Abstract
The processing properties of the wheat flour are largely determined by the structures and interactions of the grain storage proteins (also called gluten proteins) which form a continuous visco-elastic network in dough. Wheat gluten proteins are classically divided into two groups, the monomeric gliadins and the polymeric glutenins, with the latter being further classified into low molecular weight (LMW) and high molecular weight (HMW) subunits. The synthesis, folding and deposition of the gluten proteins take place within the endomembrane system of the plant cell. However, determination of the precise routes of trafficking and deposition of individual gluten proteins in developing wheat grain has been limited in the past by the difficulty of developing monospecific antibodies. To overcome this limitation, a single gluten protein (a LMW subunit) was expressed in transgenic wheat with a C-terminal epitope tag, allowing the protein to be located in the cells of the developing grain using highly specific antibodies. This approach was also combined with the use of wider specificity antibodies to compare the trafficking and deposition of different gluten protein groups within the same endosperm cells. These studies are in agreement with previous suggestions that two trafficking pathways occur in wheat, with the proteins either being transported via the Golgi apparatus into the vacuole or accumulating directly within the lumen of the ER. They also suggest that the same individual protein could be trafficked by either pathway, possibly depending on the stage of development, and that segregation of gluten proteins both between and within protein bodies may occur.
Keywords: Epitope tagging, gluten, immunolocalization, protein bodies, protein trafficking, wheat grain
Introduction
Wheat is the most widely consumed food crop in the world, being processed to give a range of breads, other baked goods, pasta, and noodles. This wide consumption is partly due to the fact that wheat is highly adaptable, giving high yields over a range of environments. However, of equal importance are the unique processing properties of wheat which result from the structures and interactions of the grain storage proteins (which together form the gluten protein fraction).
These proteins are related to those stored in other cereal grains, being members of the ‘prolamin superfamily’ (Kreis et al., 1985). Although their sole biological role is as stores of nitrogen, carbon, and sulphur, the gluten proteins also interact in dough to form a continuous network which confers the cohesive visco-elastic properties that enable the dough to be processed into noodles and pasta and to entrap carbon dioxide formed during fermentation (proofing) to give leavened bread. These properties are shared to a limited extent by the related proteins present in rye grain, but not by those in barley or other related cereals. Consequently, there is considerable interest in explaining the unique properties of the gluten proteins in relation to the structures and interactions of the individual gluten protein components.
Wheat gluten proteins are classically divided into two groups, the gliadins which are monomeric components that either lack disulphide bonds or contain only intra-chain bonds, and the glutenins which form complex polymers stabilized by inter-chain disulphide bonds. The gliadins are further classified into three types (α-, γ-, and ω-) and the component subunits of the glutenin polymers into two types called low molecular weight (LMW) and high molecular weight (HMW) subunits. The latter groups have been studied in most detail because they have the greatest impact on dough elasticity which is the major determinant of breadmaking performance (reviewed by Shewry et al., 2003).
Wheat gluten proteins are typical secretory proteins in that their synthesis, folding, and deposition take place within the endomembrane system of the plant cell. Like all proteins destined for the secretory pathway (Bollini and Chrispeels, 1979), gluten proteins are synthesized on polyribosomes attached to the endoplasmic reticulum (ER) and contain an N-terminal signal peptide sequence that mediates their passage into the lumen of the ER. This signal sequence is cleaved by signal peptidase either during or immediately after protein translocation and is therefore not present in the mature protein. Further processing of the proteins within the ER would include folding and disulphide bond formation, as well as formation of the inter-chain bonds which stabilize the glutenin polymers. Non-covalent interactions (notably hydrogen bonds) between gliadins and glutenin polymers may also be established, resulting in protein precipitation and the formation of hydrated protein particles within the secretory pathway.
It is probable that the folding and assembly of the gluten proteins is assisted by ER lumenal proteins such as the enzyme protein disulphide isomerase (PDI) and the molecular chaperone binding protein (BiP) although this is still not conclusively established (Grimwade et al., 1996; DuPont et al., 1998). The subsequent pathway of trafficking and deposition of prolamins is also unclear, since it appears to vary depending on the prolamin group, and perhaps also on the stage of endosperm development.
In fact, currently available evidence indicates that two separate pathways may operate in the developing wheat grain. Electron microscopy (EM) has provided convincing evidence for the presence of gluten proteins inside vesicles associated with the Golgi apparatus, suggesting that storage proteins may pass through the Golgi in their transport from the ER to the vacuoles (Parker, 1980, 1982; Kim et al., 1988; Bechtel et al., 1991; Loussert et al., 2008) where they form protein deposits. However, in some of these studies, proteins were also detected in small bodies surrounded by ER membrane, indicating that aggregation of storage proteins into protein bodies may also occur within the ER (Campbell, 1981; Parker, 1982), as demonstrated for prolamins of maize (Larkins and Hurkman, 1978) and rice (Krishnan et al., 1986).
Rubin et al. (1992) also provided evidence for the existence of two different types of protein body in wheat. These bodies differed in density and accumulated simultaneously and independently in wheat endosperm cells. The denser protein bodies appeared to be formed by the aggregation of storage proteins within the ER while the lighter bodies appeared to result from aggregation at a post-ER location. The authors also showed that, in young grains, most of the gliadins were present in the light protein bodies, whereas at more mature stages they were found in both protein body types. By contrast, the HMW subunits were highly enriched in the dense protein bodies during the entire period of grain development. No clear results were obtained for the LMW subunits.
It is not known why some gluten proteins accumulate within the ER while others pass via the Golgi to the vacuole and none of the wheat prolamins possess classical ER-retention signals (KDEL or HDEL). It has therefore been suggested that accumulation in the ER lumen could be a consequence of the ability of glutenins to form insoluble aggregates that would be less readily transported than the monomeric gliadins, which are initially soluble in the lumenal environment (Shewry, 1999). This is consistent with studies suggesting that ER-derived protein bodies do not form by a gradual increase in density but rather by a distinct, rapid process of storage protein aggregation (Rubin et al., 1992). However, other authors have suggested that the effective trafficking of wheat prolamins to the ER or Golgi complex may be determined by the relative strength of unidentified targeting signals within the various prolamin types (Altschuler et al., 1993).
Galili (1997) has also proposed that the protein bodies formed within the ER could subsequently become engulfed by vesicles and ‘internalized’ into vacuoles, leading to the fusion of the two protein body populations (of vacuolar and ER origin).
Determination of the locations and trafficking of individual gluten proteins in developing wheat grain has been limited in the past by the complexity of the protein mixture with a high degree of sequence similarity between some components, making it difficult to develop monospecific antibodies. In this work this limitation was overcome by expressing a single gluten protein (a LMW subunit) in transgenic wheat with a C-terminal epitope tag, allowing the protein to be located in the cells of the developing grain using highly specific antibodies. The combination of this approach with the use of wider specificity antibodies to other types of gluten protein has also allowed comparison of the deposition of different gluten protein groups within the same endosperm cells. These studies are in agreement with previous reports of the presence of two trafficking pathways and demonstrate clear segregation of some proteins in separate bodies.
Materials and methods
Plant material
Durum wheat (Triticum turgidum subsp. durum) plants of cv. Ofanto expressing a transgenic c-myc tagged B type low molecular weight glutenin subunit (Tosi et al., 2005) were grown in a greenhouse at Rothamsted Research in the conditions described by Tosi et al. (2004).
Grains for microscopic examinations were harvested at various stages post-anthesis.
Preparation of specimen for microscopy studies
For conventional chemical fixation, grains were harvested at 8, 12, 16, 20, 22, and 28 d post-anthesis (dpa). Transverse slices, approximately 1 mm thick, were cut from the wheat grains, fixed for 4 h in 3% (w/v) glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, and then rinsed in buffer. Half of the specimens were post-fixed in 1% (w/v) aqueous osmium tetroxide for 2 h, transferred to acetone, and then infiltrated and polymerized in Spurr's epoxy resin. The other half were directly dehydrated in an ethanol series and then infiltrated and polymerized in medium grade LR White resin.
Grains for high pressure freezing were collected at 8, 16, and 22 dpa. Transverse thin slices were cut with a razor blade while keeping specimens immersed in MES buffer, pH 5.5; 2 mm punches were taken, loaded into type A planchettes previously dipped in lecithin (100 mg lecithin in 1 ml chloroform) and transferred to a BAL-TEC HPM010 instrument for high-pressure freezing. Samples were stored in liquid nitrogen until the start of the freeze-substitution. Freeze-substitution was carried out in a Reichert AFS apparatus, using acetone containing 0.5% uranyl acetate for subsequent low temperature embedding in LR White resin (Agar Scientific, UK). Specimens were brought from –160 °C to –85 °C in steps of 15 °C h−1, then the freeze-substitution was started using the following program: T1 –85 °C 26 h S1 + 2 °C h−1 12.5 h; T2 –60 °C 10.5 h S2 + 2 °C h−1 15 h; T3 –30 °C 6 h. Samples were taken out of planchettes on ice and taken from acetone to methanol (1:3, 1:1, 3:1 v/v) while kept at –20 °C, then gradually embedded in LR White resin (25%, 50% 75%, 100%, v/v) over a period of 6 d. UV polymerization of the resin was carried out at –20 °C for 24 h followed by 24 h at 0 °C.
Sections for microscopy studies were prepared using a Reichert–Jung Ultracut microtome.
For light and fluorescence microscopy, sections approximately 1 μm thick were cut and collected on Poly-lysine coated multiwell slides. 0.1% (w/v) toluidine blue in 1% (w/v) borax, pH 11, was used to stain starchy endosperm and aleurone protein bodies.
For electron microscopy, ultrathin sections were collected on Formvar-coated slot or mesh nickel grids. For ultrastructural observations sections were sequentially stained with uranyl acetate (saturated solution in 50% (v/v) ethanol) and Reynold's lead citrate. The sections were examined with a JEOL 1200EX and with a JEOL JEM2010 transmission electron microscope.
Immunofluorescence microscopy
Slides with LR White embedded grain sections were pre-incubated (50 μl drop/well) in 3% (w/v) bovine serum albumin (BSA) (Fraction V, A 2153, Sigma) in PBS at pH 7.4 for 30 min, then incubated for 2 h in primary antibody. The following monoclonal and polyclonal antibodies were used, singly or in combination, diluted in PBS containing 1% BSA 0.05% Tween20: rabbit polyclonal anti-c-myc (A-14) and mouse monoclonal anti-c-myc (9E10) (Autogen Bioclear), both at 1:200 dilution; mouse monoclonal anti-α-gliadin (glia-α-9, 9-68) (Mitea et al., 2008) diluted 1:400; mouse monoclonal anti-sulphur rich prolamins IFRN0610 (Brett et al., 1999), diluted 1:400; rabbit polyclonal anti-R2-HMW (Denery-Papini et al., 1996), diluted 1:100. Slides were rinsed three times for 5 min with PBS Tween, then incubated for 2 h, in the dark, with secondary antibody (anti-rabbit Alexa 488 conjugated and/or anti-mouse Alexa 568 conjugated, Invitrogen) diluted 1:200 in PBS, 1% BSA, 0.5% Tween. Slides were then rinsed twice with PBS Tween, two times with PBS and three times with water. Samples were analysed on a Zeiss Axiophot fluorescence microscope equipped with a Retiga Exi (Qimaging) camera.
Immunogold labelling
A similar protocol was also used for the immunogold labelling of ultrathin sections from the same samples. In this case, grids were floated on 50 μl drops and the secondary antibody (goat anti-rabbit conjugated to 15 nm gold) (British BioCell International) was used at a 1:50 dilution.
Results
Characterization of the lines
The production and characterization of transgenic lines of durum wheat (cv. Ofanto) expressing a c-myc tagged form of a chromosome 1B-encoded LMW subunit under the control of the starchy endosperm-specific promoter from the HMW subunit 1D×5 gene of bread wheat have been previously described (Lamacchia et al., 2001; Tosi et al., 2004, 2005). The subunits encoded by the transgenes were expressed at levels similar to those of the endogenous LMW glutenin subunits, accounting for 7–8% and about 15% of the total glutenins in lines 1061 and 1093, respectively, and Western blotting showed that they were specifically recognized by commercial anti-c-myc antibodies (Tosi et al., 2004).
The patterns of protein deposition in these two lines were, therefore, initially compared with a control line of Ofanto using conventional EM of caryopses harvested between 8 dpa and 22 dpa, representing the major period of storage protein synthesis and deposition. The light microscopy results obtained with one of the transgenic lines are shown in Fig. 1 with the areas indicated on the cross-sections being shown at higher magnification in the right-hand panels. The results obtained with the second transgenic line and the control were essentially identical and are therefore not shown. No evidence of differences in the cell structure or pattern of protein deposition was observed between the transgenic and control lines in any of the studies carried out (by light microscopy or EM).
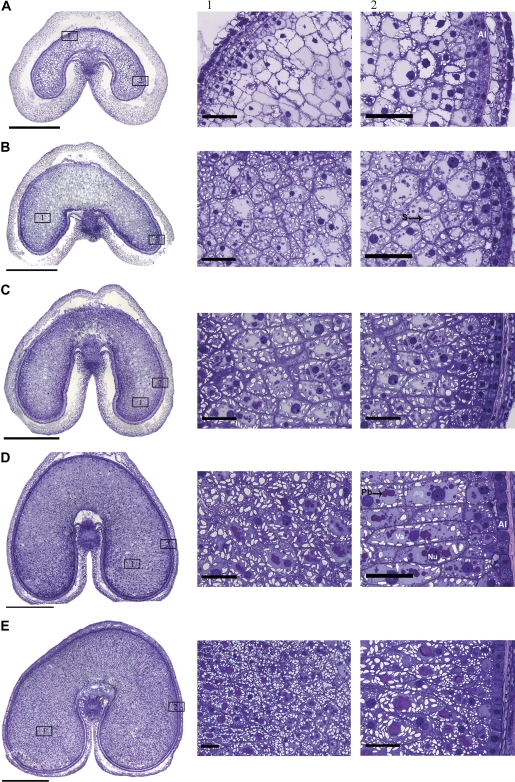
Fig. 1.
Toluidine blue-stained cross-sections and enlargements of specific areas of the wheat grain at 8 (A), 12 (B), 16 (C), 20 (D), and 22 d (E) post anthesis (dpa). Bars in cross-sections correspond to 1 mm; bars in enlargements correspond to 100 μm. Al, aleurone layer; Pb, protein bodies; S, starch granules; Nu, nucleus.
At the earliest stage (8 dpa, Fig. 1A), the aleurone cells form an outer layer with large nuclei with clearly distinguishable nucleoli and dense contents (Fig. 1A, panel 2). Because the mature wheat grain has a single layer of aleurone cells, the cells inside this layer are all destined to differentiate into starchy endosperm cells. However, at this stage, they still have a similar appearance to the aleurone cells. The inner endosperm cells differ in appearance, being typical of the starchy endosperm with large vacuoles, large nuclei, and A-type (large) starch granules present in the peripheral cytoplasm. No protein bodies are detected at this stage of development and magnification.
At 12 dpa (Fig. 1B) the aleurone layer is more clearly defined. Changes are also present in the inner endosperm cells with the central vacuole becoming divided into several smaller vacuoles which contain protein deposits. The accumulation of starch and protein in the inner endosperm cells continues to increase at 16 dpa (Fig. 1C) and 20 dpa (Fig. 1D) with most of the cell contents comprising protein and starch by 22 dpa (Fig. 1E).
Patterns of expression of the transgenic subunit
The pattern of expression of the transgenic subunit and its co-localization with other classes of gluten proteins was studied using fluorescence microscopy and double labelling with two fluorochromes, AlexaFluor conjugated 488 and 568. These dyes emit in the green and in the red region of the spectra, respectively, with no overlapping of their excitation or emission spectra. Double labelling could therefore be carried using different antibody combinations.
The commercial polyclonal antibody A-14 is specific for the c-myc tag present on the LMW subunit encoded by the transgene. This antibody was therefore used in conjunction with the gli-α-9 monoclonal antibody which is specific for a coeliac toxic motif present in α-gliadins or with the monoclonal IFRN 0610 antibody, which recognizes an epitope common to most sulphur-rich prolamins but not present in the HMW glutenins or in the tagged LMW subunit encoded by the transgene The monoclonal c-myc 9E10 antibody was also used instead of the A-14 polyclonal antibody, in conjunction with the polyclonal Anti-R2-HMW glutenin subunit antibody. Single and double labelling with these antibody combinations was carried out on sections corresponding to all five developmental stages shown in Fig. 1; none of the antibodies tested, however, gave any signal from caryopses at 8 dpa.
Labelling of the transgenic subunit with the A-14 anti-c-myc antibody showed a clear gradient in concentration, with labelling being almost absent from the cells of the subaleurone layer (Fig. 2F, green fluorescence), increasing in intensity in the second and third rows of cells inside the aleurone layer, and being strongest in the central endosperm cells. The same pattern of labelling was observed when the antibody was used alone or in combination with other antibodies for co-localization. Labelling was only observed in the protein bodies of the starchy endosperm (Figs 2, 3A, C, green fluorescence; Fig. 4A, C, magenta fluorescence) which are known to accumulate gluten proteins, but not in the protein bodies of the aleurone cells which accumulate 7S globulin (Wiley et al., 2007), and only in the transgenic grains.
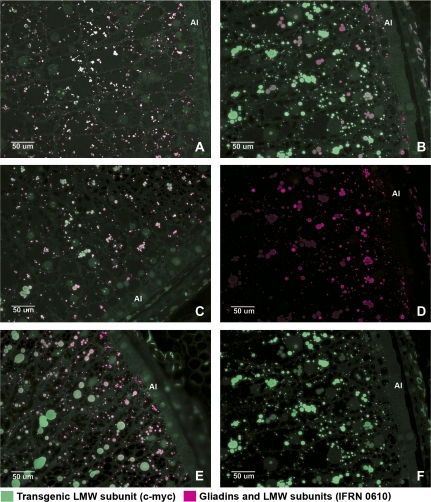
Fig. 2.
Immunofluorescence double labelling of grain sections at 12 (A), 16 (C), 20 (B, D, F), and 22 (E) dpa to show the locations of LMW glutenin subunit/gliadins and the tagged LMW subunit. Alexa 568 (magenta) was conjugated to the anti-mouse secondary antibody recognizing IFRN 0610 which is specific for a range of gliadin and LMW subunit proteins; Alexa 488 (green), was conjugated to an anti-rabbit secondary antibody recognising the A-14 antibody binding to the the c-myc tag of the LMW subunit. Micrographs (D) and (F) correspond to single channel pictures of micrograph (B). All areas shown in the micrographs correspond to those stained with toluidine blue in Fig. 1 B2, C2, D2, E2. Al, aleuronic layer.
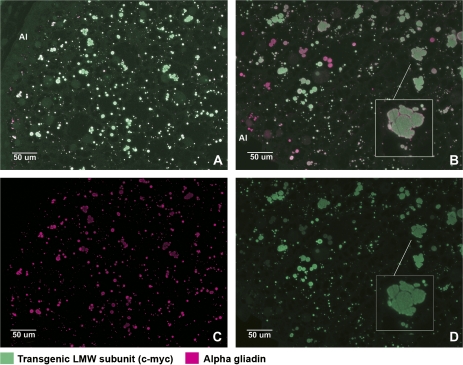
Fig. 3.
Immunofluorescence double labelling of wheat grain sections at 16 (A) and 20 (B) dpa to show the locations of alpha-gliadins and the tagged LMW subunit. Micrographs (C) and (D) show the single channel pictures corresponding to micrographs (A) and (B), respectively. Alexa 568 (magenta) was conjugated to the anti-mouse secondary antibody recognizing the anti-alpha gliadin antibody; Alexa 488 (green), was conjugated to an anti-rabbit secondary antibody recognizing the A-14 antibody binding to the c-myc tag of the LMW subunit. Areas shown in the micrographs correspond to those stained with toluidine blue in Fig. 1 C2 and D2.
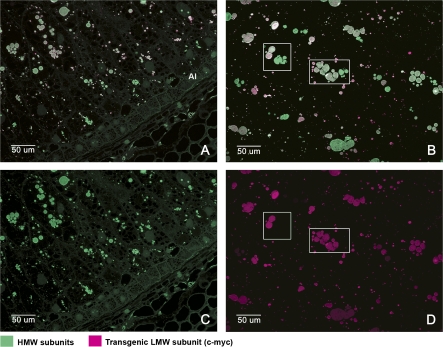
Fig. 4.
Immunofluorescence double labelling of the outer (A) and inner (B) endosperm of wheat grains at 20 dpa to show the locations of the tagged LMW subunit and HMW subunits. Alexa 568 (red) was conjugated to the anti-mouse secondary antibody recognizing the 9E10 antibody binding to the c-myc tag of the LMW subunit. Alexa 488 (green) was conjugated to an anti-rabbit secondary antibody recognizing the anti-R2-HMW antibody binding to HMW subunits. Micrographs (C) and (D) are single channel images corresponding to micrographs (A) and (B), respectively. The boxes in micrographs (B) and (D) show examples of protein bodies in the same cell which are labelled by the HMW antibody (green) but not by the anti-c-myc antibody (red). The fluorescence micrographs (A) and (C) correspond to the same region of the grain that is shown stained with toluidine blue in Fig. 1 D2. Fluorescence micrographs (B) and (D) correspond to those shown stained with toluidine blue in Fig. 1 D1.
A different pattern of labelling was observed with the 0610 antibody, with the strongest labelling being in the cells of the subaleurone layer and up to four cell layers inside the aleurone layer (Fig. 2D). The use of green labelling (to reveal the binding of the A-14 antibody to the transgenic subunit) and magenta labelling (to reveal binding of the 0610 antibody to the gliadins and LMW glutenin subunits) meant that co-localization of the two antibodies was observed as a combination of the two dyes. Co-localization was observed in most protein bodies (Fig. 2A, B, C, E) but the colour varied from light green to grey, light pink and white, indicating that the relative binding of the two antibodies varied. This presumably reflects differences in the proportions of the proteins recognized by the antibodies. This is consistent with the labelling pattern of the cells in the subaleurone layer. The protein bodies in these cells were clearly labelled magenta indicating the absence of the tagged subunit.
Labelling with the α-gliadin antibody showed a more even distribution of the protein in the different cell layers (outer and inner endosperm) (Fig. 3C). However, co-labelling with the gli-α-9 (α-gliadins) and A14 (the transgenic LMW subunit) antibodies showed that some protein bodies were labelled almost exclusively by one or the other of the two antibodies, especially at later stages of development. This is shown in Fig. 3B. Enlargement of part of this micrograph (inset Fig. 3B) also shows that merging of protein bodies enriched in α-gliadin or LMW subunits may occur, resulting in larger protein deposits of uneven shape and containing an uneven distribution of the two protein types.
The pattern of labelling observed with the Anti-R2-HMW (HMW subunit) antibody was similar to that obtained with the gli-α-9 (α-gliadin antibody), the signal being evenly distributed in the different layers of the starchy endosperm, including the subaleurone (Fig. 4A, C). Co-localization of the tagged subunit (9E10 antibody, magenta label) with the HMW glutenins (Anti-R2-HMW antibody, green label) was also observed, in both the outer (Fig. 4A) and inner (Fig. 4B) layers of the starchy endosperm but not in the subaleurone layer. However, whereas all of the protein bodies in the inner endosperm cells that contained the transgenic subunit also contained HMW glutenin subunits, other protein bodies in the same cells contained the HMW glutenin subunits but not the tagged LMW subunit (see examples in Fig. 4B, D).
Localization of the transgenic protein in cell organelles
These results from fluorescence microscopy demonstrate that different types of wheat gluten protein may differ in their pattern of deposition both between different cell layers of the endosperm and within the endosperm cells themselves. Further studies were therefore carried out to study the trafficking of the tagged LMW subunit using immunogold labelling of tissue sections for EM.
Immunogold labelling using the A14 (anti-c-myc) antibody was carried out on ultrathin sections of transgenic and null segregant seeds at 8, 12, 16, 22, and 28 dpa. Two different methods of fixation, conventional chemical fixation and high-pressure freezing, were used at each stage of development and two different resins, LR White and Spurr, were used for each type of fixation. Ultrathin sections were cut from regions corresponding to two to three cell layers from the aleurone, usually from the cheeks (see micrographs on the right side of Fig. 1).
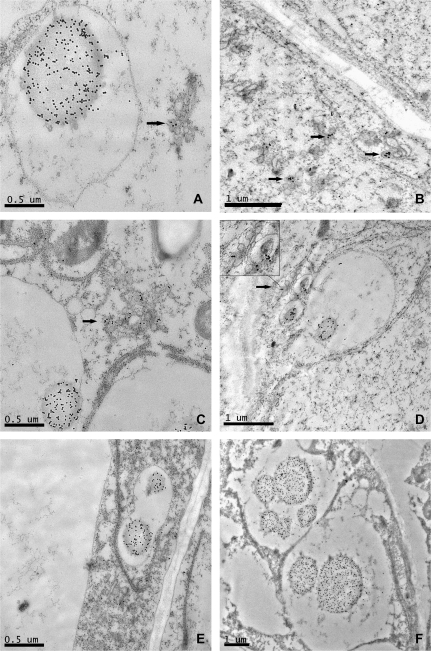
The rough endoplasmic reticulum (RER) and the Golgi apparatus were both clearly visible and well preserved in sections corresponding to 8 dpa (Fig. 5), especially in specimens prepared by high-pressure freezing. Antibody labelling was clearly observed on densely stained vesicles of about 0.2 μm associated with the Golgi apparatus (Fig. 5A, B, C, D, black arrows). However, while all the Golgi bodies in any given section showed labelling with the c-myc antibody, the labelling was restricted to one or two of the densely stained vesicles budding from each body (Fig. 5A, C). Protein bodies of diameter varying between 0.2 μm and 2 μm and surrounded by a single unit membrane were also observed (Fig. 5A, C, D, E, F) and showed heavy labelling when probed with the A14 antibody. The smallest protein bodies (0.2 μm) were often closely associated with the Golgi stacks (Fig. 5D) and surrounded by electron-lucent material enclosed within the membrane. In some cases, several protein bodies were clustered in a same vacuole-like structure (Fig. 5E, F), which may have resulted from the fusion of the surrounding membranes (Fig. 5D, E). No labelling was observed in sections prepared from null segregant seeds.
Fig. 5.
TEM micrographs showing immunogold labelling of the tagged LMW glutenin subunit in the starchy endosperm of 8dpa wheat grain using the anti-c-myc antibody. Labelling can be seen in protein bodies (A, C, D, E, F) and in Golgi vesicles (A, B, C, D, arrows).
Larger protein bodies (3–5 μm) were observed at 12 dpa (Fig. 6A), although in this case their surfaces appeared to be surrounded by small, electron-opaque vesicles. These protein bodies also contained inclusion bodies which have previously been shown to contain the storage globulin triticin (Bechtel et al., 1991). These inclusions remained as a separate phase on the surface of the protein bodies and were not labelled by the A14 antibody (Fig. 6B). Fewer Golgi bodies were observed at this stage in the starchy endosperm cells, although they continued to be observed in the subaleurone cells and the modified aleurone cells in the groove region of the grain (data not shown).
Fig. 6.
Details of sections of starchy endosperm from 12 dpa wheat grain showing labelled protein bodies and unlabelled inclusion bodies (arrows). The antibody used for labelling was the monoclonal 9E10 anti c-myc.
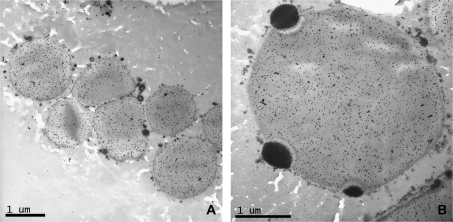
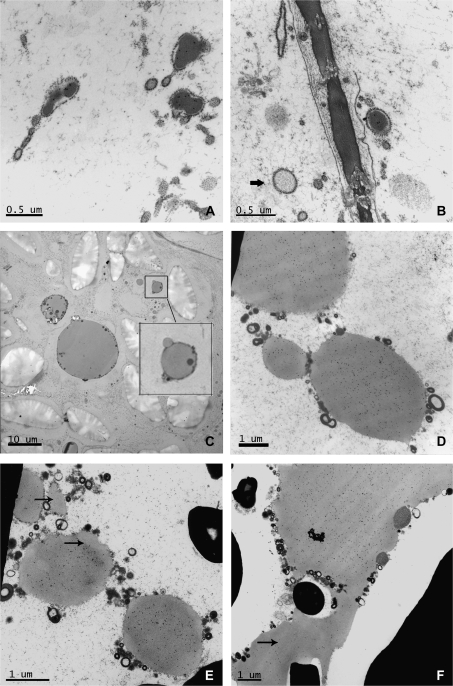
No Golgi structures with stacked cisternae were visible in the starchy endosperm cells at 16 dpa, although they were still clearly detected in the aleurone layer. Instead a new type of protein body was observed. These protein bodies were often irregular in shape and surrounded by a membrane studded on the outside with small electron-opaque bodies (Fig. 7A, B). They were therefore concluded to correspond to the RER-derived bodies as described by Campbell et al. (1981), Parker (1982), and Krishnan et al. (1991). The RER seemed to have enlarged in some points to form cisternae of up to 0.5 μm of diameter, some containing material of electron density similar to that of the gluten proteins present in the vacuolar bodies observed in an earlier stage of development (Fig. 7A, B). These RER protein bodies were labelled with the A14 antibody showing that they contained the tagged subunit. However, some of the enlarged cisternae appeared electron-translucent and showed no labelling (Fig. 7B, arrow). Fusion of small protein bodies (1 μm) with larger protein bodies was observed in several micrographs (Fig. 7C, D). Although all the protein bodies observed in the sections prepared from transgenic seeds were labelled by the A14 antibody, differences in the intensity of the labelling were noted between protein bodies with some of the larger protein bodies showing uneven patterns of labelling. For example, the protein bodies shown in Fig. 7E clearly contain phases which are less electron-opaque (unlike those containing triticin) and are not labelled with the antibody (see arrows).
Fig. 7.
Details of sections of starchy endosperm from transgenic grain at 16 (A, B, C, D), 22 (E), and 28 (F) dpa. Micrographs (A), (B), (D), (E), and (F) show immunogold labelling with the C-myc antibody. Micrograph (C) is a null control showing no labelling. (A) Labelling can be seen in the ER lumen. (B) Arrow indicates a region of enlarged ER, similar to that on the right of the picture, but with different electron density and no labelling. Micrograph (D) and the box in micrograph (C) show the fusion of protein bodies of different sizes. Arrows in (E) and (F) indicate areas of protein bodies (E) or the protein matrix (F) which show lower density of labelling than other areas.
The coalescence of protein bodies continued at 22 dpa and resulted by 28 dpa in a continuous proteinaceous matrix surrounding the starch granules (Fig. 7F). However, uneven labelling with the A14 antibody was also observed between different regions of the protein matrix at this stage (Fig. 7F, arrow), indicating that microphases of protein may be present.
Discussion
Spatial patterns of gluten protein distribution
The expression of an epitope-tagged LMW subunit in developing grain of transgenic wheat has allowed us to compare its pattern of synthesis and deposition with those of related gluten proteins.
It should be noted that the transgenic subunit was not expressed under the control of its own promoter but the promoter of an HWM subunit gene (encoding HMW subunit 1D×5) from bread wheat. Hence the expression pattern of the transgenic protein should reflect that of the HWM subunit gene and not that of the endogenous LMW subunit gene. It is therefore not surprising that the pattern differed from that of the proteins recognized by the broad specificity monoclonal antibody IFRN 0610. This antibody binds strongly to repetitive amino acid sequences containing the motif GlnGlnSerPhe (Brett et al., 1999) which are present in many gliadins and LMW subunits of glutenin, but not in the LMW subunit encoded by the transgene. The pattern of binding of the IFRN0610 antibody clearly differed from those of the A14 and 9E10 c-myc antibodies (that recognized the c-myc tag), being especially dense in the subaleurone cells. This is consistent with our knowledge that cells derived from the sub-aleurone layer may contain up to 45% protein, mainly gluten protein, in contrast to the central endosperm cells in which protein may only account for about 8% (Kent, 1966). Furthermore, Stoger et al. (2001) showed that the promoter of a LMW subunit gene conferred strong expression of the GUS reporter gene in the subaleurone and outer starchy endosperm cell layers of developing seeds when used to transform wheat. While the two classes of glutenin promoters clearly differ in their spatial patterns of expression, their timing of expression is very similar, being initiated within 2 dpa from each other (P Shewry et al., unpublished results).
The expression pattern of the LMW subunit transgene in the present study is also consistent with previous studies of the expression of the same HMW subunit 1D×5 promoter in transgenic durum wheat (Lamacchia et al., 2001). More surprising is the fact that the pattern of accumulation of the tagged subunit differed from that of the endogenous HMW subunits revealed by using the anti-R2-HMW polyclonal antibody (which was raised against a repetitive peptide present in all HMW subunits and reacts on Western blot with all HMW subunits present in bread wheat but not with gliadins or LMW subunits (Denery-Papini et al., 1996).This specificity was also confirmed by our own Western blotting with the durum cultivar used in the present study. This cultivar (Ofanto) contains only HMW subunit 1B×20 (Sapirstein et al., 2007) which is recognized by the Anti-R2-HMW antibody (results not shown). The binding of this antibody to the subaleurone cells as well as to those further into the starchy endosperm may therefore indicate that the endogenous HMW subunits present in Ofanto differ in their distribution within the grain from that of subunit 1D×5 in bread wheat.
Pathway of LMW subunit trafficking and deposition
Immunogold labelling of the starchy endosperm cells from caryopses between 8 dpa and 12 dpa showed the presence of the tagged subunit in Golgi associated vesicles. As described previously by other authors (Kim et al., 1988; Philippe et al., 2006), the Golgi at this stage consisted of several stacked cisternae and associated small (0.2 μm) vesicles differing in electron density. However, the c-myc antibody did not label all of these vesicles but only one or two of the more electron opaque vesicles in each Golgi apparatus. The densely stained immunoreactive vesicles often appeared to be enveloped in larger electrolucent vesicles which have also been described previously and proposed to represent completely distended Golgi cisternae (Kim et al., 1988). The same authors also demonstrated that some of the Golgi-associated electron opaque vesicles were labelled by an anti-gliadin antibody while others (Stenram et al., 1991; Loussert et al., 2008) showed that they also reacted with antibodies to HMW glutenin subunits and possibly to antibodies to LMW glutenin subunits. It was therefore suggested that they may represent precursors of the protein bodies. In this work, merging of the membranes surrounding these dense vesicles was observed in several micrographs and led to the fusion of the sub-micrometre protein bodies to form increasingly larger aggregates. Enlargement of wheat endosperm protein bodies by coalescence has been described by several other authors (Levanony et al., 1992; Kim et al., 1988; Arcalis et al., 2004; Loussert et al., 2008) and continues throughout the grain-filling phase of development, leading to the formation of protein bodies of several micrometres of diameter, which finally merge to form a continuous protein matrix when the seed starts to dry (Fig. 7F).
Whereas Golgi were abundant in cells up to 12 dpa, they were observed only very rarely in starchy endosperm cells from 16 dpa. Furthermore, their morphology differed in these older cells, consisting of only one or two stacks with few large, translucent vesicles (data not shown). This agrees with the observations of Kim et al. (1988). While it is possible that Golgi structures are still present at later stages of grain development (as suggested by Parker and Hawes, 1982), they would appear to have lost most of their structural organization. The accumulation of the tagged subunit within the lumen of the RER was also first observed at 16 dpa, resulting in some cases in the dilation of the ER to form small protein bodies surrounded by rough ER membranes. The accumulation of storage protein in the endoplasmic reticulum cisternae is well documented for the zeins (storage prolamins) of maize (Larkins and Hurkman, 1978) and the prolamins of rice (Krishnan et al., 1986) and several authors have also reported evidence of these RER-derived protein bodies in wheat (Campbell et al., 1981; Parker, 1982; Krishnan et al., 1991). Rubin et al. (1992) also suggested that two simultaneous and independent pathways of accumulation were active in wheat, leading to the formation of two types of protein bodies which were enriched in different type of gluten proteins and had different densities. Aggregation at the site of synthesis would lead to dense ER-derived protein bodies, enriched in HMW glutenin subunits, while the aggregation at a post-ER location (i.e. vacuole) would lead to the formation of light protein bodies rich in gliadins. However, previous studies using immunogold labelling of protein bodies at different stages of development failed to confirm that segregation in the synthesis and deposition of different types of gluten proteins occurred. Loussert et al. (2008) showed that antibodies against a range of gluten protein types labelled all protein bodies and densely stained Golgi vesicles. Although these authors failed to show any differences in intensity of labelling using antibodies with different specificities this was reported by Stenram et al. (1991), suggesting that differences occur in the proportions of different protein types in different protein bodies.
The epitope tagging approach used here allowed us to follow the deposition of a single LMW glutenin subunit. In young seeds the subunit clearly followed the Golgi–vacuole route to deposition, while during the phase of intense grain filling (from 14–16 dpa) the subunit also started to accumulate within the lumen of the RER. Evidence for differences in the proportions of different types of gluten proteins in protein bodies within the same cell was also provided by immunofluorescence using double antibody labelling. Thus, cells of the central starchy endosperm clearly contained some protein bodies which contained HMW subunits of glutenin but little or no gliadin, and this segregation may be retained even after protein body fusion and matrix formation.
The work described here makes two new contributions to our knowledge of gluten protein trafficking and deposition. Firstly, that the same protein may form protein bodies by two routes, either transport via the Golgi and Golgi-derived vesicles into the vacuole or by direct accumulation within the lumen of the ER. Gluten proteins have not been shown to have specific signals to retain them in the ER or direct them to be trafficked via the Golgi to the vacuole (reviewed by Shewry, 1999) and this is consistent with the present study in which the route of deposition appears to be determined by the developmental stage of the tissue. It is also consistent with the hypothesis that aggregation within the rough ER is favoured when the rate of protein synthesis is high as this would lead to a higher concentration of protein within the ER lumen (which would in turn favour protein aggregation).
Secondly, that segregation of protein types into specific populations of protein bodies can occur within the same cell. The mechanism for this is, again, not known, but it could result either from segregation into different populations of Golgi-derived vesicles (which is consistent with our observation that not all vesicles were labelled with our antibody) and/or by segregation of synthesis on separate sub-domains of the RER, as reported for rice prolamins and glutelins by Li et al. (1993). Irrespective of the mechanism, such segregation could have implications for the future utilization of flours produced from the grain, as the interactions of the gluten proteins are key to determining processing quality (Gupta et al., 1993, 1995; Gupta and MacRichtie, 1994; Pogna et al., 1988).
Acknowledgments
Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC) of the UK. The BBSRC is also thanked for supporting the High Pressure Freezing Facility at Oxford Brookes University. We would also thank colleagues in the Bioimaging Unit at Rothamsted, in particular Mrs Jean Devonshire, for their help and support with the TEM and LM studies and Professor Chris Hawes (Oxford Brookes University, UK) for discussions and critical comments on the manuscript. Finally, we thank Drs Yves Popineau and Sandra Denery-Papini (INRA, Nantes, France) and Professor Frits Koning (Leiden University Medical Center), for providing some of the antibodies.
References
- Altschuler Y, Rosemberg N, Harel R, Galili G. The N-and C-terminal regions regulate the transport of wheat γ-gliadin through the endoplasmic reticulum in Xenopus oocytes. The Plant Cell. 1993;5:443–450. doi: 10.1105/tpc.5.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcalis E, Sylvain M, Altmann F, Kolarich D, Drakakaki G, Fisher R, Christou P, Stoger E. Unexpected deposition patterns of recombinant proteins in post-endoplasmic reticulum compartments of wheat endosperm. Plant Physiology. 2004;136:3457–3466. doi: 10.1104/pp.104.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel DB, Wilson JD, Shewry PR. Immunocytochemical localization of the wheat storage protein triticin in developing endosperm tissue. Cereal Chemistry. 1991;68:573–577. [Google Scholar]
- Bollini R, Chrispeels MJ. The rough endoplasmic reticulum is the site of reserve-protein synthesis in developing Phaseolus vulgaris cotyledons. Planta. 1979;146:487–501. doi: 10.1007/BF00380865. [DOI] [PubMed] [Google Scholar]
- Brett GM, Mills ENC, Goodfellow BJ, Fido RJ, Tatham AS, Shewry PR, Morgan MRA. Epitope mapping studies of broad specificity monoclonal antibodies to cereal prolamins. Journal of Cereal Science. 1999;29:117–128. [Google Scholar]
- Campbell WP, Lee JW, O'Brien TP, Smart MG. Endosperm morphology and protein body formation in developing wheat grains. Australian Journal of Plant Physiology. 1981;8:5–19. [Google Scholar]
- Denery-Papini S, Popineau Y, Quillien L, Van Regenmortel MHV. Specificity of antisera raised against synthetic peptide fragments of high Mr glutenin subunits. Journal of Cereal Science. 1996;23:133–144. [Google Scholar]
- DuPont FM, Hurkman WJ, Tanaka CK, Chan R. BiP, HSP70, NDK and PDI in wheat endosperm. I. Accumulation of mRNA and protein during grain development. Physiologia Plantarum. 1998;102:70–79. [Google Scholar]
- Galili G. The prolamin storage proteins of wheat and its relatives. In: Larkins BA, Vasil IK, editors. Cellular and molecular biology of plant seed development. The Netherlands: Kluyer Academic Publishers; 1997. [Google Scholar]
- Grimwade B, Tatham AS, Freedman RB, Shewry PR, Napier JA. Comparison of the expression patterns of genes coding for wheat gluten proteins involved in the secretory pathway in developing caryopses of wheat. Plant Molecular Biology. 1996;30:1067–1073. doi: 10.1007/BF00020817. [DOI] [PubMed] [Google Scholar]
- Gupta RB, Khan K, MacRichie F. Biochemical basis of flour properties in bread wheats. I. Effects of variation in the quantity and size distribution of polymeric protein. Journal of Cereal Science. 1993;18:23–41. [Google Scholar]
- Gupta RB, MacRichtie F. Allelic variation at glutenin subunit and gliadin loci, Glu-1, Glu-3, and Gli1 of common wheats. II. Biochemical basis of the allelic effects on dough properties. Journal of Cereal Science. 1994;19:19–29. [Google Scholar]
- Gupta RB, Popineau Y, Lefebvre J, Cornec M, Lawrence GJ, MacRichie F. Biochemical basis of flour properties in bread wheats. II. Changes in polymeric protein formation and dough/gluten properties associated with the loss of low Mr or high Mr glutenin subunits. Journal of Cereal Science. 1995;21:103–116. [Google Scholar]
- Kent NL. Subaleurone endosperm cells of high protein content. Cereal Chemistry. 1966;43:585–601. [Google Scholar]
- Kim WT, Franceschi VR, Krishnan HB, Okita TW. Formation of wheat protein bodies: involvement of the Golgi apparatus in gliadin transport. Planta. 1988;176:173–182. doi: 10.1007/BF00392442. [DOI] [PubMed] [Google Scholar]
- Kreis M, Forde BG, Rahaman S, Miflin BJ, Shewry PR. Molecular evolution of the seed storage proteins of barley, rye and wheat. Journal of Molecular Biology. 1985;183:499–502. doi: 10.1016/0022-2836(85)90017-8. [DOI] [PubMed] [Google Scholar]
- Krishnan HB, Franceschi VR, Okita TW. Immunochemical studies on the role of the Golgi complex in protein-body formation in rice seeds. Planta. 1986;169:471–480. doi: 10.1007/BF00392095. [DOI] [PubMed] [Google Scholar]
- Krishnan HB, White JA, Pueppke SG. Immuonocytochemical localization of wheat prolamins in the lumen of the rough endoplasmic reticulum. Canadian Journal of Botany. 1991;69:2574–2577. [Google Scholar]
- Lamacchia C, Shewry PR, Di Fonzo N, Forsyth JL, Harris N, Lazzeri P, Napier JA, Halford NG, Barcelo P. Endosperm specific activity of a storage protein gene promoter in transgenic wheat seed. Journal of Experimental Botany. 2001;52:243–250. [PubMed] [Google Scholar]
- Larkins BA, Hurkman WJ. Synthesis and deposition of zein protein bodies of maize endosperm. Plant Physiology. 1978;62:256–263. doi: 10.1104/pp.62.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanony H, Rubin R, Altschuler Y, Galili G. Evidence for a novel route of wheat storage proteins to vacuoles. Journal of Cell Biology. 1992;119:1117–1128. doi: 10.1083/jcb.119.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Franceschi VR, Okita TW. Segregation of storage protein m-RNAs on the rough endoplasmic reticulum membranes of rice endosperm cells. Cell. 1993;72:869–879. doi: 10.1016/0092-8674(93)90576-c. [DOI] [PubMed] [Google Scholar]
- Loussert C, Popineau Y, Mangavel C. Protein bodies ontogeny and localization of prolamin comonents in the developing endosperm of wheat caryopses. Journal of Cereal Science. 2008;47:445–456. [Google Scholar]
- Mitea C, Havenaar R, Drijfhout JW, Edens L, Dekking L, Koning F. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut. 2008;57:25–32. doi: 10.1136/gut.2006.111609. [DOI] [PubMed] [Google Scholar]
- Parker ML. Protein body inclusions in developing wheat endosperm. Annals of Botany. 1980;46:29–36. [Google Scholar]
- Parker ML. Protein accumulation in the developing endosperm of a high protein line of Triticum dicoccoides. Plant, Cell and Environment. 1982;5:37–43. [Google Scholar]
- Parker ML, Hawes CR. The Golgi apparatus in developing endosperm of wheat (Triticum aestivum L.) Planta. 1982;154:277–283. doi: 10.1007/BF00387875. [DOI] [PubMed] [Google Scholar]
- Philippe S, Saulnier L, Guillon F. Arabinoxylan and (1→3), (1→4)-β-glucan deposition in cell walls during wheat endosperm development. Planta. 2006;224:449–461. doi: 10.1007/s00425-005-0209-5. [DOI] [PubMed] [Google Scholar]
- Pogna N, Lafiandra D, Feillet P, Autran JC. Evidence for a direct causal effect of low molecular weight subunits of glutenins on gluten viscoelasticity in durum wheats. Journal of Cereal Science. 1988;7:211–214. [Google Scholar]
- Rubin R, Levanony H, Galili G. Evidence for the presence of two different types of protein bodies in wheat endosperm. Plant Physiology. 1992;99:718–724. doi: 10.1104/pp.99.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapirstein HD, David P, Preston KR, Dexter JE. Durum wheat breadmaking quality: effects of gluten strength, protein composition, semolina particle size and fermentation time. Journal of Cereal Science. 2007;45:150–161. [Google Scholar]
- Shewry PR. The synthesis, processing, and deposition of gluten proteins in the developing wheat grain. Cereal Foods World. 1999;44:587–589. [Google Scholar]
- Shewry PR, Gilbert SM, Savage AWJ, Tatham AS, Wan YF, Belton PS, Wellner N, D'Ovidio R, Békés F, Halford NG. Sequence and properties of HMW subunit 1Bx20 from pasta wheat (Triticum durum) which is associated with poor end use properties. Theoretical and Applied Genetics. 2003;106:744–750. doi: 10.1007/s00122-002-1135-6. [DOI] [PubMed] [Google Scholar]
- Stenram U, Heneen WK, Skerritt JH. Immunocytochemical localization of wheat storage proteins in endosperm cells 30 days after anthesis. Journal of Experimental Botany. 1991;42:1347–1355. [Google Scholar]
- Stoger E, Parker M, Christou P, Casey R. Pea legumin overexpressed in wheat endosperm assembles into an ordered paracrystalline matrix. Plant Physiology. 2001;125:1732–1742. doi: 10.1104/pp.125.4.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi P, D'Ovidio R, Napier J, Bekes F, Shewry PR. Expression of epitope-tagged LMW glutenin subunits in the starchy endosperm of transgenic wheat and their incorporation into glutenin polymers. Theoretical and Applied Genetics. 2004;108:468–476. doi: 10.1007/s00122-003-1459-x. [DOI] [PubMed] [Google Scholar]
- Tosi P, Masci S, Giovangrossi A, D'Ovidio R, Bekes F, Larroque O, Napier J, Shewry PR. Modification of the low molecular weight (LMW) glutenin composition of transgenic durum wheat: effects on glutenin polymer size and gluten functionality. Molecular Breeding. 2005;16:113–126. [Google Scholar]
- Wiley PR, Tosi P, Evrard A, Lovegrove A, Jones HD, Shewry PR. Promoter analysis and immunolocalization show that puroindoline genes are exclusively expressed in starchy endosperm cells of wheat grain. Plant Molecular Biology. 2007;64:125–136. doi: 10.1007/s11103-007-9139-x. [DOI] [PubMed] [Google Scholar]