Abstract
Purpose
Sentinel lymph node biopsy was adopted for the staging of the axilla with the assumption that it would reduce the risk of lymphedema in women with breast cancer. The aim of this study was to determine the long-term prevalence of lymphedema after SLN biopsy (SLNB) alone and after SLNB followed by axillary lymph node dissection (SLNB/ALND).
Patients and Methods
At median follow-up of 5 years, lymphedema was assessed in 936 women with clinically node-negative breast cancer who underwent SLNB alone or SLNB/ALND. Standardized ipsilateral and contralateral measurements at baseline and follow-up were used to determine change in ipsilateral upper extremity circumference and to control for baseline asymmetry and weight change. Associations between lymphedema and potential risk factors were examined.
Results
Of the 936 women, 600 women (64%) underwent SLNB alone and 336 women (36%) underwent SLNB/ALND. Patients having SLNB alone were older than those having SLNB/ALND (56 v 52 years; P < .0001). Baseline body mass index (BMI) was similar in both groups. Arm circumference measurements documented lymphedema in 5% of SLNB alone patients, compared with 16% of SLNB/ALND patients (P < .0001). Risk factors associated with measured lymphedema were greater body weight (P < .0001), higher BMI (P < .0001), and infection (P < .0001) or injury (P = .02) in the ipsilateral arm since surgery.
Conclusion
When compared with SLNB/ALND, SLNB alone results in a significantly lower rate of lymphedema 5 years postoperatively. However, even after SLNB alone, there remains a clinically relevant risk of lymphedema. Higher body weight, infection, and injury are significant risk factors for developing lymphedema.
INTRODUCTION
Historically, lymphedema has been the most feared complication of the surgical treatment of breast cancer. With the introduction of sentinel lymph node (SLN) biopsy for axillary staging, it was assumed that SLN biopsy would be associated with minimal morbidity as compared with full axillary lymph node dissection (ALND). Several studies have suggested that SLN biopsy (SLNB) does indeed reduce, but does not eliminate, lymphedema.
However, because SLNB is a relatively recently adopted procedure, and because lymphedema can occur years after axillary surgery,1 existing studies of lymphedema after SLNB are inadequate to accurately estimate long-term risk. Approximately 25% of women ultimately developing lymphedema will do so after 3 years,1 and therefore, studies reporting prevalence of lymphedema with less than 5 years of follow-up will underestimate the ultimate prevalence. We identified only two published studies that included patients with a median follow-up of at least 2.5 years.2,3
Furthermore, baseline measurements are essential to precisely determining changes in arm size resulting from lymphedema, as opposed to baseline asymmetry between dominant and nondominant arms. In addition, because the incidence of lymphedema is thought to be small after SLNB, evaluation of a large number of patients is essential to accurately estimate the prevalence. At present, we are aware of only one study that has baseline measurements and that observes a large number of women for more than 2 years.3 We undertook this study to quantify the long-term risk of lymphedema in a large group of women undergoing SLNB alone as compared with those having SLNB followed by conventional ALND (SLNB/ALND).
PATIENTS AND METHODS
Study Design
This prospective study was designed to determine the prevalence of upper-extremity lymphedema several years after SLNB alone as compared with SLNB/ALND. The secondary objective was to explore medical and surgical factors associated with the development of lymphedema. This protocol was approved by our institutional review board.
Patients
Between June 1, 1999, and May 30, 2003, 2,703 women underwent breast cancer surgery with SLNB for clinically node-negative breast cancer, had no prior axillary surgery, had no history of breast cancer, and had baseline bilateral upper-extremity measurements performed at the time of SLNB. Between December 29, 2005, and May 31, 2007, a trained research assistant who did not have knowledge of patients’ disease or lymphedema status approached 1,087 of these eligible patients and reviewed the study objectives. A total of 1,002 patients gave written informed consent. Study participants were interviewed, answered a standardized set of questions, and had height, weight, and bilateral upper-extremity measurements repeated. Clinicopathologic characteristics of the breast primary tumor and axillary lymph nodes were obtained from the prospective service databases and medical records. Sixty-six patients were excluded from analysis because of incomplete baseline measurements (n = 48), a diagnosis of bilateral breast cancer (n = 12), subsequent additional axillary surgery (n = 5), and withdrawal of consent (n = 1). The final study population therefore consisted of 936 women, 600 (64%) of whom underwent SLNB alone and 336 (36%) of whom underwent SLNB/ALND.
Surgery
All patients underwent breast-conserving surgery or mastectomy as necessary based on tumor characteristics, surgeon recommendation, and patient choice. Patients treated with lumpectomy remained eligible if they required re-excision to achieve negative surgical margins. Patients who underwent breast-conserving surgery were referred for whole-breast radiation therapy.
The details of the SLNB protocol have been described separately elsewhere.4 Briefly, a combined dye-isotope mapping technique was used, with technetium-labeled sulfur colloid injected intradermally and isosulfan blue dye injected intraparenchymally. All blue and hot nodes were removed, as well as any palpable lymph nodes or those suggestive of abnormality identified on intraoperative examination. Frozen-section analysis of the SLN(s) was routinely performed. Any patient with an SLN metastasis identified intraoperatively underwent an immediate ALND.
Upper-Extremity Measurements
Preoperatively, at the time of SLNB, baseline measurements were obtained 10 cm above and 5 cm below the olecranon process on both the ipsilateral and contralateral upper extremities. Bilateral follow-up measurements were taken 3 to 8 years later at the same sites. The change in ipsilateral upper-extremity circumference, corrected for any change in the contralateral upper extremity, was calculated using the following formula:
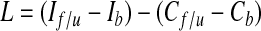 |
(1) |
where I indicates ipsilateral upper-extremity circumference, C indicates contralateral upper-extremity circumference, f/u indicates follow-up, and b indicates baseline. L was calculated for both upper arm and forearm, and lymphedema was defined as present if L > 2 cm for either location. Severe lymphedema was defined as L > 5 cm.
Patient Weight
Patients were weighed preoperatively and at the time of follow-up measurements. Weight change was defined as the difference between current and baseline measured weights. Patients gaining or losing up to 2 kg (5 lb) were considered to have no weight change.
Risk Factors
Risk factors for lymphedema were assessed through patient interview as well as review of medical records. Patients were questioned about ipsilateral breast radiation and ipsilateral upper-extremity injury or infection since their axillary surgery.
Statistical Analysis
The primary objective of the study was to measure the prevalence of lymphedema in the SLNB alone and SLNB/ALND groups based on objective arm measurements. Assuming an even distribution of patients participating in the study who had surgery between June 1, 1999, and May 30, 2003, the average follow-up time was estimated to be approximately 5 years. The literature shows that most cases of chronic lymphedema (77%) develop by 3 years after surgery.1 We therefore believed that most cases of lymphedema would be captured in our series. To determine accurate estimates of lymphedema in both surgical groups, the intended accrual was approximately 1,000 participants. We expected approximately 67% of patients with SLNB and 33% with SLNB/ALND in our cohort.
Associations between patient and disease variables and either surgery group or lymphedema status were examined using the χ2 or Fisher's exact test for categoric variables and the Wilcoxon rank sum test for continuous variables.
RESULTS
Characteristics of the study population according to type of axillary surgery are listed in Table 1. Patients having SLNB alone were older (median age at time of surgery, 56 v 52 years; P < .0001), were more likely to have breast-conserving surgery (73% v 47%; P < .0001), had smaller tumors (median size, 1.0 v 1.7 cm; P < .0001), and had an overall lower clinical stage. Of 600 women in the SLNB alone group, 571 women (95%) had N0 or N0(i+), and 29 women (5%) had N1 disease. The SLNB/ALND group was composed primarily of women with nodal metastases (292 of 336 women, 87%). The median follow-up for all patients was 5 years (range, 2.7 to 8.0 years).
Table 1.
Demographics and Disease Characteristics of 936 Patients According to Type of Axillary Surgery
| Variable | SLNB Alone (n = 600)
|
SLNB/ALND (n = 336)
|
P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | Median | Range | No. | % | Median | Range | ||
| Age at time of axillary surgery, years | 56 | 24-83 | 52 | 27-86 | < .0001 | ||||
| Age at follow-up, years | 62 | 28-90 | 56 | 31-89 | < .0001 | ||||
| Follow-up, years | 5.0 | 2.7-8.0 | 5.1 | 2.7-7.8 | .41 | ||||
| Laterality of surgery | .15 | ||||||||
| Right | 310 | 52 | 157 | 47 | |||||
| Left | 290 | 48 | 179 | 53 | |||||
| Type of surgery | < .0001 | ||||||||
| Conservation | 438 | 73 | 157 | 47 | |||||
| Mastectomy | 162 | 27 | 179 | 53 | |||||
| Tumor type | < .0001 | ||||||||
| IFDC | 420 | 70 | 296 | 88 | |||||
| IFLC | 47 | 7.8 | 27 | 8.0 | |||||
| Mucinous/papillary/medullary/tubular | 23 | 3.8 | 1 | 0.3 | |||||
| DCIS only | 79 | 13 | 8 | 2.4 | |||||
| DCIS with microinvasion | 22 | 3.7 | 1 | 0.3 | |||||
| Metaplastic | 2 | 0.3 | 0 | — | |||||
| Other | 7 | 1.2 | 3 | 0.9 | |||||
| Pathological size, cm | 1.0 | 0-5.0 | 1.7 | 0-7.5 | < .0001 | ||||
| AJCC T stage | |||||||||
| Tis | 79 | 13 | 8 | 2.4 | |||||
| T1a | 101 | 17 | 15 | 4.5 | |||||
| T1b | 158 | 26 | 52 | 15 | |||||
| T1c | 212 | 35 | 133 | 40 | |||||
| T2 | 50 | 8.3 | 118 | 34. | |||||
| T3 | 0 | — | 10 | 3.0 | |||||
| Total No. nodes excised | 3 | 1-17 | 19 | 2-65 | < .0001 | ||||
| No. of positive nodes | 0 | 0-3 | 1 | 0-32 | < .0001 | ||||
| AJCC N stage | |||||||||
| N0 | 555 | 93 | 35 | 10 | |||||
| N0(i+) | 16 | 2.7 | 9 | 3.6 | |||||
| N1mi | 18 | 3.0 | 34 | 9.2 | |||||
| N1, 1-3 node(s) positive | 11 | 1.8 | 186 | 55 | |||||
| N2, 4-9 nodes positive | 0 | — | 50 | 15 | |||||
| N3, ≥10 nodes positive | 0 | — | 22 | 6.5 | |||||
Abbreviations: SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; IFDC, infiltrating ductal carcinoma; IFLC, infiltrating lobular carcinoma; DCIS, ductal carcinoma in situ; AJCC, American Joint Commission on Cancer.
Comparisons of baseline and follow-up weights, degree of weight change, and body mass index (BMI) are listed in Table 2. Overall, patients undergoing SLNB/ALND were taller but with similar BMIs to those having SLNB alone.
Table 2.
Weight, Height, and Body Mass Index of Patients by Type of Axillary Surgery
| Variable | SLNB Alone (n = 600)
|
SLNB/ALND (n = 336)
|
P | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Current height, cm | 161 | 132-178 | 163 | 133-180 | .004 |
| Baseline weight, kg | 64 | 39-133 | 68 | 43-147 | .0003 |
| Current weight, kg | 66 | 41-131 | 69 | 39-131 | .005 |
| Baseline BMI | 25 | 15-49 | 25 | 17-54 | .02 |
| Current BMI | 25 | 17-49 | 26 | 17-48 | .07 |
| Change in weight, kg | 1.5 | −35-27 | 1.3 | −25-24 | .30 |
Abbreviations: SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; BMI, body mass index.
Lymphedema
Of patients undergoing SLNB alone, 5% (31 of 600 women) had measured lymphedema (L > 2 cm), as compared with 16% (55 of 336 women) of patients undergoing SLNB/ALND (Table 3). Severe lymphedema was found after SLNB alone in 0.5% (three of 600 women) and after SLNB/ALND in 3% (10 of 336 women) of patients. Regardless of the type of axillary surgery, lymphedema was more likely to develop in the upper arm than the forearm (Table 3).
Table 3.
Frequency of Measured Lymphedema Among Patients Undergoing SLNB Alone or SLNB/ALND
| Lymphedema | SLNB Alone (n = 600)
|
SLNB/ALND (n = 336)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Lymphedema*
|
Severe Lymphedema†
|
Lymphedema*
|
Severe Lymphedema†
|
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Frequency in either upper or lower arm | 31 | 5.2 | 3 | 0.5 | 55 | 16.4 | 10 | 3.0 |
| Upper arm only | 24 | 4.0 | 3 | 0.5 | 22 | 6.5 | 5 | 1.5 |
| Forearm only | 3 | 0.5 | 0 | — | 18 | 5.4 | 3 | 0.9 |
| Both upper and lower arm | 4 | 0.7 | 0 | — | 15 | 4.5 | 2 | 0.6 |
NOTE. L = (If/u − Ib) –(Cf/u − Cb), where I indicates ipsilateral upper-extremity circumference, C indicates contralateral upper-extremity circumference, f/u indicates follow-up, and b indicates baseline.
Abbreviations: SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
L > 2 cm.
L > 5 cm.
The median (interquartile) change in upper arm measurements for SLNB alone and SLNB/ALND, respectively, were 0 cm (range, −1.0 to 1.0 cm) and 0.5 cm (range, −0.5 to 1.0 cm; P = .0002). The median change in forearm measurements for SLNB alone and SLNB/ALND, respectively, were 0 cm (range, −0.5 to 0.5 cm) and 0 cm (range, −0.5 to 1.0 cm; P < .0001). Although the median changes were similar for both the upper and lower arm measurements, the overall changes in measurements were significantly greater for women in the SLNB/ALND group.
For women undergoing SLNB alone, the median number of nodes excised was three (range, one to 17 nodes) for those without lymphedema and four (range, one to nine nodes) for those with lymphedema (P < .0001). For patients undergoing SLNB/ALND, the median number of nodes excised was 19 (range, two to 65 nodes) for those without lymphedema and 22 (range, five to 44 nodes) for those with lymphedema (P < .0001).
Other factors associated with the development of lymphedema include greater baseline and current body weight (P < .0001), higher baseline and current BMI (P < .0001), and reported history of infection or injury (Table 4). Among the 39 patients who reported ipsilateral upper-extremity infection, 11 women (28%) had lymphedema, compared with 75 (8%) of 897 women without a history of infection (P < .0001). Similarly, patients who reported injury to their arm after axillary surgery were more likely to have lymphedema (17% v 9%; P = .02). Although patients having ipsilateral breast or chest wall irradiation had a slightly higher incidence of lymphedema, this difference was not statistically significant (10% v 8%; P = .29). In the subset of women having mastectomy and SLNB/ALND, radiation (n = 83) was associated with a 20% risk of lymphedema as compared with 11% without radiation (n = 96; P = .1). Weight gain and axillary surgery ipsilateral to the dominant arm were not significantly associated with the development of lymphedema. Type of breast surgery (breast-conserving surgery v mastectomy) was not associated with the prevalence of lymphedema when stratified by axillary procedure (P ≥ .77).
Table 4.
Factors Associated With Measured Lymphedema
| Factor | No Lymphedema* (n = 850)
|
Lymphedema† (n = 86)
|
P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | Median | Range | No. | % | Median | Range | ||
| Age at follow-up, years | 60 | 29-90 | 60 | 34-89 | .27 | ||||
| Baseline weight, kg | 65 | 39-147 | 77 | 43-134 | < .0001 | ||||
| Current weight, kg | 66 | 39-131 | 78 | 44-131 | < .0001 | ||||
| Baseline BMI | 24 | 15-54 | 29 | 20-49 | < .0001 | ||||
| Current BMI | 25 | 17-48 | 31 | 19-40 | < .0001 | ||||
| Weight gain > 2 kg | .29 | ||||||||
| No | 463 | 90 | 52 | 10 | |||||
| Yes | 387 | 92 | 34 | 8 | |||||
| Surgery in dominant arm | .46 | ||||||||
| No | 419 | 90 | 46 | 10 | |||||
| Yes | 431 | 92 | 40 | 8 | |||||
| Radiation | .29 | ||||||||
| No | 264 | 92 | 22 | 8 | |||||
| Yes | 586 | 90 | 64 | 10 | |||||
| Infection since surgery | < .0001 | ||||||||
| No | 822 | 92 | 75 | 8 | |||||
| Yes | 28 | 72 | 11 | 28 | |||||
| Injury since surgery | .02 | ||||||||
| No | 797 | 91 | 75 | 9 | |||||
| Yes | 53 | 83 | 11 | 17 | |||||
| Breast conservation | < .0001 | ||||||||
| SLNB alone | 415 | 95 | 23 | 5 | |||||
| SLNB/ALND | 130 | 83 | 27 | 17 | |||||
| Mastectomy | .001 | ||||||||
| SLNB alone | 154 | 95 | 8 | 5 | |||||
| SLNB/ALND | 151 | 84 | 28 | 16 | |||||
NOTE. L = (If/u − Ib) –(Cf/u − Cb), where I indicates ipsilateral upper-extremity circumference, C indicates contralateral upper-extremity circumference, f/u indicates follow-up, and b indicates baseline.
Abbreviations: BMI, body mass index; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
L ≤ 2 cm.
L > 2 cm.
DISCUSSION
Lymphedema represents potentially the most debilitating complication after any axillary surgery. Its true incidence can be difficult to determine, as no standardized definition of lymphedema exists. Volume displacement techniques are recognized as the gold standard in assessing lymphedema; however, this technique can be cumbersome in a busy clinical setting. Therefore, nearly all series2,3,5-17 have used circumferential measurements of the upper arm and forearm that act as a surrogate for volume change. The method of measurement, however, is not standardized, as various studies have reported arm measurement locations varying from 10 to 15 cm above the olecranon process, and from 5 to 15 cm below.3,5,11,16 Differences in measurement techniques make various studies difficult to compare.
Furthermore, defining lymphedema as a function of changes in upper-extremity circumference necessitates the availability of both baseline measurements and contralateral upper-extremity measurements. Although some groups have used the contralateral upper-extremity alone as a comparison to determine the presence of lymphedema, this method has its shortcomings. Significant differences in circumference may exist between a woman's dominant and nondominant upper extremities. Although in most women, these differences are less than 2 cm,18 a preexisting difference in the two upper-extremity circumferences could mask the detection of subsequent lymphedema in the smaller extremity. However, contralateral upper-extremity measurements are also essential. A significant change in body weight might increase the circumference of the ipsilateral upper extremity, resulting in an incorrect diagnosis of measured lymphedema if the upper-extremity measurement is only compared with the baseline measurement. Only by having comparison measurements from the contralateral upper extremity would one be able to distinguish an increase in body weight from the development of lymphedema. Thus baseline and contralateral upper-extremity measurements are essential to accurately determine a change in arm measurements and therefore the presence of lymphedema.
Two prospective randomized trials10,12 and several prospective nonrandomized series2,3,9,11,13,16,19 have concluded that lymphedema after SLNB is less common or severe than after ALND. Many of these studies do not report the actual incidence of lymphedema. Of those that do, the rates of lymphedema vary from 0% to 7% at 6 to 36 months after SLNB (Table 5).
Table 5.
Literature Reporting Measured Lymphedema After SLNB
| First Author and Year | No. of Patients
|
Follow-Up (months) | Preoperative Measurements | Measurement Method | Definition of Lymphedema | Comparison Group | Lymphedema Less After SLNB | Proportion With Lymphedema (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Entire Study | SLNB | ||||||||
| Schrenk,5 2000 | 70 | 35 | Mean 15.4 | Yes | Circumference | L as continuous variable | ALND | S | — |
| Sener,19 2001 | 420 | 303 | Median 19 | Yes | Volume displacement | — | SLNB + ALND | S | 3 |
| Burak,6 2002 | 96 | 48 | Mean 15.3 | No | Circumference | Ratio with/difference from contralateral as continuous variable | SLNB + ALND | S | — |
| Haid,7 2002 | 197 | 57 | Mean 18 | No | Circumference | > 2 cm difference from contralateral and edema | Historical ALND | S | 3.5 |
| Haid,8 2002 | 151 | 66 | Minimum 2 | No | Circumference | > 10% difference from contralateral | ALND | S | — |
| Golshan,9 2003 | 125 | 77 | Minimum 12 | No | Circumference | > 3 cm difference from contralateral | ALND | S | 2.6 |
| Veronesi,10 2003 | 200 | 100 | 24 | No | Circumference | > 2 cm difference from contralateral | SLNB + ALND RCT | S | 0 |
| Leidenius,2 2005 | 139 | 92 | Median 36 | No | Circumference | ≥ 2 cm difference from contralateral | ALND | S | 0 |
| Purushotham,12 2005 | 277 | 134 (SLNB ± ALND) | Maximum 12 | Yes | Circumference | L as continuous variable | SLNB ± ALND versus ALND RCT | S | — |
| Ronka,13 2005 | 83 | 43 | 12 | Yes | Circumference | (L/Ib) > 10% | SLNB + ALND | NS | 2 |
| Mansel,14 2006 | 816 | 413 (SLNB ± ALND or RT) | 12 | Yes | Circumference | — | SLNB ± ALND or RT versus ALND RCT | NS | — |
| Rietman,15 2006 | 181 | 57 | 24 | Yes | Circumference | Change from baseline as continuous variable | ALND ± SLNB | S | — |
| Wilke,16 2006 | 2,904 | 2,904 | 6 | Yes | Circumference | L > 2 cm | — | — | 7 |
| Langer,3 2007 | 635 | 431 | Median 31 | Yes | Circumference | ≥ 2 cm difference from baseline or contralateral, or edema | SLNB + ALND | S | 3.5 |
| Lucci,17 2007 | 821 | 411 | 12 | Yes | Circumference | L ≥ 2 cm | SLNB + ALND RCT | NS | 6 |
| Current study | 936 | 600 | Median 60 | Yes | Circumference | L > 2 cm | SLNB + ALND | S | 5 |
NOTE. L = (If/u − Ib) –(Cf/u − Cb), where I indicates ipsilateral upper-extremity circumference, C indicates contralateral upper-extremity circumference, f/u indicates follow-up, and b indicates baseline.
Abbreviations: SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; S, significant; RCT, randomized control trial; RT, radiotherapy; NS, not significant; circumference, arm circumference measurements.
The development of lymphedema is an unpredictable occurrence that can happen years after axillary surgery. Among those who develop lymphedema after ALND, the onset of symptoms occurs within 3 years in 77% of patients1 but after 3 years in approximately 25% of patients. Of women without lymphedema 3 years after ALND, the ongoing risk of developing lymphedema is approximately 1% per year for at least 20 years. With 20 years of follow-up, Petrek et al1 found lymphedema in 49% of patients, a significantly higher rate than the 6% to 30% usually reported after ALND. This suggests that the incidence of lymphedema is commonly underestimated as a result of the inadequate follow-up interval in most studies.
The development of lymphedema after SLNB is likely to follow the same pattern as for ALND. Therefore, only long follow-up will accurately predict its true incidence. Reported rates of lymphedema after SLNB of 0% to 7% with 6 to 36 months of follow-up likely represent only a fraction of patients who will ultimately be diagnosed with lymphedema. These rates are likely to increase as women are observed for longer periods of time. The current series reports the prevalence of lymphedema in women a median of 5 years postoperatively, thereby likely capturing the large majority of women who will develop lymphedema after SLNB.
It is difficult to compare our measured severe lymphedema rate of 0.5% with that of other series, as severe lymphedema after SLNB is rarely recorded or quantified as a measured value in the literature. Similar to the present study, Petrek et al1 defined severe lymphedema as a measured difference of more than 2 inches (5.08 cm). At 20 years, 13% of patients who underwent ALND were classified as having severe lymphedema. Sener et al19 defined postoperative arm volume differences of greater than 40% as severe lymphedema, but do not report its incidence. Others define it subjectively by patient report2,5,8,13 or as the need to wear a compression garment.20 To date, no series has reported severe lymphedema, as defined by arm measurements, in a patient after SLNB. However, as defined by patient report, Blanchard et al20 described the presence of subjective severe lymphedema after SLNB in four (0.6%) of 685 women, and Leidenius et al13 report it in one (1%) of 92 women. These values are similar to the observed 0.5% incidence of measured severe lymphedema in women who underwent SLNB in the present series.
In the current series, the risk factors that were significantly associated with the presence of lymphedema at a median of 5 years were greater body weight, higher BMI, infection, or injury. Obesity, increasing BMI, and weight gain are commonly recognized as risk factors for lymphedema.1,16,21 Although injury and infection have also been described as increasing the risk of lymphedema, the influence of these factors must be carefully examined. Both variables are dependent on patient recall. Although both seem to be significant risk factors, it is possible that women with lymphedema recall an inciting infection or injury more readily than a woman without lymphedema. Furthermore, it is possible that women with lymphedema are at higher risk of developing infection. Even injuries might be more commonly reported if women with lymphedema have greater difficulty recovering from injury, making it more memorable.
There are additional limitations of the current study. Because the patients were not randomly assigned to a treatment arm, the surgical procedure is almost entirely confounded by disease stage in this series. Also, as in nearly all other series, we used circumferential arm measurements, which, as opposed to volume displacement methods, will not detect those patients with isolated hand edema, therefore underestimating the incidence of lymphedema.
Circumferential arm measurements represent an objective method to determine the presence of lymphedema and cannot be influenced by sensory changes such as numbness or pain that are common after axillary surgery22 and can influence patient perceptions. However, measurements may not be the most reliable indicator of clinically significant lymphedema. An ipsilateral calculated measurement difference of more than 2 cm may be severely disfiguring in a thin woman and unnoticeable in an obese one. In addition, the influence of patient perceptions in the setting of lymphedema cannot be underestimated. It is likely that both objective upper-extremity measurements and symptom assessment are needed to determine the true prevalence of clinically significant lymphedema. We have reported our findings with regard to patient perceptions of lymphedema in an accompanying separate report.23
In conclusion, SLNB results in significantly less frequent lymphedema than ALND. At a median of 5 years after SLNB, approximately 5% of women will develop lymphedema. It is possible that this proportion will continue to increase with time, although data regarding the incidence of lymphedema after ALND suggest that the large majority of those who will ultimately develop it have done so by 5 years. The technique of SLNB has certainly reduced the morbidity associated with axillary staging for breast cancer; however, there remains a small but significant risk of measured lymphedema.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Mary J. Wright, Katherine T. Morris, Gladys L. Giron, Elyn R. Riedel, Kimberly J. Van Zee
Collection and assembly of data: Sarah A. McLaughlin, Michelle R. Sampson, Julia P. Brockway, Kimberly J. Van Zee
Data analysis and interpretation: Sarah A. McLaughlin, Karen E. Hurley, Elyn R. Riedel, Kimberly J. Van Zee
Manuscript writing: Sarah A. McLaughlin, Kimberly J. Van Zee
Final approval of manuscript: Sarah A. McLaughlin, Mary J. Wright, Katherine T. Morris, Gladys L. Giron, Michelle R. Sampson, Julia P. Brockway, Karen E. Hurley, Elyn R. Riedel, Kimberly J. Van Zee
published online ahead of print at www.jco.org on October 6, 2008.
Supported by National Cancer Institute Grant No. K07 CA109236 (K.E.H.).
Presented in part at the 60th Annual Cancer Symposium of the Society of Surgical Oncology, March 15-18, 2007, Washington, DC, and at the American Society of Clinical Oncology Breast Symposium, September 7-8, 2007, San Francisco, CA.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Petrek JA, Senie RT, Peters M, et al: Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 92:1368-1377, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Leidenius M, Leivonen M, Vironen J, et al: The consequences of long-time arm morbidity in node-negative breast cancer patients with sentinel node biopsy or axillary clearance. J Surg Oncol 92:23-31, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Langer I, Guller U, Berclaz G, et al: Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: A prospective Swiss multicenter study on 659 patients. Ann Surg 245:452-461, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cody HS 3rd, Borgen PI: State-of-the-art approaches to sentinel node biopsy for breast cancer: Study design, patient selection, technique, and quality control at Memorial Sloan-Kettering Cancer Center. Surg Oncol 8:85-91, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Schrenk P, Rieger R, Shamiyeh A, et al: Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer 88:608-614, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Burak WE, Hollenbeck ST, Zervos EE, et al: Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. Am J Surg 183:23-27, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Haid A, Koberle-Wuhrer R, Knauer M, et al: Morbidity of breast cancer patients following complete axillary dissection or sentinel node biopsy only: A comparative evaluation. Breast Cancer Res Treat 73:31-36, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Haid A, Kuehn T, Konstantiniuk P, et al: Shoulder-arm morbidity following axillary dissection and sentinel node only biopsy for breast cancer. Eur J Surg Oncol 28:705-710, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Golshan M, Martin WJ, Dowlatshahi K: Sentinel lymph node biopsy lowers the rate of lymphedema when compared with standard axillary lymph node dissection. Am Surg 69:209-211, 2003 [PubMed] [Google Scholar]
- 10.Veronesi U, Paganelli G, Viale G, et al: A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 349:546-553, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Rietman JS, Dijkstra PU, Geertzen JH, et al: Treatment-related upper limb morbidity 1 year after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast cancer. Ann Surg Oncol 11:1018-1024, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Purushotham AD, Upponi S, Klevesath MB, et al: Morbidity after sentinel lymph node biopsy in primary breast cancer: Results from a randomized controlled trial. J Clin Oncol 23:4312-4321, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Rönkä R, von Smitten K, Tasmuth T, et al: One-year morbidity after sentinel node biopsy and breast surgery. Breast 14:28-36, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Mansel RE, Fallowfield L, Kissin M, et al: Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J Natl Cancer Inst 98:599-609, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Rietman JS, Geertzen JH, Hoekstra HJ, et al: Long term treatment related upper limb morbidity and quality of life after sentinel lymph node biopsy for stage I or II breast cancer. Eur J Surg Oncol 32:148-152, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Wilke LG, McCall LM, Posther KE, et al: Surgical complications associated with sentinel lymph node biopsy: Results from a prospective international cooperative group trial. Ann Surg Oncol 13:491-500, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Lucci A, McCall LM, Beitsch PD, et al: Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol 25:3657-3663, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kissin MW, Querci della Rovere G, Easton D, et al: Risk of lymphoedema following the treatment of breast cancer. Br J Surg 73:580-584, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Sener SF, Winchester DJ, Martz CH, et al: Lymphedema after sentinel lymphadenectomy for breast carcinoma. Cancer 92:748-752, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Blanchard DK, Donohue JH, Reynolds C, et al: Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg 138:482-487, 2003; discussion 487-488 [DOI] [PubMed] [Google Scholar]
- 21.Werner RS, McCormick B, Petrek J, et al: Arm edema in conservatively managed breast cancer: Obesity is a major predictive factor. Radiology 180:177-184, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Baron RH, Fey JV, Borgen PI, et al: Eighteen sensations after breast cancer surgery: A 5-year comparison of sentinel lymph node biopsy and axillary lymph node dissection. Ann Surg Oncol 14:1653-1661, 2007 [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin S, Wright M, Morris K, et al: Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Patient perceptions and precautionary behaviors. J Clin Oncol doi: 10.1200/JCO.2008.16.3766 [DOI] [PMC free article] [PubMed]


