Abstract
Purpose
The two approved treatments for patients with metastatic melanoma, interleukin (IL)-2 and dacarbazine, mediate objective response rates of 12% to 15%. We previously reported that adoptive cell therapy (ACT) with autologous antitumor lymphocytes in lymphodepleted hosts mediated objective responses in 51% of 35 patients. Here, we update that study and evaluate the safety and efficacy of two increased-intensity myeloablative lymphodepleting regimens.
Patients and Methods
We performed two additional sequential trials of ACT with autologous tumor-infiltrating lymphocytes (TIL) in patients with metastatic melanoma. Increasing intensity of host preparative lymphodepletion consisting of cyclophosphamide and fludarabine with either 2 (25 patients) or 12 Gy (25 patients) of total-body irradiation (TBI) was administered before cell transfer. Objective response rates by Response Evaluation Criteria in Solid Tumors (RECIST) and survival were evaluated. Immunologic correlates of effective treatment were studied.
Results
Although nonmyeloablative chemotherapy alone showed an objective response rate of 49%, when 2 or 12 Gy of TBI was added, the response rates were 52% and 72% respectively. Responses were seen in all visceral sites including brain. There was one treatment-related death in the 93 patients. Host lymphodepletion was associated with increased serum levels of the lymphocyte homeostatic cytokines IL-7 and IL-15. Objective responses were correlated with the telomere length of the transferred cells.
Conclusion
Host lymphodepletion followed by autologous TIL transfer and IL-2 results in objective response rates of 50% to 70% in patients with metastatic melanoma refractory to standard therapies.
INTRODUCTION
Patients with metastatic melanoma have a median survival of 8 months and 2-year survival rates of 10% to 15%. There are two treatments approved by the US Food and Drug Administration for patients with metastatic melanoma, interleukin (IL)-2 and dacarbazine, which mediate objective response rates of approximately 15%.1
We recently reported that the adoptive transfer of autologous tumor-infiltrating lymphocytes (TIL) after lymphodepleting chemotherapy resulted in objective responses in 51% of 35 heavily pretreated patients with metastatic melanoma.2,3 This adoptive cell therapy (ACT) used tumor antigen-specific lymphocytes that were initiated in vitro from single-cell enzymatic digests or small fragments of resected tumor specimens and expanded to large numbers before infusion. TIL from metastatic melanoma lesions comprise an enriched source of tumor-antigen reactive cells.4 Optimization of methods for their isolation and expansion to large numbers for therapy5-7 have made the application of ACT logistically feasible.
In our prior clinical trial, patients received a lymphodepleting, nonmyeloablative (NMA) chemotherapy consisting of cyclophosphamide and fludarabine before cell transfer.3 In murine models, lymphodepletion seemed to enhance the antitumor effects of transferred T cells in vivo by several mechanisms including the elimination of suppressive CD4+CD25+ T-regulatory lymphocytes,8 the elimination of cellular “sinks” for homeostatic cytokines such as IL-7 and IL-15,9 and the engagement of toll-like receptors on antigen-presenting cells after damage to the integrity of the gut epithelial lining.10 In these same murine models, there was a direct relationship between the extent of lymphodepletion and the magnitude of the in vivo antitumor effect of the transferred cells.11,12
These preclinical findings led us to perform two pilot clinical trials in patients with metastatic melanoma to test whether intensifying the lymphodepletion by adding either 2 or 12 Gy of total-body irradiation (TBI) to the chemotherapy conditioning regimen would improve the therapeutic results of ACT. The results of these trials and studies of the mechanism of action of lymphodepletion form the basis of this article.
PATIENTS AND METHODS
Patients
Patients were eligible for this trial if they were 18 years of age or older with measurable metastatic melanoma and a clinical performance status of 0 or 1 on the Eastern Cooperative Oncology Group (ECOG) scale and life expectancy of greater than 3 months. Patients were excluded from participation if blood counts were outside the normal range, if systemic steroid therapy was required, or if patients had other active systemic infections, coagulation disorders or other active major medical or cardiovascular diseases or immunodeficiencies, or were positive for HIV antibody or hepatitis B or C. All patients signed an informed consent approved by the institutional review board of the National Cancer Institute.
Clinical Trial Design
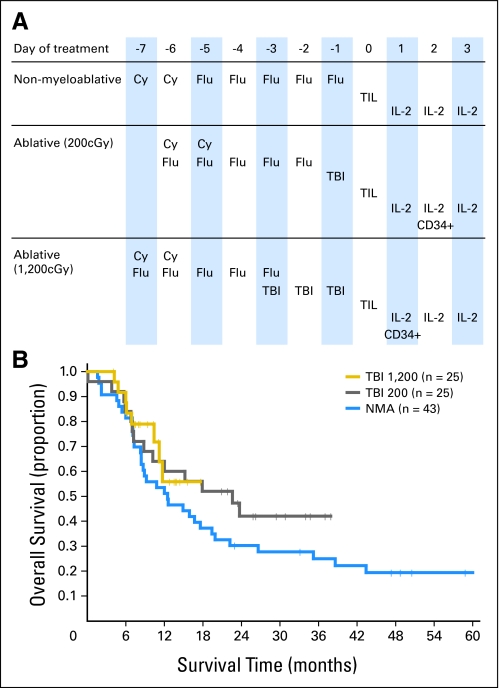
We previously reported the results of 35 patients who received TIL transfer after a NMA lymphodepleting regimen consisting of cyclophosphamide 60 mg/kg/d for 2 days and fludarabine 25 mg/m2/d for the next 5 days (Fig 1A). Two additional trials were instituted with the end points of evaluating improved response rates and survival. In the next pilot trial, 25 patients received this chemotherapy over 5 rather than 7 days followed by a single fraction of 2 Gy of TBI. In a third trial, 25 patients received the same 5 days of chemotherapy plus 12 Gy of TBI administered as 2 Gy twice a day for 3 days (Fig 1A). Some patients who met immunologic and clinical criteria were not eligible to receive TBI because of prior radiation therapy or an inability to collect adequate CD34+ stem cells. Eight additional patients were subsequently treated with the original NMA regimen without TBI, and the results in this entire cohort of 43 NMA-only patients are updated here with a median follow-up of 45 months.
Fig 1.
(A) Schedule of treatments administered for three lymphodepleting cell transfer regimens. (B) Kaplan-Meier plot showing the duration of survival of patients treated on each lymphodepleting cell transfer protocol. Vertical tick marks indicate patients who are still alive and on study. Cy, cyclophosphamide; Flu, fludarabine; TBI, total-body irradiation; CD34+, hematopoietic stem cells; TIL, tumor-infiltrating lymphocytes; IL-, interleukin.
For the 2-Gy TBI regimen, patients underwent clinical simulation for TBI that included measurement of maximum separation, typically at mid-hip. TBI was delivered with the patient positioned supine on a gurney with arms placed by the sides for lung compensation. No additional tissue compensators were used. Treatment was delivered at 2-Gy fractions with 15-MV photons delivered at a distance of 6 meters to the patient's midline at a dose rate of 0.11 Gy/min. For the 12-Gy TBI regimen, a computed tomography simulation was performed to allow planning for head, neck, and lung compensators. TBI was delivered with opposed lateral fields as described for the 2-Gy regimen with the addition of compensation. Mediastinal boosting of tissues underdosed as a result of lung compensation was accomplished with anterior-posterior and posterior-anterior 6-MV beams to obtain a final dose of 12 Gy to the total body and mediastinum while limiting the median lung dose to 6 Gy. All fields were treated twice daily with at least a 6-hour interfraction interval over 3 consecutive days. All patients received a single dose of ondansetron 0.15 mg/kg intravenously before delivery of TBI as nausea prophylaxis.
On the day after the final dose of chemotherapy or TBI, patients received a bolus intravenous infusion of TIL over 0.5 to 1 hour, and started high-dose IL-2 within 24 hours (720,000 U/kg intravenously every 8 hours to tolerance up to 15 doses). One or 2 days after TIL infusion, a minimum of 2 × 106/kg of autologous purified (Miltenyi Biotech, Bergisch Gladbach, Germany) cryopreserved CD34+ hematopoietic stem cells from a granulocyte colony-stimulating factor–mobilized pheresis were infused intravenously in only patients who received TBI.
After TIL infusion, all patients received trimethoprim, sulfamethoxazole, and fungal prophylaxis; herpes virus–seropositive patients received valacyclovir. Patients were monitored daily after TIL infusion and received platelets or packed RBCs as needed. Patient response was assessed using standard radiographic studies and physical examination at 4 weeks after cell administration and at regular intervals thereafter, and were categorized into complete, partial, or nonresponders based on Response Evaluation Criteria in Solid Tumors (RECIST) guidelines.
TIL Cultures and Immunologic Monitoring Assays
TIL cultures for patient treatment were generated as previously described.3,13 The phenotype of TIL was determined by fluorescence-activated cell sorting (FACS) analysis and the antigen specificity was assessed by cytokine-release assays using standard methods.3,13 The mean length of telomere repeats at chromosome ends in individual T-cell cultures was measured by quantitative flow-fluorescent in situ hybridization as previously described.14
Serum was collected from patients and frozen before initiation of therapy and after lymphodepletion but before cell infusion. Sera were thawed and assayed at the same time in a blinded fashion using commercially available enzyme-linked immunosorbent assay kits.
Statistical Analysis
Standard statistical methods are cited in the text, and all P values are two-tailed assuming unequal variance, with P < .05 considered significant.
RESULTS
Adoptive Cell Therapy After Lymphodepletion-Mediated Potent Antitumor Responses in Patients With Refractory Melanoma
The characteristics of the patients enrolled onto these three trials are shown in Table 1. Patients in the three clinical trials received a similar number and quality of TIL that had been selected for antitumor reactivity and then rapidly expanded to large cell numbers and infused immediately after collection.
Table 1.
Characteristics of Patients Enrolled Onto Lymphodepleting Protocols
| Characteristic | NMA (n = 43)
|
TBI-2 (n = 25)
|
TBI-12 (n = 25)
|
Total (N = 93)
|
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Sex | ||||||||
| Male | 26 | 60 | 17 | 68 | 18 | 72 | 61 | 66 |
| Female | 17 | 40 | 8 | 32 | 7 | 28 | 32 | 34 |
| Age, years | ||||||||
| 11-20 | 2 | 5 | 0 | 0 | 1 | 4 | 3 | 3 |
| 21-30 | 3 | 7 | 3 | 12 | 2 | 8 | 8 | 9 |
| 31-40 | 8 | 19 | 5 | 20 | 6 | 24 | 19 | 20 |
| 41-50 | 16 | 37 | 9 | 36 | 4 | 16 | 29 | 31 |
| 51-60 | 12 | 28 | 8 | 32 | 12 | 48 | 32 | 34 |
| 61-70 | 2 | 5 | 0 | 0 | 0 | 0 | 2 | 2 |
| ECOG PS | ||||||||
| 0 | 33 | 77 | 20 | 80 | 19 | 76 | 72 | 77 |
| 1 | 10 | 23 | 5 | 20 | 6 | 24 | 21 | 23 |
| Prior treatment | ||||||||
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery | 43 | 100 | 25 | 100 | 25 | 100 | 93 | 100 |
| Chemotherapy | 22 | 51 | 7 | 28 | 5 | 20 | 34 | 37 |
| Radiotherapy | 15 | 35 | 6 | 24 | 2 | 8 | 23 | 25 |
| Hormonal | 1 | 2 | 0 | 0 | 1 | 4 | 2 | 2 |
| Immunotherapy | 42 | 98 | 24 | 96 | 21 | 84 | 87 | 94 |
| Any 2 or more | 43 | 100 | 25 | 100 | 22 | 88 | 90 | 97 |
| Any 3 or more | 27 | 63 | 9 | 36 | 6 | 24 | 42 | 45 |
Abbreviations: NMA, nonmyeloablative group; TBI-2, 2-Gy total-body irradiation group; TBI-12, 12-Gy total-body irradiation group; ECOG PS, Eastern Cooperative Oncology Group performance status.
The objective antitumor responses and durations of response are summarized in Table 2. The response rates in the NMA trial (n = 43), the NMA plus 2-Gy trial (25 patients), and the NMA plus 12-Gy trial (n = 25) were 48.8%, 52.0%, and 72.0% respectively. Tumor regression was seen in metastases at virtually all visceral and soft tissue sites including brain. Thus, the high response rates seen in the additional 58 patients reported here who received ACT with the NMA with or without additional TBI conditioning extend and confirm the high objective response rate reported by us earlier in 35 patients who received TIL transfer after the NMA conditioning regimen.3 Among these 93 patients, 34 patients (37%) had received prior chemotherapy (the response rate in this group was 62%) and 77 patients (83%) had received prior IL-2 therapy (the response rate in this group was 56%). Among the 10 patients who achieved a complete response, there have been no relapses with a median follow-up of 31 months. According to the American Joint Committee on Cancer (AJCC) staging system, of the 10 patients who experienced a complete response, two patients were stage M1a (metastases to cutaneous, subcutaneous, and nodal sites only), four patients were stage M1b (metastases to the lung), and four patients were stage M1c (metastases to other visceral and distant sites).
Table 2.
Frequency and Duration of Objective Responses
| TBI | Total No. of Patients | PR
|
CR
|
OR
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | Duration (months) | No. | % | Duration (months) | No. | % | ||
| None* | 43 | 17 | 39.5 | 64+, 32+, 20+, 29, 28, 14, 13, 11, 8, 8, 7, 4, 3, 3, 2, 2, 2 | 4 | 9.3 | 63+, 58+, 48+, 47+ | 21 | 48.8 |
| 2 Gy | 25 | 11 | 44.0 | 33+, 29+, 23+, 14, 10, 6, 5, 5, 4, 3, 3 | 2 | 8.0 | 37+, 25+ | 13 | 52.0 |
| 12 Gy | 25 | 14 | 56.0 | 14+, 13+, 10+, 7+, 7+, 7+, 6+, 6+, 4+, 7, 6, 6, 4, 3 | 4 | 16.0 | 17+, 15+, 13+, 8+ | 18 | 72.0 |
NOTE. All patients received cyclophosphamide 60 mg/kg × 2 days + fludarabine 25 mg/m2 × 5 days.
Abbreviations: TBI, total-body irradiation; PR, partial response; CR, complete response; OR, objective response; TIL, tumor-infiltrating lymphocytes.
The median follow-up as of January 1, 2008, for the three trials was 45, 27, and 10 months, respectively. The 2-year survival rates were 30% and 42%, respectively, in the trials involving no TBI or 2-Gy TBI (Fig 1B), and follow-up was too short in the 12-Gy trial. In these sequential studies, there was no significant difference in survival among the three treatment groups (P = .13), although more prolonged follow-up is necessary.
Treatment Characteristics
The characteristics of the treatments administered to patients are shown in Table 3. All infused TIL demonstrated specific recognition of autologous melanoma or human leukocyte antigen–A2 matched melanoma lines by cytokine-release assays (Appendix Tables A1-A3, online only). Multiple features of the infused TIL cultures were evaluated for correlation with patient response, but the only treatment characteristic that was significantly correlated with clinical outcome was the number of IL-2 doses tolerated by patients. The responding patients in the 2-Gy trial tolerated significantly less IL-2 (6.9 doses) than nonresponding patients (9.3 doses; P =.01). The 52 responding patients from all three clinical trials tolerated significantly less IL-2 than the 41 nonresponding patients (P < .001). These results could suggest that TIL that are effectively engaged in antitumor responses are releasing secondary cytokines into the host's circulation that limit IL-2 tolerance compared with TIL in noneffective interactions.
Table 3.
Treatments Administered After TBI Preparative Regimens
| Treatment | TBI Dosage
|
|||
|---|---|---|---|---|
| 2 Gy
|
12 Gy
|
|||
| PR + CR (n = 13) | NR (n = 12) | PR + CR (n = 18) | NR (n = 7) | |
| Cell number | ||||
| < 3 × 1010 | 1 | 1 | 3 | 1 |
| 3 × 1010-5 × 1010 | 2 | 5 | 1 | 2 |
| 5 × 1010-7 × 1010 | 5 | 3 | 7 | 2 |
| 7 × 1010-9 × 1010 | 4 | 1 | 2 | 2 |
| > 9 × 1010 | 1 | 2 | 5 | 0 |
| % of TIL | ||||
| CD3+CD8+ | 82.1 | 74.9 | 86.0 | 60.5 |
| Standard deviation | 18.2 | 25.2 | 12.8 | 31.0 |
| CD3+CD4+ | 19.4 | 28.8 | 8.8 | 19.0 |
| Standard deviation | 18.5 | 28.0 | 10.2 | 26.3 |
| CD3–CD56+ | 0.0 | 0.0 | 2.5 | 3.1 |
| Standard deviation | 0.0 | 0.0 | 3.1 | 5.7 |
| % of CD8+ | ||||
| A2/MART-1:27-35+ | 19.6 | 3.4 | 2.8 | 1.6 |
| Standard deviation | 27.7 | 6.4 | 8.1 | 2.4 |
| A2/gp100:209-217+ | 1.4 | 1.3 | 2.2 | 6.5 |
| Standard deviation | 3.2 | 3.3 | 3.3 | 9.7 |
| Antigen specificity* | ||||
| MART-1 or gp100 | 5 | 6 | 5 | 4 |
| Autologous only | 6 | 6 | 10 | 2 |
| Both | 1 | 1 | 3 | 1 |
| HLA-A*0201 | ||||
| Yes | 9 | 8 | 13 | 6 |
| No | 3 | 4 | 5 | 1 |
| IL-2 doses, No.† | ||||
| 3-4 | 2 | 0 | 1 | 1 |
| 5-6 | 3 | 1 | 7 | 3 |
| 7-8 | 4 | 3 | 8 | 3 |
| 9-10 | 4 | 4 | 2 | 0 |
| >10 | 0 | 4 | 0 | 0 |
Abbreviations: TBI, total-body irradiation; PR, partial response; CR, complete response; NR, nonresponse; TIL, tumor-infiltrating lymphocytes; HLA, human leukocyte antigen; IL, interleukin.
TIL specificity was defined by cytokine release assay.
Responding and nonresponding patients in the 2-Gy group received statistically different amounts of IL-2 (P = .006).
Toxicities of Treatment
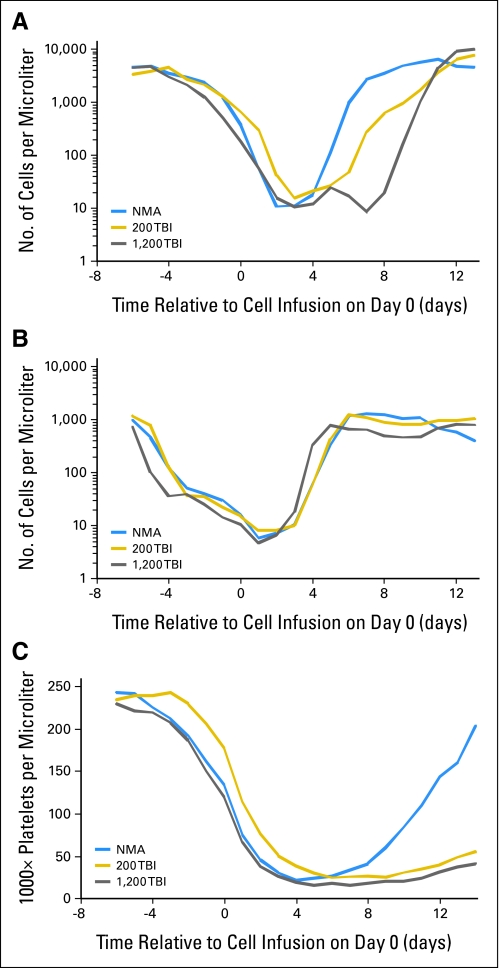
Patients exhibited the expected hematologic toxicities associated with the cyclophosphamide, fludarabine, and TBI preparative regimens. Patients recovered marrow function rapidly after cell infusion with absolute neutrophil counts greater than 500/mm3 by day 12 and sustained platelet counts greater than 20,000/mm3 by day 14 (except for four patients on the 12-Gy TBI protocol with platelet recovery on days 16, 17, 20, and 22). Patients conditioned with 12-Gy TBI demonstrated on average a 1- to 2-day delay in marrow recovery compared with other protocol patients (Appendix Fig A1, online only) and required significantly more blood-product support than patients treated with 2-Gy TBI (Table 4; P for platelets = .002; P for RBC = .05). Patients experienced the usual reversible and transient toxicities associated with IL-2 that have been extensively described elsewhere.15 The significant grade 3 and 4 treatment-related toxicities that were not associated with IL-2 administration in the two TBI protocols are shown in Table 4. Not surprisingly, both the spectrum and the intensity of toxicities were less severe in these patients who received autologous cells than those of toxicities reported in patients undergoing myeloablative conditioning regimens with allogeneic stem-cell transplantation and chronic immunosuppression. For instance, among the 50 patients who received TBI conditioning, only one patient, who received 2-Gy TBI, experienced significant pulmonary toxicity, consisting of pulmonary veno-occlusive disease refractory to steroids. This patient subsequently responded to sildenafil therapy, improved, and returned to work as a nurse, although she sometimes requires oxygen by nasal cannula when exerting. One patient who received 2-Gy TBI and four patients who received 12-Gy TBI required intubation for somnolence. Four patients who received 12-Gy TBI developed renal dysfunction attributed to thrombotic microangiopathy with elevated serum creatinine levels, though those have stabilized. There was one treatment-related death in the 2-Gy TBI protocol resulting from sepsis in a neutropenic patient who had a pre-existing but unrecognized diverticular abscess at protocol enrolment. One patient in the 12-Gy TBI group developed grade 3 posterior uveitis that was refractory to topical steroids. MART-1 tetramer-positive lymphocytes were recovered from the patient's ocular vitreous fluid during an acute uveitis phase accompanied by visual changes. The patient's uveitis symptoms resolved after intraocular steroid injections and treatment with diazoxide.
Table 4.
Transfusions and Grade 3 and 4 Nonhematologic Toxicities Associated With Nonmyeloablative Plus TBI Lymphodepleting Preparative Regimens
| Transfusions and Toxicities | No.
|
|
|---|---|---|
| 2-Gy TBI (n = 25) | 12-Gy TBI (n = 25) | |
| Transfusions administered | ||
| Platelets (6-10 U/transfusion) | 3.8 | 8.1 |
| Standard deviation | 3.4 | 4.4 |
| Packed RBCs | 4.0 | 6.2 |
| Standard deviation | 3.7 | 4.0 |
| Infection-related toxicities | ||
| CMV infection | 1 | 1 |
| Herpes zoster | 1 | 2 |
| Positive blood cultures | 2 | 4 |
| Other toxicities | ||
| Intubated for somnolence | 1 | 4 |
| Pulmonary hypertension | 1 | 0 |
| Febrile neutropenia | 12 | 16 |
| Jugular venous thrombosis | 1 | 0 |
| Autoimmune uveitis and hearing loss (transient) | 0 | 1 |
| Thrombotic microangiopathy | 0 | 4 |
| Death (bowel-perforation sepsis) | 1 | 0 |
Abbreviations: TBI, total-body irradiation; CMV, cytomegalovirus.
Cellular Correlates of Response in Patients Receiving ACT
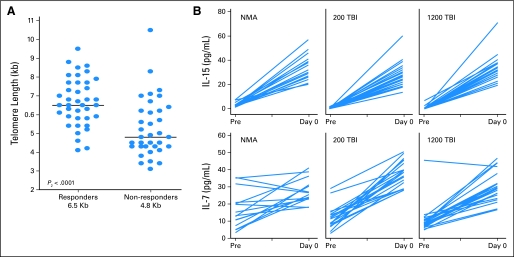
As in our prior report of patients receiving ACT with the lymphodepleting chemotherapy regimen alone, there was no significant correlation between a large number of phenotypic markers in the infused lymphocytes and clinical response. There was, however, a strong correlation between the likelihood of response across all preparative protocols and the mean telomere length of the infused cells (Fig 2A). This observation suggest that the proliferative potential of infused TIL is an important attribute of their ability to mediate tumor destruction in the patient.
Fig 2.
Adoptive cell therapy after lymphodepletion. (A) Telomere length of infused tumor-infiltrating lymphocytes (TIL) correlates with clinical response. Flow-fluorescent in situ hybridization analysis was used to quantify the mean telomere length of infused TIL for patients on all three protocols. (B) Lymphodepleting conditioning markedly increases the levels of circulating homeostatic cytokines, interleukin (IL)-7 and IL-15. Serum samples were evaluated before therapy (Pre) and immediately before TIL administration (day 0). NMA, nonmyeloablative; TBI, total-body irradiation.
Increased Lymphodepletion Was Associated With Increased Circulating Levels of the Homeostatic Cytokines IL-7 and IL-15
The role of serum cytokines in the antitumor efficacy of lymphocytes administered after lymphodepletion was investigated. Serum was sampled before initiation of the lymphodepleting preparative chemotherapy and immediately before cell administration (after lymphodepleting therapy), and circulating levels of the T cell homeostatic regulatory cytokines, IL-7, and IL-15 were quantified by enzyme-linked immunosorbent assay (Fig 2B). The starting levels of homeostatic cytokines were similar for all three populations studied. Lymphodepletion caused an elevation of the levels of IL-7 and IL-15 for all protocols. The ratio of the final concentration to the initial concentration of serum cytokines increased significantly more when patients received 12-Gy TBI than when they received NMA chemotherapy alone (P for IL-7 = .02; P for IL-15 = .005).
DISCUSSION
Nonspecific therapies including IL-2 therapy and CTLA-4 blockade can lead to durable complete responses, but these agents have low response rates (12% to 15%) in patients with melanoma. Active therapeutic immunization has been vigorously pursued in clinical trials using a host of tumor vaccines, but these have elicited disappointing response rates.16 In contrast, in mouse studies and in early clinical studies in patients with melanoma, the direct transfer of activated T cells in lymphodepleted hosts has proven to be a potent method for the treatment of established metastases.
In previous reports, we investigated the use of no or minimal immunosuppression in 86 patients,17 or NMA chemotherapy for lymphodepletion in 35 patients3 with metastatic melanoma before administration of tumor reactive TIL cells and high-dose IL-2 therapy. Without significant lymphodepletion, a response rate of 34% was seen, but many of these were of short duration. An NMA cyclophosphamide-fludarabine regimen transiently depleted circulating lymphocytes and was associated with a higher response rate as well as improved response durations. Those studies demonstrated the safety and potential efficacy of ACT after minimal or NMA lymphodepletion in patients with melanoma. However, accumulating data from animal models of tumor therapy suggested that increased levels of lymphodepletion could improve ACT efficacy.10-12,18
Here, we examined the use of increased intensity lymphodepletion with TIL administration and IL-2 therapy in two sequential phase II protocols. Initially, we evaluated the safety and efficacy of adding 2-Gy TBI to the NMA chemotherapy preparative regimen, together with autologous CD34+ hematopoietic stem cells. The 2-Gy TBI dose was chosen because the toxicities of this combination therapy were uncertain, including the engraftment of hematopoietic stem cells and hematopoietic reconstitution with concurrent high-dose IL-2. We found that 2-Gy TBI could be added to NMA with minimal additional toxicity or impact on hematopoietic recovery (Appendix Fig A1); however, overall response rate (49% v 52%) was also not significantly affected.
We then investigated the addition of 12-Gy TBI to the NMA chemotherapy preparative regimen, a myelosuppressive dose that is close to the maximum dose that can be safely administered to patients in conjunction with the chemotherapy. In 25 patients who received 12-Gy TBI with TIL plus hematopoietic stem cells and IL-2 therapy, there were no treatment-related deaths, and all patients recovered hematopoietic function. Several toxicities such as fatigue, anorexia, and weight loss were more prolonged in the patients who received the 12-Gy TBI treatment, and four patients required intubation for somnolence. Patients generally returned to normal daily routines by 2 to 3 months. Overall, these data demonstrate that for appropriately selected patients, myeloablative lymphodepleting preparative regimens can be administered safely before TIL and IL-2 therapy.
It is interesting to note that the response rate in the 12-Gy TBI trial was 72%, including four complete responders and at least two substantial partial responders who have small residual lesions that are negative on positron emission tomography scan and may convert to complete responses at longer follow-up. The 72% response rate observed in this small patient population is not significantly different from the 52% or 49% response rate seen in the groups with lesser lymphodepletion though the number of patients in each group is small. Comparison of our results with reports of others is difficult because of the potential for selection bias inherent in our nonrandomized study design.
To investigate immunologic correlates of response in the treated patients, we evaluated multiple features of the infused TIL and patient treatments. All patients received lymphocytes that were highly active and capable of human leukocyte antigen–restricted release of interferon-γ after tumor stimulation. The length of telomeres in infused TIL cells from all three protocols correlated with patient response (Fig 2A), suggesting that proliferative potential of the infused TIL is critical to the success of the treatment.
By eliminating competition for endogenous serum cytokines, lymphodepletion may directly affect the survival, persistence, and proliferation of the adoptively transferred TIL cells. We measured the γ chain receptor cytokines IL-7 and IL-15, which are homeostatic regulators of CD3+ lymphocytes, after preparative conditioning. The levels of these cytokines went up in all three protocols, but IL-7 and IL-15 serum levels were statistically higher in 12-Gy TBI group than in the NMA group. The higher levels and availability of these cytokines to TIL cells in patients conditioned with 12-Gy TBI may have caused an increased proliferation, activation, or persistence of the transferred TIL cells, or an increased functional status at the site of tumor antigen.
The results shown herein are compelling evidence that antigen-specific T cells can affect the natural history of a solid metastatic cancer in humans. Furthermore, these studies demonstrate that in some patients, appropriately selected lymphocyte cultures can impact metastatic melanoma growth and patient survival in ways that conventional therapy including surgery, radiotherapy, and chemotherapy as currently practiced cannot. Current approaches in our laboratory seek to improve the yield of TIL cultures for melanoma patients, to use vaccines to stimulate the transferred T cells in vivo, and to employ gene therapy approaches to engineer antigen specificity to T cells for patients who have no autologous reactive TIL available for ACT therapy.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Mark E. Dudley, James C. Yang, Richard Sherry, Marybeth S. Hughes, Richard Royal, Udai Kammula, Paul F. Robbins, John Wunderlich, Nicholas P. Restifo, Steven A. Rosenberg
Administrative support: Susan F. Leitman, John Wunderlich, Armen Thomasian, Stephanie G. Downey, Franz O. Smith, Jacob Klapper, Kathleen Morton, Carolyn Laurencot, Donald E. White
Provision of study materials or patients: James C. Yang, Richard Sherry, Marybeth S. Hughes, Richard Royal, Udai Kammula, Deborah E. Citrin, Susan F. Leitman, Stephanie G. Downey, Franz O. Smith, Jacob Klapper, Kathleen Morton, Steven A. Rosenberg
Collection and assembly of data: Mark E. Dudley, James C. Yang, Richard Sherry, Marybeth S. Hughes, Richard Royal, Udai Kammula, Paul F. Robbins, JianPing Huang, Deborah E. Citrin, John Wunderlich, Armen Thomasian, Kathleen Morton, Donald E. White, Steven A. Rosenberg
Data analysis and interpretation: Mark E. Dudley, James C. Yang, Richard Sherry, Paul F. Robbins, JianPing Huang, Nicholas P. Restifo, Donald E. White, Steven A. Rosenberg
Manuscript writing: Mark E. Dudley, James C. Yang, Paul F. Robbins, Deborah E. Citrin, Nicholas P. Restifo, Steven A. Rosenberg
Final approval of manuscript: Mark E. Dudley, James C. Yang, Richard Sherry, Marybeth S. Hughes, Richard Royal, Udai Kammula, Paul F. Robbins, JianPing Huang, Deborah E. Citrin, Susan F. Leitman, John Wunderlich, Nicholas P. Restifo, Armen Thomasian, Stephanie G. Downey, Franz O. Smith, Jacob Klapper, Kathleen Morton, Carolyn Laurencot, Donald E. White, Steven A. Rosenberg
Acknowledgments
We thank the members of the TIL laboratory, the immunotherapy nurses and fellows, Juan Gea-Banacloche, and the clinical staff of the Clinical Research Center.
Appendix
Fig A1.
Transient depression of hematopoietic cell counts resulting from lymphodepletion is rapidly reversed in patients on all three clinical protocols. Mean absolute counts were calculated based on daily CBCs for all patients who received lymphodepletion and tumor-infiltrating lymphocytes (TIL) therapy as their first lymphodepleting cell therapy. (A) Absolute neutrophil counts, (B) absolute lymphocyte counts, and (C) platelet counts. NMA, nonmyeloablative; TBI, total-body irradiation.
Table A1.
IFN-γ Release (pg/mL) From Representative TIL With Reactivity to Shared HLA-A2+ Tumors and Known Peptide Epitopes
| Cells | Melanoma Cell Lines
|
|||||||
|---|---|---|---|---|---|---|---|---|
| HLA-A2–
|
HLA-A2+
|
T2/Peptide*
|
||||||
| None | 888 | 938 | 526 | 624 | None | M | G | |
| Control T cells | ||||||||
| JKF6 (A2/M) | 1,341 | 887 | 921 | 20,660 | 22,720 | 2,424 | 22,400 | 0 |
| JR6C12 (A2/G) | 1,157 | 1,095 | 903 | 12,890 | 16,320 | 682 | 0 | 14,930 |
| Patient TIL | ||||||||
| 5 | 156 | 188 | 314 | 5,160 | 5,690 | 122 | 14,870 | 335 |
| 7 | 200 | 96 | 137 | 9,630 | 10,660 | 129 | 15,300 | 1,022 |
| 10 | 161 | 108 | 151 | 5,040 | 3,050 | 125 | 1,137 | 5,090 |
| 17 | 114 | 117 | 159 | 7,050 | 9,090 | 105 | 22,700 | 1,115 |
| 20 | 203 | 163 | 168 | 5,530 | 6,700 | 142 | 13,810 | 310 |
NOTE. Values shown in bold are at greater than twice background and greater than 200 pg/mL.
Abbreviations: TIL, tumor-infiltrating lymphocytes; HLA, human leukocyte antigen; M, MART-1:27-35; G, gp100:209-217.
T2 cells were pulsed with 1 μmol of peptide antigen as targets.
Table A2.
IFN-γ Release (pg/mL) From TIL Demonstrating Specificity for Autologous Melanoma Cell Lines With Unknown Peptide Antigen Specificity
| Cells | Allogeneic Melanoma Cell Line
|
Autologous Melanoma Cell Line
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| None | 888 | 938 | 526 | 624 | SB-1 | SB-2 | SB-9 | SB-11 | SB-18 | SB-23 | |
| HLA-A loci | 01 24 | 02 24 | 02 03 | 02 03 | 02 68 | 02 03 | 23 31 | 01 26 | 03 24 | 01 02 | |
| Patient TIL | |||||||||||
| 1 | 19 | 5 | 5 | 10 | 5 | 1,276 | |||||
| 2 | 0 | 0 | 0 | 98 | 38 | 3,310 | |||||
| 9 | 12 | 0 | 82 | 2 | 4 | 232 | |||||
| 11 | 6 | 4 | 14 | 7 | 5 | 11,850 | |||||
| 18 | 3 | 31 | 15 | 5 | 1 | 305 | |||||
| 23 | 0 | 14 | 12 | 0 | 3 | 1,435 | |||||
NOTE. Values shown in bold are at greater than twice background and greater than 200 pg/mL.
Abbreviation: TIL, tumor-infiltrating lymphocytes.
Table A3.
IFN-γ Release (pg/mL) From TIL Demonstrating Specificity for Autologous Single-Cell Fresh Tumor Digests With Unknown Peptide Antigen Specificity
| Cells | Allogeneic Melanoma Cell Line
|
Autologous Melanoma Single-Cell Digest
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | 888 | 938 | 526 | 624 | FrTu-4 | FrTu-16 | FrTu-19 | FrTu-22 | FrTu-24 | |
| HLA-A loci | 01 24 | 01 24 | 02 03 | 02 03 | 03 24 | 03 26 | 01 02 | 01 24 | 02 33 | |
| Patient TIL | ||||||||||
| 4 | 2 | 2 | 0 | 2 | 1 | 1,013 | ||||
| 16 | 15 | 10 | 0 | 12 | 9 | 384 | ||||
| 19 | 1 | 3 | 17 | 12 | 34 | 759 | ||||
| 22 | 1 | 105 | 431 | 5 | 82 | 1,138 | ||||
| 24 | 0 | 49 | 63 | 111 | 111 | 688 | ||||
NOTE. Values shown in bold are at greater than twice background and greater than 200 pg/mL.
Abbreviation: TIL, tumor-infiltrating lymphocytes.
published online ahead of print at www.jco.org on September 22, 2008.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00335127, NCT00096382, NCT00019942
REFERENCES
- 1.Tsao H, Atkins MB, Sober AJ: Management of cutaneous melanoma. N Engl J Med 351:998-1012, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Robbins PF, et al: Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298:850-854, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley ME, Wunderlich JR, Yang JC, et al: Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 23:2346-2357, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zippelius A, Batard P, Rubio-Godoy V, et al: Effector function of human tumor-specific CD8 T cells in melanoma lesions: A state of local functional tolerance. Cancer Res 64:2865-2873, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Topalian SL, Muul LM, Solomon D, et al: Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods 102:127-141, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Dudley ME, Wunderlich J, Nishimura MI, et al: Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother 24:363-373, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Riddell SR, Greenberg PD: The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods 128:189-201, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Antony PA, Piccirillo CA, Akpinarli A, et al: CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol 174:2591-2601, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gattinoni L, Finkelstein SE, Klebanoff CA, et al: Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 202:907-912, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulos CM, Wrzesinski C, Kaiser A, et al: Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest 117:2197-2204, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muranski P, Boni A, Wrzesinski C, et al: Increased intensity lymphodepletion and adoptive immunotherapy: How far can we go? Nat Clin Pract Oncol 3:668-681, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrzesinski C, Paulos CM, Gattinoni L, et al: Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest 117:492-501, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley ME, Wunderlich JR, Shelton TE, et al: Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 26:332-342, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Shen X, Huang J, et al: Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol 175:7046-7052, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartzentruber DJ: Guidelines for the safe administration of high-dose interleukin-2. J Immunother 24:287-293, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg SA, Yang JC, Restifo NP: Cancer immunotherapy: Moving beyond current vaccines. Nat Med 10:909-915, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg SA, Yannelli JR, Yang JC, et al: Treatment of patients with metastatic melanoma with autologous tumor- infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst 86:1159-1166, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Wrzesinski C, Restifo NP: Less is more: Lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr Opin Immunol 17:195-201, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]