Abstract
Purpose
Genetic factors are important in the etiology and pathogenesis of chronic lymphocytic leukemia (CLL), Hodgkin's lymphoma (HL), and non-Hodgkin's lymphoma (NHL). Only a few small studies have assessed clinical characteristics and prognosis for familial patients, with inconsistent findings.
Methods
Using population-based registries from Sweden and Denmark, 7,749 patients with CLL, 7,476 patients with HL, and 25,801 patients with NHL with linkable first-degree relatives were identified. Kaplan-Meier curves were constructed to compare survival in patients with lymphoma with and without a family history of lymphoma. The risk of dying was assessed using adjusted Cox proportional hazard models.
Results
We found 85 patients with CLL (1.10%), 95 patients with HL (1.28%), and 206 patients with NHL (0.80%) with a family history of any lymphoma. Five-year mortality was similar for patients with CLL (hazard ratio [HR], 1.28; 95% CI, 0.95 to 1.72), HL (HR, 0.78; 95% CI, 0.49 to 1.25), and NHL (HR, 0.91; 95% CI, 0.74 to 1.12) versus without a family history of any lymphoma. Mortality was also similar for patients with versus without a family history of the same lymphoma. T-cell/anaplastic lymphoma patients with a family history of NHL had poorer outcome 5-years after diagnosis (HR, 5.38; 95% CI, 1.65 to 17.52). Results were similar for 10 years of follow-up.
Conclusion
With the exception of T-cell/anaplastic lymphoma, survival patterns for patients with CLL, HL, and NHL with a family history of lymphoma were similar to those for sporadic patients, suggesting that most familial lymphomas do not have an altered clinical course. Our findings provide no evidence to modify therapeutic strategies for patients with CLL, HL, or NHL based solely on family history.
INTRODUCTION
A role for genetic factors in chronic lymphocytic leukemia (CLL) is unequivocal based on evidence from multiply affected families, case series, and twin, case-control, and population-based registry studies.1-11 Similar findings have been reported for Hodgkin's lymphoma (HL)12-23 and non-Hodgkin's lymphoma (NHL).1,9,20,22,24-29 Population-based studies have found CLL, HL, and NHL to co-occur in families,1,3,5,9,20,21,23-31 suggesting that shared genetic pathways may contribute to risk for multiple lymphoproliferative malignancies.32,33 Recent studies have identified polymorphisms associated with increased susceptibility to various types of lymphomas including CLL,34,35 HL,36 and NHL,32,37-39 further highlighting the importance of genetic factors in the etiology and pathogenesis of lymphomas.
Emerging evidence suggests that genetic factors might play a role in response to cancer therapy, drug toxicity, and (directly or indirectly) with potential impact on prognosis.40-47 To our knowledge, only a few small studies have assessed outcome in patients with familial lymphoma. In an observational study comparing the prognosis of patients with lymphoma, leukemia, or myeloma from 55 multiple-case and 109 single-case families, patients from multiple-case families had an 8.3% poorer survival.48 In contrast, a recent study based on 1,449 patients with CLL diagnosed, and observed, at a single center in Italy, found that a family history of hematological malignancy was not associated with survival.49
Previously, we have used large population-based databases in Sweden and Denmark to investigate the risks of lymphoproliferative malignancies in first-degree relatives of patients with CLL, HL, NHL, and multiple myeloma.5,21,27,50 As presented in Table 1, CLL, HL, and NHL were found to co-occur in families. However, although first-degree relatives of patients with multiple myeloma had a 1.7-fold increased risk of multiple myeloma, they were not at increased risk of CLL, HL, or NHL.50 In the current investigation, we used the same databases, including more than 41,000 patients with lymphoma in Sweden and Denmark, to assess clinical characteristics and prognosis in patients with and without a family history of lymphoma.
Table 1.
Risks of Lymphoproliferative Malignancies Among First-Degree Relatives of Patients With Chronic Lymphocytic Leukemia, Hodgkin's Lymphoma, Non-Hodgkin's Lymphoma, and Multiple Myeloma
| Patient's Condition | Reference | First-Degree Relative's Risk
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Chronic Lymphocytic Leukemia
|
Hodgkin's Lymphoma
|
Non-Hodgkin's Lymphoma
|
Multiple Myeloma
|
||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Chronic lymphocytic leukemia | Goldin et al5 | 7.5 | 3.6 to 15.6 | 2.4 | 1.1 to 5.1 | 1.5 | 1.0 to 2.2 | 1.1 | 0.5 to 2.4 |
| Hodgkin's lymphoma | Goldin et al21 | 2.1 | 1.2 to 3.8 | 3.1 | 1.8 to 5.3 | 1.3 | 0.9 to 1.8 | 1.0 | 0.6 to 1.8 |
| Non-Hodgkin's lymphoma | Goldin et al27 | 1.3 | 0.9 to 1.9 | 1.4 | 1.0 to 2.0 | 1.7 | 1.4 to 2.2 | 1.1 | 0.8 to 1.5 |
| Multiple myeloma | Landgren et al50 | 0.9 | 0.4 to 1.9 | 0.7 | 0.4 to 1.5 | 1.2 | 0.8 to 1.7 | 1.7 | 1.0 to 2.7 |
NOTE. Italic font indicates not significant. Bold font indicates increased risk P < .05. Table constructed using odds ratios and 95% CIs for the risk of lymphoproliferative malignancy in relatives of patients diagnosed in Sweden and Denmark with chronic lymphocytic leukemia, Hodgkin's lymphoma, non-Hodgkin's lymphoma, and multiple myeloma as reported in the respective publications.
METHODS
Swedish Family Cancer Database
The Swedish Family Cancer Database has been described in detail elsewhere.51 Briefly, all records on children born in Sweden after 1931, along with records of their registered parents at the time of birth, are maintained in the Multigenerational Register by Statistics Sweden.52 This register was linked to the Swedish Cancer Register, which contains details on all cancers diagnosed in Sweden between 1958 and 1998,53 to produce the Swedish Family Cancer Database. Demographic and vital statistic information was obtained by matching the register to the nationwide census and the death notification database, respectively. The database does not include anyone who died before 1960, and approximately half of the offspring who died before 1991 do not have linked parents and are thus excluded.
Danish Database
A similar database constructed using the Danish cancer registry and Danish Central Population Registry has been described previously.21 Briefly, a database of all patients with lymphoma was created using data from the Danish cancer registry. Patients diagnosed from April 1, 1968, to the end of 1997 in the Danish cancer registry,54 were matched to the Danish Central Population Registry that has linkages to parents for all children born since 1968 and linkages to parents for most children age 15 years or younger in 1968.54
Study Design
A cohort of patients diagnosed with lymphoma was assembled by selecting individuals with a primary diagnosis of CLL, HL, or NHL from the Swedish and Danish Cancer registries. Patients were excluded if they had more than one other primary cancer diagnosis, at or before, the first diagnosis of lymphoma. Family history was defined as any diagnosis of CLL, HL, or NHL in a parent, sibling, or offspring that occurred before the diagnosis date of the patient. Subtype information was available for 7,271 (28.2%) of the 25,801 eligible patients with NHL. Of these, 2,297 had high-grade B-cell (mainly diffuse large B-cell lymphoma), 4,359 had low-grade B-cell (mainly follicular lymphoma), and 615 had T-cell/anaplastic NHL. Follow-up started at the diagnosis date of the first lymphoma and ended at either death or censoring, whichever occurred first. Censoring events were emigration, the end of follow-up (June 1, 2001) and 5 years or 10 years postdiagnosis.
An exemption from institutional review board review was obtained from the National Institutes of Health Office of Human Subjects Research, because existing data without personal identifiers was used. Informed consent was waived, because no contact was made with study subjects.
Statistical Methods
Fisher's exact test was used to compare characteristics for CLL, HL, and NHL patients with and without a family history of any lymphoma, including sex, age group, year of diagnosis, and registry.
Survival curves for those with and without a family history of any lymphoma were estimated for CLL, HL, and NHL using the Kaplan-Meier method (MatlabR2006b, The MathWorks Inc, 1994 to 2007) and are presented stratified by age (cut-offs for CLL, HL, and NHL were 67, 45, and 62 years, respectively).
Cox proportional hazard models (PROC PHREG, SAS version 9.1; SAS Institute, Cary, NC) were used to compare 5-year and 10-year mortality in patients with and without a family history of any lymphoma. Adjusted hazard ratios (HR) and 95% CIs were estimated. Adjustment variables included in the models were sex (reference group: females), year of diagnosis in decades (reference group: earliest decade), registry (Sweden, Denmark), and age at diagnosis in quintiles for NHL and HL and median age for CLL (reference group: youngest age). The proportional hazard assumption was upheld for all covariates except for family history of lymphoma for CLL in the 10-year analyses. Therefore, an interaction term between time of follow-up and family history of lymphoma was included in the 10-year model for CLL. To ensure the stability of the model, age was categorized to below and equal to or above the median.
Cox proportional hazard models were stratified by the covariates to assess interactions. There was a significant interaction between sex and registry for NHL; this was included in the models. In a sensitivity analysis, we fitted the proportional hazard models using age as the time scale adjusting for time since diagnosis. In a separate analysis for those with a family history of lymphoma, we sought to determine if age at diagnosis, sex, and/or year of diagnosis were associated with mortality.
RESULTS
Characteristics of all CLL, HL, and NHL patients (all, high-grade B-cell, low-grade B-cell, and T-cell/anaplastic lymphoma subtypes) are presented in Table 2.
Table 2.
Characteristics of Lymphoma Patients
| Characteristic | CLL
|
HL
|
NHL
|
NHL Patients With Available Data on Histopathologic Subtype*
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High-Grade B-Cell NHL
|
Low-Grade B-Cell NHL
|
T-Cell/Anaplastic NHL
|
||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. of patients | 7,749 | 7,476 | 25,801 | 2,297 | 4,359 | 615 | ||||||
| Sex | ||||||||||||
| Male | 5,134 | 66.3 | 4,515 | 60.4 | 14,988 | 58.1 | 1,333 | 58.0 | 2,365 | 54.3 | 392 | 63.7 |
| Female | 2,615 | 33.7 | 2,961 | 39.6 | 10,813 | 41.9 | 964 | 42.0 | 1,994 | 45.7 | 223 | 36.3 |
| Age group† | ||||||||||||
| Younger patients | 3,764 | 48.6 | 4,419 | 59.1 | 12,550 | 48.6 | 1,198 | 52.2 | 2,278 | 52.3 | 302 | 49.1 |
| Older patients | 3,985 | 51.4 | 3,057 | 40.9 | 13,251 | 51.4 | 1,099 | 47.9 | 2,081 | 47.7 | 313 | 50.9 |
| Diagnosis year | ||||||||||||
| 1958-1967 | 554 | 7.1 | 852 | 11.4 | 1,277 | 4.9 | 0 | 0 | 8 | 0.2 | 0 | 0 |
| 1968-1977 | 1,130 | 14.6 | 1,905 | 25.5 | 3,522 | 13.7 | 5 | 0.2 | 16 | 0.4 | 6 | 0.98 |
| 1978-1987 | 2,448 | 31.6 | 2,179 | 29.1 | 7,309 | 28.3 | 528 | 23.0 | 1,069 | 24.5 | 89 | 14.5 |
| 1988-1998 | 3,617 | 46.7 | 2,540 | 34.0 | 13,693 | 53.1 | 1,764 | 76.8 | 3,266 | 74.9 | 520 | 84.6 |
| Registry | ||||||||||||
| Sweden | 5,927 | 76.5 | 5,047 | 67.5 | 19,651 | 76.2 | 1,054 | 45.9 | 2,480 | 56.9 | 351 | 57.1 |
| Denmark | 1,822 | 23.5 | 2,429 | 32.5 | 6,150 | 23.8 | 1,243 | 54.1 | 1,879 | 43.1 | 264 | 42.9 |
Abbreviations: CLL, chronic lymphocytic leukemia; HL, Hodgkin's lymphoma; NHL, non-Hodgkin's lymphoma.
Information on histopathologic subtype was available for 7,271 (28.2%) of 25,801 NHL patients.
Age cut-offs for CLL, HL, and NHL (including NHL subtypes) were 67, 45, and 62 years, respectively.
CLL
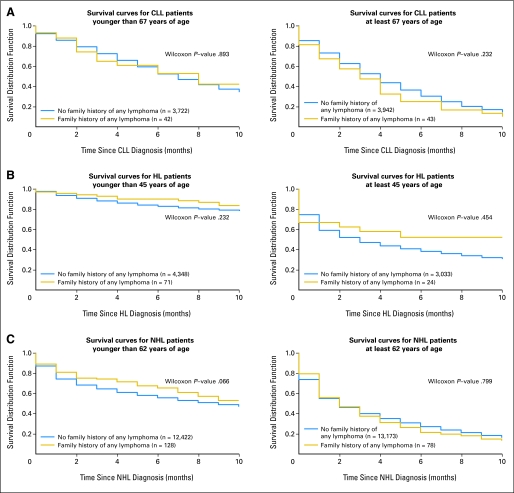
Of the 7,749 patients with CLL, 85 (1.10%) had a family history of any lymphoma (Table 3) and among these 19 had a family history of CLL. A family history of any lymphoma was not associated with sex, age, or year of diagnosis (Table 3). For clinical utility, survival curves comparing survival in patients with CLL with and without a family history of lymphoma are presented in Figure 1A. Survival was similar for patients with CLL with and without a family history of lymphoma. At 5-year follow-up, 40.4% of patients with CLL were alive; similarly, at 10-year follow-up, 36.4% were alive.
Table 3.
Comparison of Patients With Lymphoma With and Without a Family History of Any Lymphoma
| Variable | CLL
|
HL
|
NHL
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Family History
|
With Family History*
|
P | No Family History
|
With Family History*
|
P | No Family History
|
With Family History*
|
P | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||
| No. of patients | 7,664 | 85 | 7,381 | 95 | 25,595 | 206 | |||||||||
| Sex | .645 | .015 | .944 | ||||||||||||
| Male | 5,080 | 66.3 | 54 | 63.5 | 4,446 | 60.2 | 69 | 72.6 | 14,869 | 58.1 | 119 | 57.8 | |||
| Female | 2,584 | 33.7 | 31 | 36.5 | 2,935 | 39.8 | 26 | 27.4 | 10,726 | 41.9 | 87 | 42.2 | |||
| Age group† | .913 | < .001 | < .001 | ||||||||||||
| Younger patients | 3,722 | 48.6 | 42 | 49.4 | 4,348 | 58.9 | 71 | 74.7 | 12,422 | 48.5 | 128 | 62.1 | |||
| Older patients | 3,942 | 51.4 | 43 | 50.6 | 3,033 | 41.1 | 24 | 25.3 | 13,173 | 51.5 | 78 | 37.9 | |||
| Diagnosis year | .996 | .051 | .631 | ||||||||||||
| 1958-1967 | 548 | 7.2 | 6 | 7.1 | 847 | 11.5 | 5 | 5.3 | 1,268 | 5.0 | 9 | 4.4 | |||
| 1968-1977 | 1,118 | 14.6 | 12 | 14.1 | 1,873 | 25.4 | 32 | 33.7 | 3,496 | 13.7 | 26 | 12.6 | |||
| 1978-1987 | 2,420 | 31.6 | 28 | 32.9 | 2,147 | 29.1 | 32 | 33.7 | 7,242 | 28.3 | 67 | 32.5 | |||
| 1988-1998 | 3,578 | 46.7 | 39 | 45.9 | 2,514 | 34.1 | 26 | 27.4 | 13,589 | 53.1 | 104 | 50.5 | |||
| Registry | .020 | .008 | < .001 | ||||||||||||
| Sweden | 5,853 | 76.4 | 74 | 87.1 | 4,971 | 67.3 | 76 | 80.0 | 19,472 | 76.1 | 179 | 86.9 | |||
| Denmark | 1,811 | 23.6 | 11 | 12.9 | 2,410 | 32.7 | 19 | 20.0 | 6,123 | 23.9 | 27 | 13.1 | |||
| Histopathologic | |||||||||||||||
| NHL subtype | .308 | ||||||||||||||
| High-grade B cell | NA | NA | NA | NA | NA | NA | NA | NA | 2,284 | 13 | |||||
| Low-grade B cell | NA | NA | NA | NA | NA | NA | NA | NA | 4,331 | 28 | |||||
| T-cell/anaplastic | NA | NA | NA | NA | NA | NA | NA | NA | 610 | 5 | |||||
| Missing | NA | NA | NA | NA | NA | NA | NA | NA | 18,510 | 160 | |||||
Abbreviations: CLL, chronic lymphocytic leukemia; HL, Hodgkin's lymphoma; NHL, non-Hodgkin's lymphoma; NA, not available.
A known diagnosis of CLL, HL, and/or NHL in at least one first-degree relative before the diagnosis date of the patient.
Age cut-offs for CLL, HL, and NHL were 67, 45, and 62 years, respectively.
Fig 1.
(A) Survival curves for patients with chronic lymphocytic leukemia (CLL) with and without a family history of any lymphoma. Kaplan-Meier curves are presented stratified by the median age of 67 years. The solid lines represent CLL patients with no family history of lymphoma. The dotted lines represent patients with CLL with a family history of lymphoma. The number of patients with CLL with and without a family history are presented. Survival curves were compared using the generalized Wilcoxon's test; P values are presented. (B) Survival curves for patients with Hodgkin's lymphoma (HL) with and without a family history of any lymphoma. Kaplan-Meier curves are presented stratified by age 45 years. The solid lines represent patients with HL with no family history of lymphoma. The dotted lines represent patients with HL with a family history of lymphoma. The number of patients with HL with and without a family history are presented. Survival curves were compared using the generalized Wilcoxon's test; P values are presented. Median age at diagnosis was used as cut-off. (C) Survival curves for patients with non-Hodgkin's lymphoma (NHL) with and without a family history of any lymphoma. Kaplan-Meier curves are presented stratified by the median age of 62 years. The number of patients with NHL with and without a family history are presented. The solid lines represent patients with NHL with no family history of lymphoma. The dotted lines represent patients with NHL with a family history of lymphoma. Survival curves were compared using the generalized Wilcoxon's test; P values are presented.
Patients with CLL with and without a family history of lymphoma had similar 5-year and 10-year mortality (Table 4). Older age, male sex, and an earlier year of diagnosis were all predictors of worse mortality. For example, males were 1.4 times more likely to die than females at each point in time after diagnosis. There were no significant differences in prognosis by family history when the analyses were stratified by sex, age, or year of diagnosis (data not shown). In patients with CLL with a family history of lymphoma, older age at diagnosis was significantly associated with a worse outcome 5-years after diagnosis (hazard ratio [HR], 1.42; 95% CI, 1.14 to 1.75; P = .001), but sex (HR, 1.05; 95% CI, 0.57 to 1.94; P = .874) and year of diagnosis (HR, 0.64; 95% CI, 0.35 to 1.17; P = .146) were not significantly associated with mortality in these patients. Findings were similar with 10 years of follow-up (data not shown).
Table 4.
HR Estimates and 95% CIs for 5- and 10-Year Mortality
| Variable | Mortality
|
|||
|---|---|---|---|---|
| 5-Year | 10-Year | |||
| HR* | 95% CI | HR* | 95% CI | |
| CLL | ||||
| Family history of any lymphoma† | 1.28 | 0.95 to 1.72 | 1.24 | 0.93 to 1.67 |
| Male sex‡ | 1.42§ | 1.32 to 1.52 | 1.41§ | 1.33 to 1.52 |
| Age at diagnosis, ≥ 67 years¶ | 1.47§ | 1.43 to 1.51 | 2.13§ | 2.00 to 2.26 |
| Year of diagnosis, per decade‖ | 0.74§ | 0.70 to 0.76 | 0.81§ | 0.78 to 0.84 |
| HL | ||||
| Family history of any lymphoma† | 0.78 | 0.49 to 1.25 | 0.78 | 0.51 to 1.19 |
| Male sex‡ | 1.22§ | 1.12 to 1.33 | 1.22§ | 1.14 to 1.32 |
| Age at diagnosis, per quintile¶ | 2.07§ | 1.99 to 2.15 | 2.03§ | 1.96 to 2.10 |
| Year of diagnosis, per decade‖ | 0.59§ | 0.54 to 0.64 | 0.77§ | 0.74 to 0.80 |
| HL, < age 45 years | ||||
| Family history of any lymphoma† | 0.65 | 0.31 to 1.36 | 0.74 | 0.41 to 1.35 |
| Male sex‡ | 1.33** | 1.13 to 1.56 | 1.32§ | 1.14 to 1.52 |
| Age at diagnosis, ≥ 27 years¶ | 1.82§ | 1.55 to 2.14 | 1.66§ | 1.44 to 1.90 |
| Year of diagnosis, per decade‖ | 0.59§ | 0.54 to 0.64 | 0.61§ | 0.57 to 0.66 |
| HL, ≥ age 45 years | ||||
| Family history of any lymphoma† | 1.07 | 0.59 to 1.94 | 0.93 | 0.51 to 1.69 |
| Male sex‡ | 1.21** | 1.10 to 1.34 | 1.23§ | 1.12 to 1.35 |
| Age at diagnosis, ≥ 61 years¶ | 2.48§ | 2.24 to 2.75 | 2.50§ | 2.27 to 2.76 |
| Year of diagnosis, per decade‖ | 0.80§ | 0.76 to 0.84 | 0.82§ | 0.78 to 0.86 |
| NHL | ||||
| Family history of any lymphoma† | 0.91 | 0.74- to 1.12 | 0.97 | 0.80 to 1.16 |
| Male sex‡ | 1.20§ | 1.16 to 1.24 | 1.32§ | 1.23 to 1.41 |
| Age at diagnosis, per quintile¶ | 1.42§ | 1.40 to 1.44 | 1.47§ | 1.45 to 1.49 |
| Year of diagnosis, per decade‖ | 0.75§ | 0.73 to 0.78 | 0.83§ | 0.82 to 0.85 |
| High-grade B-cell lymphoma | ||||
| Family history of any lymphoma† | 0.80 | 0.33 to 1.93 | 1.02 | 0.49 to 2.16 |
| Male sex‡ | 1.24** | 1.10 to 1.39 | 1.20§ | 1.08 to 1.37 |
| Age at diagnosis, per quintile¶ | 1.39§ | 1.33 to 1.45 | 1.42§ | 1.36 to 1.48 |
| Year of diagnosis, per decade‖ | 0.84** | 0.74 to 0.95 | 0.84§ | 0.74 to 0.94 |
| Low-grade B-cell lymphoma | ||||
| Family history of any lymphoma† | 1.04 | 0.56 to 1.93 | 1.14 | 0.66 to 1.98 |
| Male sex‡ | 1.36§ | 1.23 to 1.50 | 1.32§ | 1.22 to 1.45 |
| Age at diagnosis, per quintile¶ | 1.59§ | 1.52 to 1.66 | 1.56§ | 1.51 to 1.62 |
| Year of diagnosis, per decade‖ | 0.80§ | 0.74 to 0.92 | 0.79§ | 0.72 to 0.87 |
| T-cell/anaplastic lymphoma | ||||
| Family history of any lymphoma† | 2.69 | 0.85 to 8.47 | 1.86 | 0.59 to 5.83 |
| Male sex‡ | 1.66** | 1.24 to 2.23 | 1.59§ | 1.22 to 2.08 |
| Age at diagnosis, per quintile¶ | 1.44§ | 1.31 to 1.59 | 1.45§ | 1.33 to 1.59 |
| Year of diagnosis, per decade‖ | 2.47§ | 1.66 to 3.68 | 1.88§ | 1.37 to 2.58 |
NOTE. All models are multivariable Cox regression models which include family history of any lymphoma, age at diagnosis, year of diagnosis, and country of residence (estimates not shown). Bold font indicates the risk of dying for patients with a family history of lymphoma compared with patients without a family history of lymphoma.
Abbreviations: HR, hazard ratio; CLL, chronic lymphocytic leukemia; HL, Hodgkin's lymphoma; NHL, non-Hodgkin's lymphoma.
Adjusted for registry and mutually adjusted for the other variables in Table 2.
Family history of any lymphoma: Baseline = no family history of any lymphoma. Family history of any lymphoma is defined as a known diagnosis of CLL, HL or NHL in at least one first-degree relative before the diagnosis date of the patient.
Male sex: baseline = female.
P < .001.
Age at diagnosis: baseline for CLL = patients age < 67 years; baseline for HL = patients age < 23 years (categories included: < 23, 23-31, 32-44, 45-60, 61+ years); baseline for HL = < 45 years = < 27 years; baseline for HL ≥ 45 years = < 61 years; baseline for NHL, high-grade B-cell lymphoma, low-grade B-cell lymphoma, and T-cell/anaplastic lymphoma = < 46 years (categories included: < 46, 46 to 56, 57 to 65, 66 to 73, 74+ years).
Year of diagnosis: baseline = 1958 to 1967 (categories included: 1958 to 1967, 1968 to 1977, 1978 to 1987, 1988 to 1998).
P < .05.
Likewise, patients with CLL with a family history of CLL (n = 19), had a similar outcome to patients with CLL without a family history of CLL (5-year: HR, 0.74; 95% CI, 0.40 to 1.39; 10-year: HR, 0.81; 95% CI, 0.48 to 1.37).
HL
A family history of any lymphoma was observed in 95 (1.28%) of the 7,476 patients with HL. Patients with HL with a family history of lymphoma were more likely to be male and were younger than patients without a family history of lymphoma (Table 3). Survival curves comparing survival in patients with HL with and without a family history of lymphoma are presented in Figure 1B. No significant difference in 10-year survival was observed. Overall, 63.7% and 61.6% of patients with HL were alive at 5-year and 10-year follow-up, respectively.
Mortality (5-year and 10-year) was similar for patients with HL with and without a family history of lymphoma when adjusted for age, sex, and year of diagnosis (Table 4). Because age at diagnosis of HL is an important determinant of survival, Cox regression models were stratified by age of diagnosis younger than 45 years or 45 years or older (Table 4). Older age, male sex, and an earlier year of diagnosis were all predictors of worse outcome in the overall and age stratified analyses. Stratifying by sex and year of HL diagnosis resulted in similar HRs (data not shown). In patients with HL with a family history of any lymphoma, older age at diagnosis was associated with worse outcome 5-years after diagnosis (HR, 3.26; 95% CI, 1.98 to 5.38; P < .001), while sex (HR, 0.79; 95% CI, 0.28 to 2.65; P = .787) and calendar period of diagnosis (HR, 0.58; 95% CI, 0.22 to 1.54, P = .272) were not significantly associated with mortality in these patients. Similar findings were observed with 10 years of follow-up (data not shown).
Likewise, patients with HL with a family history of HL (n = 21), had a similar outcome to patients with HL without a family history of HL (5-year: HR, 0.74; 95% CI, 0.24 to 2.29; 10-year: HR, 0.92; 95% CI, 0.38 to 2.21).
NHL
Of the 25,801 patients with NHL, 206 (0.80%) had a family history of any lymphoma (Table 3). Family history of any lymphoma was significantly associated with a younger age at diagnosis, but not with sex or year of NHL diagnosis (Table 2). Survival curves comparing survival in patients with NHL with and without a family history of lymphoma are shown in Figure 1C. No significant differences in survival were observed between patients with and without a family history of lymphoma. Overall, 42.3% and 39.4% of patients with NHL were alive at 5-year and 10-year follow-up, respectively.
Mortality (5 year and 10 year) was similar for patients with NHL with and without a family history of any lymphoma (Table 4). Older age, male sex, and earlier year of diagnosis were associated with worse outcome. Family history of any lymphoma did not impact prognosis in the analyses stratified by sex, median age, or median year of diagnosis of NHL (data not shown). In patients with NHL with a family history of any lymphoma, older age at diagnosis was associated with poorer outcome (HR, 1.55; 95% CI, 1.33 to 1.81; P < .001). Sex (HR, 1.07; 95% CI, 0.71 to 1.64; P = .740) and calendar period (HR, 1.07; 95% CI, 0.70 to 1.62; P = .759) were not significantly associated with outcome. Having a family history of NHL (n = 101) did not impact prognosis (5 year: HR, 0.96; 95% CI, 0.72 to 1.27; 10 year: HR, 0.99; 95% CI, 0.76 to 1.28).
Family history of any lymphoma was observed in 13 (0.57%) high-grade B-cell, 28 (0.64%) low-grade B-cell, and five (0.81%) T-cell/anaplastic lymphoma patients and was not associated with outcome (Table 4). Family history of NHL was reported in eight (0.35%) high-grade B-cell, 11 (0.25%) low-grade B-cell, and three (0.49%) T-cell/anaplastic lymphoma cases. For high- and low-grade B-cell lymphoma patients 5-year mortality did not differ according to family history of NHL (HR, 0.89; 95% CI, 0.29 to 2.78 and HR, 1.48, 95% CI, 0.61 to 3.56, respectively). However, outcome was worse in patients with T-cell/anaplastic lymphoma who had a family history of NHL (HR, 5.38; 95% CI, 1.65 to 17.52). Similar patterns were observed with 10 years of follow-up (data not shown).
DISCUSSION
In this large population-based study, including more than 41,000 patients with CLL, HL, and NHL, we found no overall difference in survival patterns between patients with versus without a family history of lymphoma. Outcome was worse for patients with T-cell/anaplastic lymphoma with a family history of NHL. These findings add significantly to the restricted literature on the prognosis of patients with familial CLL, HL, and NHL.
Family history of lymphoma has repeatedly been reported as a risk factor for CLL in the literature.1-3,5,8-10 However, few studies have investigated whether having a family history of lymphoma impacts prognosis. A study based on 126 patients with CLL reported that mean survival of patients with CLL with a family history of malignancy was higher compared with patients without a family history of malignancy55; however, the investigators failed to characterize malignancy type in the family members. Conversely, in a small study of patients from 55 multiple-case and 109 single-case families with leukemia, lymphoma, or myeloma, the investigators suggested that patients in multiple-case families had poorer prognosis, although no formal survival analyses were conducted.48
Our study is the largest to date to investigate survival differences between patients with CLL with and without a family history of lymphoma. Our finding of no overall difference in prognosis replicates the results of an Italian case-control study of 1,449 patients with CLL, where similar 10-year survival probabilities in sporadic (67%) and familial (66%) patients were reported.49 The prevalence of a family history of lymphoma in first degree relatives (1.15%) was similar to that found in our study (1.10%); however, prevalence of a family history of CLL was higher (0.80% v 0.25%).
In the Italian case-control study, patients with CLL with male sex, older age, advanced Rai stage, and those who required treatment had poorer survival.49 We also found increasing age and male sex to be significant predictors of worse outcome; however, we did not have information about the stage of disease or treatment. Although we were unable to adjust for these variables in the survival analyses, the lack of a significant difference between familial and sporadic cases with respect to these variables in the Italian study49 suggests that adjusting for these factors would not strongly affect our findings.
The risk of HL has been reported to be two to three times higher in patients with a family history of lymphoma.20,21,23 To our knowledge, no previous studies have investigated prognosis in patients with familial HL. We found no difference in prognosis between patients with HL with versus without a family history of any lymphoma or HL after 5 or 10 years of follow-up. Although age at diagnosis of HL is an important determinant of survival, we found no difference in outcome between patients with HL with and without a family history of lymphoma in those younger than age 45 or 45 years or older.
Patients with a family history of lymphoma have a two- to four-fold increased risk of NHL.1,9,20,22,24-29 To our knowledge, no studies have investigated the impact of having a family history of lymphoma on prognosis.
We previously reported an increased risk of NHL in relatives of patients with aggressive, high-grade B-cell NHL compared with relatives of all patients with NHL in Scandinavia.27 Despite this, and the fact that aggressive and indolent NHL have different treatment regimes56 and prognoses,57 we found no difference in 5-year or 10-year mortality between patients with NHL with versus without a family history of any lymphoma or NHL.
Although subtype information was only available for 28.2% of the patients with NHL, outcome was similar for patients with and without a family history of any lymphoma in the subtype specific analyses. However, the small sample sizes limited the power to detect differences in mortality and meant we were unable to investigate differences in survival patterns for rarer NHL subtypes.
While survival for both low- and high-grade B-cell lymphoma improved over time, this was not the case for T-cell/anaplastic NHL. In addition, the risk of dying was higher (at 5-year and 10-year follow-up) in patients with T-cell/anaplastic lymphoma who had a family history of NHL. Despite the limitations of small sample size (there were only three patients with T-cell lymphoma with a family history of NHL) and multiple testing, the heterogeneity of NHL suggests that future investigations should look at the impact of family history on prognosis by NHL subtypes.
Our study is the largest to date to address the pertinent question about whether or not having a family history of lymphoma adversely affects survival for patients with CLL, HL, and NHL. The main strength of our study is the use of large population-based, multigenerational registers which eliminate the problems associated with ascertainment and recall bias in case-control studies. These databases have high completeness of case ascertainment and diagnostic accuracy for lymphomas from the national cancer registries.54,58
One potential limitation of our study is incomplete ascertainment of family history of lymphoma. We were unable to capture information on lymphoma diagnoses for relatives before 1958 in Sweden and 1943 in Denmark, because no national cancer registration systems were available.51 If survival differed in patients with relatives diagnosed with lymphoma before these dates our results would be biased toward showing no overall difference in outcome. However, when we restricted our analyses to persons diagnosed in the later time periods (after 1987), where ascertainment of family history was likely complete, no differences in 5-year or 10-year mortality were observed between patients with versus without a family history of lymphoma. Because the Swedish database had more years of overlap between patients with lymphoma and first-degree relatives than the Danish database, we found somewhat more familial lymphoproliferative malignancies in the Swedish database. However, when we conducted survival subanalyses restricted to Sweden and Denmark only, the results were very similar (not shown).
Currently, there is an evolving interest in molecular predictors of outcome in lymphomas.40-46 With the introduction of novel therapies, these efforts will ultimately allow clinicians to increasingly focus on more individualized approaches, with the aim to tailor treatment. Family history of lymphoma is a well-known risk factor for developing lymphoma.1-11 The influence of family history on lymphoma outcome has, until recently, been obscure and based on small series.48,49,55 Our finding of no difference in prognosis between familial and sporadic lymphomas, with the possible exception of T-cell/anaplastic lymphoma, suggests the same phenotype and natural course. However, it cannot be ruled out that there are subsets of patients in whom genes predisposing to familial lymphoma impact prognosis. Future work is needed to explore whether pathways associated with increased risk of lymphoma also impact clonal progression, survival, and dissemination.
In conclusion, overall 5-year and 10-year survival patterns were similar for patients with CLL, HL, and NHL with versus without a family history of any lymphoma, suggesting that familial lymphomas do not have an altered clinical course. With one exception, T-cell/anaplastic lymphoma, outcome was also similar for patients with versus without a family history of the same lymphoma. At this time, our findings provide no evidence to alter therapeutic strategies for patients with CLL, HL, or NHL based solely on family history.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Ola Landgren
Administrative support: Gloria Gridley
Provision of study materials or patients: Lene Mellemkjaer, Kari Hemminki, Magnus Björkholm
Collection and assembly of data: Ruth M. Pfeiffer, Joshua S. Rapkin, Gloria Gridley, Lene Mellemkjaer, Kari Hemminki, Magnus Björkholm, Neil E. Caporaso, Ola Landgren
Data analysis and interpretation: Lesley A. Anderson, Ruth M. Pfeiffer, Ola Landgren
Manuscript writing: Lesley A. Anderson, Ola Landgren
Final approval of manuscript: Lesley A. Anderson, Ruth M. Pfeiffer, Joshua S. Rapkin, Gloria Gridley, Lene Mellemkjaer, Kari Hemminki, Magnus Björkholm, Neil E. Caporaso, Ola Landgren
Acknowledgments
We thank Emily Steplowski of Information Management Services Inc, Silver Spring, MD, for computer programming support and David Check from the Biostatistics Branch, National Cancer Institute, for constructing the Kaplan-Meier graphs.
published online ahead of print at www.jco.org on July 7, 2008.
Supported by the intramural program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics, Bethesda, MD. The Research and Development Office, Northern Ireland sponsored L.A.A. to participate in the Cancer Prevention Fellowship Program, Office of Preventative Oncology, Division of Cancer Prevention, National Cancer Institute through the Ireland-Northern Ireland-USA Consortium.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Cannon-Albright LA, Thomas A, Goldgar DE, et al: Familiality of cancer in Utah. Cancer Res 54:2378-2385, 1994 [PubMed] [Google Scholar]
- 2.Capalbo S, Trerotoli P, Ciancio A, et al: Increased risk of lymphoproliferative disorders in relatives of patients with B-cell chronic lymphocytic leukemia: Relevance of the degree of familial linkage. Eur J Haematol 65:114-117, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Cartwright RA, Bernard SM, Bird CC, et al: Chronic lymphocytic leukaemia: Case control epidemiological study in Yorkshire. Br J Cancer 56:79-82, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang ET, Smedby KE, Hjalgrim H, et al: Family history of hematopoietic malignancy and risk of lymphoma. J Natl Cancer Inst 97:1466-1474, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Goldin LR, Pfeiffer RM, Li X, et al: Familial risk of lymphoproliferative tumors in families of patients with chronic lymphocytic leukemia: Results from the Swedish Family-Cancer Database. Blood 104:1850-1854, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Ishibe N, Sgambati MT, Fontaine L, et al: Clinical characteristics of familial B-CLL in the National Cancer Institute Familial Registry. Leuk Lymphoma 42:99-108, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Kerber RA, O'Brien E: A cohort study of cancer risk in relation to family histories of cancer in the Utah population database. Cancer 103:1906-1915, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Linet MS, Van Natta ML, Brookmeyer R, et al: Familial cancer history and chronic lymphocytic leukemia: A case-control study. Am J Epidemiol 130:655-664, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Pottern LM, Linet M, Blair A, et al: Familial cancers associated with subtypes of leukemia and non-Hodgkin's lymphoma. Leuk Res 15:305-314, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Radovanovic Z, Markovic-Denic L, Jankovic S: Cancer mortality of family members of patients with chronic lymphocytic leukemia. Eur J Epidemiol 10:211-213, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Yuille MR, Matutes E, Marossy A, et al: Familial chronic lymphocytic leukaemia: A survey and review of published studies. Br J Haematol 109:794-799, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Lindelof B, Eklund G: Analysis of hereditary component of cancer by use of a familial index by site. Lancet 358:1696-1698, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Shugart YY, Hemminki K, Vaittinen P, et al: A genetic study of Hodgkin's lymphoma: An estimate of heritability and anticipation based on the familial cancer database in Sweden. Hum Genet 106:553-556, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Westergaard T, Melbye M, Pedersen JB, et al: Birth order, sibship size and risk of Hodgkin's disease in children and young adults: A population-based study of 31 million person-years. Int J Cancer 72:977-981, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Mack TM, Cozen W, Shibata DK, et al: Concordance for Hodgkin's disease in identical twins suggesting genetic susceptibility to the young-adult form of the disease. N Engl J Med 332:413-418, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Kerzin-Storrar L, Faed MJ, MacGillivray JB, et al: Incidence of familial Hodgkin's disease. Br J Cancer 47:707-712, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haim N, Cohen Y, Robinson E: Malignant lymphoma in first-degree blood relatives.Cancer 49:2197-2200, 1982 [DOI] [PubMed] [Google Scholar]
- 18.Bernard SM, Cartwright RA, Darwin CM, et al: Hodgkin's disease: Case control epidemiological study in Yorkshire. Br J Cancer 55:85-90, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grufferman S, Cole P, Smith PG, et al: Hodgkin's disease in siblings. N Engl J Med 296:248-250, 1977 [DOI] [PubMed] [Google Scholar]
- 20.Goldgar DE, Easton DF, Cannon-Albright LA, et al: Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst 86:1600-1608, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Goldin LR, Pfeiffer RM, Gridley G, et al: Familial aggregation of Hodgkin lymphoma and related tumors. Cancer 100:1902-1908, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Hemminki K, Li X, Czene K: Familial risk of cancer: Data for clinical counseling and cancer genetics. Int J Cancer 108:109-114, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Paltiel O, Schmit T, Adler B, et al: The incidence of lymphoma in first-degree relatives of patients with Hodgkin disease and non-Hodgkin lymphoma: Results and limitations of a registry-linked study. Cancer 88:2357-2366, 2000 [PubMed] [Google Scholar]
- 24.Altieri A, Bermejo JL, Hemminki K: Familial risk for non-Hodgkin lymphoma and other lymphoproliferative malignancies by histopathologic subtype: The Swedish Family-Cancer Database. Blood 106:668-672, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Cartwright RA, McKinney PA, O'Brien C, et al: Non-Hodgkin's lymphoma: Case control epidemiological study in Yorkshire. Leuk Res 12:81-88, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee N, Hartge P, Cerhan JR, et al: Risk of non-Hodgkin's lymphoma and family history of lymphatic, hematologic, and other cancers. Cancer Epidemiol Biomarkers Prev 13:1415-1421, 2004 [PubMed] [Google Scholar]
- 27.Goldin LR, Landgren O, McMaster ML, et al: Familial aggregation and heterogeneity of non-Hodgkin lymphoma in population-based samples. Cancer Epidemiol Biomarkers Prev 14:2402-2406, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Mensah FK, Willett EV, Ansell P, et al: Non-Hodgkin's lymphoma and family history of hematologic malignancy. Am J Epidemiol 165:126-133, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Zhu K, Levine RS, Gu Y, et al: Non-Hodgkin's lymphoma and family history of malignant tumors in a case-control study (United States). Cancer Causes Control 9:77-82, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Casey R, Brennan P, Becker N, et al: Influence of familial cancer history on lymphoid neoplasms risk validated in the large European case-control study epilymph. Eur J Cancer 42:2570-2576, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Cuttner J: Increased incidence of hematologic malignancies in first-degree relatives of patients with chronic lymphocytic leukemia. Cancer Invest 10:103-109, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Skibola CF, Curry JD, Nieters A: Genetic susceptibility to lymphoma. Haematologica 92:960-969, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldin LR, McMaster ML, Ter-Minassian M, et al: A genome screen of families at high risk for Hodgkin lymphoma: Evidence for a susceptibility gene on chromosome 4. J Med Genet 42:595-601, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudd MF, Sellick GS, Webb EL, et al: Variants in the ATM-BRCA2-CHEK2 axis predispose to chronic lymphocytic leukemia. Blood 108:638-644, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Jamroziak K, Balcerczak E, Smolewski P, et al: MDR1 (ABCB1) gene polymorphism C3435T is associated with P-glycoprotein activity in B-cell chronic lymphocytic leukemia. Pharmacol Rep 58:720-728, 2006 [PubMed] [Google Scholar]
- 36.Hohaus S, Massini G, D‘Alo’ F, et al: Association between glutathione S-transferase genotypes and Hodgkin's lymphoma risk and prognosis. Clin Cancer Res 9:3435-3440, 2003 [PubMed] [Google Scholar]
- 37.Lan Q, Zheng T, Shen M, et al: Genetic polymorphisms in the oxidative stress pathway and susceptibility to non-Hodgkin lymphoma. Hum Genet 121:161-168, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Purdue MP, Lan Q, Kricker A, et al: Polymorphisms in immune function genes and risk of non-Hodgkin lymphoma: Findings from the New South Wales Non-Hodgkin Lymphoma study. Carcinogenesis 28:704-712, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Shen M, Zheng T, Lan Q, et al: Polymorphisms in DNA repair genes and risk of non-Hodgkin lymphoma among women in Connecticut. Hum Genet 119:659-668, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Lee JJ, Kim DH, Lee NY, et al: Interleukin-10 gene polymorphism influences the prognosis of T-cell non-Hodgkin lymphomas. Br J Haematol 137:329-336, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Warzocha K, Ribeiro P, Bienvenu J, et al: Genetic polymorphisms in the tumor necrosis factor locus influence non-Hodgkin's lymphoma outcome. Blood 91:3574-3581, 1998 [PubMed] [Google Scholar]
- 42.Juszczynski P, Kalinka E, Bienvenu J, et al: Human leukocyte antigens class II and tumor necrosis factor genetic polymorphisms are independent predictors of non-Hodgkin lymphoma outcome. Blood 100:3037-3040, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Lech-Maranda E, Baseggio L, Bienvenu J, et al: Interleukin-10 gene promoter polymorphisms influence the clinical outcome of diffuse large B-cell lymphoma. Blood 103:3529-3534, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Caporaso NE: Integrative study designs–next step in the evolution of molecular epidemiology? Cancer Epidemiol Biomarkers Prev 16:365-366, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Staudt LM: Gene expression profiling of lymphoid malignancies. Annu Rev Med 53:303-318, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Li L: Survival prediction of diffuse large-B-cell lymphoma based on both clinical and gene expression information. Bioinformatics 22:466-471, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Gemmati D, Ongaro A, Tognazzo S, et al: Methylenetetrahydrofolate reductase C677T and A1298C gene variants in adult non-Hodgkin's lymphoma patients: Association with toxicity and survival. Haematologica 92:478-485, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Rosenlof RC, Lemon HM, Rigby PG: Familial factors relating to prognosis of leukemia and lymphoma. Natl Cancer Inst Monogr 34:283-289, 1971 [PubMed] [Google Scholar]
- 49.Mauro FR, Giammartini E, Gentile M, et al: Clinical features and outcome of familial chronic lymphocytic leukemia. Haematologica 91:1117-1120, 2006 [PubMed] [Google Scholar]
- 50.Landgren O, Linet MS, McMaster ML, et al: Familial characteristics of autoimmune and hematologic disorders in 8,406 multiple myeloma patients: A population-based case-control study. Int J Cancer 118:3095-3098, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Hemminki K, Li X, Plna K, et al: The nation-wide Swedish family-cancer database–updated structure and familial rates. Acta Oncol 40:772-777, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Skarle A: Flergenerationsregistret. Stockholm, Sweden, Statistics Sweden, Population Statistics, 2001
- 53.The National Board of Health and Welfare: Cancer Incidence in Sweden 2003. Stockholm, Sweden, National Board of Health and Welfare, 2004
- 54.Storm HH, Michelsen EV, Clemmensen IH, et al: The Danish Cancer Registry–history, content, quality and use. Dan Med Bull 44:535-539, 1997 [PubMed] [Google Scholar]
- 55.Nenova IS, Karnolski IN, Mateva NG, et al: Familial study of chronic lymphocytic leukemia: Aggregation of different malignant processes in families with individuals affected with chronic lymphocytic leukemia. Folia Med (Plovdiv) 48:11-16, 2006 [PubMed] [Google Scholar]
- 56.Zinzani PL: Traditional treatment approaches in B-cell non-Hodgkin's lymphoma. Leuk Lymphoma 44:S6-S14, 2003. (suppl 4) [DOI] [PubMed] [Google Scholar]
- 57.Varterasian ML, Graff JJ, Severson RK, et al: Non-Hodgkin's lymphoma: An analysis of the Metropolitan Detroit SEER database. Cancer Invest 18:303-308, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Turesson I, Linet MS, Bjorkholm M, et al: Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964-2003. Int J Cancer 121:2260-2266, 2007 [DOI] [PubMed] [Google Scholar]