Abstract
There are only a handful of concepts concerning cancer and carcinogenesis that are currently beyond dispute. One such dogma is the adenoma-carcinoma sequence and that a multistep accumulation of genetic alterations is required for transformation from a benign to a neoplastic tissue. The inevitable derivative of this dogma is that every invasive carcinoma is in fact a missed intraepithelial tumor, and furthermore, a late evolutionary stage in the sequence of development from a precursor lesion. Until fairly recently, high-grade serous ovarian carcinoma seemed to be one of the only known deviants of these concepts. In this article, we discuss the emergence of the fallopian tube fimbria as a field of origin for high-grade serous carcinomas and present a binary model of ovarian cancer pathogenesis that takes into consideration prior epidemiologic, morphologic, and genetic data. With the rise of the fallopian tube secretory epithelial cell as a cell of origin for high-grade pelvic serous carcinomas, the need to develop tools and model systems to characterize the biology and physiology of this cell is recognized.
INTRODUCTION
The body of literature on the involvement of oncogenes and tumor suppressors in the pathogenesis of many epithelial tumors has grown tremendously over the past two decades. Despite the diversity of aberrant pathways, it is now possible to begin to sketch out common themes for many epithelial cancers and their precursor lesions. For instance, recent studies demonstrate that in most epithelial tumors, one of the earliest identifiable changes in precursor lesions is the activation of the DNA damage repair (DDR) machinery and nuclear accumulation of p53 as recently reviewed by Halazonetis et al.1 In this model, the DDR pathway is activated by replicative stress and is considered a barrier to malignant transformation by inducing cell cycle arrest, senescence,2 and apoptosis.
Most epithelial tumors also follow the adenoma-carcinoma sequence3 scheme whereby a normal epithelial cell undergoes genetic alterations of increasing complexity resulting in a morphological evolution toward a state that is phenotypically consistent with a tumor cell.4 This pathway has been described in most epithelial tumors including colorectal carcinoma, where this sequence was first described by Jackman and Mayo in 1951,1 breast, thyroid, prostate, bladder,5 gastric, esophageal, pancreatic, cervical, endometrial, head and neck squamous cell carcinoma, skin,6 and non–small-cell lung carcinoma.6 A subset of low-grade adenocarcinomas of the ovary, specifically endometrioid and mucinous tumors, also follow a similar sequence.7 Therefore, the two models of epithelial carcinogenesis, the DDR pathway and the adenoma-carcinoma pathway, are active in most cancers studied. However, high-grade serous ovarian carcinomas (HGSOC), which typically emerge in the absence of recognizable pre-existing conditions, have been one important exception. In fact, the reproducible identification of a precursor and preinvasive lesion for this tumor has eluded clinicians and scientists for decades.
The apparent defiance of serous carcinomas to conform to the adenoma-carcinoma or DDR models is likely linked to the propensity of this tumor to spread early in its course. The dramatically rapid spread of this malignancy in the pelvis makes early-stage detection a highly rare event, and even in such cases finding a clear spectrum of continuous development from hyperproliferative to neoplastic tissue requires deliberate rigorous pathologic examination. Is it possible that serous ovarian carcinoma is indeed a fundamentally different entity, with exceptional biologic behavior, or have we been looking in the wrong place all along?
TWO CLASSES OF OVARIAN CANCER
Recent molecular understanding correlated well with what was known by oncologists and pathologists for a long time: high-grade serous tumors differ from all other ovarian carcinomas in terms of their development, prognosis, pathologic findings, and underlying genetic alterations. This leads to the classification of ovarian cancers into type 1 tumors which are low grade and slowly developing (including endometrioid, mucinous and low grade serous); and type 2 tumors which are rapidly progressing high-grade serous carcinomas.8-10 Expression profiling studies have shown that high-grade tumors cluster separately from low-grade carcinomas and borderline tumors.11,12 Moreover, the former is associated strongly with TP53 mutations, whereas the latter are associated with mutations in KRAS, BRAF,13 PTEN,14 and CTNNB1/β-catenin.15
The serous subtype of ovarian carcinoma accounts for approximately 60% to 80% of ovarian cancer cases and is by far the most aggressive histology. Fewer than 25% of the cases are detected at an early stage (stages I and II), a statistic which reflects grimly on the survival figures.16,17 Nearly 22,500 women are diagnosed with ovarian cancer every year in the United States alone, with approximately 200,000 new cases worldwide, and more than 50% die of this disease.18-20 High-grade serous carcinoma involves the surface of the ovary, often bilaterally, and the peritoneal membranes, with rapid onset of carcinomatosis, a fact that restricts the surgical options to debulking only. Despite the introduction of taxanes to the therapeutic protocols and the prolonged survival with intraperitoneal chemotherapy administration, there has been little progress in improving cure rates, a parameter that is still solely dependent on the disease stage at time of presentation. Certain targeted therapeutics have advanced into clinical trials in the past few years, but most of them have met with little success.
Several germ-line mutations and copy number variations that harbor increased risk for HGSOC have been reported, BRCA1 and BRCA2 being the most prevalent ones, accounting for 5% to 10% of the cases with up to 54% lifetime risk.21 Women recognized to have BRCA mutations are currently treated with risk-reducing (prophylactic) excision of the adnexa (bilateral salpingo-oophorectomy [BSO]). This practice, which targets healthy women, has provided most of the emerging data in the study of early serous carcinomas, as will be discussed later.
The ongoing debate about the cell of origin of pelvic serous carcinomas (defined as tumors of serous histology arising in the ovary, fallopian tube, or peritoneum) was previously comprehensively reviewed by Piek et al,22 and from a pathological point of view by Crum et al.23-25 An updated review of the genetic pathways to ovarian carcinogenesis, including the serous subtype was recently published by Landen et al.8 This review aims to confront the traditional models for serous carcinogenesis with accumulating data about the fallopian tube being the site-of-origin of a large proportion of high-grade pelvic serous carcinomas. We will focus on the proposed precursor lesions, and show that they comply with the established models of epithelial carcinogenesis. We will discuss the need to develop experimental model systems to study this epithelium and will raise questions, both clinical and biologic, that for the first time can actually be addressed as the fog surrounding the origin of this disease begins to clear.
OVARIAN SURFACE EPITHELIUM AND THE CORTICAL INCLUSION CYST
The single layer of ovarian surface epithelium (OSE) constitutes less than 1% of the total ovarian mass, yet more than 90% of ovarian cancers are described as epithelial in origin. The OSE cells are uncommitted mesothelial cells that express both epithelial and mesenchymal markers.26-28 However, human OSE normally does not express certain markers of ovarian carcinomas such as E-cadherin, CA-125, and human epididymis protein 4 (HE4).29,30
The OSE has endured as the field of origin for ovarian cancers since the dawn of the incessant ovulation hypothesis, coined by Fathalla in 1971.31 This hypothesis focuses on the OSE because of its exposure to repeated trauma and repair processes with every ovulatory cycle. It combines observations about spontaneous ovarian cancer in animals, specifically hyperovulated hens32 and epidemiological data about risk factors in humans,33 and hypothesizes that the greater the total number of lifetime ovulatory cycles the greater the risk for derangement of the repair mechanism of the OSE, leading to ovarian carcinoma. Over the years this model was expanded to account for the contribution of inflammation to ovarian carcinogenesis.28 In this scenario, normal ovulation is seen as an inflammatory response, involving cellular infiltration as well as cytokines’ and chemokines’ release.34,35 The main mediators are nitric oxide, prostaglandins, IL-1β, IL-6, IL-8, tumor necrosis factor-α, and neutrophil elastase.35-41 Presumably these factors may induce DNA damage in OSE cells, which along with the tissue repair processes proposed by Fathalla may result in neoplastic transformation.
Two other hypotheses relate to the hormone responsiveness of the OSE, which expresses receptors for gonadotrophin-releasing hormone, luteinizing hormone, follicle-stimulating hormone, estrogen, androgen, and progesterone. The gonadotrophin hypothesis suggests that the exposure to excessive hormone concentrations may be an independent facilitator of malignant transformation of the OSE.42-44 High gonadotrophin levels are characteristic in menopause, the polycystic ovary syndrome,45 infertility treatments or low parity and in women that do not use oral contraceptives,46 all of which are established risk factors for ovarian cancer.47 The hormone stimulation hypothesis regards elevated androgens as a predisposing factor, while progesterone it is a protective factor.46 It reconciles observations about increased risk in polycystic ovary syndrome, and protection conferred by gestation and oral contraception. It is likely that a combination of various aspects of these models contribute to the transformation of the OSE and the development of ovarian carcinomas.
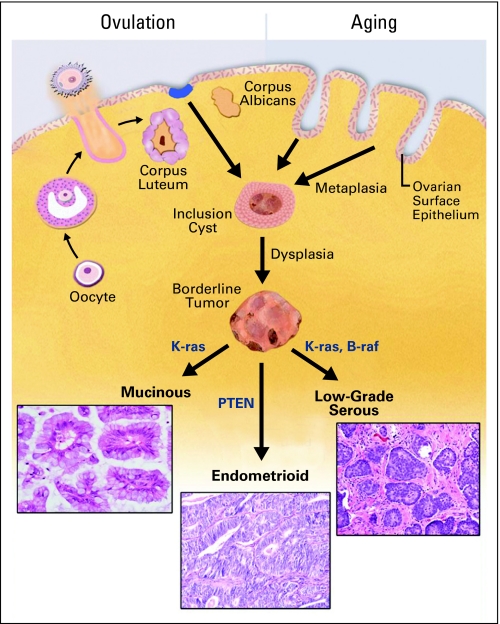
There is strong evidence that many of the tumors of borderline malignancy and low-grade carcinomas of the ovary arise from cortical inclusion cysts (CICs) within the ovarian parenchyma underlying the OSE. These benign cysts are composed of Müllerian epithelium that closely resembles the fallopian tube. One proposed origin of CICs is invaginations of OSE into the stroma of the ovary as a result of ovulation and aging, with acquisition of a Müllerian epithelial phenotype through metaplasia.27,28,48 Another possible source is shedding of tubal epithelium (endosalpingiosis). Irrespective of the mechanism, CICs are lined with Müllerian epithelium that is exposed to the ovarian cortical stromal milieu. Presumably, as a result of hormone exposure and wound remodeling that occurs in response to ovulation, these cells are rendered susceptible to neoplastic transformation and in some cases give rise to mucinous, endometrioid and low-grade serous carcinomas (Fig 1).49-52 CICs often occur adjacent to benign epithelial tumors, suggesting lineage continuity. Many of the endometrioid and mucinous carcinomas are associated with these benign tumors, a convincing link between CICs and these malignancies. In contrast to these tumors, a morphologic continuum from OSE to HGSOC has not been mapped and there has been scant evidence for the existence of a precursor lesion in the OSE or the CICs. Moreover, the majority of ovarian carcinomas (serous, mucinous, and endometrioid) seem to bear little resemblance to their proposed cell of origin (the OSE), recapitulating the histological features of Müllerian epithelia of the fallopian tube, cervix, and endometrium, respectively.53,54
Fig 1.
Transformation of ovarian surface epithelium (OSE). The OSE undergoes cyclic ovulation-induced rupture, leading to formation of cortical inclusion cysts (CICs). Entrapped within the ovarian cortex, the OSE undergoes Müllerian metaplasia, and is exposed to hormone and inflammatory stimuli that induce replicative stress and DNA damage which can lead to defined mutations and transformation into mucinous, endometrioid, and low-grade serous carcinomas.
Several studies have extensively examined the epithelium of ovaries removed as part of risk-reducing BSO procedures. Their findings have not established a consistent relationship between CICs and serous carcinomas. While some groups reported an increase in the number of inclusion cysts, metaplastic changes in these cysts, and cellular abnormalities55-57 others publications disputed these findings.58-62 The most recent study by Folkins et al, examining the largest cohort reported thus far, extensively studied 75 cases of prophylactic BSO from women who were BRCA1/2 mutation positive. Only one case harbored a questionable precursor lesion on the OSE, characterized by p53 staining.63 These observations raise the following questions: is the origin of HGSOC fundamentally different from other ovarian carcinomas? Is there a serous carcinogenic sequence? If so, where does this sequence occur?
EMERGENCE OF THE FALLOPIAN TUBE AS A SITE OF ORIGIN
Extrauterine serous carcinoma is presumed to arise in three different locations in the female pelvis: the ovary (serous ovarian carcinoma), the endosalpinx (serous fallopian tube carcinoma [FTSC]), and on the peritoneal surface (primary peritoneal serous carcinoma). The assignment of tumor origin by pathologists has been predicated on pathologic criteria that focus on the location of the bulk of the disease. Because most serous carcinomas present as large ovarian masses, ovarian cases outnumber the other two by about a 50:1 ratio. The only site with a recognizable “early” carcinoma is the fallopian tube. A diagnosis of FTSC requires that four criteria be met:64-66 the main tumor is in the fallopian tube and arises from the endosalpinx, histology matches the tubal phenotype, if the tubal wall is invaded there has to be an evident transition between normal and malignant tubal epithelium, and the fallopian tube should contain more tumor than the ovary or endometrium. It is noteworthy that tumors meeting these criteria are uncommon, such that FTSC is decidedly a rare diagnosis. This is in part due to two facts. The first is that tumors presenting as large tubal masses are uncommon. The second is that pathologists do not customarily examine the entire fallopian tube in cases of pelvic serous carcinoma, but rather only a perfunctory section of the central fallopian tube. In retrospect, it is this practice that delayed the appreciation of the role of the distal fallopian tube—the fimbria—in serous carcinogenesis.
The aforementioned facts notwithstanding, the role of the fallopian tube in serous carcinogenesis was not ignored. Although the reported average annual incidence of FTSC in the United States is believed to be 0.3 per 100,000 women per year (compared with 16 per 100,000 per year in ovarian cancer),67 some investigators appreciated that BRCA1 and BRCA2 germ-line mutations confer an increased risk for FTSC. In a prospective study of 381 BRCA1 mutation carriers, Brose et al reported a 120-fold increased risk of fallopian tube cancer compared with the general population.68 Bannatyne and Russell69 commented that rigorous sectioning is essential to detect early tubal carcinomas. They anticipated finding tubal intraepithelial carcinoma (TIC) in 5% to 10% of serous ovarian cancer cases and suggested this phenomenon signified multifocal serous neoplasia. A reassessment of the incidence and the prognostic and clinicopathological features of this malignancy became possible with the widespread practice of prophylactic BSO for management of patients with familial high risk. In contrast to the standard pathologic sampling of the ampullary region of the fallopian tube in ovarian cancer cases, the more extensive complete sectioning of the tube highlighted the relative abundance of lesions specifically in the fimbria. Table 170-86 summarizes the results of published studies looking at the incidence of fallopian tube intraepithelial or invasive carcinoma in patients with the diagnosis of pelvic serous carcinoma or in patients undergoing risk-reducing BSO.
Table 1.
Involvement of the FT in Patients With Invasive Pelvic Carcinomas and in High-Risk Population Undergoing Prophylactic BSO
| Study | No. of Patients |
BRCA Mutations Status
|
Pelvic Carcinoma
|
Involvement of FT
|
Extensive Sectioning | Comments | |||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||||
| Pelvic carcinoma | |||||||||
| Agoff et al70 | 7 | 4/7 | 57 | 7 | 100 | 5/7 | 71 | + | Diagnosis of two FT malignancies required peritoneal lavage |
| Piek et al71 | 50 | NA | 50/50 | 100 | 3/50 | 6 | NA | ||
| Kindelberger et al72 | 55 | NA | 55/55 | 100 | 41/55 | 75 | + | 93% of TICs occurred in the fimbria | |
| Carlson et al73 | 45 | NA | 45/45 Primary peritoneal carcinoma | 100 | 28/45 | 62 | ± | 26 cases had incomplete pathologic examination and the rate of tubal involvement was 50%, while the next 19 cases, which underwent extensive sectioning had tubal involvement rate of 79% | |
| High-risk prophylactic BSO v general population | |||||||||
| Carcangiu et al74 | 26 high-risk v 49 control BSOs | BRCA1, 22 | 85 | 2/26 | 7.7 | 2/2 | 100 | NA | |
| BRCA, 24 | 15 | ||||||||
| Medeiros et al75 | 13 v 13 control BSO | NA | 5/13 | 38 | 5/5 | 100 | + | 4/5 (80%) of cases involved the fimbria | |
| High-risk prophylactic BSO | |||||||||
| Lu et al76 | 50 | NA | 4/50 | 8 | 0/4 | + (37/50 cases) | |||
| Kauff et al77 | 98 | BRCA1, 56 | 57 | 0 | 0 | − | One case of primary peritoneal carcinoma 16.3 months after BSO | ||
| BRCA2, 42 | 43 | ||||||||
| Leeper et al78 | 30 | BRCA1, 19 | 63.5 | 5/30 | 16.6 | 3/5 | 60 | + | |
| BRCA2, 4 | 13.5 | ||||||||
| No mutation detected, 7 | 23 | ||||||||
| Colgan et al79 | 60 | 5/60 | 8 | 2/5 | 40 | + | |||
| Olopade et al80 | 98 | 98/98 | 3/98 | 3 | 0/3 | − | |||
| Olivier et al81 | 90 | BRCA1, 58 | 64 | 5/90 | 5.5 | 3/5 | 60 | + | Three additional cases of primary peritoneal cancer detected in follow-up; 38 additional high-risk patients underwent only prophylactic oophorectomy—no malignancies found |
| BRCA2, 6 | 7 | ||||||||
| Both BRCA1/2, 1 | 1 | ||||||||
| Not specified, 25 | 28 | ||||||||
| Powell et al82 | 67 | BRCA1, 43 | 64 | 7/41 | 17 | 4/7 | 57 | + (41/67 cases) | No abnormalities detected in 25 standard protocol cases; two additional cases of primary peritoneal cancer detected in follow-up |
| BRCA2, 24 | 36 | ||||||||
| Meeuwissen et al83 | 133 | BRCA1/2, 86 | 65 | 1/133 | 0.75 | 1/1 | 100 | − | One additional case of primary peritoneal cancer detected in follow-up |
| Unknown, 47 | 35 | ||||||||
| Lamb et al84 | 113 | BRCA1, 40 | 35 | 7/113 | 6.2 | 5/7 | 71 | + | One of the two cases of malignancies that did not involve the FT was diagnosed as ovarian borderline adenofibroma with epithelial cytological atypia |
| BRCA2, 22 | 19 | ||||||||
| Not tested or no mutation, 51 | 45 | ||||||||
| Finch et al85 | 159 | BRCA1, 94 | 59 | 7/159 | 4.4 | 6/7 | 85 | + | |
| BRCA2, 65 | 41 | ||||||||
| Callahan et al86 | 122 | BRCA1, 60 | 49 | 7/122 | 5.7 | 7/7 | 100 | + | 6/7 (85%) of findings occurred in the fimbria, 1/7 involved the ampulla |
| BRCA2, 60 | 49 | ||||||||
| Not specified, 2 | 2 | ||||||||
Abbreviations: FT, fallopian tube; BSO, bilateral salpingo-oophorectomy; TIC, tubal intraepithelial carcinoma; NA, not available.
What have emerged from this data are two realizations. First, although the majority of serous carcinomas in symptomatic women with BRCA 1/2 mutations are attributed to the ovary, a majority of early serous malignancies (TICs) detected in risk-reducing BSOs of healthy women are localized to the distal fallopian tube. Second, in women with pelvic serous carcinoma whose BRCA status is unknown, TICs can be detected in approximately 50% of cases. Moreover, analysis of TP53 mutations in the TICs and the adjacent bulky carcinomas in these cases invariably reveal shared mutations, further supporting an origin in the distal fallopian tube.72
The conclusions from these works are that the initial modified criteria by Hu et al64-66 for the diagnosis of FTSC detects only a portion of FTSCs and that the fallopian tube is a major site of origin for pelvic serous cancer irrespective of BRCA status.
MISSING PRECURSOR LESION
The classical adenoma-carcinoma sequence model not only outlines the transition from an intraepithelial to an invasive lesion, but also highlights the latent precursor phase, that may or may not eventually progress to a neoplasm. In the distal fallopian tube, TICs cannot be considered precursor lesions, as they are widely viewed as frank malignancies that will eventually spread if not detected. What has been missing in the serous carcinogenesis sequence has been a bonafide precursor with limited potential of progressing to malignancy. Thus, the question has been whether one could find the precursor that preceded TIC and its lethal consequence, pelvic serous carcinoma.
Piek et al87 were the first to describe dysplastic changes in the fallopian tubes of 12 patients with familial high risk for ovarian cancer undergoing prophylactic BSO, and 13 control cases without increased risk. The dysplastic regions were pure secretory cell segments and displayed a higher proliferative index (demonstrated by positive Ki-67 staining). However, only one of these lesions displayed increase nuclear p53 staining.87 Carcangiu et al reported atypical hyperplastic lesions in two of 22 (9%) prophylactically resected fallopian tubes of BRCA1 mutation carriers.74 These studies, however, did not identify a discrete entity that was associated with the most common genetic defect of HGSOC—TP53 mutations. Resolving the earliest step in the TP53 mutation pathway was needed to bring forward a novel precursor to pelvic serous cancer.
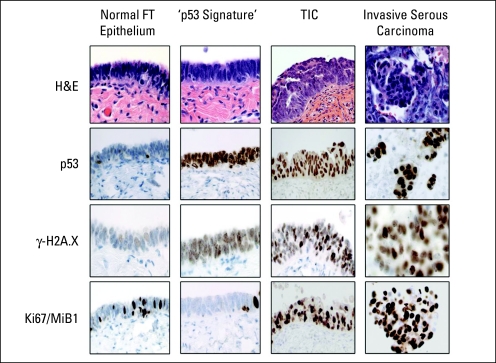
The mentioned studies focused on the secretory epithelial cells of the fallopian tube (FTSECs). While the normal fallopian tube epithelium is a mixture of ciliated and secretory cells, the TIC and the invasive FTSC as well as HGSOC are comprised of only secretory cells. Using a protocol for sectioning and extensively examining the fallopian tube fimbria, termed the SEE-FIM protocol,75 Lee et al88 detected discrete segments of secretory cells with strong nuclear p53 immunostaining in benign-appearing tubal mucosa, which were termed p53 signatures. Interestingly, the p53 signatures displayed evidence for genome-wide DNA damage (as measured by positive immunohistochemical staining for phoshorylated histone H2A.X), a finding common to other epithelial precursors described by Bartkova et al5 and Gorgoulis et al.6 TP53 gene mutations were reliably detected in eight of 14 p53 signatures tested (57%).88 Figure 2 shows the histological characteristics of the spectrum of carcinogenic changes in the fallopian tube epithelium, ranging from normal to p53 signature, TIC, and invasive HGSOC.
Fig 2.
Pathologic features of the fallopian tube (FT) carcinogenesis spectrum. While normal FT epithelium contains both ciliated and secretory cells, p53 signature—the proposed precursor lesion—is characterized by normal tissue morphology with p53-positive secretory cells harboring DNA damage (γ-H2A.X staining). Tubal intraepithelial carcinoma (TIC) shares these features but has acquired a proliferative advantage (increased Ki-67/MiB1 staining). Invasive serous carcinoma shows increased proliferation and disruption of the basement membrane.
The same group compared 41 BRCA mutation carriers versus 58 cases undergoing BSO for benign indications and 17 patients with TICs. The p53 signatures occurred in 37% and 33% of fallopian tubes of these cases, respectively. Approximately 80% were detected in the fimbria. In fallopian tubes harboring TICs, p53 signatures were detected in 53% of the cases. Occasionally direct continuity was detected.89 Rarely p53-positive foci with features intermediate between p53 signatures and TICs were identified. These transition lesions, like the dysplasias previously reported by Piek et al,87 have an increased proliferation index (represented by high Ki-67/MiB1 scores) compared with p53 signatures but less so than TICs. No similar findings were found on the surface of ovaries removed in risk-reducing BSOs or for other benign indications.63
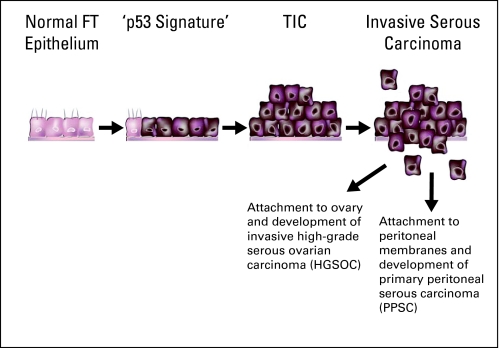
In summary, the findings from these recent studies reveal a spectrum of visible epithelial alterations that offer a plausible serous carcinogenic model with three stages: the p53 signature, the in situ carcinoma (TIC), and the invasive carcinoma. All of the components of this spectrum share the following features with frankly malignant serous carcinoma: secretory-cell phenotype, evidence of genome-wide DNA damage, TP53 gene mutations, and a predominant fimbrial localization (Figs 3 and 4). The p53 signature precursors are similarly prevalent in women with familial high risk for ovarian cancer and in the general population. One mystery that remains to be unraveled is why the fallopian tube is invariably linked to BRCA1- and BRCA2-associated malignancies and to only one half of HGSOC in the general population. Conceivably, BRCA mutation carriers are either more susceptible to the aberrations in the pathways that lead to fallopian tube transformation, or conversely, less susceptible to transformation associated with CICs. Deciphering the answer to this question requires a thorough understanding of the carcinogenic pathways involving the two sites of origin.
Fig 3.
High-grade serous carcinogenic sequence. The spectrum of fallopian tube (FT) epithelial transformation ranges from normal epithelium, through p53 signature, an intraepithelial, and eventually, an invasive carcinoma. Exfoliation into the peritoneal cavity is an early event. This model suggests that all types of pelvic serous tumors are the same entity, the majority of which originate from the FT fimbria. TIC, tubal intraepithelial carcinoma.
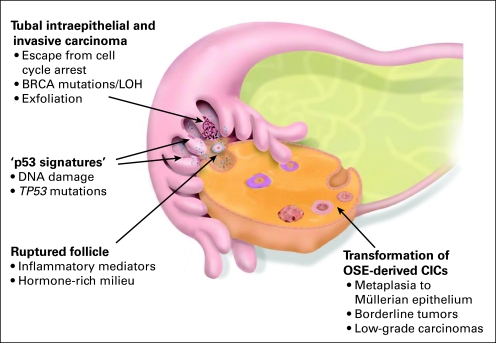
Fig 4.
An integrated model of high-grade serous carcinogenesis. This model integrates the data about the stepwise development of serous carcinoma in the fimbria of the fallopian tube (FT) and in the ovarian surface epithelium (OSE) –derived cortical inclusion cysts (CICs). The hormone stimulation and the inflammatory mediators involved in ovulation are believed to have similar carcinogenic effect in both pathways.
FTSEC: THE PRIME SUSPECT EMERGES
Identification of the field of origin of serous carcinogenesis, immediately positions the FTSEC in the spotlight as being the cell of origin. The current knowledge about the normal biology of the fallopian tube is very limited, and most of the data originates from the discipline of assisted reproduction. As previously mentioned, the normal epithelium of the fallopian tube is comprised of two cell types: ciliated and secretory. The ciliated cells of the fallopian tube play a major role in the transport of the ovum, the sperm cells, and the zygote. Several markers uniquely distinguish the ciliated cells from the FTSEC: LhS28 (marks 9+2 ciliary basal bodies),90 CDKN1A/p21/WAF (cell cycle–related protein),91 and Foxj1 (transcription marker expressed during ciliogenesis).92,93
The secretory cells secrete mucus that slows the progression of the spermatozoa through the fallopian tube, preserves their viability, and facilitates their appropriate capacitation and activation.94,95 Markers reported to be exclusively of the FTSEC subpopulation include: human milk fat globule 2,90 Bcl-2 (mitochondrial suppressor of apoptosis),91 and Pax-8 (transcription factor, regulator of thyroid, and urogenital tract development).96
The ability to conduct in-depth studies of the cell of origin, beyond the descriptive histopathological research described earlier, depends on adequate in vitro culture systems. Several studies reported culturing human fallopian tube epithelial cells.97-101 However, these culture systems fail to present us with the proper tools to investigate the complexity of the carcinogenic process since, in these systems, the cells senesce and dedifferentiate after a short time in culture. Specifically, the enigmatically exclusive transformation of the FTSEC which spawns the serous tumors in a setting of a mixed cell population warrants insight. One report, by Kervancioglu et al, highlights the fundamental advantage of polarized epithelial culture in recapitulating the in vivo tissue biology.102 With a similar approach in mind our group developed a fallopian tube epithelium culture system based on previous experience with polarized airway epithelium cultures.103,104 We are now able to mimic the normal histology of the fallopian tube fimbria epithelium, with ex vivo cocultures of ciliated and secretory cells. Furthermore, this model system mimics the in vivo profile of the two cell populations with regards to some of the immunomarkers mentioned earlier, and can be, for the first time, utilized to more closely examine serous carcinogenesis (Levanon and Drapkin, unpublished). With the identification of the FTSEC as a cell of origin for serous cancers and the emergence of in vitro culture systems, the time is now ripe to ask questions and apply well-established investigational approaches to the problem of pelvic serous carcinoma.
IMPLICATIONS AND FUTURE DIRECTIONS
The standard of care for pelvic serous carcinomas has not become integrated into the era of targeted therapy. The lack of insight into mechanisms of serous carcinogenesis reflects our inability to utilize the newer biologic therapies. It is tempting to speculate that an unprecedented rate of progress is now possible because the fundamental hurdle of identified the correct field of origin and precursor lesion has been resolved. Clinical issues that must be addressed include the following points.
Should prophylactic surgery be limited to the fallopian tubes, or can one or both ovaries be spared in BRCA mutations carriers? Currently there is no data to support or reject such a practice. It carries an obvious decrease in early menopause–related morbidity and an improvement in quality of life, including preservation of fertility, but evidence supporting this approach is still limited in terms of the number of prospectively accrued cases. Irrespective of this, the pathologic practice for assessing BSO specimens from high-risk cases should be universally updated to include extensive sectioning of the fallopian tube with specific attention to the fimbria.
Is there hope for early detection of intraepithelial carcinomas as one strategy to reduce serous cancer mortality? One might expect that the practice of more complete fallopian tube examination will uncover more early serous carcinomas and provide more information as to their prognosis. Whether such tumors can be identified by noninvasive (serologic) assays or imaging will not be known until such techniques are developed. Of the few cases of stage 0 (intraepithelial) FTSCs diagnosed prospectively in BRCA-positive women, no recurrences have been documented.105,106 However, higher stage FTSC is associated with relatively poor prognosis despite adjuvant therapy.107,108 Larger studies devoted to detailed examination of the distal fallopian tube will expand the database of stage 0 FTSC cases and provide greater insight into the potential value of early detection, the prognosis, and the appropriateness of postoperative therapy in these cases.
Is fimbriectomy a viable alternative to tubal ligation that will significantly reduce serous cancer risk? The current data indicates that a significant percentage of serous carcinomas arise from a precursor condition in the distal fallopian tube. It is reasonable to expect that sterilization practices that targeted the fimbria would maximize the protective effect of tubal sterilization on serous cancer prevention.109 However, the value of such a practice must be evaluated in the context of the safety and feasibility of fimbriectomy and the fact that such a strategy precludes so-called tubal reversal procedures in the future for the patient. Nevertheless, barring the emergence of a successful chemopreventive or early detection algorithm, removing fimbrial tissue is the most obvious, albeit yet unproven, surgical approach to pelvic serous cancer prevention.
The identification of the cell of origin for serous carcinogenesis also paves the road to solving several urgent biologic questions.
What are the pathways that lead to serous carcinogenesis? One pathway that consistently distinguishes HGSOC from other histological subtypes of ovarian cancer is the involvement of TP53. It is either mutated or nonfunctional in 50% to 80% of tumors.8 Another oncogene that has attracted significant attention is the EGFR/HER2 pathway.110 What other factors or pathways do these proteins interact with? Isolation of FTSEC in the laboratory will make it possible to determine the consequences of defined genetic alterations in this epithelium. Work in progress is not only focused on defining the important pathways in serous carcinogenesis but determining which may be amenable to therapeutic intervention with currently available drugs. In addition, genome-scale analyses comparing serous carcinomas with the FTSEC, rather than the OSE, may yield new candidate targets for further investigation and eventually for therapeutic intervention.
Can we identify more reliable early-detection biomarkers for HGSOCs? Work in our group and others has identified a number of promising biomarker including HE4, mesothelin, and kallikreins among others.111-117 Using similar approaches, we can now refocus current efforts in this area and look for membrane and secreted proteins that distinguish the normal fallopian tube epithelium from its malignant counterpart. This is an essential step in the development of diagnostic and prognostic biomarkers for this disease.
Can we use the emerging knowledge to develop better animal models? Previously, animal models of ovarian cancer were based on transformed OSE xenografted into mice. The resulting tumors were typically poorly differentiated, and rarely reflected the histologic spectrum seen in human ovarian cancer. Elucidating the candidate pathways involved in serous carcinogenesis and having the cell of origin in hand, will hopefully lead to more clinically relevant in vitro experimental models, and eventually also more authentic mouse models.
In additional to the aforementioned clinical and biologic issues that can now be addressed, cell-based culture systems represent a novel opportunity to define the unique biology of the fallopian tube fimbrial epithelium. For instance, differences in DDR patterns in normal basal and luminal mammary epithelial cells were recently reported,118 implying that the basal cell type may be more susceptible to DNA damage in the setting of a BRCA1 mutation than the luminal cell. Our preliminary studies suggest that a similar difference exists between the two cell types in the fallopian tube (Levanon and Drapkin, unpublished). Similar culture systems can be used to probe the relation between cumulative lifetime ovulation cycles and the risk for HGSOC. Does periodic exposure of the FTSEC to hormonal and inflammatory mediators of ovulation participate in the carcinogenesis of this cell type? If so, one can imagine future studies aimed at moderating the risk of some of these insults in high-risk individuals.
The genetic aberrations responsible for the adenoma-carcinoma sequence of HGSOC can now be better described. We can now shed some light on the endogenous and exogenous risk factors for pelvic serous carcinomas. One possible question may be why is it that the incidence of precursors in BRCA1 and BRCA2 mutation carriers is similar to the general population,88 but the risk for cancer is significantly increased. A retrospective genetic epidemiological study enrolling patients who underwent prophylactic BSO and were diagnosed as having precursor lesions but not invasive cancer compared with HGSOC patients may help shed light, though the cohort size is still small.
Finally, it is the hope that with the evolution of molecular imaging (reviewed by Weissleder),119 translation of the above noted genomic and phenotypic studies on serous precursor lesions, will result in the development of new imaging modality or probes that will make early detection a reality.
Last but not least, perhaps it is time to refine the nomenclature and ascribe the credit to the true organ of origin. Naming the tumor pelvic serous carcinoma rather than ovarian serous carcinoma may not only be a matter of semantics, but hopefully the first step toward its eradication.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Keren Levanon, Christopher Crum, Ronny Drapkin
Financial support: Christopher Crum, Ronny Drapkin
Provision of study materials or patients: Christopher Crum, Ronny Drapkin
Collection and assembly of data: Keren Levanon, Ronny Drapkin
Manuscript writing: Keren Levanon, Christopher Crum, Ronny Drapkin
Final approval of manuscript: Christopher Crum, Ronny Drapkin
published online ahead of print at www.jco.org on October 13, 2008
Supported by Grants No. P50 CA105009, K08 CA108748, R21 CA12468 from the National Cancer Institute, Ovarian Cancer Research Fund (individual investigator award and program project development award), Phi Beta Psi Sorority Charitable Trust, Fannie E. Ripple Foundation, Robert and Deborah First Fund, Randi and Joel Cutler Ovarian Cancer Research Fund, and the Columbia Hospital for Women Research Foundation.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Halazonetis TD, Gorgoulis VG, Bartek J: An oncogene-induced DNA damage model for cancer development. Science 319:1352-1355, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bartkova J, Rezaei N, Liontos M, et al: Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444:633-637, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Jackman RJ, Mayo CW: The adenoma-carcinoma sequence in cancer of the colon. Surg Gynecol Obstet 93:327-330, 1951 [PubMed] [Google Scholar]
- 4.Vogelstein B, Kinzler KW: The multistep nature of cancer. Trends Genet 9:138-141, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Bartkova J, Horejsi Z, Koed K, et al: DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434:864-870, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Gorgoulis VG, Vassiliou LV, Karakaidos P, et al: Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434:907-913, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Smith Sehdev AE, Sehdev PS, Kurman RJ: Noninvasive and invasive micropapillary (low-grade) serous carcinoma of the ovary: A clinicopathologic analysis of 135 cases. Am J Surg Pathol 27:725-736, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Landen CN Jr, Birrer MJ, Sood AK: Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol 26:995-1005, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Shih IeM, Kurman RJ: Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am J Pathol 164:1511-1518, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shih IeM, Kurman RJ: Molecular pathogenesis of ovarian borderline tumors: New insights and old challenges. Clin Cancer Res 11:7273-7279, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Bonome T, Lee JY, Park DC, et al: Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res 65:10602-10612, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Zorn KK, Bonome T, Gangi L, et al: Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res 11:6422-6430, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Singer G, Oldt R III, Cohen Y, et al: Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst 95:484-486, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Obata K, Morland SJ, Watson RH, et al: Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res 58:2095-2097, 1998 [PubMed] [Google Scholar]
- 15.Wu R, Zhai Y, Fearon ER, et al: Diverse mechanisms of beta-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res 61:8247-8255, 2001 [PubMed] [Google Scholar]
- 16.Scully R, Young RH, Clement PB: Tumors of the Ovary, Maldeveloped Gonads, Fallopian Tube, & Broad Ligament. Washington, DC, Armed Forces Institute of Pathology, 1998
- 17.Seidman JD, Horkayne-Szakaly I, Haiba M, et al: The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol 23:41-44, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Cannistra SA: Cancer of the ovary. N Engl J Med 351:2519-2529, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Quirk JT, Natarajan N, Mettlin CJ: Age-specific ovarian cancer incidence rate patterns in the United States. Gynecol Oncol 99:248-250, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Ozols RF, Bookman MA, Connolly DC, et al: Focus on epithelial ovarian cancer. Cancer Cell 5:19-24, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Struewing JP, Hartge P, Wacholder S, et al: The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 336:1401-1408, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Piek JM, Kenemans P, Verheijen RH: Intraperitoneal serous adenocarcinoma: A critical appraisal of three hypotheses on its cause. Am J Obstet Gynecol 191:718-732, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Crum CP, Drapkin R, Kindelberger D, et al: Lessons from BRCA: The tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res 5:35-44, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crum CP, Drapkin R, Miron A, et al: The distal fallopian tube: A new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol 19:3-9, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Jarboe EA, Folkins AK, Drapkin R, et al: Tubal and ovarian pathways to pelvic epithelial cancer: A pathological perspective. Histopathology 53:127-138, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Auersperg N, Wong AS, Choi KC, et al: Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr Rev 22:255-288, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Drapkin R, Hecht JL: The origins of ovarian cancer: Hurdles and progress. Women's Oncol Rev 2:261-268, 2002 [Google Scholar]
- 28.Fleming JS, Beaugie CR, Haviv I, et al: Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: Revisiting old hypotheses. Mol Cell Endocrinol 247:4-21, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Piek JM, Dorsman JC, Shvarts A, et al: Cultures of ovarian surface epithelium from women with and without a hereditary predisposition to develop female adnexal carcinoma. Gynecol Oncol 92:819-826, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Maines-Bandiera SL, Auersperg N: Increased E-cadherin expression in ovarian surface epithelium: An early step in metaplasia and dysplasia? Int J Gynecol Pathol 16:250-255, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Fathalla MF: Incessant ovulation–a factor in ovarian neoplasia? Lancet 298:163, 1971 [DOI] [PubMed] [Google Scholar]
- 32.Fredrickson TN: Ovarian tumors of the hen. Environ Health Perspect 73:35-51, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purdie DM, Bain CJ, Siskind V, et al: Ovulation and risk of epithelial ovarian cancer. Int J Cancer 104:228-232, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Bonello N, McKie K, Jasper M, et al: Inhibition of nitric oxide: Effects on interleukin-1 beta-enhanced ovulation rate, steroid hormones, and ovarian leukocyte distribution at ovulation in the rat. Biol Reprod 54:436-445, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Ness RB, Cottreau C: Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst 91:1459-1467, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Buscher U, Chen FC, Kentenich H, et al: Cytokines in the follicular fluid of stimulated and non-stimulated human ovaries: Is ovulation a suppressed inflammatory reaction? Hum Reprod 14:162-166, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Natarajan M, Gibbons CF, Mohan S, et al: Oxidative stress signalling: A potential mediator of tumour necrosis factor alpha-induced genomic instability in primary vascular endothelial cells. Br J Radiol 80:S13-S22, 2007. (Spec No 1) [DOI] [PubMed] [Google Scholar]
- 38.Babbar N, Casero RA Jr: Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: A potential mechanism for inflammation-induced carcinogenesis. Cancer Res 66:11125-11130, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Yan B, Wang H, Rabbani ZN, et al: Tumor necrosis factor-alpha is a potent endogenous mutagen that promotes cellular transformation. Cancer Res 66:11565-11570, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Coussens LM, Werb Z: Inflammation and cancer. Nature 420:860-867, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson SC, Coussens LM: Soluble mediators of inflammation during tumor development. Adv Cancer Res 93:159-187, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Cramer DW, Welch WR: Determinants of ovarian cancer risk: II. Inferences regarding pathogenesis. J Natl Cancer Inst 71:717-721, 1983 [PubMed] [Google Scholar]
- 43.Cramer DW, Hutchison GB, Welch WR, et al: Determinants of ovarian cancer risk: I. Reproductive experiences and family history. J Natl Cancer Inst 71:711-716, 1983 [PubMed] [Google Scholar]
- 44.Cramer DW, Welch WR, Cassells S, et al: Mumps, menarche, menopause, and ovarian cancer. Am J Obstet Gynecol 147:1-6, 1983 [DOI] [PubMed] [Google Scholar]
- 45.Schildkraut JM, Schwingl PJ, Bastos E, et al: Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol 88:554-559, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Risch HA: Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst 90:1774-1786, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Rossing MA, Daling JR, Weiss NS, et al: Ovarian tumors in a cohort of infertile women. N Engl J Med 331:771-776, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Drapkin RI, Hecht JL: Pathogenesis of ovarian cancer. Philadelphia, PA, WB Saunders, 2006
- 49.Auersperg N, Ota T, Mitchell GW: Early events in ovarian epithelial carcinogenesis: Progress and problems in experimental approaches. Int J Gynecol Cancer 12:691-703, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Wong AS, Auersperg N: Ovarian surface epithelium: Family history and early events in ovarian cancer. Reprod Biol Endocrinol 1:70, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deligdisch L, Gil J, Kerner H, et al: Ovarian dysplasia in prophylactic oophorectomy specimens: Cytogenetic and morphometric correlations. Cancer 86:1544-1550, 1999 [PubMed] [Google Scholar]
- 52.Bell DA, Scully RE: Early de novo ovarian carcinoma: A study of fourteen cases. Cancer 73:1859-1864, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Scully RE: Ovarian tumors: A review. Am J Pathol 87:686-720, 1977 [PMC free article] [PubMed] [Google Scholar]
- 54.Dubeau L: The cell of origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: Does the emperor have no clothes? Gynecol Oncol 72:437-442, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Resta L, Russo S, Colucci GA, et al: Morphologic precursors of ovarian epithelial tumors. Obstet Gynecol 82:181-186, 1993 [PubMed] [Google Scholar]
- 56.Okamura H, Katabuchi H: Detailed morphology of human ovarian surface epithelium focusing on its metaplastic and neoplastic capability. Ital J Anat Embryol 106:263-276, 2001 [PubMed] [Google Scholar]
- 57.Salazar H, Godwin AK, Daly MB, et al: Microscopic benign and invasive malignant neoplasms and a cancer-prone phenotype in prophylactic oophorectomies. J Natl Cancer Inst 88:1810-1820, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Werness BA, Afify AM, Bielat KL, et al: Altered surface and cyst epithelium of ovaries removed prophylactically from women with a family history of ovarian cancer. Hum Pathol 30:151-157, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Piek JM, Verheijen RH, Menko FH, et al: Expression of differentiation and proliferation related proteins in epithelium of prophylactically removed ovaries from women with a hereditary female adnexal cancer predisposition. Histopathology 43:26-32, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Casey MJ, Bewtra C, Hoehne LL, et al: Histology of prophylactically removed ovaries from BRCA1 and BRCA2 mutation carriers compared with noncarriers in hereditary breast ovarian cancer syndrome kindreds. Gynecol Oncol 78:278-287, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Stratton JF, Buckley CH, Lowe D, et al: Comparison of prophylactic oophorectomy specimens from carriers and noncarriers of a BRCA1 or BRCA2 gene mutation: United Kingdom Coordinating Committee on Cancer Res (UKCCCR) Familial Ovarian Cancer Study Group. J Natl Cancer Inst 91:626-628, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Barakat RR, Federici MG, Saigo PE, et al: Absence of premalignant histologic, molecular, or cell biologic alterations in prophylactic oophorectomy specimens from BRCA1 heterozygotes. Cancer 89:383-390, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Folkins AK, Jarboe EA, Saleemuddin A, et al: A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol 109:168-173, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu CY, Taymor ML, Hertig AT: Primary carcinoma of the fallopian tube. Am J Obstet Gynecol 59:58-67, 1950 [DOI] [PubMed] [Google Scholar]
- 65.Sedlis A: Primary carcinoma of the fallopian tube. Obstet Gynecol Surv 16:209-226, 1961 [DOI] [PubMed] [Google Scholar]
- 66.Yoonessi M: Carcinoma of the fallopian tube. Obstet Gynecol Surv 34:257-270, 1979 [PubMed] [Google Scholar]
- 67.Rosenblatt KA, Weiss NS, Schwartz SM: Incidence of malignant fallopian tube tumors. Gynecol Oncol 35:236-239, 1989 [DOI] [PubMed] [Google Scholar]
- 68.Brose MS, Rebbeck TR, Calzone KA, et al: Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst 94:1365-1372, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Bannatyne P, Russell P: Early adenocarcinoma of the fallopian tubes: A case for multifocal tumorigenesis. Diagn Gynecol Obstet 3:49-60, 1981 [PubMed] [Google Scholar]
- 70.Agoff SN, Mendelin JE, Grieco VS, et al: Unexpected gynecologic neoplasms in patients with proven or suspected BRCA-1 or -2 mutations: Implications for gross examination, cytology, and clinical follow-up. Am J Surg Pathol 26:171-178, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Piek JM, Torrenga B, Hermsen B, et al: Histopathological characteristics of BRCA1- and BRCA2-associated intraperitoneal cancer: A clinic-based study. Fam Cancer 2:73-78, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Kindelberger DW, Lee Y, Miron A, et al: Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol 31:161-169, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Carlson JW, Miron A, Jarboe EA, et al: Serous tubal intraepithelial carcinoma: Its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol 26:4160-4165, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carcangiu ML, Radice P, Manoukian S, et al: Atypical epithelial proliferation in fallopian tubes in prophylactic salpingo-oophorectomy specimens from BRCA1 and BRCA2 germline mutation carriers. Int J Gynecol Pathol 23:35-40, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Medeiros F, Muto MG, Lee Y, et al: The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol 30:230-236, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Lu KH, Garber JE, Cramer DW, et al: Occult ovarian tumors in women with BRCA1 or BRCA2 mutations undergoing prophylactic oophorectomy. J Clin Oncol 18:2728-2732, 2000 [DOI] [PubMed] [Google Scholar]
- 77.Kauff ND, Satagopan JM, Robson ME, et al: Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 346:1609-1615, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Leeper K, Garcia R, Swisher E, et al: Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol Oncol 87:52-56, 2002 [DOI] [PubMed] [Google Scholar]
- 79.Colgan TJ, Murphy J, Cole DE, et al: Occult carcinoma in prophylactic oophorectomy specimens: Prevalence and association with BRCA germline mutation status. Am J Surg Pathol 25:1283-1289, 2001 [DOI] [PubMed] [Google Scholar]
- 80.Olopade OI, Artioli G: Efficacy of risk-reducing salpingo-oophorectomy in women with BRCA-1 and BRCA-2 mutations. Breast J 10:S5-S9, 2004. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 81.Olivier RI, van Beurden M, Lubsen MA, et al: Clinical outcome of prophylactic oophorectomy in BRCA1/BRCA2 mutation carriers and events during follow-up. Br J Cancer 90:1492-1497, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Powell CB, Kenley E, Chen LM, et al: Risk-reducing salpingo-oophorectomy in BRCA mutation carriers: Role of serial sectioning in the detection of occult malignancy. J Clin Oncol 23:127-132, 2005 [DOI] [PubMed] [Google Scholar]
- 83.Meeuwissen PA, Seynaeve C, Brekelmans CT, et al: Outcome of surveillance and prophylactic salpingo-oophorectomy in asymptomatic women at high risk for ovarian cancer. Gynecol Oncol 97:476-482, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Lamb JD, Garcia RL, Goff BA, et al: Predictors of occult neoplasia in women undergoing risk-reducing salpingo-oophorectomy. Am J Obstet Gynecol 194:1702-1709, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Finch A, Shaw P, Rosen B, et al: Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol 100:58-64, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Callahan MJ, Crum CP, Medeiros F, et al: Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol 25:3985-3990, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Piek JM, van Diest PJ, Zweemer RP, et al: Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol 195:451-456, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Lee Y, Miron A, Drapkin R, et al: A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol 211:26-35, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Jarboe E, Folkins A, Nucci MR, et al: Serous carcinogenesis in the fallopian tube: A descriptive classification. Int J Gynecol Pathol 27:1-9, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Comer MT, Leese HJ, Southgate J: Induction of a differentiated ciliated cell phenotype in primary cultures of Fallopian tube epithelium. Hum Reprod 13:3114-3120, 1998 [DOI] [PubMed] [Google Scholar]
- 91.Piek JM, van Diest PJ, Verheijen RH, et al: Cell cycle-related proteins p21 and bcl-2: markers of differentiation in the human fallopian tube. Histopathology 38:481-482, 2001 [DOI] [PubMed] [Google Scholar]
- 92.Okada A, Ohta Y, Brody SL, et al: Role of foxj1 and estrogen receptor alpha in ciliated epithelial cell differentiation of the neonatal oviduct. J Mol Endocrinol 32:615-625, 2004 [DOI] [PubMed] [Google Scholar]
- 93.Gomperts BN, Gong-Cooper X, Hackett BP: Foxj1 regulates basal body anchoring to the cytoskeleton of ciliated pulmonary epithelial cells. J Cell Sci 117:1329-1337, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Kervancioglu ME, Djahanbakhch O, Aitken RJ: Epithelial cell coculture and the induction of sperm capacitation. Fertil Steril 61:1103-1108, 1994 [DOI] [PubMed] [Google Scholar]
- 95.Hunter RH, Rodriguez-Martinez H: Capacitation of mammalian spermatozoa in vivo, with a specific focus on events in the fallopian tubes. Mol Reprod Dev 67:243-250, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Bowen NJ, Logani S, Dickerson EB, et al: Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol 104:331-337, 2007 [DOI] [PubMed] [Google Scholar]
- 97.Henriksen T, Tanbo T, Abyholm T, et al: Epithelial cells from human fallopian tube in culture. Hum Reprod 5:25-31, 1990 [DOI] [PubMed] [Google Scholar]
- 98.Yeung WS, Ho PC, Lau EY, et al: Improved development of human embryos in vitro by a human oviductal cell co-culture system. Hum Reprod 7:1144-1149, 1992 [DOI] [PubMed] [Google Scholar]
- 99.Ando H, Kobayashi M, Toda S, et al: Establishment of a ciliated epithelial cell line from human fallopian tube. Hum Reprod 15:1597-1603, 2000 [DOI] [PubMed] [Google Scholar]
- 100.Bongso A, Ng SC, Sathananthan H, et al: Establishment of human ampullary cell cultures. Hum Reprod 4:486-494, 1989 [DOI] [PubMed] [Google Scholar]
- 101.Ouhibi N, Menezo Y, Benet G, et al: Culture of epithelial cells derived from the oviduct of different species. Hum Reprod 4:229-235, 1989 [DOI] [PubMed] [Google Scholar]
- 102.Kervancioglu ME, Saridogan E, Martin JE, et al: A simple technique for the long-term non-polarised and polarised culture of human fallopian tube epithelial cells. Biol Cell 82:103-107, 1994 [DOI] [PubMed] [Google Scholar]
- 103.Vermeer PD, Einwalter LA, Moninger TO, et al: Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 422:322-326, 2003 [DOI] [PubMed] [Google Scholar]
- 104.Welsh MJ: Ion transport by primary cultures of canine tracheal epithelium: Methodology, morphology, and electrophysiology. J Membr Biol 88:149-163, 1985 [DOI] [PubMed] [Google Scholar]
- 105.Agoff SN, Garcia RL, Goff B, et al: Follow-up of in situ and early-stage fallopian tube carcinoma in patients undergoing prophylactic surgery for proven or suspected BRCA-1 or BRCA-2 mutations. Am J Surg Pathol 28:1112-1114, 2004 [DOI] [PubMed] [Google Scholar]
- 106.Paley PJ, Swisher EM, Garcia RL, et al: Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: A case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol 80:176-180, 2001 [DOI] [PubMed] [Google Scholar]
- 107.Klein M, Rosen A, Lahousen M, et al: The relevance of adjuvant therapy in primary carcinoma of the fallopian tube, stages I and II: Irradiation vs. chemotherapy. Int J Radiat Oncol Biol Phys 48:1427-1431, 2000 [DOI] [PubMed] [Google Scholar]
- 108.Rosen AC, Klein M, Hafner E, et al: Management and prognosis of primary fallopian tube carcinoma: Austrian Cooperative Study Group for Fallopian Tube Carcinoma. Gynecol Obstet Invest 47:45-51, 1999 [DOI] [PubMed] [Google Scholar]
- 109.Narod SA, Sun P, Ghadirian P, et al: Tubal ligation and risk of ovarian cancer in carriers of BRCA1 or BRCA2 mutations: A case-control study. Lancet 357:1467-1470, 2001 [DOI] [PubMed] [Google Scholar]
- 110.Afify AM, Werness BA, Mark HF: HER-2/neu oncogene amplification in stage I and stage III ovarian papillary serous carcinoma. Exp Mol Pathol 66:163-169, 1999 [DOI] [PubMed] [Google Scholar]
- 111.Bast RC Jr, Badgwell D, Lu Z, et al: New tumor markers: CA125 and beyond. Int J Gynecol Cancer 15:274-281, 2005. (suppl 3) [DOI] [PubMed] [Google Scholar]
- 112.Bast RC Jr, Brewer M, Zou C, et al: Prevention and early detection of ovarian cancer: Mission impossible? Recent Results Cancer Res 174:91-100, 2007 [DOI] [PubMed] [Google Scholar]
- 113.Drapkin R, von Horsten HH, Lin Y, et al: Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res 65:2162-2169, 2005 [DOI] [PubMed] [Google Scholar]
- 114.Diamandis EP, Scorilas A, Fracchioli S, et al: Human kallikrein 6 (hK6): A new potential serum biomarker for diagnosis and prognosis of ovarian carcinoma. J Clin Oncol 21:1035-1043, 2003 [DOI] [PubMed] [Google Scholar]
- 115.Hellstrom I, Raycraft J, Hayden-Ledbetter M, et al: The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res 63:3695-3700, 2003 [PubMed] [Google Scholar]
- 116.Hibbs K, Skubitz KM, Pambuccian SE, et al: Differential gene expression in ovarian carcinoma: Identification of potential biomarkers. Am J Pathol 165:397-414, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Drapkin R, Crum CP, Hecht JL: Expression of candidate tumor markers in ovarian carcinoma and benign ovary: Evidence for a link between epithelial phenotype and neoplasia. Hum Pathol 35:1014-1021, 2004 [DOI] [PubMed] [Google Scholar]
- 118.Huper G, Marks JR: Isogenic normal basal and luminal mammary epithelial isolated by a novel method show a differential response to ionizing radiation. Cancer Res 67:2990-3001, 2007 [DOI] [PubMed] [Google Scholar]
- 119.Weissleder R: Molecular imaging in cancer. Science 312:1168-1171, 2006 [DOI] [PubMed] [Google Scholar]