;67:5546. doi: 10.1021/jo011192z. [DOI] [PubMed] [Google Scholar]
- 15.See Supporting Information
- 16.Buenadicha FL, Bartolome MT, Aguirre MJ, Avendano C, Sollhuber M. Tetrahedron: Asymmetry. 1998;9:483. [Google Scholar]
- 17.Miki Y, Hachiken H, Kashima Y, Sugimura W, Yanase N. Heterocycles. 1998;48:1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details, spectra, and spectral data of all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.