Abstract
Purpose
Adjuvant chemotherapy for resected non–small-cell lung cancer (NSCLC) is now accepted on the basis of several randomized clinical trials (RCTs) that demonstrated improved survival. Although there is strong evidence that adjuvant chemotherapy is effective in stages II and IIIA NSCLC, its utility in stage IB disease is unclear. This report provides a mature analysis of Cancer and Leukemia Group B (CALGB) 9633, the only RCT designed specifically for stage IB NSCLC.
Patients and Methods
Within 4 to 8 weeks of resection, patients were randomly assigned to adjuvant chemotherapy or observation. Eligible patients had pathologically confirmed T2N0 NSCLC and had undergone lobectomy or pneumonectomy. Chemotherapy consisted of paclitaxel 200 mg/m2 intravenously over 3 hours and carboplatin at an area under the curve dose of 6 mg/mL per minute intravenously over 45 to 60 minutes every 3 weeks for four cycles. The primary end point was overall survival.
Results
Three hundred-forty-four patients were randomly assigned. Median follow-up was 74 months. Groups were well-balanced with regard to demographics, histology, and extent of surgery. Grades 3 to 4 neutropenia were the predominant toxicity; there were no treatment-related deaths. Survival was not significantly different (hazard ratio [HR], 0.83; CI, 0.64 to 1.08; P = .12). However, exploratory analysis demonstrated a significant survival difference in favor of adjuvant chemotherapy for patients who had tumors ≥ 4 cm in diameter (HR, 0.69; CI, 0.48 to 0.99; P = .043).
Conclusion
Because a significant survival advantage was not observed across the entire cohort, adjuvant chemotherapy should not be considered standard care in stage IB NSCLC. Given the magnitude of observed survival differences, CALGB 9633 was underpowered to detect small but clinically meaningful improvements. A statistically significant survival advantage for patients who had tumors ≥ 4 cm supports consideration of adjuvant paclitaxel/carboplatin for stage IB patients who have large tumors.
INTRODUCTION
The use of adjuvant chemotherapy in non–small-cell lung cancer (NSCLC) has changed dramatically in recent years. Until the report of the International Adjuvant Lung Trial (IALT) in 2003,1 there was little convincing evidence that adjuvant chemotherapy improved outcome in NSCLC. Since then, five randomized trials reported that adjuvant chemotherapy improves survival in resected NSCLC.2-6
In 2004, we reported preliminary results of Cancer and Leukemia Group B (CALGB) 9633, a randomized clinical trial (RCT) designed to study adjuvant paclitaxel/carboplatin in stage IB NSCLC. Preliminary results indicated that adjuvant chemotherapy improved overall survival (OS) and disease-free survival (DFS).5 Indeed, accrual to CALGB 9633 was stopped early by the Data and Safety Monitoring Board after a planned interim analysis in November 2003 demonstrated that OS had crossed a prespecified stopping boundary for efficacy. The hazard ratio (HR) for OS was the lowest reported in any RCT (HR, 0.62; 90% CI, 0.44 to 0.89; P = .014, one tailed).
When CALGB 9633 was under development in the early 1990s, our objective was to study adjuvant chemotherapy in high-risk, stage I NSCLC. We hoped to define high risk on the basis of a number of clinicopathologic and molecular markers. However, we concluded that it was not then possible to utilize clinical/molecular markers to define risk in a uniform and reproducible manner.
Accordingly, we defined high risk on the presence of T2N0 disease. In stage I NSCLC, large tumor size has been the most consistent determinant of survival. Among nine series that included greater than 2,000 patients with T2N0 disease, 5-year survival after resection ranged from 45% to 68%.7 On the basis of such data, the International Staging System subdivided stage I into A and B subcategories in 1997, which defined stage IB NSCLC as T2N0M0.8
We chose paclitaxel/carboplatin on the basis of two phase II studies that indicated response rates of 62% in advanced NSCLC.9,10 Although superiority to other combinations was not confirmed in subsequent RCTs11-13 or meta-analyses,14,15 paclitaxel/carboplatin remains one of the most widely used regimens in the United States. Moreover, toxicity compares favorably to cisplatin-based doublets, and no standard chemotherapy regimen had been established in the adjuvant setting.16
Although our 2004 presentation demonstrated considerable efficacy in stage IB NSCLC, median follow-up then was only 34 months. In addition, survival comparisons were based on only 57% (88 of 155) of deaths required for final analysis.5
Since 2004, three larger trials with broader inclusion criteria (ie, IALT, National Cancer Institute of Canada Clinical Trials Group [NCIC-CTG] JBR10 trial, and Adjuvant Navelbine International Trialists Association [ANITA]) each reported significant OS advantages with cisplatin-based doublets, but they failed to demonstrate improved survival in stage IB subsets.2,4,6 In addition, Lung Adjuvant Cisplatin Evaluation (LACE), a pooled analysis of five RCTs that included 4,584 patients, also showed a significant OS advantage for all patients, but it failed to demonstrate efficacy for the 30% who had stage IB disease.17 The objective of this report is to provide a mature analysis for CALGB 9633.
METHODS
Study Design
Random assignment was performed within 4 to 8 weeks of resection and was stratified on the basis of histology (squamous v nonsquamous), tumor differentiation (poor v others), and mediastinoscopy (performed v not performed). Participants were randomly assigned to adjuvant chemotherapy or observation. Chemotherapy consisted of paclitaxel (Taxol; Bristol-Myers Squibb, Princeton, NJ) 200 mg/m2 intravenously over 3 hours and carboplatin (Paraplatin; Bristol-Myers Squibb), at an area under the curve (AUC) dose of 6 mg/mL per minute intravenously over 45 to 60 minutes. Treatment was repeated every 3 weeks for four cycles. CALGB 9633 was approved by institutional review boards in accordance with Department of Health and Human Services regulations.
Eligibility
Eligibility required age ≥ 18 years; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 1; histologically proven NSCLC; T2 tumor; pathologically negative lymph nodes at mediastinoscopy and/or surgery; and resection that consisted of lobectomy or pneumonectomy.
Statistical Analysis
The primary objective of the study was to determine if adjuvant chemotherapy improved OS after resection of stage IB NSCLC. Secondary objectives were to assess the impact of chemotherapy on DFS and to assess toxicities of adjuvant paclitaxel and carboplatin.
On the basis of available literature, we projected 50% 5-year survival for surgery alone in stage IB NSCLC. The study was designed in 1996 to determine whether adjuvant chemotherapy resulted in a 13% absolute improvement in 5-year survival (from 50% to 63%), with 80% power and with a two-tailed log-rank test conducted at the .05 significance level. With an assumption that survival was exponentially distributed, this improvement corresponded to an HR of 0.67. Although the accrual target was initially 500 patients, accrual was less than 50% of expected. As an alternative to protocol termination, we elected to reduce the accrual target from 500 to 384 patients in 2000. We reasoned that slow accrual allowed longer observation times for each patient. Although the protocol originally was designed for two-sided hypothesis testing, it was converted to one-sided testing (α = .05) to maintain feasibility and statistical power when the sample size was reduced. The magnitude of effect size that we were seeking with 80% power did not change when the required number of deaths was reduced from 200 to 155.
OS and DFS were calculated by using the Kaplan-Meier life-table method. OS was defined as time from random assignment to death from any cause. DFS was defined as time from random assignment to recurrence or death. Subgroup comparisons of OS and DFS were performed by using the log-rank test and the Cox proportional hazards model. Exploratory analyses were conducted with the Cox model to explore the effect of tumor size on OS and DFS and to determine whether treatment differences were consistent between men and women and among ethnicities.
Accordingly, all P values reported herein are one-sided (unless otherwise specified). Reported confidence intervals are two-sided 90% confidence intervals, which best correspond to one-tailed P values. Analyses reflect the CALGB database as of April 6, 2007.
Descriptive statistics were tabulated and summarized with SAS software (Version 9.1; SAS Institute, Cary, NC). Survival analysis was performed with S-Plus (Version 3.3; Statistical Sciences, Seattle, WA) software. Analyses were performed by using the intent-to-treat principle, which included all eligible, ineligible, and canceled patients.
Interim Monitoring and Early Stopping
In accordance with CALGB policy, the study was reviewed semiannually by an independent data safety monitoring board (DSMB). Early termination was considered if the P value of the log-rank test was less than a nominal significance level calculated with the use of the Lan–DeMets α spending function18,19 with O’Brien–Fleming boundaries.20 Accrual was stopped in November 2003, when survival results were less than the prespecified stopping boundary.
RESULTS
Participants
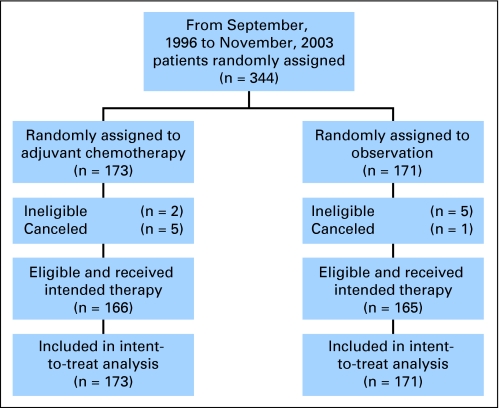
The protocol was activated on September 5, 1996 and was closed on November 26, 2003 (Fig 1). At closure, 344 patients had been enrolled, which represented 90% of target accrual. One hundred seventy-three patients were randomly assigned to chemotherapy, and 171 were randomly assigned to observation.
Fig 1.
Consort diagram.
Among 344 patients, seven were retrospectively determined to be ineligible (two in the chemotherapy and five in the observation group), and six were cancelled and never received treatment (five in the chemotherapy and one in the observation group). Two patients were ineligible because they did not have NSCLC, and five were because they did not have stage IB disease. Thus, 331 patients were eligible and received their intended treatment. However, all 344 patients are included in the intent-to-treat analysis.
Demographics
As listed in Table 1, groups were well balanced with regard to age, sex, PS, symptoms at diagnosis, tumor size, histology, and extent of resection. Median follow-up was 74 months. Patients in both groups were predominantly white men who had a PS of 0.
Table 1.
Baseline Patient Characteristics
| Variable | Chemotherapy (n = 173)
|
Control (n = 171)
|
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sex | |||||
| Male | 112 | 65 | 108 | 63 | .76 |
| Female | 61 | 35 | 63 | 37 | |
| Age, years | |||||
| Median | 61 | 62 | .40 | ||
| Range | 34-78 | 40-81 | |||
| Age distribution, years | .24 | ||||
| 30-39 | 3 | 2 | 0 | 0 | |
| 40-49 | 27 | 16 | 20 | 12 | |
| 50-59 | 48 | 28 | 56 | 33 | |
| 60-69 | 56 | 32 | 62 | 36 | |
| ≥ 70 | 39 | 23 | 33 | 19 | |
| Ethnicity | |||||
| White | 157 | 91 | 150 | 88 | .36 |
| Nonwhite | 16 | 9 | 21 | 12 | |
| Performance status | |||||
| 0 | 94 | 56 | 98 | 58 | .84 |
| 1 | 74 | 44 | 70 | 41 | |
| 2 | 1 | 1 | 1 | 1 | |
| Unknown | 2 | 1 | 4 | 2 | |
| Weight loss, % | |||||
| < 5 | 125 | 80 | 126 | 80 | .77 |
| 5-10 | 20 | 13 | 22 | 14 | |
| > 10 | 11 | 7 | 10 | 6 | |
| Unknown | 0 | 0 | 1 | 1 | |
| Symptoms present | |||||
| No | 39 | 23 | 45 | 26 | .47 |
| Yes | 131 | 77 | 126 | 74 | |
| Symptom duration, months | |||||
| < 3 | 101 | 67 | 94 | 64 | |
| 3-6 | 31 | 21 | 30 | 21 | .82 |
| > 6 | 19 | 13 | 22 | 15 | |
| Tumor diameter, cm† | |||||
| Mean | 4.59 | 4.50 | .69 | ||
| Median | 4.0 | 4.0 | |||
| Range | 0-14 | 1-12 | |||
| Histology | |||||
| Adenocarcinoma | 90 | 54 | 84 | 49 | .47 |
| Squamous | 58 | 35 | 58 | 34 | |
| Other | 20 | 12 | 28 | 16 | |
| Tumor differentiation | |||||
| Well or moderate | 86 | 50 | 85 | 50 | .99 |
| Poor | 87 | 50 | 86 | 50 | |
| Mediastinoscopy | |||||
| Yes | 139 | 80 | 135 | 79 | .75 |
| No | 34 | 20 | 36 | 21 | |
| Surgical procedure | |||||
| Thoracotomy | 158 | 95 | 157 | 93 | .48 |
| Thoracoscopy | 8 | 5 | 11 | 7 | |
| Extent of resection | |||||
| Lobectomy | 146 | 88 | 151 | 89 | .34 |
| Pneumonectomy | 19 | 12 | 18 | 11 | |
P values are two-sided.
Statistics for tumor diameter are based on 162 and 168 patients in chemotherapy and control groups, respectively.
The mean tumor diameter was 4.59 cm and 4.50 cm in the experimental and control groups, respectively. The median tumor diameter was 4.0 cm in both groups. (This apparent discrepancy is based on the fact that there were 51 patients whose tumor diameters were coded as 4.0 cm.) Overall, 59% of participants had tumors ≥ 4.0 cm in diameter.
The predominant histology was adenocarcinoma, which represented 51% of tumors. Preoperative mediastinoscopy was performed on 80%. Resection consisted of lobectomy in 89% and pneumonectomy in 11%.
Toxicity and Delivery of Chemotherapy
Toxicity data are available on 158 (91%) of 173 patients who received chemotherapy. Chemotherapy was well tolerated, and there were no treatment-related toxic deaths. The predominant toxicity was grade 3 to 4 neutropenia, which was observed in 35% (Table 2).
Table 2.
Toxicity of Adjuvant Chemotherapy
| Toxicity | Toxicity Grade
|
|||||
|---|---|---|---|---|---|---|
| 3 (severe)
|
4 (life-threatening)
|
5 (lethal)
|
||||
| No. | % | No. | % | No. | % | |
| Neutropenia | 18 | 11 | 38 | 24 | 0 | 0 |
| Thrombocytopenia | 9 | 6 | 0 | 0 | 0 | 0 |
| Anemia | 4 | 3 | 0 | 0 | 0 | 0 |
| Nausea and vomiting | 9 | 6 | 0 | 0 | 0 | 0 |
| Infection | 9 | 6 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 23 | 15 | 1 | 1 | 0 | 0 |
| Myalgias/arthralgias | 9 | 6 | 0 | 0 | 0 | 0 |
| Malaise/fatigue | 5 | 3 | 1 | 1 | 0 | 0 |
| Sensory neuropathy | 8 | 5 | 0 | 0 | 0 | 0 |
| Anorexia | 2 | 1 | 0 | 0 | 0 | 0 |
| Dyspnea | 5 | 3 | 2 | 1 | 0 | 0 |
| Hypotension | 0 | 0 | 1 | 1 | 0 | 0 |
| Phlebitis | 1 | 1 | 1 | 1 | 0 | 0 |
| Pain | 7 | 4 | 0 | 0 | 0 | 0 |
| Weight loss | 1 | 1 | 0 | 0 | 0 | 0 |
| Maximum toxicity | 64 | 41 | 45 | 28 | 0 | 0 |
NOTE. Data were available on 158 of 173 patients randomly assigned to adjuvant chemotherapy.
Data are available on 136 (79%) of 173 patients regarding delivery of chemotherapy. Eighty-six percent (117 of 136) received all four cycles of adjuvant chemotherapy. Among those given four cycles, 66% (77 of 117) received full-dose chemotherapy, and 34% (40 of 117) required a dose reduction at some point. Fifty-seven percent (77 of 136) received four cycles of chemotherapy at full dose.
OS, DFS, and Competing Risk Analysis
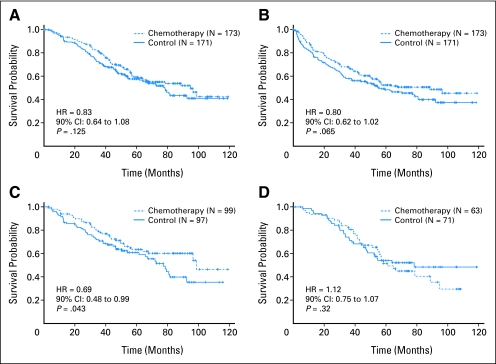
Table 3 includes data on OS and DFS for all 344 patients. With 74 deaths in the chemotherapy arm and 81 in the control arm, the difference in OS was not statistically significant (HR, 0.83; 90% CI, 0.64 to 1.08; P = .125; Fig 2A). The median survival times were 95 months and 78 months in chemotherapy and observation groups, respectively. Lack of differences were consistent between men and women (P = .29) and among ethnicity groups (P = .28).
Table 3.
Overall and Disease-Free Survival for All Patients Stratified by Tumor Size
| Survival Outcome | Survival Analyses
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall
|
Disease-Free
|
|||||||||
| Adjuvant Chemotherapy | Control | P | HR | 90% CI | Adjuvant Chemotherapy | Control | P | HR | 90% CI | |
| Intent-to-treat analysis of all randomly assigned patients | ||||||||||
| No. of patients | 173 | 171 | 173 | 171 | ||||||
| Recurrence or death | .125 | 0.83 | 0.64 to 1.08 | .065 | 0.80 | 0.62 to 1.02 | ||||
| No. | 74 | 81 | 81 | 92 | ||||||
| % | 43 | 47 | 47 | 54 | ||||||
| 1-year | ||||||||||
| % | 94 | 94 | .50 | 85 | 81 | .079 | ||||
| 90% CI, % | 91 to 98 | 91 to 97 | 80 to 90 | 75 to 87 | ||||||
| 2-year | ||||||||||
| % | 90 | 84 | .053 | 75 | 68 | .048 | ||||
| 90% CI, % | 86 to 95 | 79 to 90 | 68 to 81 | 62 to 76 | ||||||
| 3-year | ||||||||||
| % | 80 | 73 | .020 | 67 | 58 | .0048 | ||||
| 90% CI, % | 74 to 86 | 65 to 79 | 60 to 74 | 51 to 66 | ||||||
| 4-year | ||||||||||
| % | 70 | 62 | .045 | 60 | 54 | .060 | ||||
| 90% CI, % | 63 to 77 | 55 to 70 | 53 to 68 | 47 to 62 | ||||||
| 5-year | ||||||||||
| % | 60 | 58 | .190 | 52 | 48 | .117 | ||||
| 90% CI, % | 52 to 68 | 51 to 66 | 45 to 61 | 41 to 57 | ||||||
| 6-year | ||||||||||
| % | 55 | 53 | .353 | 51 | 46 | .094 | ||||
| 90% CI, % | 48 to 64 | 45 to 62 | 43 to 59 | 38 to 54 | ||||||
| Tumor size ≥ 4 cm in diameter | ||||||||||
| No. of patients | 99 | 97 | 99 | 97 | ||||||
| Recurrence or death | ||||||||||
| No. | 37 | 53 | .042 | 0.69 | 0.48 to 0.99 | 42 | 53 | .035 | 0.69 | 0.49 to 0.97 |
| % | 38 | 55 | 42 | 55 | ||||||
| 1-year | ||||||||||
| % | 94 | 92 | .284 | 82 | 76 | .182 | ||||
| 90% CI, % | 90 to 98 | 87 to 97 | 74 to 90 | 68 to 85 | ||||||
| 2-year | ||||||||||
| % | 90 | 81 | .047 | 71 | 67 | .259 | ||||
| 90% CI, % | 85 to 95 | 75 to 88 | 63 to 81 | 58 to 77 | ||||||
| 3-year | ||||||||||
| % | 78 | 71 | .126 | 66 | 56 | .085 | ||||
| 90% CI, % | 71 to 85 | 64 to 79 | 57 to 76 | 47 to 67 | ||||||
| 4-year | ||||||||||
| % | 71 | 64 | .141 | 63 | 54 | .105 | ||||
| 90% CI, % | 60 to 79 | 56 to 73 | 53 to 73 | 44 to 65 | ||||||
| 5-year | ||||||||||
| % | 64 | 61 | .355 | 59 | 51 | .141 | ||||
| 90% CI, % | 56 to 73 | 53 to 70 | 49 to 70 | 41 to 62 | ||||||
| 6-year | ||||||||||
| % | 60 | 54 | .197 | 55 | 47 | .137 | ||||
| 90% CI, % | 52 to 70 | 45 to 64 | 46 to 67 | 37 to 59 | ||||||
| Tumor size < 4 cm in diameter | ||||||||||
| No. of patients | 63 | 71 | 63 | 71 | ||||||
| Recurrence or death | ||||||||||
| No. | 34 | 33 | .32 | 1.12 | 0.75 to 1.67 | 36 | 38 | .49 | 1.01 | 0.69 to 1.48 |
| % | 54 | 46 | 57 | 54 | ||||||
| 1-year | ||||||||||
| % | 94 | 97 | .165 | 89 | 86 | .308 | ||||
| 90% CI, % | 89 to 99 | 94 to 100 | 81 to 97 | 78 to 94 | ||||||
| 2-year | ||||||||||
| % | 90 | 87 | .280 | 79 | 70 | .119 | ||||
| 90% CI, % | 84 to 97 | 91 to 94 | 70 to 90 | 60 to 82 | ||||||
| 3-year | ||||||||||
| % | 81 | 73 | .141 | 66 | 60 | .234 | ||||
| 90% CI, % | 73 to 89 | 64 to 82 | 55 to 79 | 50 to 73 | ||||||
| 4-year | ||||||||||
| % | 67 | 62 | .272 | 56 | 55 | .477 | ||||
| 90% CI, % | 58 to 78 | 53 to 73 | 45 to 70 | 45 to 69 | ||||||
| 5-year | ||||||||||
| % | 52 | 54 | .392 | 40 | 46 | .278 | ||||
| 90% CI, % | 42 to 64 | 45 to 65 | 29 to 55 | 35 to 59 | ||||||
| 6-year | ||||||||||
| % | 45 | 52 | .221 | 40 | 44 | .353 | ||||
| 90% CI, % | 35 to 58 | 43 to 63 | 29 to 55 | 33 to 58 | ||||||
Abbreviation: HR, hazard ratio.
Fig 2.
Kaplan-Meier estimates of survival among patients who received adjuvant paclitaxel and carboplatin and those who underwent observation alone. (A) Overall survival, all patients; (B) disease-free survival, all patients; (C) tumor size ≥ 4 cm in diameter; and (D) tumor size < 4 cm in diameter.
The difference in DFS did not reach statistical significance (HR, 0.80; 90% CI, 0.62 to 1.02; P = .065; Fig 2B). The median DFS times were 89 months and 56 months in chemotherapy and observation groups, respectively. Lack of treatment differences were consistent between men and women (P = .64) and between white and nonwhite ethnicities (P = .17).
Cumulative incidence models were used to estimate the probability of death from lung cancer versus death as a result of other causes. As listed in Table 4, there was a 28% reduction in mortality as a result of lung cancer (HR, 0.72; 90% CI, 0.50 to 1.02). However, this difference was not significant (P = .059). Death as a result of other causes was similar (HR, 1.02; 90% CI, 0.68 to 1.53; P = .47).
Table 4.
Competing Risk Analysis
| Cause of Death | Treatment Group
|
Analysis
|
|||||
|---|---|---|---|---|---|---|---|
| Adjuvant Chemotherapy (n = 173)
|
Control (n = 171)
|
||||||
| No. | % | No. | % | P | HR | 90% CI | |
| Lung cancer | 39 | 22.5 | 50 | 29.2 | .059 | 0.72 | 0.50 to 1.02 |
| Other | 35 | 20.2 | 31 | 18.1 | .47 | 1.02 | .68 to 1.53 |
| All | 74 | 42.7 | 81 | 47.4 | .125 | 0.83 | 0.64 to 1.08 |
Abbreviation: HR, hazard ratio.
Exploratory Analysis: Relationship Between Adjuvant Chemotherapy and Tumor Size
Because tumor size is so well established as a prognostic factor in stage I NSCLC, we conducted an exploratory analysis to determine whether patients who had large tumors derived benefit from adjuvant chemotherapy. We chose to dichotomize at 4.0 cm because of mounting evidence that 4.0 cm represents a better threshold than the traditional 3.0 cm cutoff for subdividision of stage I patients into prognostically meaningful groups.21-24
Tumor size was available in 96% (330 of 344) of patients. Fifty-nine percent (196 of 330) had tumors ≥ 4.0 cm in diameter (Table 3). Among those with larger tumors, the mean tumor diameters were 5.77 cm and 5.80 cm in chemotherapy and control groups, respectively (median diameter, 5.0 cm in both groups). In this subgroup, there were significant advantages in OS and DFS for adjuvant chemotherapy. There was a 31% reduction in risk of death, as measured by OS analysis, among those with tumors ≥ 4 cm (HR, 0.69; 90% CI, 0.48 to 0.99; P = .043; Fig 2C) who received chemotherapy. Median survival times were 99 months and 77 months in chemotherapy and control groups, respectively. There was also a significant, 31% improvement in DFS that favored the chemotherapy group (HR, 0.69; 90% CI, 0.49 to 0.97; P = .035) in the median DFS times of 96 and 63 months, respectively.
Forty-one percent (134 of 330) had tumors less than 4.0 cm in diameter (Table 3). Among patients who had tumors less than 4.0 cm, mean tumor diameters were 2.73 cm and 2.71 cm in chemotherapy and control groups, respectively (median, 3.0 cm in both groups). In this subgroup (Fig 2D), there was a trend toward inferior OS in the chemotherapy group (HR, 1.12; 90% CI, 0.75 to 1.67; P = .32). Median survivals were 61 months and 78 months in chemotherapy and controls groups, respectively. There was no difference in DFS (HR, 1.01; 90% CI, 0.69 to 1.48; P = .49), as median DFS times were 55 months and 53 months in chemotherapy and control groups, respectively.
DISCUSSION
The last 5 years have seen a dramatic shift in the standard of care regarding adjuvant chemotherapy of NSCLC. Beginning with the report of a significant survival difference from IALT, five multi-institutional RCTs have been reported that demonstrate statistically significant survival advantages associated with adjuvant chemotherapy for early-stage NSCLC.
These five positive RCTs include the preliminary 2004 report of CALGB 9633.1 This was the only RCT designed specifically for stage IB NSCLC. However, stage IB patients were eligible to participate in each trial. Although mature results of CALGB 9633 no longer demonstrate a significant OS advantage for adjuvant chemotherapy across the entire cohort, the other four RCTs have retained significance in the entire study population. Because stage IB patients were eligible to participate in each positive trial, one question is whether sufficient evidence exists to routinely recommend adjuvant chemotherapy for patients with stage IB NSCLC, despite the results reported here. The answer would appear to be no.
Three of these four RCTs utilized a cisplatin-based doublet as the adjuvant chemotherapy. In IALT, patients were randomly assigned either to observation or to three or four cycles of cisplatin (Platinol; Bristol-Myers Squibb) combined with either vinorelbine (Navelbine; GlaxoSmithKline, Research Triangle Park, NC), vinblastine (Velban; Eli Lilly & Co, Indianapolis, IN), vindesine, or etoposide (Vepesid; Bristol-Myers Squibb). All resectable patients (including stages IA to IIIB) were eligible to participate in IALT.2
In both NCIC-CTG-JBR-10 and ANITA, patients were randomly assigned either to observation or to four cycles of cisplatin/vinorelbine. Eligibility in NCIC-CTG-JBR-10 included stages IB and II NSCLC,4 whereas stagesIB, II, and IIIA were eligible in in ANITA.6 The fourth RCT was the Japan Lung Cancer Research Group (JLCRG) study, in which patients with stage IA or IB lung adenocarcinoma were randomly assigned to 2 years of adjuvant chemotherapy with oral uracil/tegafur (UFT) or observation.3
Although each study showed a significant OS advantage for chemotherapy, no cisplatin-based RCT demonstrated a significant OS advantage for the stage IB subset. In IALT, there was no survival advantage among 681 stage I patients (HR, 0.95; 95% CI, 0.74 to 1.23), 73% of whom had stage IB NSCLC.2,25 ANITA demonstrated a significant OS advantage for adjuvant cisplatin/vinorelbine among all patients and among patients in the stages II and IIIA subsets, although not in the stage IB subset (HR, 1.10; 95% CI, 0.76 to 1.67).6 Similarly, NCIC-CTG-JBR-10 demonstrated a significant OS advantage for adjuvant cisplatin/vinorelbine in all patients and in the stage II subset.4 However, there was no survival advantage in stage IB disease (P = .79).
The only other multi-institutional RCT that did show benefit in stage IB disease is JLCRG, which utilized adjuvant UFT in stage I lung adenocarcinoma. Results indicate a significant survival improvement for all 979 patients with stage IA or IB disease (HR, 0.71; 95% CI, 0.52 to 0.98).3 Among the 27% of participants with stage IB disease, there was a significant survival advantage (HR, 0.48; 95% CI, 0.29 to 0.81). In contrast, there was no benefit for UFT within the much larger stage IA subset (HR, 0.97; 95% CI, 0.64 to 1.46). There is no experience with adjuvant UFT outside Japan, and this agent is not available in Europe or North America for NSCLC.
The only other data that supports efficacy for adjuvant chemotherapy in stage IB NSCLC comes from a small, single-institutional RCT from Italy.26 In this trial, 140 stage IB patients were randomly assigned after resection to six cycles of adjuvant cisplatin/etoposide or observation. Results demonstrate large and significant OS/DFS advantages in the adjuvant arm. Five-year, 10-year, and median survivals were 62%, 44%, and 84.8 months versus 42%, 20%, and 41.6 months, respectively (P = .02). Although impressive, the small sample size and the large effect size introduce questions about the reproducibility of these findings.
Accordingly, results of CALGB 9633 remain highly relevant to the question of the effect of adjuvant chemotherapy in stage IB NSCLC. Unfortunately, with longer follow-up, our encouraging preliminary findings have not been sustained. Nonetheless, the HR of 0.83 reported in our current analysis is similar to overall HRs observed in several positive trials. For example, in IALT and in ANITA, statistically significant survival advantages for adjuvant chemotherapy indicate HRs of 0.86, and 0.80, respectively. Moreover, LACE, a pooled analysis of five RCTs, reports a significant modest OS advantage (HR, 0.89; 95% CI, 0.82 to 0.96).17
Accordingly, the 17% reduction in risk of death observed in updated results from our study is similar in magnitude to survival advantages observed in several positive trials. However, with only 344 participants, CALGB 9633 is considerably smaller than any of the other RCTs. Given the smaller sample size and the lower event rates, our study had only 31% power to demonstrate a significant survival difference with HR = 0.83. Similarly, the 28% reduction in lung cancer mortality would suggest a benefit for adjuvant chemotherapy, although with only 89 deaths as a result of lung cancer, there was insufficient power to show a statistically significant difference.
Clearly, our results do not support routine use of adjuvant chemotherapy as standard of care in stage IB NSCLC. Recent American Society of Clinical Oncology guidelines assert that “adjuvant chemotherapy is not recommended for routine use for patients with completely resected stage IB NSCLC,”27 a conclusion with which we concur.
Nonetheless, CALGB 9633 does demonstrate a trend in favor of use of adjuvant chemotherapy. Moreover, exploratory analysis suggests that the adjuvant chemotherapy significantly improves survival for patients who had tumors ≥ 4.0 cm.
Although the analysis on the basis of on tumor size represents an unplanned subgroup analysis, the finding that adjuvant chemotherapy is effective for stage IB patients with large tumors is biologically plausible,21-24 and, if confirmed, would represent an observation that could substantially impact clinical practice. In this regard, after our updated 2006 presentation,28 investigators from NCIC-CTG-JBR-10 analyzed their data and also found a significant survival advantage for stage IB patients with greater than 4-cm tumors (Frances Shepherd, personal communication, April 19, 2007). We currently are participating in a pooled analysis in the context of an expanded LACE analysis in which this observation will be further explored.
We believe that our results support consideration for adjuvant chemotherapy in stage IB patients for those who had tumors ≥ 4.0 cm, which comprised 59% of patients in our study. The finding of a significant 31% mortality reduction provides a basis for considering adjuvant treatment in this subgroup, despite the fact that this was not an a priori objective of our trial. Indeed, the vast majority of such patients would be classified as having stage II tumors on the basis of a proposed new staging system for NSCLC.23,24
In terms of treatment regimen, our results suggest that adjuvant paclitaxel/carboplatin was at least comparable to cisplatin-based combinations for stage IB disease.29 Although LACE reported a significant survival advantage for all treated patients (HR, 0.89; 95% CI, 0.82 to 0.96) and in stage II and III patients (HR, = 0.83; 95% CI, 0.73 to 0.95), there was no significant advantage in stage IB NSCLC (HR, 0.93; 95% CI, 0.78 to 1.10).17
It also should be emphasized that adjuvant paclitaxel plus carboplatin was well tolerated, and there were no chemotherapy-related toxic deaths. Although compliance with adjuvant chemotherapy has been difficult in all RCTs, it was less problematic in CALGB 9633 than in cisplatin-based trials.30,31 Accordingly, paclitaxel plus carboplatin could be considered as a treatment option in selected stage IB patients who had tumors ≥ 4.0 cm in diameter. We believe that our results support the need to at least discuss the potential role of adjuvant chemotherapy with stage IB patients who have large tumors.
At this time, our study indicates that routine use of adjuvant chemotherapy is not justified for all patients with stage IB NSCLC. Nonetheless, results of CALGB 9633 (and confirmatory findings from NCIC-CTG-JBR-10) support consideration for adjuvant chemotherapy in stage IB patients who have tumors ≥ 4.0 cm in diameter.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Gary M. Strauss, Bristol-Myers Squibb; Everett E. Vokes, Bristol-Myers Squibb Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Gary M. Strauss, James E. Herndon, Michael A. Maddaus, David H. Harpole, David J. Sugarbaker, Everett E. Vokes, Mark R. Green
Administrative support: David J. Sugarbaker, Richard L. Schilsky, Everett E. Vokes, Mark R. Green
Provision of study materials or patients: David W. Johnstone, Heidi H. Gillenwater, David J. Sugarbaker
Collection and assembly of data: Dorothy M. Watson
Data analysis and interpretation: Gary M. Strauss, James E. Herndon, Michael A. Maddaus, Elizabeth A. Johnson, David H. Harpole, Dorothy M. Watson, Richard L. Schilsky, Everett E. Vokes, Mark R. Green
Manuscript writing: Gary M. Strauss, James E. Herndon, Michael A. Maddaus, Elizabeth A. Johnson, David H. Harpole, Richard L. Schilsky, Everett E. Vokes, Mark R. Green
Final approval of manuscript: Gary M. Strauss, James E. Herndon, Michael A. Maddaus, David W. Johnstone, Elizabeth A. Johnson, David H. Harpole, David J. Sugarbaker, Richard L. Schilsky, Everett E. Vokes, Mark R. Green
Appendix
Interim analyses and changing results over time in cancer and leukemia group B study 9633
In accordance with Cancer and Leukemia Group B (CALGB) policies and procedures, the status and progress of CALGB 9633 was reviewed semiannually by the CALGB Data and Safety Monitoring Board (DSMB). As part of the CALGB quality assurance program, members of the Data Audit Committee visit all participating institutions at least every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such onsite review of medical records was performed in 160 (46.5%) of 344 patients on this study.
Reports to the DSMB regularly summarized reported toxicities. After 30 deaths (ie, approximately 20% of the needed number of deaths) were observed, formal efficacy analyses were initiated and reported to the DSMB on a semiannual basis. Interim monitoring of the study was conducted to allow for early stopping with rejection of the null or alternative hypothesis. For each pairwise comparison, methodology developed by Lan and DeMets18 and extended by Pampallona and Tsiatis19 were used to determine boundary significance levels at each of the interim analyses. Boundaries analogous to those proposed by O’Brien and Fleming20 and truncated at a nominal P value of .001 were used. The methodology of Lan and DeMets and of Pampallona and Tsiatis allow the type I and II errors to be used up as a function of the accumulating information to maintain overall type I and II errors.
Appendix Table A1 lists some details about the interim efficacy analyses that were generated and reported to the DSMB. The results observed at the time of interim analysis 3 flirted with the stopping boundary without crossing it; however, by the time of interim analysis 4, the stopping boundary had been crossed. The DSMB decided to terminate patient accrual and to release the unblinded data to the study team.
By the time accrual was terminated, 171 and 173 patients had been enrolled onto the observation and chemotherapy arms, respectively. The Kaplan-Meier curves observed when accrual was terminated are presented in Appendix Figure A1 and associated statistics are presented in Appendix Table A2.
Though accrual had been terminated, patient follow-up continued. The trend in treatment efficacy changed with additional follow-up. Though early survival differences remained, the magnitude of treatment differences was mitigated slightly. The tails of the log-rank test statistic retreated back across the O’Brien Fleming boundary—a phenomena that is not a violation of the underlying theory for random walks.
We believe that the deterioration of the proportional hazards assumption with larger survival times is the primary reason why interim and final results summarized here differed. To better understand the lack of proportionality, a step function has been used within the context of a Cox model to describe the effect of treatment. Appendix Table A3 summarizes analyses in which the treatment effect remains constant during each 12-month period. The hazard ratios associated with the first 3 years favor chemotherapy treatment; however, with additional follow-up during years 4 and 5, that survival advantage is eliminated (Fig 2A).
Tumor diameter in stage IB NSCLC, adjuvant chemotherapy, and biological plausibility.
With regard to the question of the biologic plausibility of adjuvant chemotherapy effectiveness in stage IB NSCLC among patients who have tumors ≥ 4.0 cm in diameter, emerging data from numerous sources support that the traditional 3.0 cm cutoff does not represent an appropriate prognostic threshold for subdividing stage I patients.21 For example, a Surveillance Epidemiology End Results analysis of 9,191 stage I patients found that survival was similar in tumors between 2.0 to 2.9 cm and 3.0 to 3.9 cm and in tumors between 4.0 to 4.9 cm and 5.0 to 5.9 cm. This led the authors to recommend that the T descriptor for NSCLC be modified to classify T1 tumors as less than 2 cm and to subclassify T2 tumors into T2A, which could be defined as tumors from 2.0 to 3.9 cm, and T2B, which could be defined as tumors ≥ 4 cm in diameter.22
Moreover, the International Association for Study of Lung Cancer Lung Project, which is based on data of 100,869 patients from around the world, has recommended major revisions for staging in the forthcoming seventh edition of the TNM classification for lung cancer.23,24 Specifically with regard to the T descriptor that is based on tumor size, recommendations for change are as follows: T1a, less than 2 cm; T1b, greater than 2 to 3 cm; T2a, greater than 3 to 5 cm; T2b, greater than 5 to 7 cm; and T3, greater than 7 cm. With regard to stage groupings, T1aN0 and T1bN0 tumors will be classified as stage IA, T2aN0 as stage IB, T2bN0 as stage IIA, and T3N0 as stage IIB. The results of our study provide further justification that a modification to the staging system is needed. Indeed, tumors currently classified as stage IB NSCLC would be classified as stage IB, IIA, or IIB tumors in the new system.
Finally, with regard to biologic plausibility, it is appropriate to point out that mean tumor volume in our subset with larger tumors was almost an order of magnitude greater than in the subset with smaller tumors. In our study, mean tumor diameter was 5.78 cm among those who had tumors ≥ 4.0 cm compared with 2.72 cm for the subset who had tumors less than 4.0 cm. Accordingly, the ratio of diameters was approximately two-fold (5.78 ÷ 2.72 = 2.125). However, because volume is proportional to diameter raised to the third power, the group of patients with larger tumors in our study had a mean tumor volume that was 9.6-fold greater than the group of patients who had smaller tumors (assuming spherical shape).
Molecular markers and adjuvant chemotherapy in NSCLC.
Eligibility for all adjuvant randomized controlled trials (including CALGB 9633) was determined exclusively by stage and resectability. However, evidence is rapidly accumulating that risk assessment on the basis of conventional clinical criteria has major limitations in early NSCLC (Herbst RS, Lippman SM. N Engl J Med 356:76-78, 2007).
For example, a recent companion study to the International Adjuvant Lung Trial demonstrated that excision repair cross-complementation group 1 protein serves as a prognostic marker in early-stage NSCLC as well as a predictive marker with regard to the selection of patients likely to achieve improved survival from adjuvant cisplatin-based therapy (Olaussen K, Dunant A, Fouret P, et al. N Engl J Med 355: 983-991, 2006). Two recent reports of gene expression profiling in early-stage NSCLC demonstrate that gene expression profiling technology is capable of predicting recurrence and survival with greater accuracy than conventional clinical criteria (Potti A, Mukherjee S, Petersen R, et al. N Engl J Med 355:570-580, 2006; Chen HY, Yu SL, Chen CH, et al. N Engl J Med 356:11-20, 2007). Another study demonstrated that methylation of the promoter region of four specific genes predicts early recurrence after resection of stage I NSCLC (Brock MV, Hooker CM, Ota-Machida E, et al. N Engl J Med 358:1118-1128, 2008).
Evolving data on molecular markers provide the opportunity to design a new generation of adjuvant trials in NSCLC, which could provide evidence for adjuvant therapy to be targeted toward individual patients who are most likely to recur and to tailor therapy from which individual patients are most likely to derive benefit.
Influence of CALGB 9633 on current trial design.
The finding of a significant survival advantage in CALGB 9633 for adjuvant paclitaxel/carboplatin among patients who have tumors ≥ 4 cm in diameter has directly influenced the design of the only ongoing North American randomized trial on adjuvant chemotherapy in early-stage NSCLC. Eastern Cooperative Oncology Group (ECOG) Study 1505 is designed to address the incremental effectiveness of bevacizumab in combination with adjuvant chemotherapy in stages IB, II, and IIIA NSCLC. Its rationale is based on the results of a randomized controlled trial conducted by ECOG in stage IIIB and IV NSCLC, which demonstrated that combined bevacizumab and paclitaxel/carboplatin were superior to paclitaxel/carboplatin alone (Sandler A, Gray R, Perry MC, et al. N Engl J Med 355:2542-2550, 2006).
Because overall survival results in CALGB 9633 were not significantly different, but because there was a significant survival advantage among patients who had tumors ≥ 4 cm in diameter, two modifications in the protocol were made (Wakelee H, Schiller J, Gandara D. Clinical Lung Cancer 8:18-21, 2006). The original protocol design permitted the individual investigator to choose the adjuvant chemotherapy regimen from four regimens that have similar efficacy in advanced NSCLC.12 These four regimens included three cisplatin-based doublets (ie, vinorelbine/cisplatin, docetaxel/cisplatin, or gemcitabine/cisplatin) or the paclitaxel/carboplatin regimen as utilized in CALGB 9633. However, on the basis of our results, the permitted chemotherapy was modified to include only one of the three cisplatin-based doublets but not the paclitaxel/carboplatin combination. The second modification in the protocol is that stage IB patients would only be eligible for ECOG 1505 if they had tumors ≥ 4 cm in diameter.
List of participating institutions for CALGB 9633.
The following institutions participated in this study: Dana-Farber Cancer Institute, Boston, MA—Eric P. Winer, MD, supported by CA32291; Dartmouth Medical School—Norris Cotton Cancer Center, Lebanon, NH—Marc S. Ernstoff, MD, supported by CA04326; Duke University Medical Center, Durham, NC—Jeffrey Crawford, MD, supported by CA47577; Long Island Jewish Medical Center, Lake Success, NY—Marc Citron, MD, supported by CA11028; Massachusetts General Hospital, Boston, MA—Michael L. Grossbard, MD, supported by CA12449; Medical University of South Carolina, Charleston, SC—Mark Green, MD, supported by CA03927; Missouri Baptist Medical Center, St. Louis, MO, Alan P. Lyss, MD, supported by CA11455802; Mount Sinai Medical Center, Miami, FL—Rogerio Lilenbaum, MD, supported by CA45564; Mount Sinai School of Medicine, New York, NY—Lewis R. Silverman, MD, supported by CA04457; Northern Indiana Cancer Research Consortium CCOP, South Bend, IN—Rafat Ansari, MD, supported by CA86726; Rhode Island Hospital, Providence, RI—William Sikov, MD, supported by CA08025; Roswell Park Cancer Institute, Buffalo, NY—Ellis Levine, MD, supported by CA02599; Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC—James N. Atkins, MD, supported by CA45808. State University of New York Upstate Medical University, Syracuse, NY—Stephen L. Graziano, MD, supported by CA21060; The Ohio State University Medical Center, Columbus, OH—Clara D. Bloomfield, MD, supported by CA77658; University of California at San Diego, San Diego, CA—Joanne Mortimer, MD, supported by CA11789; University of California at San Francisco, San Francisco, CA—Alan P. Venook, MD, supported by CA60138; University of Chicago, Chicago, IL—Gini Fleming, MD, supported by CA41287; University of Illinois MBCCOP, Chicago, IL—Lawrence E. Feldman, MD, supported by CA74811; University of Iowa, Iowa City, IA—Gerald Clamon, MD, supported by CA47642; University of Maryland Greenebaum Cancer Center, Baltimore, MD—Martin Edelman, MD, supported by CA31983; University of Massachusetts Medical School, Worcester, MA—William V. Walsh, MD, supported by CA37135; University of Minnesota, Minneapolis, MN—Bruce A. Peterson, MD, supported by CA16450; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO—Michael C. Perry, MD, supported by CA12046; University of Nebraska Medical Center, Omaha, NE—Anne Kessinger, MD, supported by CA77298; University of North Carolina at Chapel Hill, Chapel Hill, NC—Thomas C. Shea, MD, supported by CA47559; University of Tennessee Memphis, Memphis, TN—Harvey B. Niell, MD, supported by CA47555; Vermont Cancer Center, Burlington, VT—Hyman B. Muss, MD, supported by CA77406; Wake Forest University School of Medicine, Winston-Salem, NC—David D. Hurd, MD, supported by CA03927; Walter Reed Army Medical Center, Washington, DC—Thomas Reid, MD, supported by CA26806; Washington University School of Medicine, St. Louis, MO—Nancy Bartlett, MD, supported by CA77440; and Weill Medical College of Cornell University, New York, NY—Scott Wadler, MD, supported by CA07968.
Fig A1.
Survival curve at the time of accrual termination.
Table A1.
Interim Efficacy Analyses
| Analysis Group | No. of Deaths per Treatment Group
|
P | P Threshold for Statistical Significance† | |||
|---|---|---|---|---|---|---|
| Observation | Chemotherapy | Total*
|
||||
| No. | % | |||||
| 1 | 22 | 13 | 35 | 23 | .115 | |
| 2 | 26 | 19 | 45 | 29 | .127 | |
| 3 | 42 | 24 | 66 | 43 | .008 | < .0028 |
| 4 | 49 | 30 | 79 | 51 | .005 | < .0094 |
Percentage of the required number of deaths for the study to be fully mature.
Calculated according to the O’Brien-Fleming decision rule and computed with S+ SeqTrial (S+ SeqTrial User's Manual 2000, Data Analysis Products Division; MathSoft, Seattle, WA).
Table A2.
Survival Statistics at Accrual Termination
| Variable | Treatment Group
|
|
|---|---|---|
| Observation | Chemotherapy | |
| No. of patients | 171 | 173 |
| No. of deaths | 49 | 30 |
| Survival, months | ||
| Median | 54.1 | NE |
| 95% CI | 46.3 to ∞ | NE |
| 1-year | 0.93 | 0.95 |
| 95% CI | 0.89 to 0.97 | 0.91 to 0.98 |
| 2-year | 0.82 | 0.94 |
| 95% CI | 0.76 to 0.89 | 0.90 to 0.98 |
| 3-year | 0.68 | 0.83 |
| 95% CI | 0.60 to 0.77 | 0.76 to 0.90 |
Abbreviation: NE, not estimable.
Table A3.
Estimated Hazard Ratios by 12-Month Intervals
| Time period, months | Analyses
|
|
|---|---|---|
| Hazard Ratio | 95% CI | |
| 0-12 | 0.996 | 0.414 to 2.392 |
| 12-24 | 0.400 | 0.166 to 0.964 |
| 24-36 | 0.742 | 0.391 to 1.406 |
| 36-48 | 1.117 | 0.537 to 2.322 |
| 48-60 | 1.685 | 0.680 to 4.176 |
| ≥ 60 | 0.649 | 0.284 to 1.481 |
Hazard Ratio < 1 favors the chemotherapy group.
published online ahead of print at www.jco.org on September 22, 2008
Supported in part by Grants. No. CA08025; CA33601 (S.G.); CA47577; CA16450; CA41287; CA45808; and CA31946 (R.L.S.) from the National Cancer Institute; and by Grant No. U10 CA21661 and Grant No. CA-25224.
Presented in part at the 40th Annual Meeting of the American Society of Clinical Oncology, June 5-8, 2004, New Orleans, LA, and at the 42nd Annual Meeting of the American Society of Clinical Oncology, June 2-6, 2006, Atlanta, GA.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Le Chevalier T, et al: Results of the Randomized International Adjuvant Lung Cancer Trial (IALT): cisplatin-based chemotherapy (CT) versus no CT in 1867 patients with resected non–small-cell lung cancer. Proc Am Soc Clin Oncol 22:2, 2003. (abstr 6) [Google Scholar]
- 2.Arriagada R, Bergman B, et al: Cisplatin-based adjuvant chemotherapy in patients with completely resected non–small-cell lung cancer: The International Adjuvant Lung Cancer Trial (IALT) Collaborative Group. N Engl J Med 350:351-360, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Kato H, Ichinose Y, Ohta M, et al: A randomized trial of adjuvant chemotherapy with Uracil-Tegafur for adenocarcinoma of the lung. N Engl J Med 350:1713-1721, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Winton T, Livingston R, Johnson D, et al: Vinorelbine plus cisplatin vs. observation in resected non–small-cell lung cancer. N Engl J Med 352:2589-2597, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Strauss GM, Herndon JE, Maddaus MA, et al: Randomized Clinical Trial of adjuvant chemotherapy with paclitaxel and carboplatin following resection in Stage IB non–small-cell lung cancer (NSCLC): Report of Cancer and Leukemia Group B (CALGB) Protocol 9633. J Clin Oncol 22:621s, 2004. (suppl; abstr 7019) [Google Scholar]
- 6.Douillard JY, Rosell R, De Lena M, et al: Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non–small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomized controlled trial. Lancet Oncology 7:719-727, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Strauss GM, Rathore R: Lung cancer, in Crapo JD, Glassroth J, Karlinsky JB, et al (eds): Baum's Textbook of Pulmonary Diseases. Philadelphia, PA, Lippincott Williams & Wilkins, 2004, pp 787-857
- 8.Mountain CE: Revisions in the international system for staging lung cancer. Chest 111:1710-1717, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Natale R: Preliminary results of a phase I/II clinical trial of paclitaxel and carboplatin in non–small-cell lung cancer. Semin Oncol 23:51-54, 1996. (suppl 16) [PubMed] [Google Scholar]
- 10.Langer C, Leighton J, Comis R, et al: Paclitaxel and carboplatin in combination in the treatment of advanced non–small-cell lung cancer: A phase II toxicity, response and survival analysis. J Clin Oncol 13:1860-1870, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Kelly K, Crowley J, Bunn P Jr, et al: Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small-cell lung cancer: A Southwest Oncology Group trial. J Clin Oncol 19:3210-3218, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Schiller JH, Harrington A, Belani C, et al: Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med 346:92-98, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Lilenbaum RC, Herndon JE Jr, List MA, et al: Single-agent versus combination chemotherapy in advanced non–small-cell lung cancer: The cancer and leukemia group B (study 9730). J Clin Oncol 23:190-196, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Hotta K, Matsuo K, Ueoka H, et al: Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non–small-cell lung cancer. J Clin Oncol 22:3852-3859, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Ardizzoni A, Boni L, Tiseo M, et al: Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non–small-cell lung cancer: An individual patient data meta-analysis. J Natl Cancer Inst 99:847-857, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Scagliotti G, De Marinis F, Rinaldi M, et al: Phase III randomized trial comparing three platinum-based doublets in advanced non–small-cell lung cancer. J Clin Oncol 20:4285-4291, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Pignon J, Tribodet H, Scagliotti J, et al: Lung Adjuvant Cisplatin Evaluation (LACE): A pooled analysis of five randomized clinical trials including 4,584 patients. J Clin Oncol 24:366s, 2006. (suppl; abstr 7008) [Google Scholar]
- 18.Lan GGK, DeMets DL: Discrete sequential boundaries for clinical trials. Biometrika 70:659-663, 1983 [Google Scholar]
- 19.Pampallona S, Tsiatis A: Group sequential designs for one-sided and two-sided hypothesis testing with provision for early stopping in favor of the null hypothesis. J Stat Plan Inference 42:19-35, 1994 [Google Scholar]
- 20.O’Brien P, Fleming TR: A multiple testing procedure for clinical trials. Biometrics 35:549-556, 1979 [PubMed] [Google Scholar]
- 21.Lopez-Encuentra A, Duque-Medina J, Rami-Porta R, et al: Is 3 cm a prognostic threshold in pathologic stage I non–small-cell lung cancer? A multicenter study of 1,020 patients. Chest 121:1515-1520, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Mery CM, Pappas AN, Burt BM, et al: Diameter of non–small-cell lung cancer correlates with long-term survival: Implications for T stage. Chest 128:3255-3260, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Rami-Porta R, Ball D, Crowley J, et al: The IASLC Lung Cancer Staging Project: Proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2:593-602, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Goldstraw P, Crowley J, Chansky K, et al: The IASLC Lung Cancer Staging Project: Proposals for the revision of the tnm stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2:706-714, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Strauss G: Management of early-stage lung cancer: Past, present, and future adjuvant trials. Oncology 20:1651-1663, 2006 [PubMed] [Google Scholar]
- 26.Roselli M, Mariotti S, Ferroni P, et al: Postsurgical chemotherapy in stage IB non–small-cell lung cancer: Long-term survival in a randomized study. Int J Cancer 119:955-960, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Pisters KM, Evans WK, Azzoli CG, et al: Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non–small-cell lung cancer guideline. J Clin Oncol 25:5506-5518, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Strauss GM, Herndon JE, Maddaus MA, et al: Adjuvant chemotherapy in stage IB non–small-cell lung cancer (NSCLC): Update of Cancer and Leukemia Group B (CALGB) Protocol 9633. J Clin Oncol 24:365s, 2006. (abstr 7007) [Google Scholar]
- 29.Pignon JP, Massard C: Adjuvant therapy for early lung cancer: Reflections and perspectives. Oncology 20:1669-1673, 2006 [Google Scholar]
- 30.Dunant A, Pignon JP, Le Chevalier T, et al: Adjuvant chemotherapy for non–small-cell lung cancer: Contribution of the International Adjuvant Lung Trial. Clinical Cancer Res 11:5017s-5021s, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Langer CL: The role of adjuvant chemotherapy for elderly patients with non–small-cell lung cancer, in Corey J. Langer (eds): American Society of Clinical Oncology 2006 Educational Book. Alexandria, VA, American Society of Clinical Oncology, 2006, pp 289-292