Abstract
Thaxtomin A, a phytotoxin produced by Streptomyces eubacteria, is suspected to act as a natural cellulose synthesis inhibitor. This view is confirmed by the results obtained from new chemical, molecular, and microscopic analyses of Arabidopsis thaliana seedlings treated with thaxtomin A. Cell wall analysis shows that thaxtomin A reduces crystalline cellulose, and increases pectins and hemicellulose in the cell wall. Treatment with thaxtomin A also changes the expression of genes involved in primary and secondary cellulose synthesis as well as genes associated with pectin metabolism and cell wall remodelling, in a manner nearly identical to isoxaben. In addition, it induces the expression of several defence-related genes and leads to callose deposition. Defects in cellulose synthesis cause ectopic lignification phenotypes in A. thaliana, and it is shown that lignification is also triggered by thaxtomin A, although in a pattern different from isoxaben. Spinning disc confocal microscopy further reveals that thaxtomin A depletes cellulose synthase complexes from the plasma membrane and results in the accumulation of these particles in a small microtubule-associated compartment. The results provide new and clear evidence for thaxtomin A having a strong impact on cellulose synthesis, thus suggesting that this is its primary mode of action.
Keywords: Arabidopsis thaliana, callose, cellulose, defence response, isoxaben, lignin, microtubule, phytotoxin, Streptomyces, thaxtomin
Introduction
Cellulose is the major component of the plant cell wall (CW). The CW provides general plant strength (Shedletzky et al., 1992) and is important for the protection of cells against pathogens, dehydration, and other environmental factors (Braam, 1999; Vorwerk et al., 2004). In addition, the primary CW contributes to the determination of plant development, cell shape (Delmer and Amor, 1995), and cell–cell interactions. Once expansion growth has ceased, some cell types deposit a thick secondary CW (Turner et al., 2001), which is mainly composed of highly aligned, crystalline cellulose and xylans (Ebringerova and Heinze, 2000; Brown et al., 2005), and which can also be lignified in order to provide further strength. Defects or irregularities in cellulose deposition and CW integrity have been shown to trigger defence pathways in both primary and secondary CW mutants (Caño-Delgado et al., 2000; Ellis et al., 2002; Hernandez-Blanco et al., 2007).
Synthetic chemical compounds, for example, isoxaben or dichlobenil (DCB), that specifically inhibit CW synthesis have been identified previously (Hogetsu et al., 1974; Heim et al., 1990). Pinpointing such a compound's molecular and cellular effects on CW synthesis and identification of the genetic modifications in mutants resistant or hypersensitive to such compounds can advance our understanding of CW biology. Genetic studies revealed that the herbicide isoxaben has a direct effect on cellulose synthases (CESA), i.e. CESA3 and CESA6 (Scheible et al., 2001; Desprez et al., 2002) as well as CESA2 and CESA5 (Desprez et al., 2007). Isoxaben has also been reported to prevent incorporation of glucose into the CW and to inhibit the assembly of hemicelluloses (Heim et al., 1990, 1998). More recently, isoxaben and DCB were also shown to cause aberrant CESA complex (CESA-C) patterns in the plasma membrane (PM) (Paredez et al., 2006; DeBolt et al., 2007) by changing CESA motility and to affect cortical microtubule arrays (Lazzaro et al., 2003; Paredez et al., 2006, 2008; DeBolt et al., 2007).
The cyclic dipeptide thaxtomin A is the only somewhat familiar natural compound for which inhibition of cellulose synthesis has been suggested (King et al., 2001; Fry and Loria, 2002; Scheible et al., 2003). Thaxtomin A is produced by plant-pathogenic soil bacteria of the genus Streptomyces (King and Lawrence, 1996) implying that cellulose synthesis is a natural target in plant–pathogen interactions. The molecular mode of action of thaxtomin A remains unknown. It was noted that nanomolar concentrations of thaxtomin A cause swelling of hypocotyls and reduced seedling growth in A. thaliana (Scheible et al., 2003). In tobacco suspension cultures, it causes radial cell swelling in dividing or expanding cells, but does not affect mitotic and cortical microtubules (Fry and Loria, 2002). Thaxtomin A also inhibits normal cell elongation of tobacco suspension cells in a manner that suggests an effect on primary CW development (Fry and Loria, 2002), and the incorporation of [14C]-glucose into the acid-insoluble CW fraction (i.e. cellulose) in A. thaliana hypocotyls (Scheible et al., 2003). Furthermore, etiolated A. thaliana seedlings treated with nanomolar concentrations of thaxtomin A display a Fourier-transform infrared (FT-IR) spectral phenotype that is most related to those of A. thaliana rsw1/cesa1 mutant seedlings or A. thaliana seedlings treated with cellulose-synthesis inhibitors like DCB, isoxaben, or flupoxam (Scheible et al., 2003; Robert et al., 2004).
In addition to thaxtomin A's putative role as a cellulose synthesis inhibitor, its application to plants was proposed to trigger an early Ca2+ signalling cascade that might be crucial for plant-pathogen interactions (Tegg et al., 2005). However, although thaxtomin A leads to programmed cell death, no evidence for an activation of defence responses like oxidative burst, rapid medium alkalization or an activation of defence genes has been reported (Duval et al., 2005).
In this work, further effects of thaxtomin A on A. thaliana are described, confirming its role as a natural cellulose synthesis inhibitor. These include a more detailed CW fractionation study to reveal further insights into the nature of non-cellulosic CW fractions, something that was not addressed in previous reports. Thaxtomin A's impact on the expression of CW and defence response-related genes was also investigated and it is shown that thaxtomin A triggers lignification in A. thaliana seedlings, in a pattern that differs from the one of isoxaben. Finally, it is demonstrated that thaxtomin A has clear impact on the motility of CESA-Cs as well as their distribution in the PM.
Materials and methods
Seedling growth conditions and thaxtomin A treatment
A. thaliana wild-type Col-0 seeds from an in-house collection, cesa3je5::GFP-CESA3 seeds (Desprez et al., 2007) and cesa6prc::GFP-TUA6 seeds (Ueda et al., 1999) were surface-sterilized and stratified at 4 °C for 3 d, before they were exposed to white light for 3 h to induce and synchronize germination, and then grown in sterile liquid cultures as previously described by Scheible et al. (2004). Thaxtomin A was purified from 4–7-d-old oatmeal broth cultures of Streptomyces scabies by ethyl acetate extraction and reverse phase thin-layer chromatography, essentially as described by King and Lawrence (1996). Purified thaxtomin A (or isoxaben) was dissolved in methanol and added to the A. thaliana seedling cultures from a 20 μM (or 0.5 μM) stock solution to a final concentration of 200 nM (or 5 nM). Seedlings were treated 4 d after transfer to liquid media and were harvested for analyses 2 d after the treatment. Etiolated seedlings cultures were dark-grown in flasks wrapped with aluminium foil, and treated with thaxtomin A (or isoxaben) as above.
Cellulose [14C]-sucrose incorporation assay and CW fractionation
The radiolabel incorporation assay was performed according to Scheible et al. (2003) and Fagard et al. (2000), however, [U-14C]-sucrose (Amersham) was used instead of [14C]-glucose. CW fractionation was performed according to Peng et al. (2000). Fractions analysed for their 14C incorporation were the (i) chloroform fraction, (ii) ammonium-oxalate fraction, (iii) 0.1 M KOH fraction, (iv) 4 M KOH fraction, (v) the acid-soluble fraction, and (vi) the acid-insoluble cellulosic fraction (Peng et al., 2000).
RNA isolation, DNase I treatment, cDNA synthesis, qRT-PCR and primers
All experiments were performed as described by Czechowski et al. (2005) and Udvardi et al. (2008). RNA from whole seedlings was isolated using the Qiagen Plant RNeasy Mini Kit. After RNA quantification, 5 μg total RNA was digested with DNase I (Sigma) according to the manufacturer's instructions. After checking for the absence of genomic DNA, cDNA was synthesized from DNA-free RNA using Supercript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions. QRT–PCR was carried out in 10 μl reaction volumes using an ABI Prism® HT-7900 Sequence detection system (Applied Biosystems). Primer sequences are presented in Supplementary Table S1 at JXB online. Results were normalized with the internal reference gene At4g05320 (UBQ10).
Lignin and callose staining
Seedlings were put on a microscope slide and were treated with two drops of freshly prepared phloroglucinol solution, consisting of 25 mg phloroglucinol dissolved in 25 ml 100% methanol and 25 ml 37% hydrochloric acid. After incubation for 2 min, seedlings were observed using a Leica MZ 12.5 stereo microscope. Callose staining with aniline blue was performed on 6-d-old etiolated seedlings as described previously (http://commonweb.unifr.ch/biol/pub/mauchgroup/staining.html; Stone et al., 1985). Aniline blue fluorescence was observed with an Olympus BX51 epi-fluorescence stereo microscope.
Spinning disc microscopy
Seedlings expressing GFP::CESA3 and GFP::TUA6 fusion proteins were cultivated as described by Chan et al. (2005). Imaging of GFP-CESA3 and GFP-TUA6 was conducted as follows. Hypocotyls of 3-d-old etiolated seedlings were analysed on an Axiovert 200M microscope (Zeiss, Thornwood, NY), equipped with a Yokogawa CSU22 spinning disc, Zeiss 100/1.4 N.A. oil objective and Andor EMCCD iXon DU 895 camera (Plateforme d'Imagerie Dynamique, Institut Pasteur, Paris, France). A 488 nm diode pumped solid-state laser was used for excitation, and emission collected using a BP 488/25 filter (Semrock, Rochester, NY). Particle velocities were calculated from kymographs created in Image J (W Rasband, National Institutes of Health, Besthesda, MD, USA). Three-day-old, chamber-cultivated A. thaliana seedlings were treated with or without 200 nM thaxtomin A.
Results
Thaxtomin A alters cell wall [14C]-isotope partitioning
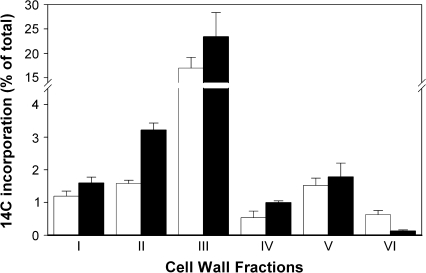
It has previously been shown that thaxtomin A treatment results in a reduced level of [14C]-isotope incorporation into the cellulosic fraction (Scheible et al., 2003). This reduction of radiolabel incorporated into cellulose was shown to be compensated for by an increase in the non-cellulosic fraction, while the uptake of radiolabel per se was unchanged by thaxtomin A. Because A. thaliana CWs contain three major classes of polysaccharides (cellulose, hemicelluloses, and pectins), thaxtomin A's effect on the de novo synthesis of these polysaccharides was analysed in more detail. The incorporation of [14C]-isotope, provided as [U-14C]-sucrose, into several CW fractions was determined, as obtained by sequential extractions with (i) chloroform, (ii) ammonium-oxalate, (iii) 0.1 M KOH, (iv) 4 M KOH, and (v) acetic/nitric acid (acid-soluble fraction). In addition, the acid-insoluble fraction was analysed (vi). These CW fractions are considered to contain (i) lipids, (ii) neutral polysaccharides and pectins, (iii, iv) hemicelluloses/pectins, (v) residual pectins, non-crystalline cellulose, and hemicelluloses, and (vi) pure, crystalline cellulose, respectively (Peng et al., 2000). Thaxtomin A application induced significant changes in [14C]-isotope incorporation within several CW fractions (Fig. 1). In agreement with Scheible et al. (2003), etiolated A. thaliana seedlings treated with 200 nM thaxtomin A showed a strongly reduced level of incorporated isotope label into the cellulosic fraction and FTIR analyses confirmed that thaxtomin A-treated seedlings clustered with cellulose-deficient mutants (Scheible et al., 2003). Compared with the untreated control, the ammonium-oxalate fraction, representing mainly pectins, showed a significant increase in [14C]-isotope. The [14C]-isotope in the chloroform fraction (lipids), the 0.1 M KOH and 4 M KOH fractions, respectively (hemicelluloses/pectins) and the acid-soluble fraction (hemicelluloses) were slightly increased.
Fig. 1.
Thaxtomin A alters 14C incorporation in cell wall fractions. 14C incorporation in 6-d-old, liquid culture-grown, etiolated Arabidopsis seedlings treated with 200 nM thaxtomin A for 2 d (black bars) and control seedlings (white bars) is shown for different cell wall fractions as follows: (i) chloroform fraction, (ii) ammonium-oxalate fraction, (iii) 0.1 M KOH fraction, (iv) 4 M KOH fraction, (v) acetic/nitric acid soluble fraction, and (vi) acid insoluble fraction. 14C incorporation in each fraction is expressed as a percentage of total 14C uptake. Each bar represents the mean ±SE from four biological replicates.
Expression analysis of CW-related genes
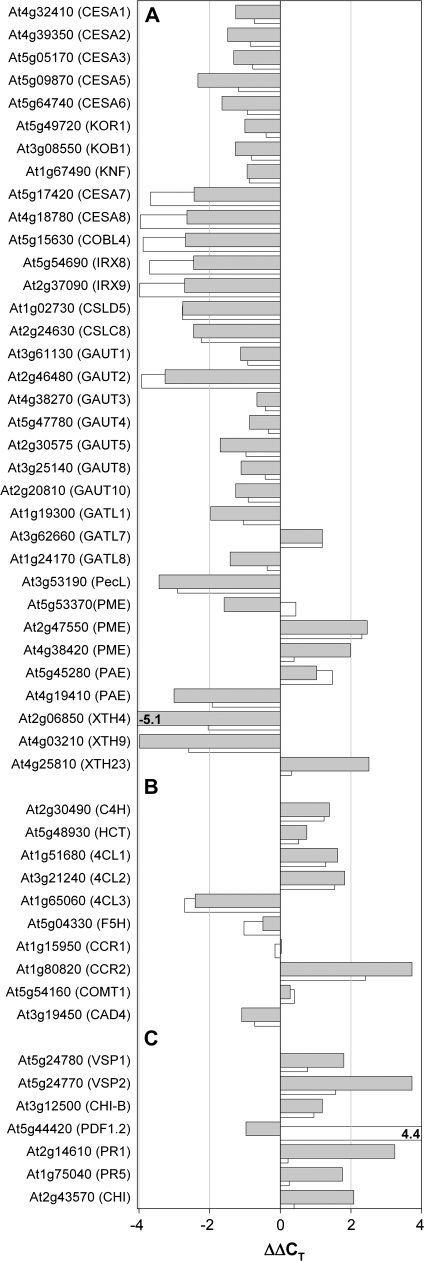
It was intended to investigate whether the observed changes in CW polysaccharide composition induced by thaxtomin A application to A. thaliana seedlings are also accompanied by transcriptional changes. Therefore a number of CW genes were chosen (Fig. 2A; see Supplementary Table S1 at JXB online) that are either involved in cellulose or pectin synthesis or CW remodelling or were previously shown to respond to isoxaben treatment in A. thaliana suspension-cultured cells (Manfield et al., 2004), and their expression levels were determined by quantitative real-time RT-PCR. Thaxtomin A application resulted in a clear repression of 15 genes and the induction of three genes (see Supplementary Table S1 at JXB online; Fig. 2A), whereas 25 CW genes were less or not affected, based on a ΔΔCT value <2 or >–2 (i.e. less than 3–4-fold induction/repression). Thaxtomin A treatment resulted in a consistent but mild repression of genes involved in primary cellulose biosynthesis such as CESA1, CESA2, CESA3, CESA5, CESA6 or KORRIGAN, KOBITO1, and KNOPF (Fig. 2A), which could be due to the fact that elongation/expansion growth of the primary CW was advanced in 6-d-old etiolated seedlings. It is known that KORRIGAN, KOBITO1, and KNOPF affect primary wall deposition during cell elongation/expansion and seed development (Nicol et al., 1998; Pagant et al., 2002; Gillmor et al., 2002). Genes involved in secondary CW synthesis such as CESA7 and CESA8 consistently showed a stronger repression (ΔΔCT value <–2; Fig. 2A; see Supplementary Table S1 at JXB online). Inhibition of secondary wall synthesis is also reflected in repression of COBRA-like 4 (COBL4) (Brown et al., 2005), IRX8 and IRX9 (Fig. 2A). IRX8 (GAUT12) and IRX9 are important in glucuronoxylan- and homogalacturonan biosynthesis during secondary CW formation (Persson et al., 2005, 2007; Brown et al., 2005; Pena et al., 2007). Our set of surveyed genes also contained two CESA-like genes, CslC8 and CslD5. The latter was recently found to be important for xylan synthesis (Bernal et al., 2007), while for the former a biosynthetic role in CW polymer formation is so far only predicted (Richmond and Somerville, 2000) although a close homologue, CslC4, was recently shown to encode a β-1,4 glucan synthase (Cocuron et al., 2007). Both these genes were also found to be strongly repressed in thaxtomin A-treated seedlings (Fig. 2A).
Fig. 2.
Changes in gene expression in thaxtomin A and isoxaben-treated seedlings. (A) Cell wall genes, (B) genes involved in lignin synthesis, and (C) genes involved in defence responses. The expression levels are given expressed as ΔΔCT (i.e. a logarithmic scale), where ΔΔCT is the difference in normalized qRT-PCR threshold cycle number of the respective gene between thaxtomin- or isoxaben-treated seedlings and control seedlings. Negative and positive numbers thus represent repression and induction in thaxtomin-treated seedlings, respectively. Normalization was preformed using the UBQ10 (At4g05320) as a reference. Data for thaxtomin A-treated seedlings are shown as grey bars, data for seedlings treated with isoxaben as slightly displaced white bars at the back. Each value represents the mean of four biological replicas from two independent experiments. Standard errors of CT values are small and not shown.
Transcript levels for a number of genes encoding pectin synthetic/modifying enzymes were also changed after thaxtomin A application. Several members of the family of galacturonosyltransferase genes (Sterling et al., 2006) and especially GAUT2 displayed reduced transcript levels, whereas the transcript for GATL7 was increased (Fig. 2A; see discussion for more information). In our set of examined CW genes, strong repression of a pectate lyase-like gene (At3g53190) and of a pectin acetylesterase (At4g19410) was also found, while the expression of two pectin methylesterases (At2g47550, At4g38420) was increased and one (At5g53370) was slightly decreased (Fig. 2A; see Supplementary Table S1 at JXB online). Strong transcript changes were also observed for three genes from the family of xyloglucan endotransglucosylase/hydrolases (XTH) (Fig. 2A; see Supplementary Table S1 at JXB online), which are presumably involved in wall loosening, resulting in CW extension (Smith and Fry, 1991; Vissenberg et al., 2005). Expression of two XTH genes (XTH4 and XTH19) was repressed in thaxtomin A treated seedlings, whereas XTH23 was induced. Interestingly, the transcript changes shown in Fig. 2A and others, that are only shown in Supplementary Table S1 at JXB online, were also recapitulated in a similar or nearly identical manner in isoxaben-treated seedlings (cf. white bars in Fig. 2A), suggesting that thaxtomin A and isoxaben affect A. thaliana seedlings in the same or a very similar manner and, possibly, also have a similar mode of action at the molecular level.
Thaxtomin A invokes ectopic lignification and induction of defence-response genes
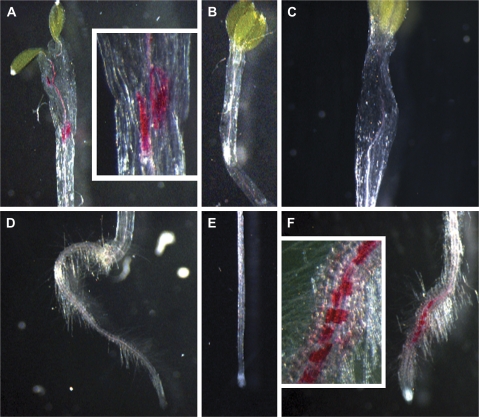
A previous study showed that A. thaliana eli1/cesa3 mutant seedlings and seedlings treated with the cellulose-synthesis inhibitor isoxaben exhibit strong ectopic lignification (Caño-Delgado et al., 2000) and up-regulation of defence response genes. To analyse whether thaxtomin A phenocopies isoxaben in this respect, lignification was investigated in 6-d-old seedlings. In contrast to methanol-treated control seedlings, which displayed no ectopic lignin (Fig. 3B, E), seedlings treated with 200 nM thaxtomin A showed strong lignification in the hypocotyls (Fig. 3A), whereas, unlike isoxaben-treated seedlings (Caño-Delgado et al., 2003; Fig. 3F), they had no ectopic lignin in the root (Fig. 3D). The lignification pattern in light-grown seedlings was similar to the pattern found in etiolated seedlings (data not shown).
Fig. 3.
Thaxtomin A causes ectopic lignification in etiolated Arabidopsis seedlings. Lignification was analysed in 6-d-old, liquid culture-grown, etiolated Arabidopsis seedlings treated with 200 nM thaxtomin A for 2 d (A, D), methanol-treated control seedlings (B, E) or seedlings treated with 5 nM isoxaben (C, F). Typical staining patterns are shown. Insets in (A) and (F) show magnifications of strongly lignified regions.
Ectopic lignification in thaxtomin A-treated seedlings is in agreement with the altered expression of genes involved in the lignin biosynthetic pathway (Raes et al., 2003) as depicted in Fig. 2B. Thaxtomin A treatment resulted in strong up-regulation of the cinnamoyl CoA reductase gene CCR2. Likewise, a cinnamate-4-hydroxylase gene (C4H), two genes encoding isoforms of 4-coumarate:CoA ligase (4CL1, 4CL2), and a hydroxycinnamoyltransferase gene (HCT) were induced, while a third 4-coumarate:CoA ligase gene (4CL3) was repressed. AtCCR2 is known to be expressed during pathogenic attacks and stress and, together with cinnamyl alcohol reductase, leads to monolignol precursors of lignin (Humphreys and Chapple, 2002) via a reduction of phenylpropanoid cinnamoyl CoA-ester intermediates. The expression of other genes like CCR1, COMT, F5H, or CAD4 did not differ much from the one in control seedlings. Very similar results were also obtained with isoxaben-treated A. thaliana plantlets (Fig. 2B; Caño-Delgado et al., 2003).
As ectopic lignification and induction of defence genes are a consequence of perturbations in cellulose synthesis or reduced CW integrity (Ellis et al., 2002; Caño-Delgado et al., 2003), the expression of several candidate genes involved in plant defence responses were also analysed (Fig. 2C; see Supplementary Table S1 at JXB online). In this respect, and in addition to CCR2, thaxtomin A was found to induce significantly the expression of VSP1, VSP2, and CHI-B, three marker genes of the jasmonic acid-dependent defence (Turner et al., 2002), PR1 and PR5, which are markers for the salicylic acid-dependent defence (Turner et al., 2002), and also the chitinase At2g43570 (Pastori et al., 2003). Interestingly PDF1.2 (At5g44420) was unaffected in thaxtomin A-treated seedlings, whereas this ethylene and jasmonate-responsive plant defensin gene is strongly induced in isoxaben-treated etiolated seedlings (Fig. 2C) or isoxaben-treated, light-grown A. thaliana plantlets (Caño-Delgado et al., 2003).
As with lignification and the expression of defence genes, callose deposition in places other than the nascent cell plate or pollen tube cell walls, is regarded as a marker of the plant defence response (Carpita and McCann, 2000). Hence the deposition of callose in thaxtomin A and isoxaben-treated etiolated Arabidopsis seedlings was analysed by aniline blue staining (Stone et al., 1985). Treatment with one or the other inhibitor again led to similar results (see Supplementary Fig. S1 at JXB online). Both inhibitors led to ectopic callose in the elongating root zone just above the root tip, and thaxtomin A also resulted in callose staining in the upper, swollen part of the etiolated hypocotyl, which is reminiscent of the observed lignification patterns (Fig. 3). In addition, thaxtomin A was found to induce callose deposition in cotelydons of light-grown seedlings, which again recapitulates the lignification pattern observed in such growth conditions (results not shown). The fact that thaxtomin A or isoxaben-treated seedlings are able to produce callose also suggests that the inhibitors do not affect the synthesis of UDP-glucose, which is the substrate for the synthesis of cellulose and callose at the PM surface thus suggesting that the inhibitory effect is specific for cellulose.
Thaxtomin A causes depletion of CESA complexes from the PM
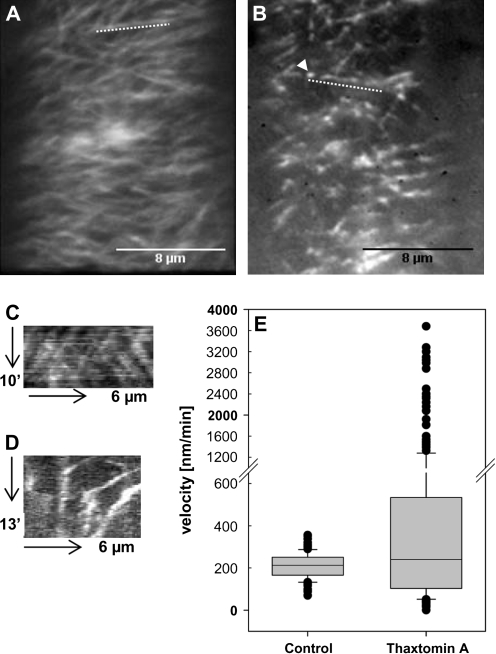
Short-term thaxtomin A treatments (2.5 h) of seedlings expressing GFP-CESA3 (Desprez et al., 2007) were performed to study the effect of the phytotoxin on the density and velocity of PM-localized CESA-Cs and on cortical microtubule organization. Thaxtomin A strongly compromised CESA-C density and -motility (Fig. 4). Thaxtomin A treatment largely decreased the intensity of GFP-CESA3 signals at the PM in cells of the upper hypocotyl (Fig. 4B) as compared to hypocotyls of untreated control seedlings (Fig. 4A). From this it is concluded that thaxtomin A, like isoxaben (Paredez et al., 2006), also clears CESA-Cs from the PM. CESA-Cs then accumulated in a novel intracellular small microtubule-associated compartment termed MASC (Fig. 4B, arrow) (EF Crowell, V Bischoff, T Desprez, A Rolland, JD Stierhof, K Schumacher, M Gonneau, H Höfte, S Vernhettes; unpublished results). It was observed that the particles display erratic movement; particles show steady and fast, sometimes accelerating and in some cases slowing down movement, generally along linear tracks (Fig. 4D; see Video S1 at JXB online). The velocity pattern of the MASCs is different from the normal GFP-CESA3 particles of untreated control seedlings that usually follow linear and bidirectional trajectories with constant velocities (Paredez et al., 2006; Fig. 4C; see Video S2 at JXB online) and had similar velocities to those reported in the literature (Desprez et al., 2007) (207±66 nm min−1; range 69–351 nm min−1) (Fig. 4E). The average velocity of MASC particles in thaxtomin A-treated upper hypocotyl cells was as high as 638±558 nm min−1 (range 16–3679 nm min−1), or sometimes even reached 10,500 nm min−1 (data not shown). Besides the effects on CESA-C density and movement, treatment of seedlings with thaxtomin A did not cause any significant effects on cortical microtubule orientation (see Supplementary Fig. S2 at JXB online).
Fig. 4.
Thaxtomin A affects CESA-C abundance and motility. (A, B) Optical sections of plasma membrane in upper hypocotyl cells of 3-d-old etiolated cesa3je5:GFP-CESA3 seedlings. Images were acquired by spinning disc confocal microscopy. Average projections of 39 and 54 frames acquired in 15 s intervals in the plane of the cell membrane of control (A) and thaxtomin A-treated seedlings (B), respectively are shown. Average projections illustrate the movement of labelled particles along linear tracks. (C) Kymograph of the region marked by the dotted line in (A) displaying steady and bidirectional particle translocation. (D) Kymograph of region marked by the dotted line in (B) displaying steady, slow, accelerated or very fast particle translocation. (E) Box plot diagram of particle velocities calculated from 140 or 300 particles of control or thaxtomin A-treated seedlings, respectively. Particle velocity was measured in six cells from four seedlings and was determined by manual tracking.
Discussion
Thaxtomin A has previously been described as a putative cellulose synthesis inhibitor (King et al., 2001; Fry and Loria, 2002; Scheible et al., 2003). Additional effects of thaxtomin A on A. thaliana seedlings were studied to determine its role in this regard. By analysis of radiolabelled CW fractions it was possible to show that, in particular, the synthesis of pectins, and hemicelluloses (non-cellulosic carbohydrate fractions) was stimulated after thaxtomin A treatment, whereas the synthesis of cellulose was strongly reduced. This study provides more detailed evidence of thaxtomin A's effects on CW composition than has previously been known (Scheible et al., 2003). Based on these results, it is suggested that thaxtomin A-treated plants compensate for their decreased cellulose synthesis by producing other wall polysaccharides. Similar changes have been reported in CW mutants (Burton et al., 2000; Fagard et al, 2000; Zhong et al., 2003) and in plants treated with cellulose biosynthesis inhibitors such as isoxaben (Heim et al., 1990, 1998).
The biochemical changes in CW composition invoked by thaxtomin A were accompanied by changes in CW gene expression. Primary CESA genes and additional primary CW synthesis genes (KORRIGAN and KOBITO1) were repressed. The repression was even more pronounced for genes involved in secondary CW synthesis (CESA7, CESA8, COBRA-like 4, IRX8, and IRX9). From this, it is concluded that, besides its known effects on primary cellulose synthesis (Fagard et al., 2000; Fry and Loria, 2002), thaxtomin A also affects secondary CW synthesis, possibly due to the inability of thaxtomin A-treated seedlings to properly initiate this developmental program when primary wall deposition is altered or incomplete. This interpretation is also mirrored in the strongly reduced expression in thaxtomin A-treated seedlings of a gene named CAD9 (cf. Table S1 at JXB online), which, according to Kim et al. (2007), encodes a dehydrogenase of unknown function with predominant expression in xylem-forming vascular tissue.
Thaxtomin A also led to changes in the expression of additional CW-related genes. Some putative galacturonosyltransferases genes, proposed to be necessary for pectin synthesis (Sterling et al., 2006), were slightly repressed, and one was more strongly repressed. This seems contradictory to the increase in radiolabelled pectins, but might be explained by the fact that only a few genes suspected of being involved in pectin synthesis have been proved to be, for example Qua1 (Bouton et al., 2002) or Qua2 (Mouille et al., 2007), and others were not investigated in this study. Also transcriptional regulation of pectin synthetic/modifying genes might be overcompensated for by post-transcriptional/post-translational levels of control. The transcriptional regulation of these genes could be more important during development when co-expression of large sets of CW genes is required for proper and largely simultaneous synthesis of CW polymers.
De-esterification of pectins is a prerequisite for the formation of extensive Ca2+-bridged junction zones (Powell et al., 1982; Brett and Waldron, 1990) and of cross-links between free carboxyl groups, for example, between pectins and hemicelluloses or pectins and lignin (Carpita and McCann, 2000). A pectin acetylesterase and two pectin methylesterases were found to be up-regulated by thaxtomin A treatment. Together with the observed strong decrease of a pectate lyase transcript this could signify a higher degree of crosslinking of the pectic network and hence result in the strengthening of the CW as a compensatory response to inhibition of cellulose synthesis by thaxtomin A. This interpretation is supported by previous data on compensation of the loss of cellulose by an increased amount of pectin with a lower degree of esterification (Burton et al., 2000).
Thaxtomin A treatment also resulted in the strong repression of two xyloglucan endotransglucosylase/hydrolases (XTH), and the weaker induction of a XTH-related gene. The role of individual XTHs is often unknown. Some XTHs have been proposed to catalyse a type of wall-loosening that leads to CW extension both by cutting and restructuring the existing wall-bound xyloglucans (Rose et al., 2002). Repression of such XTH genes might thus be a strategy to prevent CW loosening when cellulose synthesis is inhibited. Some XTHs also function in the incorporation of newly secreted xyloglucan chains into the CW (Smith and Fry, 1991; Vissenberg et al., 2005), and the induction of genes encoding these XTHs might strengthen the CW.
While the observed changes in CW gene expression are (i) not always easily understood due to the lack of knowledge of the precise biological functions of the encoded proteins and (ii) not necessarily transferable to the observed changes in CW composition (Fig. 1), the qRT-PCR results support the general idea of extensive CW remodelling to compensate for reduced cellulose in thaxtomin A-treated seedlings. More importantly, the comparison of thaxtomin A and isoxaben clearly reveals that both agents lead to identical qualitative and very similar quantitative effects on CW gene expression, making a strong case for thaxtomin A acting as a cellulose synthesis inhibitor in a manner comparable to isoxaben, namely the inhibition of cellulose synthase per se (Scheible et al., 2001; Desprez et al., 2002).
A similar conclusion is reached by the study of ectopic lignification and the analysis of genes involved in lignin biosynthesis. Our data reveal that, when applied at low inhibiting concentrations, thaxtomin A, like isoxaben (Caño-Delgado et al., 2003), invokes ectopic lignification. However, thaxtomin A results in lignification exclusively in the hypocotyl, whereas isoxaben treatment results in lignification in the roots of etiolated A. thaliana seedlings (Fig. 3; Desprez et al., 2002). This suggests that thaxtomin A either affects different tissues and/or molecular targets than isoxaben, or that the uptake of the two agents happens in different tissues. Thaxtomin A-induced lignification might represent another avenue that plant cells can choose to stabilize the compromised CW. Lignin molecules can form entire new CW networks or can be bound to de-esterified carboxyl groups of pectins (see above). The analysis of genes involved in lignin biosynthesis revealed that CCR2 was highly up-regulated. CCR (cinnamoyl-CoA reductase) proteins play a major role in all proposed lignin pathways (Boerjan et al., 2003; Fan et al., 2006) as they convert CoA intermediates into their aldehydes. In particular, CCR2 is active during lignification in response to pathogenic attacks (Lauvergeat et al., 2001) and also seems to have a key role in thaxtomin A- and isoxaben-induced ectopic lignification. Up-regulation of 4CL1 and 4CL2 which encode the isoforms involved in lignin formation (Ehlting et al., 1999) and C4H further support the induction of lignin biosynthesis at the molecular level. Repression of 4CL3 is irrelevant in this regard as it participates in the biosynthetic pathway leading to flavonoids.
Thaxtomin A and isoxaben-treatments also resulted in the strong up-regulation of several well-known defence genes (Caño-Delgado et al., 2003). However, the overlap between the two agents was not nearly as good as for the CW- or lignin biosynthesis genes (Fig. 2C). It seems likely that induction of defence genes by both agents is the consequence of perturbed cellulose synthesis, but that the spectrum of genes and their quantitative response is related to the place of action (root versus hypocotyl; cf. Fig. 3). It also cannot be ruled out that parts of the defence response represent a more direct response of plants to thaxtomin A in a function similar to that of a bacterial elicitor.
Cellulose synthesis inhibitors like isoxaben and DCB also cause altered CESA accumulation patterns and affect cortical microtubule arrays (DeBolt et al., 2007; Paredez et al., 2006, 2008; Lazzaro et al., 2003). Isoxaben treatments cause loosening of the radial microtubule array in pollen tubes (Lazzaro et al., 2003), a shift of cortical microtubule alignment from transverse to oblique (Paredez et al., 2008) and a release of CESA-C from the PM. Seedlings treated with DCB revealed a reduced CESA-C motility and an accumulation of CESA-C at the PM (DeBolt et al., 2007). No drastic DCB effects on microtubule organization have been demonstrated so far. This study revealed that thaxtomin A, like isoxaben, cleared the CESA3 proteins off the PM. The loss of CESA3 from the PM was accompanied by an accumulation of CESA3-containing particles, which were identified as a novel microtubule-dependent intracellular compartment described previously (EF Crowell, V Bischoff, T Desprez, A Rolland, JD Stierhof, K Schumacher, M Gonneau, H Höfte, S Vernhettes; unpublished results). These particles, named MASC, accumulate CESA proteins and are of a much higher velocity than regular GFP-CESA3 proteins but their function remains unclear. Because of the lack of significant effects on the cortical microtubule array, we propose that thaxtomin A directly affects the CESA-C stability and this reduces CESA-C density in the PM. Similar effects have been found following treatments with other CW inhibitors (isoxaben, DCB, CGA615′325 (Paredez et al., 2006; DeBolt et al., 2007; EF Crowell, V Bischoff, T Desprez, A Rolland, JD Stierhof, K Schumacher, M Gonneau, H Höfte, S Vernhettes; unpublished results), but direct molecular targets have only been proposed for isoxaben (Scheible et al., 2001, Desprez et al., 2002). It seems unlikely that thaxtomin A targets a cellular component that interacts with both CESAs and the cortical microtubule array as proposed for morlin (DeBolt et al., 2007). We propose that thaxtomin A's effect on CESA-C density in the PM directly contributes to the reported reduction in crystalline cellulose biosynthesis (Scheible et al., 2003).
In conclusion, it is suggested that the loss of crystalline cellulose following thaxtomin A treatment is directly linked to the release of CESA-C from the PM. As seen from the transcript profiling data and the [14C]-sucrose incorporation assay, thaxtomin A is very comparable to isoxaben and leads to major changes in CW composition towards the production of pectins and hemicelluloses, and results in additional CW reinforcement by triggering ectopic lignification. In summary, this work provides new and, so far, the most advanced insights into the mode of action of the phytotoxin thaxtomin A, and its cellular and molecular effects on CW biosynthesis.
Supplementary data
The following supplementary data are available at JXB online.
Table S1. Quantitative real-time RT-PCR results and primer sequences.
Fig. S1. Aniline blue staining of Arabidopsis seedlings treated with thaxtomin A or isoxaben.
Fig. S2. Effect of thaxtomin A on GFP-TUA6-labelled cortical microtubules.
Video S1. Distribution and motility of MASC in the cell membrane.
Video S2. Distribution and motility of GFP-CESA3 in the cell membrane.
Supplementary Material
Acknowledgments
We are grateful to Samantha Vernhettes, Elizabeth Crowell (INRA, Versailles) and Christophe Machu (Institut Pasteur, Paris) for assistance with spinning disc microscopy, Dana Schindelasch and Eugenia Maximova (Max-Planck-Institute of Molecular Plant Physiology, MPI-MPP) for assistance with fluorescence microscopy, and Thierry Desprez (INRA, Versailles) and Jordi Chan (John Innes Centre, Norwich, UK) for seeds of the cesa3::GFP-CESA3 and GFP-TUA6 lines, respectively. We also thank Samantha Vernhettes and Staffan Persson (MPI-MPP) for proofreading. The work was supported by a grant to W-RS from the German Science Foundation (DFG, SCHE 548/3), and by the Max-Planck Society.
Glossary
Abbreviations
- 4CL
4-coumarate:CoA ligase
- C4H
cinnamate-4-hydroxylase
- CAD
cinnamyl alcohol reductase
- CCR
cinnamoyl CoA reductase
- CESA
cellulose synthase
- CESA-C
cellulose synthase complex
- DCB
dichlobenil
- FT-IR
Fourier-transform infrared
- GFP
green fluorescent protein
- HG
homogalacturonan
- MASC
microtubule-associated cellulose synthase compartment
- PM
plasma membrane
- XTH
xyloglucan endotransglucosylase/hydrolases
References
- Bernal AJ, Jensen JK, Harholt J, et al. Disruption of ATCSLD5 results in reduced growth, reduced xylan and homogalacturonan synthase activity and altered xylan occurrence in Arabidopsis. The Plant Journal. 2007;52:791–802. doi: 10.1111/j.1365-313X.2007.03281.x. [DOI] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annual Review of Plant Biology. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- Bouton S, Leboeuf E, Mouille G, Leydecker MT, Talbotec J, Granier F, Lahaye M, Hofte H, Truong HN. Quasimodo1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis. The Plant Cell. 2002;14:2577–2590. doi: 10.1105/tpc.004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J. If walls could talk. Current Opinion in Plant Biology. 1999;2:521–524. doi: 10.1016/s1369-5266(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Brett C, Waldron K. Physiology and biochemistry of plant cell walls. In: Black M, Chapman J, editors. Topics in plant physiology. London: Unwin Hyman; 1990. pp. 6–57. [Google Scholar]
- Brown DM, Zeef LAH, Ellis J, Goodacre R, Turner SR. Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. The Plant Cell. 2005;17:2281–2295. doi: 10.1105/tpc.105.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Gibeaut DM, Bacic A, Findlay K, Roberts K, Hamilton A, Baulcombe DC, Fincher GB. Virus-induced silencing of a plant cellulose synthase gene. The Plant Cell. 2000;12:691–705. doi: 10.1105/tpc.12.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caño-Delgado A, Penfield S, Smith C, Catley M, Bevan M. Reduced cellulose synthesis invokes lignification and defence responses in Arabidopsis thaliana. The Plant Journal. 2003;34:351–362. doi: 10.1046/j.1365-313x.2003.01729.x. [DOI] [PubMed] [Google Scholar]
- Caño-Delgado AI, Metzlaff K, Bevan MW. The eli1 mutation reveals a link between cell expansion and secondary cell wall formation in Arabidopsis thaliana. Development. 2000;127:3395–3405. doi: 10.1242/dev.127.15.3395. [DOI] [PubMed] [Google Scholar]
- Carpita NC, McCann M. The cell wall. In: Buchanan B, Gruissem R, Jones R, editors. Biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Physiologists; 2000. [Google Scholar]
- Chan J, Calder G, Fox S, Lloyd C. Localization of the microtubule end binding protein EB1 reveals alternative pathways of spindle development in Arabidopsis suspension cells. The Plant Cell. 2005;17:1737–1748. doi: 10.1105/tpc.105.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocuron JC, Lerouxel O, Drakakaki G, Alonso AP, Liepman AH, Keegstra K, Raikhel N, Wilkerson CG. A gene from the cellulose synthase-like C family encodes a beta-1,4 glucan synthase. Proceedings of the National Academy of Sciences, USA. 2007;104:8550–8555. doi: 10.1073/pnas.0703133104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBolt S, Gutierrez R, Ehrhardt DW, Melo CV, Ross L, Cutler SR, Somerville C, Bonetta D. Morlin, an inhibitor of cortical microtubule dynamics and cellulose synthase movement. Proceedings of the National Academy of Sciences, USA. 2007;104:5854–5859. doi: 10.1073/pnas.0700789104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer DP, Amor Y. Cellulose biosynthesis. The Plant Cell. 1995;7:987–1000. doi: 10.1105/tpc.7.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez T, Juraniec M, Crowell EF, Jouy H, Pochylova Z, Parcy F, Hofte H, Gonneau M, Vernhettes S. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 2007;104:15572–15577. doi: 10.1073/pnas.0706569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez T, Vernhettes S, Fagard M, Refregier G, Desnos T, Aletti E, Py N, Pelletier S, Hofte H. Resistance against herbicide isoxaben and cellulose deficiency caused by distinct mutations in same cellulose synthase isoform CESA6. Plant Physiology. 2002;128:482–490. doi: 10.1104/pp.010822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval I, Brochu V, Simard M, Beaulieu C, Beaudoin N. Thaxtomin A induces programmed cell death in Arabidopsis thaliana suspension-cultured cells. Planta. 2005;222:820–831. doi: 10.1007/s00425-005-0016-z. [DOI] [PubMed] [Google Scholar]
- Ebringerova A, Heinze T. Naturally occurring xylans: structures, isolation procedures and properties. Macromolecular Rapid Communications. 2000;21:542–556. [Google Scholar]
- Ehlting J, Büttner D, Wang Q, Douglas CJ, Somssich IE, Kombrink E. Three 4-coumarate:coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms. The Plant Journal. 1999;19:9–20. doi: 10.1046/j.1365-313x.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis I, Wasternack C, Turner JG. The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. The Plant Cell. 2002;14:1557–1566. doi: 10.1105/tpc.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard M, Desnos T, Desprez T, Goubet F, Refregier G, Mouille G, McCann M, Rayon C, Vernhettes S, Hofte H. PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. The Plant Cell. 2000;12:2409–2423. doi: 10.1105/tpc.12.12.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Linker R, Gepstein S, Tanimoto E, Yamamoto R, Neumann PM. Progressive inhibition by water deficit of cell wall extensibility and growth along the elongation zone of maize roots is related to increased lignin metabolism and progressive stelar accumulation of wall phenolics. Plant Physiology. 2006;140:603–612. doi: 10.1104/pp.105.073130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry BA, Loria R. Thaxtomin A: evidence for a plant cell wall target. Physiological and Molecular Plant Pathology. 2002;60:1–8. [Google Scholar]
- Gillmor CS, Poindexter P, Lorieau J, Palcic MM, Somerville C. Alpha-glucosidase I is required for cellulose biosynthesis and morphogenesis in Arabidopsis. Journal of Cell Biology. 2002;156:1003–1013. doi: 10.1083/jcb.200111093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim DR, Larrinua IM, Murdoch MG, Roberts JL. Triazofenamide is a cellulose biosynthesis inhibitor. Pesticide Biochemistry and Physiology. 1998;59:163–168. [Google Scholar]
- Heim DR, Skomp JR, Tschabold EE, Larrinua IM. Isoxaben inhibits the synthesis of acid insoluble cell-wall materials in Arabidopsis thaliana. Plant Physiology. 1990;93:695–700. doi: 10.1104/pp.93.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Blanco C, Feng DX, Hu J, et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. The Plant Cell. 2007;19:890–903. doi: 10.1105/tpc.106.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogetsu T, Shibaoka H, Shimokor M. Involvement of cellulose synthesis in actions of gibberellin and kinetin on cell expansion: 2,6-dichlorobenzonitrile as a new cellulose synthesis inhibitor. Plant and Cell Physiology. 1974;15:389–393. [Google Scholar]
- Humphreys JM, Chapple C. Rewriting the lignin roadmap. Current Opinion in Plant Biology. 2002;5:224–229. doi: 10.1016/s1369-5266(02)00257-1. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim KW, Cho MH, Franceschi VR, Davin LB, Lewis NG. Expression of cinnamyl alcohol dehydrogenases and their putative homologues during Arabidopsis thaliana growth and development: lessons for database annotations? Phytochemistry. 2007;68:1957–1974. doi: 10.1016/j.phytochem.2007.02.032. [DOI] [PubMed] [Google Scholar]
- King RR, Lawrence CH. Characterization of new thaxtomin A analogues generated in vitro by Streptomyces scabies. Journal of Agricultural and Food Chemistry. 1996;44:1108–1110. [Google Scholar]
- King RR, Lawrence CH, Gray JA. Herbicidal properties of the thaxtomin group of phytotoxins. Journal of Agricultural and Food Chemistry. 2001;49:2298–2301. doi: 10.1021/jf0012998. [DOI] [PubMed] [Google Scholar]
- Lauvergeat V, Lacomme C, Lacombe E, Lasserre E, Roby D, Grima-Pettenati J. Two cinnamoyl-CoA reductase (CCR) genes from Arabidopsis thaliana are differentially expressed during development and in response to infection with pathogenic bacteria. Phytochemistry. 2001;57:1187–1195. doi: 10.1016/s0031-9422(01)00053-x. [DOI] [PubMed] [Google Scholar]
- Lazzaro MD, Donohue JM, Soodavar FM. Disruption of cellulose synthesis by isoxaben causes tip swelling and disorganizes cortical microtubules in elongating conifer pollen tubes. Protoplasma. 2003;220:201–207. doi: 10.1007/s00709-002-0042-7. [DOI] [PubMed] [Google Scholar]
- Manfield IW, Orfila C, McCartney L, Harholt J, Bernal AJ, Scheller HV, Gilmartin PM, Mikkelsen JD, Knox JP, Willats WGT. Novel cell wall architecture of isoxaben-habituated Arabidopsis suspension-cultured cells: global transcript profiling and cellular analysis. The Plant Journal. 2004;40:260–275. doi: 10.1111/j.1365-313X.2004.02208.x. [DOI] [PubMed] [Google Scholar]
- Mouille G, Ralet MC, Cavelier C, et al. Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. The Plant Journal. 2007;50:605–614. doi: 10.1111/j.1365-313X.2007.03086.x. [DOI] [PubMed] [Google Scholar]
- Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Hofte H. A plasma membrane-bound putative endo-1,4-β-D-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO Journal. 1998;17:5563–5576. doi: 10.1093/emboj/17.19.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagant S, Bichet A, Sugimoto K, Lerouxel O, Desprez T, McCann M, Lerouge P, Vernhettes S, Hofte H. KOBITO1 encodes a novel plasma membrane protein necessary for normal synthesis of cellulose during cell expansion in Arabidopsis. The Plant Cell. 2002;14:2001–2013. doi: 10.1105/tpc.002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez AR, Persson S, Ehrhardt DW, Somerville CR. Genetic evidence that cellulose synthase activity influences microtubule cortical array organization. Plant Physiology. 2008;147:1723–1734. doi: 10.1104/pp.108.120196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez AR, Somerville CR, Ehrhardt DW. Visualization of cellulose synthase demonstrates functional association with microtubules. Science. 2006;312:1491–1495. doi: 10.1126/science.1126551. [DOI] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH. Leaf vitamin C contents modulate plant defence transcripts and regulate genes that control development through hormone signaling. The Plant Cell. 2003;15:939–951. doi: 10.1105/tpc.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena MJ, Zhong RQ, Zhou GK, Richardson EA, O'Neill MA, Darvill AG, York WS, Ye ZH. Arabidopsis irregular xylem8 and irregular xylem9: Implications for the complexity of glucuronoxylan biosynthesis. The Plant Cell. 2007;19:549–563. doi: 10.1105/tpc.106.049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng LC, Hocart CH, Redmond JW, Williamson RE. Fractionation of carbohydrates in Arabidopsis root cell walls shows that three radial swelling loci are specifically involved in cellulose production. Planta. 2000;211:406–414. doi: 10.1007/s004250000301. [DOI] [PubMed] [Google Scholar]
- Persson S, Caffall KH, Freshour G, Hilley MT, Bauer S, Poindexter P, Hahn MG, Mohnen D, Somerville C. The Arabidopsis irregular xylem8 mutant is deficient in glucuronoxylan and homogalacturonan, which are essential for secondary cell wall integrity. The Plant Cell. 2007;19:237–255. doi: 10.1105/tpc.106.047720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, Wei HR, Milne J, Page GP, Somerville CR. Identification of genes required for cellulose synthesis by regression analysis of public microarray data sets. Proceedings of the National Academy of Sciences, USA. 2005;102:8633–8638. doi: 10.1073/pnas.0503392102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DA, Morris ER, Gidley MJ, Rees DA. Conformations and interactions of pectins. II. Influence of residue sequence on chain association in calcium pectate gels. Journal of Molecular Biology. 1982;155:517–531. doi: 10.1016/0022-2836(82)90485-5. [DOI] [PubMed] [Google Scholar]
- Raes J, Rohde A, Christensen JH, Van de Peer Y, Boerjan W. Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiology. 2003;133:1051–1071. doi: 10.1104/pp.103.026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond TA, Somerville CR. The cellulose synthase superfamily. Plant Physiology. 2000;124:495–498. doi: 10.1104/pp.124.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Mouille G, Höfte H. The mechanism and regulation of cellulose synthesis in primary walls: lessons from cellulose-deficient Arabidopsis mutants. Cellulose. 2004;11:351–364. [Google Scholar]
- Rose JKC, Braam J, Fry SC, Nishitani K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant and Cell Physiology. 2002;43:1421–1435. doi: 10.1093/pcp/pcf171. [DOI] [PubMed] [Google Scholar]
- Scheible WR, Eshed R, Richmond T, Delmer D, Somerville C. Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis ixr1 mutants. Proceedings of the National Academy of Sciences, USA. 2001;98:10079–10084. doi: 10.1073/pnas.191361598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Fry B, Kochevenko A, Schindelasch D, Zimmerli L, Somerville S, Loria R, Somerville CR. An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. The Plant Cell. 2003;15:1781–1794. doi: 10.1105/tpc.013342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiology. 2004;136:2483–2499. doi: 10.1104/pp.104.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedletzky E, Shmuel M, Trainin T, Kalman S, Delmer D. Cell wall structure in cells adapted to growth on the cellulose-synthesis inhibitor 2,6-dichlorobenzonitrile: a comparison between two dicotyledonous plants and a graminaceous monocot. Plant Physiology. 1992;100:120–130. doi: 10.1104/pp.100.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Fry SC. Endotransglycosylation of xyloglucans in plant cell suspension cultures. Biochemical Journal. 1991;279:529–535. doi: 10.1042/bj2790529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling JD, Atmodjo MA, Inwood SE, Kolli VSK, Quigley HF, Hahn MG, Mohnen D. Functional identification of an Arabidopsis pectin biosynthetic homogalacturonan galacturonosyltransferase. Proceedings of the National Academy of Sciences, USA. 2006;103:5236–5241. doi: 10.1073/pnas.0600120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BA, Evans NA, Bonig I, Clarke AE. The application of Sirofluor, a chemically defined fluorochrome from aniline blue for the histochemical detection of callose. Protoplasma. 1985;122:191–195. [Google Scholar]
- Tegg RS, Melian L, Wilson CR, Shabala S. Plant cell growth and ion flux responses to the streptomycete phytotoxin thaxtomin A: calcium and hydrogen flux patterns revealed by the non-invasive MIFE technique. Plant and Cell Physiology. 2005;46:638–648. doi: 10.1093/pcp/pci069. [DOI] [PubMed] [Google Scholar]
- Turner JG, Ellis G, Devoto A. The jasmonate signal pathway. The Plant Cell. 2002;14:S153–S164. doi: 10.1105/tpc.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SR, Taylor N, Jones L. Mutations of the secondary cell wall. Plant Molecular Biology. 2001;47:209–219. [PubMed] [Google Scholar]
- Udvardi MK, Czechowski T, Scheible WR. Eleven golden rules of quantitative RT-PCR. The Plant Cell. 2008;20:1736–1737. doi: 10.1105/tpc.108.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Matsuyama T, Hashimoto T. Visualization of microtubules in living cells of transgenic Arabidopsis thaliana. Protoplasma. 1999;206:201–206. [Google Scholar]
- Vissenberg K, Fry SC, Pauly M, Höfte H, Verbelen JP. XTH acts at the microfibril-matrix interface during cell elongation. Journal of Experimental Botany. 2005;56:673–683. doi: 10.1093/jxb/eri048. [DOI] [PubMed] [Google Scholar]
- Vorwerk S, Somerville S, Somerville C. The role of plant cell wall polysaccharide composition in disease resistance. Trends in Plant Science. 2004;9:203–209. doi: 10.1016/j.tplants.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Zhong RQ, Morrison WH, Freshour GD, Hahn MG, Ye ZH. Expression of a mutant form of cellulose synthase AtCesA7 causes dominant negative effect on cellulose biosynthesis. Plant Physiology. 2003;132:786–795. doi: 10.1104/pp.102.019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.