Abstract
Purpose
A phase I/II trial was performed to evaluate the safety and immunogenicity of a novel melanoma vaccine comprising six melanoma-associated peptides defined as antigenic targets for melanoma-reactive helper T cells. Source proteins for these peptides include MAGE proteins, MART-1/MelanA, gp100, and tyrosinase.
Patients and Methods
Thirty-nine patients with stage IIIB to IV melanoma were vaccinated with this six-peptide mixture weekly at three dose levels, with a preceding phase I dose escalation and subsequent random assignment among the dose levels. Helper T-lymphocyte responses were assessed by in vitro proliferation assay and delayed-type hypersensitivity skin testing. Patients with measurable disease were evaluated for objective clinical response by Response Evaluation Criteria in Solid Tumors.
Results
Vaccination with the helper peptide vaccine was well tolerated. Proliferation assays revealed induction of T-cell responses to the melanoma helper peptides in 81% of patients. Among 17 patients with measurable disease, objective clinical responses were observed in two patients (12%), with response durations of 1 and 3.9+ years. Durable stable disease was observed in two additional patients for periods of 1.8 and 4.6+ years.
Conclusion
Results of this study support the safety and immunogenicity of a vaccine comprised of six melanoma helper peptides. There is also early evidence of clinical activity.
INTRODUCTION
CD4+ T lymphocytes are critical in generating effector T-cell responses, licensing dendritic cells, and maintaining immunologic memory.1-4 However, their role in melanoma has not been well defined, and cancer vaccine trials have largely ignored the impact of vaccination on helper T cells and, instead, have focused on epitopes for CD8+ cytotoxic T lymphocytes. In prior trials, we incorporated a nonspecific helper peptide from tetanus toxoid, restricted by multiple HLA-DR molecules, that induces helper T-cell responses when administered in an emulsion with Montanide ISA-51 adjuvant (Seppic Inc, Fairfield, NJ) and granulocyte-macrophage colony-stimulating factor.5,6 However, nonspecific helper T-cell responses may not be optimal for induction of antitumor immunity.
HLA-DR–restricted peptides have been identified from melanoma-associated proteins,7-13 but there is little in vivo human experience with them. Prior studies used only one or two peptides and limited enrollment to a single major histocompatibility complex class II allele.14-16 Immune responses were disappointing for most peptides tested. The intent of the present study was to evaluate the safety and immunogenicity of a vaccine comprising six melanoma-associated helper peptides derived from cancer-testis antigens and from melanocytic differentiation proteins. A secondary aim was to measure clinical outcome.
PATIENTS AND METHODS
Patients
Patients with American Joint Committee on Cancer stage IIIB to IV melanoma, with or without measurable disease, were eligible. Candidates were required to express at least one of the five HLA-DR alleles by which CD4 T-cell recognition for the six peptides had been defined (HLA-DR1, -DR4, -DR11, -DR13, or -DR15; approximately 90% of patients screened, data not shown). Other inclusion criteria included age 18 years or older, Eastern Cooperative Oncology Group performance status of 0 to 1, adequate liver and renal function, and ability to provide informed consent. Exclusion criteria included ocular melanoma; pregnancy; cytotoxic chemotherapy, interferon, or radiation within the preceding month; known or suspected allergies to vaccine components; multiple brain metastases; use of corticosteroids; class III or IV heart disease; systemic autoimmune disease with visceral involvement; and uncontrolled diabetes (hemoglobin A1C ≥ 7%). Patients were studied after informed consent and with institutional review board (HIC No. 10464) and US Food and Drug Administration approval (BB-IND No. 10825).
Clinical Trial Design
The main objectives of this study were to assess the safety of administering six class II major histocompatibility complex–restricted synthetic melanoma peptides in a vaccine at increasing doses and to select the dose level (200, 400, or 800 μg of each peptide) that produced the greatest magnitude of immune response with high probability. The trial was monitored for treatment-related adverse events, and a modified dose-finding scheme was used to assess safety. For the initial phase, the first nine patients were accrued to increasing dose levels in cohorts of three patients. Subsequent patients were randomly assigned to one of the three dose regimens. Assuming that safety criteria would be satisfied for two or more dose levels, this study was designed to select the best treatment worthy of further investigation in single-factor Bernoulli response experiments using the ranking and selection procedure, BSH.16a Sample size was established to ensure that, if the difference between the immune response rates was at least δ = 25%, then the higher one will be selected with high probability. Accrual of 12 eligible patients per dose, regardless of selecting between two or three dose levels, satisfied the minimum criteria of P ≥ .80.
Toxicity Assessment and Stopping Rules
The trial was monitored continuously for treatment-related adverse events using National Cancer Institute Common Toxicity Criteria version 2. Toxicities were recorded by patients using a daily toxicity diary, which was reviewed by interview with a study clinician weekly. Dose-limiting toxicity was defined as an unexpected adverse event possibly, probably, or definitely related to treatment and ≥ grade 4 metabolic/hematologic toxicity, ≥ grade 3 nonhematologic/metabolic toxicity, ≥ grade 2 allergic reaction, or ≥ grade 1 ocular adverse event. Visual flashing lights/floaters needed to occur more than twice as nonisolated events to be considered a dose-limiting toxicity. Patients with unequivocal disease progression were removed from treatment.
End Points for the Study
The study had two primary end points, safety and immunogenicity. The primary immunologic end point was T-cell response to the six melanoma helper peptides, as measured by proliferation assay in the lymph node draining the vaccine site (sentinel immunized node [SIN]17). Secondary end points included T-cell responses to helper peptides in peripheral-blood mononuclear cells (PBMC), delayed-type hypersensitivity (DTH) testing, and clinical outcome. Patients with measurable disease were observed for objective clinical response using Response Evaluation Criteria in Solid Tumors. All patients were observed for progression-free/disease-free and overall survival.
Vaccine Composition
All patients received a vaccine comprising the following six melanoma peptides reported to be restricted by one or more HLA-DR molecules: AQNILLSNAPLGPQFP (Tyrosinase56-70, HLA-DR4),7 FLLHHAFVDSIFEQWLQRHRP (Tyrosinase386-406, HLA-DR15),8 RNGYRALMDKSLHVGTQCALTRR (Melan-A/MART-151-73, HLA-DR4),9 TSYVKVLHHMVKISG (MAGE-3281-295, HLA-DR11),10 LLKYRAREPVTKAE (MAGE-1,2,3,6121-134, HLA-DR13),11 and WNRQLYPEWTEAQRLD (gp10044-59, HLA-DR4 and HLA-DR1).12,13 An alanine residue has been added at the N terminus of AQNILLSNAPLGPQFP (Tyrosinase56-70) to prevent cyclization of the N-terminal glutamine residue in the originally described sequence QNILLSNAPLGPQFP.7,18
The peptides for vaccines were synthesized and purified (> 95%) under Good Manufacturing Practices conditions by Multiple Peptide Systems (now NeoMPS, San Diego, CA). After solubilization, each peptide was sterile filtered, mixed, vialed, and lyophilized under Good Manufacturing Practices conditions by Merck Biosciences AG Clinalfa (Läufelingen, Switzerland) in single-use vials. Vials were submitted to quality assurance studies including sterility, identity, purity, potency, general safety, pyrogenicity, and stability in accordance with Code of Federal Regulations guidelines and BB-IND No. 10825. In addition, a tetanus helper peptide, AQYIKANSKFIGITEL, was used in laboratory analyses.5
Collection of PBMCs
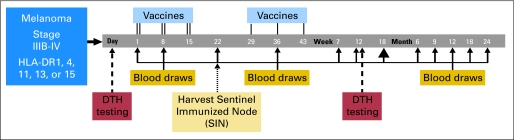
Blood (80 to 100 mL) was drawn at weeks 0, 1, 3, 5, 7, 12, and 18 and at months 6, 9, 12, 18, and 24 (Fig 1). Lymphocytes were isolated using Ficoll gradient centrifugation and cryopreserved in 10% dimethyl sulfoxide/90% serum.
Fig 1.
Flow diagram for Mel41 clinical trial. For each vaccine, the peptides were administered with granulocyte-macrophage colony-stimulating factor 110 μg in a stable emulsion with Montanide ISA-51 adjuvant 1 mL on days 1, 8, 15, 29, 36, and 43 (weeks 0, 1, 2, 4, 5, and 6). The first three vaccinations were divided between two injection sites (primary and replicate), and the last three were delivered to the primary injection site only. At each injection site, half of the vaccination was administered subcutaneously, and half was administered intradermally. DTH, delayed-type hypersensitivity.
Harvest of the SIN
On day 22 (week 3), the lymph node draining the replicate immunization site (SIN) was localized and harvested under local anesthesia, as reported.17 A central slice was preserved in formalin, and the remainder was dissociated mechanically into a single-cell suspension and cryopreserved by the University of Virginia's Tissue Procurement Facility.
Proliferation Assays
Responses to the six melanoma helper peptides were assessed in vitro by measuring proliferation after antigen exposure. PBMCs were thawed in RPMI 1640 medium with 5% heat-inactivated human AB serum (Gemini Bio-Products, West Sacramento, CA) and DNAse (Worthington Biochemical Corporation, Lakewood, NJ, Catalogue No. LS002139; 100 mg, 2,430 units/mg dry weight) at 100 U/mL and washed twice. Cells were adjusted to 1 × 106 cells/mL in tissue culture medium consisting of AIM V (Invitrogen Corporation/GIBCO, Carlsbad, CA) with heat-inactivated 10% human AB serum plus each of the following 11 conditions: media only; bovine serum albumin (BSA; Sigma-Aldrich, St Louis, MO; 10 μg/mL); tetanus peptide (10 μg/mL); each of the six melanoma helper peptides (10 μg/mL, individually tested); six melanoma helper pool (6MHP; all six peptides, each 10 μg/mL); and phytohemagluttinin (PHA; Sigma; 5 μg/mL). PBMCs from two normal donors (Virginia Blood Services, Richmond, VA) were included as controls in each of following 13 conditions: media only; BSA; tetanus peptide; six-melanoma peptide pool; PHA; influenza lysate; lysate control (Microbix Biosystems Inc, Toronto, Ontario, Canada); and PHA. Cultures were prepared in triplicate in 96-well, flat bottom cluster dishes and incubated for 5 days at 37°C and 5% carbon dioxide. On day 5, 1 μCi/well of tritiated thymidine was added for 8 hours before harvesting. Background stimulation was determined as the mean of media-only and BSA-stimulated cultures and was used to determine fold-increase or stimulation index based on the following definitions: Nvax = stimulation in vaccine peptide; Nneg = stimulation in negative control; and stimulation index = Rvax = Nvax/Nneg.
For evaluations of PBMCs, a patient is considered to have a proliferation response to vaccination only if all of the following criteria have been met: Rvax ≥ 4; (Nvax − 1 standard deviation) ≥ (Nneg + 1 standard deviation); and Rvax after vaccination ≥ 4 × Rvax before vaccine. Prevaccination lymph node samples were not routinely evaluated; thus, the third criterion was not applied to SIN. Fold-increases less than one were converted to one to prevent inflation of adjusted fold-increases as a result of prevaccine ratios less than one and division by zero, while not affecting the determination of response. Influenza lysate controls in normal donors provided an assessment of interassay reproducibility; the calculated interassay coefficient of variation was 48%.
DTH Testing
At least 2 days before the first vaccination, patients were injected intradermally (usually on a forearm) with a panel of recall antigens (tuberculin, tetanus toxoid [when available], Trichophyton, and Candida) and with the 6MHP (100 μg each). The diameter of induration was read at 24 hours by the patients and at 48 hours by a licensed practitioner. Tests were repeated at week 12 (day 85) and were considered positive if, at 24 or 48 hours, the diameter of induration was at least 5 mm greater than the maximum prevaccine diameter.
Immunologic Reagents for Flow Cytometry
The fluorescent tagged antibodies used were specific for CD3 allophycocyanin (APC), CD3 APC-cyanine7 (Cy7), CD4 phycoerythrin (PE; BD Biosciences, San Jose, CA), CD8 PE-Cy7 (Beckman Coulter, Miami, FL), FoxP3 (rat, clone PCH101) and its isotype control (eBiosciences, San Diego, CA), and CD25-PE (Miltenyi Biotec Inc, Auburn, CA).
Phenotypic Analysis of Peptide-Stimulated Cells
After 5 days of incubation, 6MHP-stimulated carboxyfluorescein diacetate succinimidyl ester–labeled PBMC were stained with CD3-APC, CD4-PE, and CD8-PE-Cy7 antibodies. Cells were fixed in 0.5% paraformaldehyde (Sigma-Aldrich) and collected on a Becton Dickinson FACSCalibur with dual lasers (488 and 635 nm; BD Biosciences) capable of seven-parameter (four-color) analysis. Data were analyzed with FloJo software (Treestar, Ashland, OR).
Intracellular Detection of FoxP3
Cryopreserved PBMC and SIN lymphocytes were thawed in prewarmed thaw medium and then surface labeled for CD4 (PE; BD Biosciences) and CD25 (Miltenyi Biotec). Labeled cells were fixed, permeabilized, and stained using APC-conjugated antihuman FoxP3 or its isotype control. Data were collected on a Becton Dickinson FACSCalibur and analyzed using FloJo software.
RESULTS
Eligibility Review
Thirty-nine patients were enrolled; however, two were found to be ineligible on postreview (incorrect staging [stage IIIA] and uncontrolled diabetes at study entry). Their data are included for toxicity and adverse event assessments but are excluded from analyses of immunologic and clinical outcomes. Patient demographics and clinical presentations were similar across study groups (Table 1).
Table 1.
Demographics and Clinical Characteristics of Eligible Patients* by Treatment Group
| Characteristic | Arm and Peptide Dose (No. of patients)
|
Total (N =37)
|
|||
|---|---|---|---|---|---|
| Arm A, 200 μg (n = 12) | Arm B, 400 μg (n = 12) | Arm C, 800 μg (n = 13) | No. of Patients | % | |
| Female | 3 | 6 | 7 | 16 | 43 |
| Race | |||||
| Non-Hispanic white | 12 | 12 | 11 | 35 | 95 |
| Egyptian | 0 | 0 | 1 | 1 | 3 |
| Hispanic | 0 | 0 | 1 | 1 | 3 |
| Age, years | |||||
| Median | 63 | 58 | 62 | 59 | |
| Range | 36-84 | 34-78 | 42-79 | 34-84 | |
| Performance status = 0 | 9 | 8 | 7 | 24 | 65 |
| Disease stage | |||||
| IIIB, IIIC | 3 | 5 | 3 | 11 | 30 |
| IV | 9 | 7 | 10 | 26 | 70 |
| Disease status | |||||
| Clinically NED | 5 | 8 | 6 | 19 | 51 |
| Nonmeasurable disease | 0 | 1 | 0 | 1 | 3 |
| Measurable disease | 7 | 3 | 7 | 17 | 46 |
| HLA-DR type† | |||||
| 1 | 1 | 1 | 3 | 5 | |
| 4 | 2 | 8 | 6 | 16 | |
| 11 | 4 | 2 | 4 | 10 | |
| 13 | 8 | 0 | 4 | 12 | |
| 15 | 3 | 5 | 3 | 11 | |
| 7 | 1 | 2 | 3 | 6 | |
| 12 | 1 | 1 | 0 | 2 | |
| 14 | 1 | 0 | 1 | 2 | |
| 17 | 3 | 1 | 0 | 4 | |
Abbreviation: NED, no evidence of disease.
One ineligible patient, who was treated in the 200-μg group, was a 38-year-old white male with measurable stage IV disease who was ineligible because of uncontrolled diabetes (hemoglobin A1C = 7.7%). The other patient, who was treated in the 400-μg group, was a 56-year-old white male with stage IIIA disease who was incorrectly staged as IIIB.
Two additional patients had second HLA-DR alleles that could not be defined with certainty.
Summary of Clinical Toxicities
Treatment-related adverse events are listed for all 39 patients in Appendix Table A1 (online only). There were no grade 4 toxicities, no deaths on study, and no dose-limiting toxicities at any dose. Toxicity profiles were comparable across dose levels (data not shown). Grade 1 and 2 flu-like symptoms were common and usually limited to 24 hours after each vaccine. All patients developed vaccine injection site reactions. Other common treatment-related grade 1 and 2 toxicities were fatigue (85%), headache (41%), myalgias (28%), rigors/chills (28%), arthralgias (18%), nausea (21%), and dizziness/lightheadedness (21%). Common treatment-related laboratory abnormalities included grade 1 hyperkalemia (33%), decreases in hemoglobin (28%), and lymphopenia (13%). Hyperglycemia was recorded in 49% of patients, but blood samples were not fasting, so the hyperglycemia is not likely to be clinically relevant. There was one treatment-related grade 3 toxicity (injection site reaction in a patient in group A, which was the group treated with 200 μg). The following unexpected treatment-related adverse events required institution review board reporting in four patients: grade 2 autoimmune reaction (group B, 400 μg), grade 1 blurred vision (group C, 800 μg), grade 2 vomiting and grade 2 anorexia (group C), and grade 2 edema and grade 2 skin erythema (group C).
Autoimmune Toxicities
Autoimmune toxicities were observed in eight patients (21%), including four patients in group A, three patients in group B, and one patient in group C (maximum grade was grade 1 in seven patients and grade 2 in one patient). Four patients (10%) developed treatment-related vitiligo; four patients (10%) had asymptomatic elevations of rheumatoid factor, and one patient (3%) had asymptomatic elevation of antinuclear antibody. Details are listed in Appendix Table A2 (online only).
Immune Response Data in PBMC and SIN: Proliferation Assay
All 37 eligible patients were assessable for immune responses in PBMCs; 36 were assessable in SIN. Prevaccine proliferative responses to these peptides (> four-fold the negative controls) were detectable in PBMC in two patients (5.2-fold to MAGE-1,2,3,6121-134 and 4.5-fold to 6MHP pool and 4.3-fold to MAGE-3281-295, data not shown). This represents 5% of patients; thus, criteria used for proliferation assay responses result in 95% of patients being negative before vaccination.
Immune responses induced by the six melanoma helper peptides.
Vaccines induced immune response to multiple antigens (Fig 2A). Reactivity to the mixture of six helper peptides (6MHP) correlated well with the sum of reactivities to individual peptides (Spearman r = 0.77 in PBMC and r = 0.96 in SIN, both P < .001; Fig 2B), so reactivity to the 6MHP mixture was used for comparisons across study arms. Reactivity in PBMC was first evident 3 weeks after the first vaccine and persisted through week 7 (Fig 2C), with a slow decline in reactivity through week 39. For each patient, a median of four PBMC samples (range, one to eight samples) were evaluated through a median of 12 weeks (range, 3 to 52 weeks); among patients with a detectable proliferative response, a median of three PBMC samples tested positive. Twenty-one patients had immune responses to 6MHP in the PBMC, of whom 20 had assessable SIN. Nineteen of these patients (95%) had immune responses detected in the SIN. The one exception had a weak response in the SIN (4.15×, data not shown). There were nine additional patients with immune responses in the SIN without immune response in PBMC.
Fig 2.
Cellular proliferative responses to six melanoma helper peptides (6MHP) in peripheral-blood mononuclear cells (PBMCs) and the sentinel immunized node (SIN). (A) Proliferation data are shown for one patient VMM699 at 5 days after stimulation with the 6MHP pool, individual peptides, or an irrelevant tetanus helper peptide. Calculated stimulation index (SI) data are shown; SIs above the red line (SI > 4) are considered positive. The patient is HLA-DR4 and HLA-DR12 positive; described HLA-DR restrictions for peptides with responses (TSY, WNR, FLL, and AQN) are DR11, DR4 and DR1, DR15, and DR4, respectively. W, weeks; T, vaccine treatment; SIN W = 3, sentinel immunized node harvested at week 3. (B) SIs reported for the pool of 6MHP correlated with the sum of maximum SIs for each peptide at all time points evaluated in the PBMC or SIN. (C) Time course for proliferation assay SI data over time for all evaluated patients, with box plots (boxes = 25th to 75th percentiles; vertical lines = minimum to maximum). Numbers at the top of each bar are the number of assessable patients at the time shown. W, weeks from study initiation; M, months from study initiation; T, treatment/vaccine number; SIN, day of SIN biopsy; NA, not available. These are PBMC data only. Blood was not drawn at weeks 2, 4, and 6 and was not evaluated at month 12. (D) Box plot of SIs for immune responders, through week 12, in the PBMC and SIN for each study group (groups A, B, and C = 200, 400, and 800 μg dose, respectively).
Immune responses were not dose dependent across the dose range tested in this study.
Vaccination induced immune reactivity to 6MHP in 57% of patients in PBMC, 78% of patients in SIN, and 81% of patients in PBMC or SIN (Table 2). Results did not differ by study group in χ2 analyses. Furthermore, the magnitudes of response to the 6MHP pool were comparable across peptide doses (Fig 2D).
Table 2.
Proliferative Immune Responses to Peptides After Vaccination
| Patient Group | No. of Patients | % of Patients With Proliferative Responses*
|
||
|---|---|---|---|---|
| PBMC (n = 37) | SIN (n = 36) | Overall (N = 37) | ||
| Arm A, 200 μg | 12 | 67 | 83 | 83 |
| Arm B, 400 μg | 12 | 42 | 91 | 92 |
| Arm C, 800 μg | 13 | 62 | 62 | 69 |
| Overall | 37 | 57 | 78 | 81 |
Abbreviations: PBMC, peripheral-blood mononuclear cell; SIN, sentinel immunized node.
No difference was noted among study groups when examined as a continuous response.
Responding cells are CD4+ T cells.
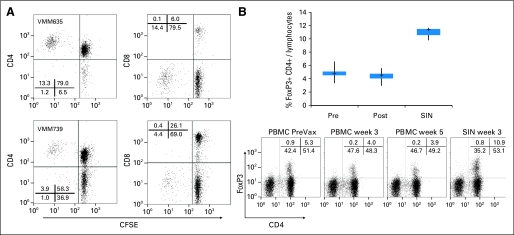
The melanoma helper peptides were originally identified as epitopes for CD4+ T lymphocytes; however, such peptides could contain embedded CD8 T-cell epitopes. To determine whether responding T cells were CD4+ or CD8+ cells, mononuclear cells were stained with carboxyfluorescein diacetate succinimidyl ester, cultured 5 days with 6MHP, and evaluated by flow cytometry. Proliferating cells were CD4+; there was no significant proliferation of CD8+ cells (Fig 3A).
Fig 3.
Identification and characterization of the cells responding to the six melanoma helper peptides (6MHP). (A) Two patients were evaluated for proliferative response to the 6MHP pool after labeling with carboxyfluorescein diacetate succinimidyl ester (CFSE). The proliferative response was observed among the CD4 T-cell population in both patients. (B) The proportion of CD4+ FoxP3-positive cells among circulating peripheral-blood mononuclear cells (PBMCs) or lymphocytes from the sentinel immunized node (SIN) was evaluated for five patients on the Mel41 trial. Flow cytometry plots are shown for one patient (VMM681); summary data (n = 5) are shown in a box plot, in which the box represents the 25th to 75th percentiles, the vertical lines represent minimum and maximum, and plus signs represent medians. PBMC data are for patients VMM625, VMM677, VMM681, VMM683, and VMM699; SIN data are for four of those patients, plus patient VMM727 instead of patient VMM677. Postvaccine data are inclusive of PBMC data analyzed at weeks 3, 5, 7, and 26. PreVax, before vaccine.
FoxP3-Positive CD4+ Cells Are Not Increased by Vaccination With Melanoma Helper Peptides
We evaluated whether vaccination with 6MHP may expand circulating CD4+ CD25HI FoxP3-positive regulatory T cells by staining PBMCs from five patients (Fig 3B). The proportion of FoxP3-positive cells did not increase during or after vaccination. The proportion of FoxP3-positive cells was higher in SIN than in PBMCs; we have observed this result in prior vaccine studies. Findings shown here are for FoxP3-positive CD4+ cells, but the same conclusion is drawn by evaluation of CD25HI CD4+ cells (data not shown).
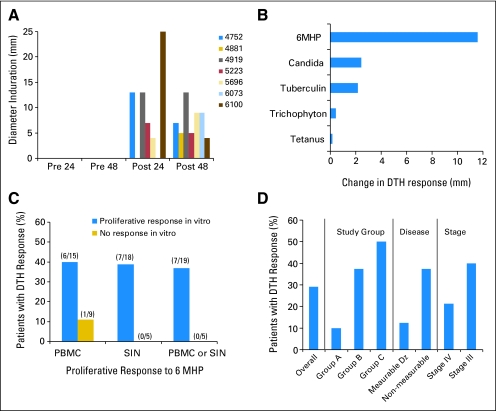
DTH Testing
Seven (29%) of 24 assessable patients developed DTH responses to 6MHP after vaccination (Fig 4A). Reasons for not being assessable for DTH included early disease progression, noncompliance, and failure to place DTH tests at week 12. Decreased reactivity to 6MHP was observed in only one patient. For the seven patients with DTH reactivity, increases in mean DTH reactivity to 6MHP were greater than changes in DTH reactivity to a panel of standard recall antigens (Fig 4B).
Fig 4.
Delayed-type hypersensitivity (DTH) assay data. DTH reactivity was identified in seven of 24 assessable patients. (A) The diameter of induration is shown at 24 and 48 hours for those seven patients, both before vaccine and at 12 weeks (post). (B) The change, from before vaccine to week 12, in induration diameter (maximum of 24 hours and 48 hours, subtracting maximum prevaccine value, averaged for all seven patients) is shown for reactions to the six melanoma helper peptides (6MHP) and to the standard recall antigens. (C) The association between DTH reactivity and proliferative responses is shown in peripheral-blood mononuclear cell (PBMC) and sentinel immunized node (SIN). (D) Associations between DTH reactivity and study group, disease status, and disease stage are presented based on the proportions of patients in each subgroup with DTH reactivity. The denominators for subgroups are as follows: overall (n = 24), group A (n = 10), group B (n = 8), group C (n = 6), measurable disease (Dz; n = 8), nonmeasurable disease (n = 16), stage IV (n = 14), and stage III (n = 10).
Among the 24 assessable patients, 37% of those with T-cell proliferation responses in vitro had DTH positivity, but all nonresponders were DTH negative (Fig 4C). There were trends toward associations between DTH reactivity and peptide dose, clinical disease status, and stage, but because of the small numbers, these trends cannot be meaningfully assessed for statistical significance (Fig 4D).
Clinical Outcomes
This study included 17 eligible patients with measurable disease (Table 1), among whom two patients experienced objective clinical tumor regressions (response rate = 12%) and two (12%) experienced durable stable disease. One objective response has been durable and ongoing for 3.9 years, and one response was durable for 1 year, after which surgical resection of a single site of progression has been followed by 1.5 additional years of stable disease. One patient with stable disease remains alive and free of progression at 4.6 years, whereas the other patient is alive at 2.4 years after having experienced progression at 1.8 years (Appendix Table A3, online only). Survival estimates at 18 months are 55% (95% CI, 30% to 74%) for patients with evidence of disease, 89% (95% CI, 63% to 97%) for patients clinically free of disease, and 72% (95% CI, 54% to 84%) for all patients (Appendix Table A4, online only).
DISCUSSION
To our knowledge, this is the first evaluation of this vaccine preparation, comprised of six peptides from melanocytic differentiation antigens and cancer testis antigens, and of four of the peptides within it to be performed in humans. The findings demonstrate safety and immunogenicity. Immune responses to the 6MHP pool were detected in more than 80% of patients (Table 2) across a wide range of HLA-DR molecules, suggesting that these peptides may be broadly relevant to the immune response to melanoma. Findings suggest promiscuity of these helper peptides across a wider range of HLA-DR molecules than originally reported (Fig 2A); a full analysis of this promiscuity is being prepared separately (manuscript in preparation). The immune responses were transient in some patients and persistent in others (Figs 2A and 2C). That some responses were detectable through week 39, more than 6 months after the last vaccine, suggests the possibility of memory T-cell induction. However, the transience of some responses also suggests immune regulatory processes that should be identified and targeted for combination immunotherapy in the future. A follow-up trial with these six helper peptides includes booster vaccines, which will be evaluated for maintaining responses.
In addition to in vitro evidence of immunogenicity of this helper peptide vaccine, we also observed in vivo evidence of immune reactivity based on DTH responses in seven (29%) of 24 assessable patients. There were also autoimmune reactivities in 21% of patients, including vitiligo in 10%, without associated symptoms. Clinical responses were observed in two of 17 patients with measurable disease, and durable disease stabilization occurred in two additional patients. Immune responses were identified to 6MHP for all patients with DTH responses, all patients with autoimmune toxicities, and all four patients with partial clinical responses or stable disease. Together, these data suggest both biologic activity and evidence of clinical activity. This phase I/II trial provides data that support larger studies of this six–helper peptide mixture with or without immunogens to stimulate CD8+ T cells.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Craig L. Slingluff Jr, Immatics (C), Bristol-Myers Squibb Co (C) Stock Ownership: None Honoraria: None Research Funding: Craig L. Slingluff Jr, Berlex/Bayer; William W. Grosh, Berlex/Bayer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Craig L. Slingluff Jr, Gina R. Petroni, William W. Grosh, Kimberly A. Chianese-Bullock, Robyn Fink, James W. Patterson
Administrative support: Craig L. Slingluff Jr, Gina R. Petroni, William W. Grosh, Mark Smolkin, Kimberly A. Chianese-Bullock, Donna H. Deacon, Robyn Fink, Patrice K. Rehm
Provision of study materials or patients: Craig L. Slingluff Jr, William W. Grosh, Patrice Y. Neese, Carmel Nail, Priscilla Merrill, James W. Patterson, Patrice K. Rehm
Collection and assembly of data: Craig L. Slingluff Jr, Gina R. Petroni, Walter Olson, Andrea Czarkowski, William W. Grosh, Mark Smolkin, Kimberly A. Chianese-Bullock, Patrice Y. Neese, Donna H. Deacon, Carmel Nail, Priscilla Merrill, Robyn Fink, Patrice K. Rehm
Data analysis and interpretation: Craig L. Slingluff Jr, Gina R. Petroni, Walter Olson, Andrea Czarkowski, Mark Smolkin, Kimberly A. Chianese-Bullock
Manuscript writing: Craig L. Slingluff Jr, Gina R. Petroni, Walter Olson, Andrea Czarkowski, Mark Smolkin
Final approval of manuscript: Craig L. Slingluff Jr, Gina R. Petroni, Walter Olson, Andrea Czarkowski, William W. Grosh, Mark Smolkin, Kimberly A. Chianese-Bullock, Patrice Y. Neese, Donna H. Deacon, Carmel Nail, Priscilla Merrill, Robyn Fink, James W. Patterson, Patrice K. Rehm
Appendix
Methods of Peptide Solubilization
Methods for solubilization in buffered aqueous solutions without dimethyl sulfoxide and creation of a stable sterile peptide mixture were determined at the University of Virginia. The peptide Tyrosinase386-406, FLLHHAFVDSIFEQWLQRHRP, was soluble in aqueous solution, but when reconstituted with the others at appropriate pH for their solubility, it partially precipitated, leading to a slightly cloudy solution.
Table A1.
Treatment-Related Toxicities of Melanoma Helper Peptide Vaccine
| Toxicity | Total Patients
|
Grade (No. of patients)
|
|||
|---|---|---|---|---|---|
| No. | % | 1 | 2 | 3 | |
| All patients, maximum toxicity grade | 39 | 100 | 4 | 34 | 1 |
| Allergy/immunology | 7 | 18 | 6 | 1 | — |
| Allergic rhinitis, nasal symptoms | 2 | 5 | 2 | — | — |
| Autoimmune reaction | 5 | 13 | 4 | 1 | — |
| Blood/bone marrow | 19 | 49 | 18 | 1 | — |
| Hemoglobin | 11 | 28 | 11 | — | — |
| Leukocytes (total WBC) | 2 | 5 | 2 | — | — |
| Lymphopenia | 5 | 13 | 5 | — | — |
| Neutrophils/granulocytes | 2 | 5 | 1 | 1 | — |
| Cardiovascular (general): edema | 1 | 3 | — | 1 | — |
| Coagulation: prothrombin time | 1 | 3 | 1 | — | — |
| Constitutional symptoms | 35 | 90 | 33 | 2 | — |
| Fatigue (lethargy, malaise, asthenia) | 33 | 85 | 31 | 2 | — |
| Fever | 7 | 18 | 7 | — | — |
| Rigors, chills | 11 | 28 | 11 | — | — |
| Sweating (diaphoresis) | 5 | 13 | 5 | — | — |
| Dermatology/skin | 39 | 100 | 4 | 34 | 1 |
| Dermatology/skin, other | 1 | 3 | 1 | — | — |
| Erythema | 1 | 3 | — | 1 | — |
| Flushing | 5 | 13 | 5 | — | — |
| Injection site reaction | 39 | 100 | 4 | 34 | 1 |
| Pigmentation changes (eg, vitiligo) | 4 | 10 | 4 | — | — |
| Pruritus | 2 | 5 | 2 | — | — |
| Rash/desquamation | 1 | 3 | 1 | — | — |
| Wound, noninfectious | 9 | 23 | 9 | — | — |
| GI | 12 | 31 | 11 | 1 | — |
| Anorexia | 2 | 5 | 1 | 1 | — |
| Constipation | 1 | 3 | 1 | — | — |
| Diarrhea | 3 | 8 | 3 | — | — |
| Flatulence | 1 | 3 | 1 | — | — |
| Mouth dryness | 1 | 3 | 1 | — | — |
| Nausea | 8 | 21 | 7 | 1 | — |
| Stomatitis/pharyngitis | 2 | 5 | 2 | — | — |
| Taste disturbance (dysgeusia) | 1 | 3 | 1 | — | — |
| Vomiting | 2 | 5 | 1 | 1 | — |
| Hepatic: bilirubin, AST, or ALT | 4 | 10 | 4 | — | — |
| Metabolic/laboratory | 32 | 82 | 32 | — | — |
| Hyperglycemia | 19 | 49 | 19 | — | — |
| Hyperkalemia | 13 | 33 | 13 | — | — |
| Hypernatremia | 1 | 3 | 1 | — | — |
| Hypocalcemia | 3 | 8 | 3 | — | — |
| Hypoglycemia | 4 | 10 | 4 | — | — |
| Hypokalemia | 3 | 8 | 3 | — | — |
| Hypomagnesemia | 3 | 8 | 3 | — | — |
| Hyponatremia | 6 | 15 | 6 | — | — |
| Hypophosphatemia | 1 | 3 | 1 | — | — |
| Neurology: dizziness/lightheadedness | 8 | 21 | 8 | — | — |
| Ocular/visual: vision, blurred vision | 1 | 3 | 1 | — | — |
| Pain | 20 | 51 | 18 | 2 | — |
| Abdominal pain or cramping | 2 | 5 | 2 | — | — |
| Arthralgia (joint pain) | 7 | 18 | 7 | — | — |
| Headache | 16 | 41 | 16 | — | — |
| Myalgia (muscle pain) | 11 | 28 | 11 | — | — |
| Pain, other | 2 | 5 | — | 2 | — |
| Pulmonary | 5 | 13 | 5 | — | — |
| Cough | 1 | 3 | 1 | — | — |
| Dyspnea (shortness of breath) | 2 | 5 | 2 | — | — |
| Voice changes/stridor/larynx | 3 | 8 | 3 | — | — |
| Renal/genitourinary: creatinine | 5 | 13 | 5 | — | — |
Table A2.
Patients With Autoimmune Findings
| Patient Registration No. | Study Group | Vitiligo | Maximum No. of Weeks ANA and RF Were Evaluated | ANA | RF | Prior Immunotherapy (or treatment other than surgery) | Proliferative Response |
|---|---|---|---|---|---|---|---|
| 2302 | A | No | 52 | Negative | 5 weeks: 117*; 12 weeks: 126*; 1 year: 203* | None | Yes |
| 2611 | B | No | 12 | Negative | Pretreatment: < 20; 12 weeks: 96.9* | None | Yes |
| 4502 | B | Yes | 52 | Negative | Pretreatment: < 20; 7 weeks: 30*; 12 weeks: < 20; 1 year: < 20 | None | Yes |
| 4652 | C | No | 12 | Negative | Pretreatment: 25.5; 5 weeks: 39.1*; 12 weeks: 26.7 | None | Yes |
| 4924 | B | Yes | 12 | Negative | Pretreatment: 34.2; 5 weeks: < 20; 12 weeks: < 20 | Mel43 peptide vaccine trial | Yes |
| 5270 | A | Yes | 12 | Negative | Negative | Mel43 peptide vaccine trial | Yes |
| 5500 | A | No | 12 | Pretreatment: ANA negative; 12 weeks: ANA positive 1:160* | Negative | IFN | Yes |
| 5813 | A | Yes | 52 | Negative | Negative | Intratumoral BCG injections; Mel36 peptide vaccine trial | Yes |
Abbreviations: ANA, antinuclear antibody (measured as a titer); RF, rheumatoid factor (measured in units); IFN, interferon; BCG, Bacille Calmette-Guérin.
Abnormal value.
Table A3.
Clinical Response Summary
| Patient Registration No. | Study Arm | Best Response | PFS (years) | Overall Survival (years) | Immune Response* SIN | Immune Response* PBMC |
|---|---|---|---|---|---|---|
| 2302 | A | SD | 4.6† | 4.6 | Yes | Yes |
| 4366 | A | PR | 3.9† | 3.9 | Yes | No |
| 5185 | C | PR | 1.0‡ | 2.9 | Yes | Yes |
| 5850 | C | SD | 1.8‡ | 2.3 | No | Yes |
Abbreviations: PFS, progression-free survival; SD, stable disease; PR, partial response; SIN, sentinel immunized node; PBMC, peripheral-blood mononuclear cell.
Immune response indicates immune response detected in vitro by proliferative response to the six melanoma helper pool.
Patient remains clinically free of progression at last follow-up.
Progression of disease at this interval.
Table A4.
Survival Information by Clinical Disease Status
| Parameter | Clinical Evidence of Disease | No Clinical Evidence of Disease | All Eligible Patients |
|---|---|---|---|
| No. of patients eligible and assessable | 18 | 19 | 37 |
| Median survival estimate, months | 18.7 | Too early to tell | 36.2 |
| Median follow-up time, months | 17.6 | 24 | 22.4 |
| Survival estimates | |||
| 6 months | |||
| % | 89 | 95 | 92 |
| 95% CI, % | 62 to 97 | 68 to 99 | 77 to 97 |
| 12 months | |||
| % | 72 | 95 | 84 |
| 95% CI, % | 46 to 87 | 68 to 99 | 67 to 92 |
| 18 months | |||
| % | 55 | 89 | 72 |
| 95% CI, % | 30 to 74 | 63 to 97 | 54 to 84 |
published online ahead of print at www.jco.org on September 22, 2008
Supported by National Institutes of Health (NIH)/National Cancer Institute (NCI) Grant No. R21 CA105777 (C.L.S). Support was also provided by a University of Virginia (UVA) Cancer Center Support grant (Grant No. NIH/NCI P30 CA44579, Clinical Trials Office, Tissue Procurement Facility, Flow Cytometry Core, and Biomolecular Core Facility) and the UVA General Clinical Research Center (Grant No. NIH M01 RR00847). Peptides used in this vaccine were prepared with philanthropic support from Alice T. and William H. Goodwin Jr. Granulocyte-macrophage colony-stimulating factor (Berlex, now Bayer) and Montanide ISA-51 (Seppic Inc) were used in the vaccines in this trial, but these were paid for by UVA.
Presented in part at the 21st Annual Meeting of the International Society for the Biological Therapy of Cancer, October 26-29, 2006, Los Angeles, CA.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00089219
REFERENCES
- 1.Mortara L, Gras-Masse H, Rommens C, et al: Type 1 CD4(+) T-cell help is required for induction of antipeptide multispecific cytotoxic T lymphocytes by a lipopeptidic vaccine in rhesus macaques. J Virol 73:4447-4451, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoenberger SP, Toes RE, van der Voort EL, et al: T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480-483, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Hung K, Hayashi R, Lafond-Walker A, et al: The central role of CD4+ T-cells in the antitumor immune response. J Exp Med 188:2357-2368, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui S, Ahlers JD, Vortmeyer AO, et al: A model for CD8+ CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. J Immunol 163:184-193, 1999 [PubMed] [Google Scholar]
- 5.Slingluff CL Jr, Yamshchikov G, Neese P, et al: Phase I trial of a melanoma vaccine with gp100(280-288) peptide and tetanus helper peptide in adjuvant: Immunologic and clinical outcomes. Clin Cancer Res 7:3012-3024, 2001 [PubMed] [Google Scholar]
- 6.Slingluff CL Jr, Chianese-Bullock KA, Bullock TN, et al: Immunity to melanoma antigens: From self-tolerance to immunotherapy. Adv Immunol 90:243-295, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Topalian SL, Gonzales MI, Parkhurst M, et al: Melanoma-specific CD4+ T cells recognize nonmutated HLA-DR-restricted tyrosinase epitopes. J Exp Med 183:1965-1971, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi H, Kokubo T, Sato K, et al: CD4+ T cells from peripheral blood of a melanoma patient recognize peptides derived from nonmutated tyrosinase. Cancer Res 58:296-301, 1998 [PubMed] [Google Scholar]
- 9.Zarour HM, Kirkwood JM, Kierstead LS, et al: Melan-A/MART-1(51-73) represents an immunogenic HLA-DR4-restricted epitope recognized by melanoma-reactive CD4(+) T cells. Proc Natl Acad Sci U S A 97:400-405, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manici S, Sturniolo T, Imro MA, et al: Melanoma cells present a MAGE-3 epitope to CD4(+) cytotoxic T cells in association with histocompatibility leukocyte antigen DR11. J Exp Med 189:871-876, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaux P, Vantomme V, Stroobant V, et al: Identification of MAGE-3 epitopes presented by HLA-DR molecules to CD4(+) T lymphocytes. J Exp Med 189:767-778, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halder T, Pawelec G, Kirkin AF, et al: Isolation of novel HLA-DR restricted potential tumor-associated antigens from the melanoma cell line FM3. Cancer Res 57:3238-3244, 1997 [PubMed] [Google Scholar]
- 13.Li K, Adibzadeh M, Halder T, et al: Tumour-specific MHC-class-II-restricted responses after in vitro sensitization to synthetic peptides corresponding to gp100 and Annexin II eluted from melanoma cells. Cancer Immunol Immunother 47:32-38, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khong HT, Yang JC, Topalian SL, et al: Immunization of HLA-A*0201 and/or HLA-DPbeta1*04 patients with metastatic melanoma using epitopes from the NY-ESO-1 antigen. J Immunother 27:472-477, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phan GQ, Touloukian CE, Yang JC, et al: Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother 26:349-356, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong R, Lau R, Chang J, et al: Immune responses to a class II helper peptide epitope in patients with stage III/IV resected melanoma. Clin Cancer Res 10:5004-5013, 2004 [DOI] [PubMed] [Google Scholar]
- 16a.Bechhofer RE, Santner TJ, Goldman DM: Design and Analysis of Experiments for Statistical Selection, Screening, and Multiple Comparisons. New York, NY, Wiley, 1995
- 17.Yamshchikov GV, Barnd DL, Eastham S, et al: Evaluation of peptide vaccine immunogenicity in draining lymph nodes and blood of melanoma patients. Int J Cancer 92:703-711, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Thompson LW, Hogan KT, Caldwell JA, et al: Preventing the spontaneous modification of an HLA-A2-restricted peptide at an N-terminal glutamine or an internal cysteine residue enhances peptide antigenicity. J Immunother 27:177-183, 2004 [DOI] [PubMed] [Google Scholar]