Abstract
Purpose
Sentinel lymph node (SLN) biopsy was adopted for the staging of the axilla with the assumption that it would reduce the risk of lymphedema in women with breast cancer. This study was undertaken to examine patient perceptions of lymphedema and use of precautionary behaviors several years after axillary surgery.
Patients and Methods
Nine hundred thirty-six women who underwent SLN biopsy (SLNB) alone or SLNB followed by axillary lymph node dissection (SLNB/ALND) between June 1, 1999, and May 30, 2003, were evaluated at a median of 5 years after surgery. Patient-perceived lymphedema and avoidant behaviors were assessed through interview and administered a validated instrument, and compared with arm measurements.
Results
Current arm swelling was reported in 3% of patients who received SLNB alone versus 27% of patients who received SLNB/ALND (P < .0001), as compared with 5% and 16%, respectively, with measured lymphedema. Only 41% of patients reporting arm swelling had measured lymphedema, and 5% of patients reporting no arm swelling had measured lymphedema. Risk factors associated with reported arm swelling were greater body weight (P < .0001), higher body mass index (P < .0001), infection (P < .0001), and injury (P = .007) in the ipsilateral arm since surgery. Patients followed more precautions if they had measured or perceived lymphedema.
Conclusion
Body weight, infection, and injury are significant risk factors for perceiving lymphedema. There is significant discordance between the presence of measured and patient-perceived lymphedema. When compared to SLNB/ALND, SLNB-alone results in a significantly lower rate of patient-perceived arm swelling 5 years postoperatively, and is perceived by fewer women than are measured to have it.
INTRODUCTION
Lymphedema is the most debilitating complication of the surgical treatment of breast cancer. The technique of sentinel lymph node biopsy (SLNB) has reduced the morbidity of axillary staging for breast cancer, but is still associated with a smaller but significant risk of paresthesias,1,2 limited shoulder mobility,3-5 and lymphedema.
Documenting the benefits of SLNB is challenging because lymphedema encompasses both physical changes assessed by objective measures (eg, arm swelling) and sensations assessed by patient report (eg, pain, tightness). Existing series quantifying lymphedema after SLNB have used circumferential arm measurements,6-9 patient questionnaires,4,10-12 or both3,13-20 to document its presence. The reported rates of lymphedema may be influenced by each patient's interpretation of their own arm swelling and concomitantly by existing sensory changes. Therefore, the definition and prevalence of what constitutes clinically significant lymphedema is unclear.
Furthermore, approximately 25% of women ultimately developing lymphedema after axillary lymph node dissection (ALND) will do so after 3 years.21 Therefore, in order to assess the long-term prevalence of lymphedema in this population that generally has a very long survival, it is necessary to evaluate women several years after axillary surgery. The current published literature regarding lymphedema after SLNB reports only short-term outcomes (≤ 3 years).
This prospective study was designed to determine the prevalence of patient-perceived lymphedema and objectively measured lymphedema in a large population of women several years after SLNB alone as compared with SLNB followed by conventional axillary lymph node dissection (SLNB/ALND). Measured lymphedema was found in 5% of patients who underwent SLNB alone and 16% of patients who underwent SLNB/ALND, and is reported separately.22 The primary aim of this analysis is to examine the frequency of patient-perceived arm swelling through interview and with a validated instrument, and to compare these subjective reports to arm measurements.
In addition, although women undergoing SLNB are at low risk of lymphedema, many still pursue arm-hand precautions in an attempt to reduce their risk of arm swelling. The list of activities women are told to avoid by both medical personnel and the lay press is extensive and inconsistent. A Google search of the world wide web using the search terms “lymphedema prevention” and “breast cancer” retrieves 1,420 sites (January 3, 2008); some are medical (American Cancer Society), some are sales related (eg, selling compression sleeves via the Internet),23 and some are patient created.24 While the professionally maintained sites generally state, “There are no scientific studies showing women can prevent lymphedema”25 many of the lay press sites do not. 26 The list of restrictions varies from sensible avoidance of cuts and infections to recommendations to maintain a low-salt diet. Recent publications have begun to assess the validity of the more commonly listed precautions (such as avoidance of exercise and air travel) and found no relationship between these precautionary behaviors and the development of lymphedema.27,28 Therefore, we also evaluated the precautionary behaviors that patients followed in an attempt to avoid developing lymphedema.
PATIENTS AND METHODS
Study Design
This prospective study was designed to determine the prevalence of patient-perceived upper-extremity lymphedema several years after SLNB alone as compared with SLNB/ALND. Secondary objectives were: to explore medical and surgical factors associated with the development of patient-perceived lymphedema, to explore avoidant behaviors employed by women, and to compare objective upper extremity measurements with patient perceptions of lymphedema. This protocol was approved by our institutional review board.
Patients
Between June 1, 1999, and May 30, 2003, 2,703 women underwent breast cancer surgery with unilateral SLNB for clinically node-negative breast cancer, had no prior axillary surgery, had no history of breast cancer, and had baseline bilateral upper extremity measurements performed at the time of SLNB. Between December 29, 2005 and May 31, 2007, a trained research assistant who did not have knowledge of patients’ disease or lymphedema status approached 1,087 of these eligible patients and reviewed the study objectives. One thousand two patients gave written informed consent. Study participants were interviewed, answered a standardized set of questions, and had height, weight, and bilateral upper extremity measurements repeated. Clinicopathologic characteristics of the breast primary tumor and axillary lymph nodes were obtained from the prospective service databases and medical records. Sixty-six patients were excluded from analysis due to incomplete baseline measurements (n = 48), a diagnosis of bilateral breast cancer (n = 12), additional axillary surgery (n = 5), and withdrawal of consent (n = 1). The final study population therefore consisted of 936 women—600 (64%) of whom had SLNB alone and 336 (36%) of whom had SLNB/ALND.
Arm Measurements and Weights
Details of our measurement calculations of upper arm and forearm are described separately.22 Using the formula:
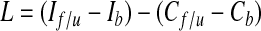 |
where I indicates ipsilateral upper-extremity circumference, C indicates contralateral upper-extremity circumference, f/u indicates follow-up, and b indicates baseline; lymphedema was defined as present if L > 2 cm for either upper arm or forearm location. Patient weights were obtained at baseline and follow-up visits.
Patient Interview
Patient perceptions and symptoms of lymphedema were assessed during a standardized interview. Patients were specifically asked if they had any current swelling in the upper extremity ipsilateral to their axillary surgery, as well as questions about ipsilateral breast or chest wall radiation, ipsilateral upper extremity injury or infection, and change in body weight. In addition, patients were administered an adaptation of the Lymphedema and Breast Cancer Questionnaire,29 a validated instrument that assesses symptoms indicative of lymphedema. Patient use of precautionary behaviors to reduce the risk of lymphedema was also assessed by standardized interview.
Statistical Analysis
Details of the study design are described separately.22 Briefly, the intended accrual was approximately 1,000 patients. Patient responses from the interview were summarized and compared by axillary surgery group, the presence of objectively measured lymphedema, and other patient characteristics. Differences between groups were assessed using the χ2 test for categoric variables and the Wilcoxon rank sum test or Kruskal-Wallis test for continuous variables. McNemar's test was used to assess whether measured and perceived lymphedema were concordant. All analyses were done using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Characteristics of the study population according to the axillary surgery performed are described in detail in a separate report.22 Patients having SLNB alone were older, more likely to have successful breast conserving surgery, had smaller tumors, and had an overall lower clinical stage.22 The median follow-up for all patients was 5 years (range, 2.7 to 8.0).
Interview Data
The presence of current ipsilateral arm swelling was reported by 3% (18 of 600) of patients with SLNB alone and 27% (91 of 336) of patients with SLNB/ALND (Table 1). Fewer SLNB-alone patients reported a history of ipsilateral upper extremity infection (2% v 8%; P < .0001) or injury (6% v 9%; P = .03) compared with the SLNB/ALND group. Rates of breast or chest wall radiation were similar in both groups (70% v 69%; P = .84).
Table 1.
Interview Data
| Variable | SLNB Alone (n = 600)
|
SLNB/ALND (n = 336)
|
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Dominant arm, right | 543 | 90.5 | 301 | 89.6 | .65 |
| Current arm swelling | 18 | 3.0 | 91 | 27.1 | < .0001 |
| History of infections in arm since surgery | 13 | 2.2 | 26 | 7.7 | < .0001 |
| History of injury in arm since surgery | 33 | 5.5 | 31 | 9.2 | .03 |
| Breast or chest wall radiation | 418 | 69.7 | 232 | 69.0 | .84 |
Abbreviations: SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
Validated Instrument Data
Results from the adapted Lymphedema and Breast Cancer Questionnaire demonstrate that fewer SLNB-alone than SLNB/ALND patients perceived an increase in their arm size or tighter-fitting sleeves (4% v 16%; P < .0001; Table 2). Similarly, only 2% of SLNB-alone compared with 9% of SLNB/ALND patients felt their sleeve cuffs fit more tightly (P < .0001). Furthermore, SLNB-alone patients perceived significantly less arm firmness or tightness (20% v 40%; P < .0001), heaviness (7.3% v 23%; P < .0001), numbness (21% v 52%; P < .0001), tenderness (17% v 24%; P = .005), or aching (24% v 35%; P = .0005). Overall, patients undergoing SLNB alone experienced fewer symptoms (mean, 1.1 v 2.3; P < .0001) than those undergoing SLNB/ALND.
Table 2.
Adaptation of Lymphedema and Breast Cancer Questionnaire
| Variable | SLNB Alone (n = 600)
|
SLNB/ALND (n = 336)
|
P | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean | SD | Median | Range | No. | % | Mean | SD | Median | Range | ||
| Arm size larger | 24 | 4.0 | 53 | 16 | < .0001 | ||||||||
| Neck size larger | 12 | 2.0 | 5 | 1.5 | .57 | ||||||||
| Shoulder size larger | 11 | 1.8 | 4 | 1.2 | .45 | ||||||||
| Sleeve fits tighter | 22 | 3.7 | 53 | 16 | < .0001 | ||||||||
| Sleeve cuff fits tighter | 10 | 1.7 | 30 | 8.9 | < .0001 | ||||||||
| Swelling with pitting | 5 | 0.8 | 15 | 4.5 | .0002 | ||||||||
| Firmness/tightness | 119 | 20 | 134 | 40 | < .0001 | ||||||||
| Heaviness | 44 | 7.3 | 77 | 23 | < .0001 | ||||||||
| Numbness | 127 | 21 | 176 | 52 | < .0001 | ||||||||
| Tenderness | 99 | 17 | 81 | 24 | .005 | ||||||||
| Aching | 145 | 24 | 117 | 35 | .0005 | ||||||||
| Breast swelling | 27 | 4.5 | 17 | 5.1 | .70 | ||||||||
| No. of symptoms reported per person | 1.1 | 1.4 | 1 | 0-9 | 2.3 | 2.0 | 2 | 0-10 | < .0001 | ||||
Abbreviations: SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; SD, standard deviation.
Risk Factors
Factors associated with patient-perceived lymphedema include greater baseline and current body weight (P < .0001), higher baseline and current body mass index (P < .0001), reported history of infection or injury, and type of axillary surgery (Table 3). Among the 39 patients who reported ipsilateral upper extremity infection, 16 (41%) reported lymphedema compared with 93 (10%) of 897 patients without a history of infection (P < .0001). Similarly, patients who reported injury to their arm after axillary surgery were more likely to report swelling (23% v 11%; P = .002). Although patients having ipsilateral breast or chest wall irradiation had a somewhat higher prevalence of perceived current arm swelling, this difference was not statistically significant (13% v 9%; P = .16). Weight gain since surgery and axillary surgery ipsilateral to the dominant arm were similarly not significantly associated with patient-reported arm swelling (P = .22 and .15, respectively).
Table 3.
Factors Associated With Perceived Current Arm Swelling
| Variable | Patient-Reported Current Swelling
|
P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No (n = 827)
|
Yes (n = 109)
|
||||||||
| No. | % | Median | Range | No. | % | Median | Range | ||
| Age at follow-up, years | 60 | 28-90 | 56 | 33-89 | .12 | ||||
| Weight, kg | |||||||||
| Baseline | 64 | 39-133 | 71 | 44-147 | < .0001 | ||||
| Current | 66 | 39-131 | 74 | 44-141 | < .0001 | ||||
| BMI | |||||||||
| Baseline | 25 | 15-49 | 26 | 19-54 | < .0001 | ||||
| Current | 25 | 17-49 | 28 | 19-48 | < .0001 | ||||
| Weight gain > 2 kg | |||||||||
| No | 461 | 89 | 54 | 10 | .22 | ||||
| Yes | 336 | 87 | 55 | 13 | |||||
| Surgery in dominant arm | |||||||||
| No | 418 | 90 | 47 | 10 | .15 | ||||
| Yes | 409 | 87 | 62 | 13 | |||||
| Radiation | |||||||||
| No | 259 | 91 | 27 | 9 | .16 | ||||
| Yes | 568 | 87 | 82 | 13 | |||||
| Infection since surgery | |||||||||
| No | 804 | 90 | 93 | 10 | <.0001 | ||||
| Yes | 23 | 59 | 16 | 41 | |||||
| Injury since surgery | |||||||||
| No | 778 | 89 | 94 | 11 | .002 | ||||
| Yes | 49 | 77 | 15 | 23 | |||||
| Procedure | |||||||||
| SNLB alone | 582 | 97 | 18 | 3 | < .0001 | ||||
| SNLB/ALND | 245 | 73 | 91 | 27 | |||||
Abbreviations: SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; BMI, body mass index.
Patient Perceptions of Current Arm Swelling and Measured Lymphedema
Patients were categorized as follows: no lymphedema, 786 of 936 (84%; no perceived swelling and L ≤ 2 cm); asymptomatic lymphedema, 41 of 936 (4%; no perceived swelling but L > 2 cm); symptomatic nonlymphedema, 64 of 936 (7%; perceived swelling but L ≤ 2 cm); and symptomatic lymphedema, 45 of 936 (5%; perceived swelling and L > 2 cm; Table 4). Of 109 women with symptoms of arm swelling, only 45 (41%) demonstrated measured lymphedema.
Table 4.
Association of Measured Lymphedema (L > 2 cm) and Patient-Reported Current Arm Swelling by Arm Dominance and Type of Axillary Surgery
| Variable | No. of Patients | Patient-Reported Current Swelling
|
P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No
|
Yes
|
|||||||||
| No Lymphedema (L ≤ 2 cm)
|
Asymptomatic Lymphedema (L > 2 cm)
|
Symptomatic Nonlymphedema (L ≤ 2 cm)
|
Symptomatic Lymphedema (L > 2 cm)
|
|||||||
| No. | % | No. | % | No. | % | No. | % | |||
| All patients | 936 | 786 | 84 | 41 | 4.3 | 64 | 6.8 | 45 | 4.8 | .02 |
| Dominant arm | 471 | 394 | 84 | 15 | 3.2 | 37 | 7.9 | 25 | 5.3 | .002 |
| Non-dominant arm | 465 | 392 | 84 | 26 | 5.6 | 27 | 5.8 | 20 | 4.3 | .89 |
| SLNB alone | 600 | 557 | 93 | 25 | 4.2 | 12 | 2.0 | 6 | 1.0 | .03 |
| SLNB/ALND | 336 | 229 | 68 | 16 | 4.8 | 52 | 15 | 39 | 12 | < .0001 |
NOTE. L > 2 cm, definition of measured lymphedema as calculated by formula L = (If/u−Ib) –(Cf/u−Cb) where I indicates ipsilateral upper-extremity circumference, C indicates contralateral upper-extremity circumference, f/u indicates follow-up, and b indicates baseline.
Abbreviations: ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy.
Overall, 471 patients had axillary surgery ipsilateral to their dominant upper extremity, and of these, 419 (89%) reported perceptions concordant with arm measurements (no lymphedema or symptomatic lymphedema; Table 4). The remaining 52 women with surgery in the dominant arm were more likely to report symptomatic nonlymphedema than asymptomatic lymphedema (37 v 15; P = .002). Similarly, of 465 women having surgery ipsilateral to their nondominant arm, 412 (89%) reported perceptions concordant (no lymphedema or symptomatic lymphedema). However, the remaining 53 women having surgery in the nondominant arm were equally likely to report symptomatic nonlymphedema or asymptomatic lymphedema (27 v 26, P = .89).
The majority of patients undergoing either SLNB alone or SLNB/ALND reported symptoms concordant with measurements (ie, no lymphedema or symptomatic lymphedema; Table 4). However, when patient perception and L > 2 cm were discordant, patients having SLNB/ALND were more likely to have symptomatic nonlymphedema instead of asymptomatic lymphedema (52 v 16; P < .0001). In contrast, patients who underwent SLNB alone were more likely to have asymptomatic lymphedema than symptomatic nonlymphedema (25 v 12; P = .03).
Precautionary Behaviors
The large majority of women undergoing either SLNB alone or SLNB/ALND practiced precautionary behaviors to reduce their subsequent risk of lymphedema (Table 5). However, women in the SLNB/ALND group practiced more avoidant behaviors than did women in the SLNB-alone group (mean, 5.1 v 4.3; P < .0001; Table 6). When precautionary behaviors were assessed by presence of lymphedema as determined by either L > 2 cm or patient perception, patients with lymphedema followed significantly more precautionary behaviors (Table 6). In addition, symptomatic lymphedema and symptomatic nonlymphedema patients followed more precautions than nonsymptomatic patients (P < .0001). Arm dominance did not significantly influence the number of precautionary behaviors practiced.
Table 5.
Comparison of Precautionary Behaviors Adopted by Patients Undergoing SLNB Alone or SLNB/ALND
| Behavior | SLNB Alone (n = 600)
|
SLNB/ALND (n = 336)
|
P | ||
|---|---|---|---|---|---|
| No. | %* | No. | %* | ||
| Avoids IVs in the affected arm | 498 | 84 | 331 | 99 | < .0001 |
| Avoids blood pressure cuff inflation on the affected arm | 507 | 85 | 331 | 99 | < .0001 |
| Avoids blood draws in the affected arm | 507 | 85 | 333 | 99 | < .0001 |
| Avoids tennis or other racquet sports | 29 | 25 | 18 | 36 | .19 |
| Avoids playing golf | 14 | 16 | 4 | 10 | .42 |
| Avoids lifting weight over 15 pounds | 169 | 57 | 97 | 61 | .49 |
| Avoids swimming | 17 | 7.1 | 7 | 4.8 | .39 |
| Avoids skiing | 21 | 22 | 8 | 17 | .52 |
| Avoids other athletic pursuits | 15 | 3.7 | 9 | 4.1 | .83 |
| Avoids picking up children | 63 | 17 | 45 | 24 | .05 |
| Avoids housework | 23 | 4.2 | 15 | 4.9 | .73 |
| Avoids gardening | 26 | 8.2 | 13 | 7.4 | .86 |
| Wears gloves when gardening or doing housework | 241 | 69 | 159 | 74 | .18 |
| Avoids lifting suitcases or purse carrying | 203 | 36 | 147 | 47 | .002 |
| Wears compression sleeve when flying | 7 | 1.2 | 52 | 16 | < .0001 |
| Has changed pattern with flying | 56 | 9.3 | 44 | 13 | .08 |
| Avoids flying | 18 | 3.0 | 18 | 5.4 | .08 |
| Only takes short flights ≤ 1 hour in length | 4 | 0.7 | 2 | 0.6 | .99 |
Abbreviations: SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; IVs, intravenous catheters.
Women who answered the question with “not applicable” were excluded from the denominator.
Table 6.
No. of Precautions Followed by Surgical Procedure, Arm Dominance, and Lymphedema Category
| Parameter | No. of Patients | Mean | SD | Median | Range | P |
|---|---|---|---|---|---|---|
| SLNB | ||||||
| Alone | 600 | 4.3 | 1.9 | 4 | 0-13 | < .0001 |
| Followed by ALND | 336 | 5.1 | 1.5 | 5 | 1-11 | |
| Arm | ||||||
| Dominant | 471 | 4.7 | 1.8 | 5 | 0-13 | .24 |
| Non-dominant | 465 | 4.5 | 1.9 | 4 | 0-13 | |
| L > 2 cm | ||||||
| No | 850 | 4.5 | 1.8 | 4 | 0-13 | < .0001 |
| Yes | 86 | 5.3 | 1.9 | 5 | 0-11 | |
| Patient-reported current swelling | ||||||
| No | 827 | 4.4 | 1.8 | 4 | 0-13 | < .0001 |
| Yes | 109 | 5.7 | 1.8 | 5 | 1-11 | |
| Swelling by LBCQ | ||||||
| No | 859 | 4.5 | 1.8 | 4 | 0-13 | < .0001 |
| Yes | 77 | 5.5 | 2.0 | 5 | 0-11 | |
| Lymphedema | ||||||
| No* | 786 | 4.4 | 1.8 | 4 | 0-13 | < .0001 |
| Asymptomatic* | 41 | 4.7 | 1.7 | 5 | 0-8 | |
| Symptomatic nonlymphedema* | 64 | 5.5 | 1.8 | 5 | 1-10 | |
| Symptomatic* | 45 | 5.9 | 1.9 | 6 | 3-11 |
NOTE. L > 2 cm, definition of measured lymphedema as calculated by formula L = (If/u−Ib) –(Cf/u−Cb) where I indicates ipsilateral upper-extremity circumference, C indicates contralateral upper-extremity circumference, f/u indicates follow-up, and b indicates baseline.
Abbreviations: SD, standard deviation; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; LBCQ, Lymphedema and Breast Cancer Questionnaire; No lymphedema, no perceived swelling and L ≤ 2 cm; asymptomatic lymphedema, no perceived swelling but L > 2 cm; symptomatic non-lymphedema, perceived swelling but L ≤ 2 cm; symptomatic lymphedema, perceived swelling and L > 2 cm.
The most commonly avoided behaviors were ipsilateral placement of intravenous catheters, inflation of blood pressure cuffs, and percutaneous venipuncture (Table 5). Patients with measured or perceived lymphedema more frequently avoided carrying heavy suitcases or picking up children, and more frequently wore a compression garment prophylactically when flying (data not shown). Few patients limited their recreational activities (tennis, golfing, swimming, skiing, housework, gardening) to a significant degree (Table 5).
DISCUSSION
While L > 2 cm is an accepted threshold for classifying lymphedema, it is an arbitrary definition that does not assess patient perceptions. The American Cancer Society acknowledges the difficulties in defining lymphedema and suggests that patient perceptions are important and may be present in the absence of any objective changes.30 We undertook this study of patient-centered outcomes with the premise that a patient's perceptions are ultimately the most important outcome to that individual patient.
Data from this present study reveal considerable discordance between objective and subjective measures of lymphedema. Using standardized arm measurements, patient interview data, and a validated instrument, we demonstrate that rates of lymphedema differ depending on how it is defined. This variation suggests one method is not adequate to accurately define the prevalence of lymphedema after axillary surgery.
There is significant variation in patient-reported lymphedema ranging from 7% to 77% after ALND, and from 0% to 13% after SLNB.4,10,11,13,15,17,31 In this series, 3% of patients 5 years after SLNB alone perceived arm swelling, similar to both the recent Axillary Lymphatic Mapping Against Nodal Axillary Clearance (ALMANAC)18 and American College of Surgeons Z001120 trials documenting patient-reported lymphedema in 5% and 2%, respectively, of SLNB patients 1 year postsurgery.
Despite the importance of patient perceptions, lymphedema defined solely by patient perceptions is also problematic. Sensory changes are well documented after axillary surgery. At 5 years follow-up, numbness remains the most prevalent, severe, and distressing sensory change occurring in up to 55% of SLNB/ALND and 22% of SLNB-alone patients.2 It is possible that women mistakenly interpret this common sensory change as arm swelling even though a measurement difference does not exist. Furthermore, at our institution, all women undergoing ALND participate in an organized class learning shoulder mobility exercises and techniques to limit their risk of lymphedema. Simply attending this class may heighten one's awareness of the earliest perceived arm changes before measurement changes are evident. Finally, patient age may be a confounding factor in patient-perceived lymphedema. Younger patients seem to be more sensitive to the discomfort caused by axillary surgery.1 Although this may be physiologic, it may also be due to age-related postoperative expectations or previous life experiences. In this series, women undergoing ALND experienced more numbness, had more education about lymphedema, and were younger. All of these factors might explain why patient-reported arm swelling was more common than measured lymphedema (27% v 16%) for the SLNB/ALND group, while patient-perceived swelling was less common than measured lymphedema (3% v 5%) for SLNB-alone patients.
Similar to measured lymphedema,22 significant factors associated with the presence of patient-perceived lymphedema include obesity, infection, and injury. Although arm dominance was not a significant risk factor predicting women who might develop measured lymphedema, it was significant in predicting which women would perceive it. Women with symptomatic nonlymphedema were more likely to have had surgery ipsilateral to the dominant arm. Patients comprising this group may represent those who unknowingly at baseline had asymmetry between the two arms, which was recognized only after surgery in the context of experiencing common postoperative sensory changes. Instead of realizing the dominant arm can naturally be larger than the nondominant arm, this size discrepancy and common arm sensations after axillary surgery could be misinterpreted as new onset lymphedema. Alternatively, symptomatic nonlymphedema may include patients with a subclinical form of lymphedema who do not yet meet objective measurement criteria. Women may be more sensitive to this occurrence in their dominant arm.
Women with asymptomatic lymphedema more commonly had surgery in their nondominant arm. It is possible that lymphedema in the nondominant extremity is less bothersome than on the dominant side, and therefore patients are less likely to report symptoms. Perhaps if at baseline the dominant arm was larger, but unrecognized as such, then measured lymphedema was not perceived in the nondominant arm because current arm measurements and perceptions in clothes were similar between the two arms. These discrepancies between patient perceptions and measurements suggest that further prospective study is needed to determine which symptomatic nonlymphedema patients will develop measured lymphedema, and when or if patients with nonsymptomatic lymphedema will develop symptoms.
It is not surprising that patients with measured or perceived lymphedema practice more precautionary behaviors as they are likely more focused on protecting their arm. It is interesting, however, to note that even though SLNB is associated with a lower risk of lymphedema and fewer sensory changes than ALND,1,2,10,11,18,32 more than 80% of SLNB-alone patients practice similar precautionary behaviors as those having ALND. Although Peintinger et al32 demonstrated through a validated questionnaire that the extent of axillary surgery did not influence quality of life, the influence of these precautions on overall quality of life remains unclear and should be studied further. Furthermore, in the setting of SLNB, it is possible that such lifestyle modifications may not be warranted for such little benefit.
Ideally, both objective measurements and symptom assessment are needed to determine the prevalence of clinically significant lymphedema. The clinical relevance of arm measurements must be interpreted in the context of patient symptoms to determine how lymphedema affects one's quality of life. By rigid measurement definitions, a woman may demonstrate measurement change but have no symptoms. Arguably, if her quality of life is not restricted, her measurement difference may not be clinically relevant. Conversely, one who has measurement differences below the accepted threshold for lymphedema may be severely symptomatic. Clearly, patient perceptions of arm swelling are important to the patient.
In summary, we found that by both objective and subjective measures, lymphedema is less prevalent after SLNB alone than after SLNB/ALND, but there is only moderate concordance between the two types of measurements. Risk factors for patient-perceived arm swelling, body weight, injury, and infection are the same as those for measured lymphedema. Further work is needed to determine the extent to which patient-reported arm symptoms may represent an early manifestation of, or signal increased risk for, subsequent arm swelling. Most women undergoing SLNB alone, despite being at low risk for lymphedema, actively avoid behaviors thought to increase their risk. Patient-reported arm symptoms, whether or not they were accompanied by measurable arm swelling, appear to prompt more precautionary behavior. To enable the population of breast cancer survivors to further improve the quality of their lives, additional study is needed to better understand how objective measures and subjective perceptions of lymphedema are related to each other, and whether avoidant behaviors reduce the risk of lymphedema.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Karen E. Hurley, Elyn R. Riedel, Katherine T. Morris, Mary J. Wright, Kimberly J. Van Zee
Collection and assembly of data: Sarah A. McLaughlin, Michelle R. Sampson, Julia P. Brockway
Data analysis and interpretation: Sarah A. McLaughlin, Mary J. Wright, Karen E. Hurley, Elyn R. Riedel, Kimberly J. Van Zee
Manuscript writing: Sarah A. McLaughlin, Kimberly J. Van Zee, Karen E. Hurley, Elyn R. Riedel
Final approval of manuscript: Sarah A. McLaughlin, Mary J. Wright, Katherine T. Morris, Michelle R. Sampson, Julia P. Brockway, Karen E. Hurley, Elyn R. Riedel, Kimberly J. Van Zee
published online ahead of print at www.jco.org on October 6, 2008.
Supported by National Cancer Institute Grant No. K07 CA109236 (K.E.H.).
Presented in part at the 60th Annual Cancer Symposium of the Society of Surgical Oncology, March 15–18, 2007, Washington, DC, and at the American Society of Clinical Oncology Breast Symposium, September 7–8, 2007, San Francisco, CA.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Temple LK, Baron R, Cody HS III, et al: Sensory morbidity after sentinel lymph node biopsy and axillary dissection: A prospective study of 233 women. Ann Surg Oncol 9:654-662, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Baron RH, Fey JV, Borgen PI, et al: Eighteen sensations after breast cancer surgery: A 5-year comparison of sentinel lymph node biopsy and axillary lymph node dissection. Ann Surg Oncol 14:1653-1661, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Haid A, Kuehn T, Konstantiniuk P, et al: Shoulder-arm morbidity following axillary dissection and sentinel node only biopsy for breast cancer. Eur J Surg Oncol 28:705-710, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Schijven MP, Vingerhoets AJ, Rutten HJ, et al: Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol 29:341-350, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Rietman JS, Geertzen JH, Hoekstra HJ, et al: Long term treatment related upper limb morbidity and quality of life after sentinel lymph node biopsy for stage I or II breast cancer. Eur J Surg Oncol 32:148-152, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Sener SF, Winchester DJ, Martz CH, et al: Lymphedema after sentinel lymphadenectomy for breast carcinoma. Cancer 92:748-752, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U, Paganelli G, Viale G, et al: A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 349:546-553, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Rietman JS, Dijkstra PU, Geertzen JH, et al: Treatment-related upper limb morbidity 1 year after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast cancer. Ann Surg Oncol 11:1018-1024, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Wilke LG, McCall LM, Posther KE, et al: Surgical complications associated with sentinel lymph node biopsy: Results from a prospective International Cooperative Group trial. Ann Surg Oncol 13:491-500, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Swenson KK, Nissen MJ, Ceronsky C, et al: Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Ann Surg Oncol 9:745-753, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Blanchard DK, Donohue JH, Reynolds C, et al: Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg 138:482-487, 2003; discussion 487-8, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Barranger E, Dubernard G, Fleurence J, et al: Subjective morbidity and quality of life after sentinel node biopsy and axillary lymph node dissection for breast cancer. J Surg Oncol 92:17-22, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Schrenk P, Rieger R, Shamiyeh A, et al: Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer 88:608-614, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Burak WE, Hollenbeck ST, Zervos EE, et al: Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. Am J Surg 183:23-27, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Leidenius M, Leivonen M, Vironen J, et al: The consequences of long-time arm morbidity in node-negative breast cancer patients with sentinel node biopsy or axillary clearance. J Surg Oncol 92:23-31, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Purushotham AD, Upponi S, Klevesath MB, et al: Morbidity after sentinel lymph node biopsy in primary breast cancer: Results from a randomized controlled trial. J Clin Oncol 23:4312-4321, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Ronka R, von Smitten K, Tasmuth T, et al: One-year morbidity after sentinel node biopsy and breast surgery. Breast 14:28-36, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Mansel RE, Fallowfield L, Kissin M, et al: Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC trial. J Natl Cancer Inst 98:599-609, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Langer I, Guller U, Berclaz G, et al: Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: A prospective Swiss multicenter study on 659 patients. Ann Surg 245:452-461, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucci A, McCall LM, Beitsch PD, et al: Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol 25:3657-3663, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Petrek JA, Senie RT, Peters M, et al: Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 92:1368-1377, 2001 [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin S, Wright MJ, Morris KT, et al: Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J Clin Oncol 2008. doi; 10.1200/JCO.2008.16.3725 [DOI] [PMC free article] [PubMed]
- 23.LymphaCare: The Lymphedema Resource. www.lymphacare.com
- 24.A Breast in the West. http://www.abreastinthewest.ca/resources_lymphdema.cfm
- 25.Werner RS, McCormick B, Petrek J, et al: Arm edema in conservatively managed breast cancer: Obesity is a major predictive factor. Radiology 180:177-184, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Pink ribbon International. http://pinkribbon.com/
- 27.Harris SR, Niesen-Vertommen SL: Challenging the myth of exercise-induced lymphedema following breast cancer: A series of case reports. J Surg Oncol 74:95-98, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Graham PH: Compression prophylaxis may increase the potential for flight-associated lymphoedema after breast cancer treatment. Breast 11:66-71, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Armer J, Fu MR, Wainstock JM, et al: Lymphedema following breast cancer treatment, including sentinel lymph node biopsy. Lymphology 37:73-91, 2004 [PubMed] [Google Scholar]
- 30.Rockson SG, Miller LT, Senie R, et al: American Cancer Society Lymphedema Workshop: Workgroup III: Diagnosis and management of lymphedema. Cancer 83:2882-2885, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Haid A, Koberle-Wuhrer R, Knauer M, et al: Morbidity of breast cancer patients following complete axillary dissection or sentinel node biopsy only: A comparative evaluation. Breast Cancer Res Treat 73:31-36, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Peintinger F, Reitsamer R, Stranzl H, et al: Comparison of quality of life and arm complaints after axillary lymph node dissection vs sentinel lymph node biopsy in breast cancer patients. Br J Cancer 89:648-652, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]


