Abstract
Purpose
This study compares non–small-cell lung cancer (NSCLC) treatments provided to older patients (age ≥ 66 years) who are dually eligible for Medicare and Medicaid with treatments provided to similar patients who are insured by Medicare. We extend the analysis to include a comparison of survival rates between Medicare and dually eligible patients. Dual eligibility is associated with low socioeconomic status. However, Medicaid coverage in addition to Medicare removes many financial barriers to care.
Patients and Methods
The sample included 2,626 older patients with local and regional stage NSCLC diagnosed between 1997 and 2000. Four outcomes were studied: the likelihood of receiving resection, chemotherapy, radiation therapy, and survival (perioperative and longer-term). Logistic regression was used to predict the likelihood of treatment, and stratified and multivariate analyses were used to evaluate differences in survival.
Results
Dually eligible patients were half as likely to undergo resection as Medicare patients (P < .001) and were more likely to receive radiation than Medicare patients. Stratified and multivariate analyses showed that surgically treated dually eligible patients had slightly inferior survival as compared with that of Medicare patients. Survival was equivalent among patients who did not undergo resection, regardless of insurance coverage.
Conclusion
Older dually eligible patients with NSCLC had a lower likelihood of undergoing resection despite controls for socioeconomic factors and comorbidities. However, if such patients were surgically treated, survival improved substantially, but it remained inferior to the survival of Medicare patients. Additional research is needed to understand why resection rates were substantially lower among dually eligible patients.
INTRODUCTION
Lung cancer is the leading cause of cancer-related death in the United States1 and is the most prevalent cancer among Medicare- and Medicaid-insured patients.2 Although the annual lung cancer mortality rate has been decreasing overall,1 it remains high, particularly among racial and ethnic minorities.2 Improved survival rates for patients with non–small-cell lung cancer (NSCLC), the most common form of lung cancer, can be achieved when the disease is diagnosed early and treated aggressively.3 Resection is generally regarded as the most effective treatment for persons with stage I or II disease. Adjuvant chemotherapy has also been shown to provide a statistically significant survival advantage for patients with completely resected NSCLC.4-6 For patients for whom surgery is not an option, radiotherapy is regarded as a life-prolonging alternative.7,8
Treatment delivery often falls short of recommended NSCLC care as a result of clinical reasons, including inoperable conditions, comorbid conditions, patient refusal, and advanced age,3,7,9 and as a result of nonclinical reasons, such as those related to minority race and low socioeconomic status.10,11 For nearly a decade, studies have reported that black patients are less likely to undergo surgical resection and adjuvant chemotherapy than white patients, and correspondingly, black patients have a lower 5-year survival rate in comparison with white patients.10-13
This study examines how Medicaid insurance in addition to Medicare insurance (referred to as dual eligibility) influences the treatment and survival of older patients with NSCLC. More than 60% of dually eligible beneficiaries live below the poverty level, and almost all live 200% of poverty level.14 They are more likely than Medicare beneficiaries to be from a minority population, to be unmarried, to live alone, to be institutionalized, and to have lower educational attainment as compared with Medicare beneficiaries.15
Four outcomes were compared between dually eligible and Medicare patients. First, we examined the likelihood that patients with stage I, II, and IIIA disease undergo surgical resection. Second, we predicted the likelihood that patients received chemotherapy. Third, we examined the likelihood of radiation delivery. Finally, we compared survival differences, both perioperative and longer-term, between dually eligible patients and Medicare patients.
The influence of dual eligibility on treatment and survival is a novel aspect of our research and is important because Medicaid recipients often embody the characteristics associated with disparate cancer outcomes. For example, to qualify for Medicaid benefits, persons age ≥ 65 years must be considered medically needy or be unable to pay their medical bills.16 Nevertheless, Medicaid covers the majority of medical expenses that are not covered by Medicare, such as copayments and deductibles. Therefore, these dually eligible patients may have fewer financial barriers to medical care than patients insured by Medicare alone. We hypothesize that disparate treatment will explain differences in survival between these two groups.
PATIENTS AND METHODS
Data Sources
We used statewide Medicaid and Medicare data merged with the Michigan Tumor Registry to extract a sample of patients with a first primary cancer diagnosis. The Michigan Cancer Surveillance Program, which maintains the Michigan Tumor Registry, is greater than 95% complete based on external audit findings. This study was approved by institutional review boards at the Michigan Department of Community Health, Michigan State University, East Lansing, MI, and Virginia Commonwealth University, Richmond, VA.
Patients were matched to the Michigan state segment of the Medicare Denominator file from January 1, 1997, through December 31, 2000, using the patient's Social Security number. Survival status was available on all patients through December 31, 2003. Medicaid-insured patients were identified by matching the Medicaid eligibility files against the Tumor Registry, using deterministic and probabilistic methods. We extracted, from statewide Medicare files, all claims for inpatient, outpatient, and physician services during the study period for all patients that correctly matched to the Michigan state segment of the Medicare Denominator file (approximately 89% of patients). The process for linking the Tumor Registry, Medicare, and Medicaid data sets is described fully elsewhere.17
Study Cohort
The study cohort consists of patients age 66 years and older diagnosed with NSCLC using tumor site codes 34.0 to 34.9 and International Classification of Diseases (ICD)-O-2 morphology codes 8010 to 8040, 8050 to 8076, 8140, 8143, 8250 to 8260, 8310, 8320, 8323, 8470 to 8490, 8550 to 8573, 8980, and 8981. We limited the sample to patients with a Surveillance, Epidemiology, and End Results summary stage of in situ or local or TNM stage I, II, or III (n = 3,765).
We excluded patients who resided in a nursing home (n = 43) because they were generally poor candidates for surgery. Nursing home patients were identified from the Medicaid eligibility file. Private pay nursing home patients remained in the Medicare sample because Medicare claims files do not adequately identify nursing home patients. We removed patients diagnosed with Alzheimer's disease and/or dementia using the following ICD, version 9, codes: 331.0, 331.x, 290.0, and 797 (n = 55). This exclusion likely reduced the number of nursing home residents in the Medicare sample and removed patients for whom cancer treatment may be inappropriate. We also excluded patients with race designated as “other” or “unknown” (n = 88).
The minimum observation period for each patient was 30 days of claims data from the date of diagnosis. Therefore, we excluded patients diagnosed in December 2000 (n = 59) and those who died within 30 days after diagnosis (n = 96). We then excluded the following patients: those not enrolled in Parts A and B (n = 45), those enrolled in a managed care plan (n = 273), and patient without any health care claims (n = 12). We believe that claims data for these patients were incomplete. The remaining sample size was 3,094 patients. Approximately 11% of them were dually eligible.
We removed patients with stage IIIB NSCLC from the full sample for the estimations that predicted the likelihood of a resection and survival; this sample size was 2,626 patients. We then removed patients from the full sample who had less than 6 months of claims data after diagnosis in estimations of the likelihood of chemotherapy or radiation (n = 2,348).
Identification of Surgical Procedures
We defined surgery as having a claim, within 6 months after diagnosis, in the inpatient, outpatient, or physician file for local resection, segmentectomy, partial or radical pneumonectomy, lobectomy, sleeve resection, and wedge resection. The ICD-9 and Current Procedural Terminology (CPT) codes used to identify surgery were 32.50, 32.60, and CPT 32440, 32442, 32445, 32488, and 32500 (pneumonectomy); ICD9-CM 32.40 and CPT 32480, 32482, and 32486 (lobectomy); 32484 (bilobectomy); and ICD9-CM 32.29 and 32.30 and CPT 32520 (wedge resection).
Identification of Chemotherapy and Radiation
Patients with a chemotherapy claim within 6 months after the date of diagnosis were considered to have initiated chemotherapy. Chemotherapy claims were identified from the inpatient, outpatient, and physician claim files using the following codes: CPT codes 96400 through 96599; Health Care Common Procedural Codes Q0083 through Q0085, J8510, J8520, J8521, J8530 through J8999, J9000 through J9999, and J0640; ICD-9 procedure code 99.25, ICD-9 diagnosis codes E0781, E9331, and V58.1. Radiation therapy within 6 months after diagnosis was also examined. Radiotherapy was identified from the inpatient, outpatient, and physician claim files using the following ICD-9 codes: V58.0, V66.1, V67.1, and 92.21 to 92.29 and CPT codes 77260 through 77999.
Survival
Survival time was defined as the number of months from the month of diagnosis to the month of death or from the month of diagnosis to December 31, 2003. The majority of patients (79%) died before December 31, 2003. All-cause mortality was used in the survival analysis.
Perioperative mortality was another measure of survival for those patients who had surgery. It was defined as a dichotomous variable to indicate whether death occurred within 30 days after the date of surgery.
Control Variables
Data on patient age, race, and sex were obtained from the Michigan Tumor Registry. Age was grouped into the following categories: 66 to 70 years, 71 to 74 years, 75 to 79 years, and 80 years and older. Race was categorized as white or African American. In addition to these variables, we had information on patients’ census tract median household income, which we included in the models. The household income categories were less than $35,000 and ≥ $35,000. We also included variables that indicated whether the patient lived in a metropolitan area, urban area adjacent to a metropolitan area, urban area not adjacent to a metropolitan area, rural area adjacent to a metropolitan area, or an isolated rural area. Address information was not available for 5% of the patients. Therefore, we used monotone multiple imputations method with logistic regression to impute the missing categorical variables for both census tract median income and rural and urban residence.18 Analyses were conducted with and without imputed values, and the findings were nearly identical.
Comorbidity burden is an important prognostic factor in patients with NSCLC and is a statistically significant predictor of surgical resection.10,11 To estimate patient comorbidity burden, we used the Deyo et al19 and Klablunde et al20 adaptation of the Charlson Comorbidity Index,21 which has been used to explain the probability and extent of cancer treatment.20,22 We counted comorbidities by using all inpatient, outpatient, and physician claims for services rendered to patients in the year before diagnosis. We classified comorbidity scores into three groups: 0, 1, ≥ 2. We also examined the prevalence of the following comorbid conditions: myocardial infarction, congestive heart failure (CHF), peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), paralysis, diabetes, chronic renal failure, cirrhosis, liver disease, ulcers, and rheumatoid disorders. We found a higher prevalence of CHF, COPD, and ulcers among dually eligible patients relative to Medicare patients. CHF and COPD can be counter-indications for surgical resection. When we included a dichotomous variable for each of these conditions in the estimations that predicted the likelihood of resection (results not shown), CHF and COPD were statistically significant and negatively associated with the likelihood of resection, but these variables were also highly correlated with the prevalence of other comorbid conditions. Therefore, we report models with a count of comorbid conditions. The coefficient for dual eligibility remained stable regardless of how we specified comorbidity.
In the estimations predicting the likelihood of a resection, we included variables for TNM stage I or II versus stage IIIA. A variable for stage IIIB was added to estimations predicting the likelihood of chemotherapy and radiation therapy. In all estimations, we included variables for histology, which were adenocarcinomas, large-cell carcinomas, squamous cell carcinomas, and other or unknown.
Statistical Analysis
We described the characteristics of all patients with lung cancer by Medicare and Medicaid enrollment and used χ2 tests to test for statistical differences between the samples. Adjusted logistic regression was then used to measure the relationship between the independent variables and resection, chemotherapy initiation, and radiation initiation. Adjusted logistic regression was also used to predict the likelihood of perioperative mortality for patients who underwent surgery. We reported odds ratios (OR) and 95% CIs and P values. P values were derived from likelihood ratio tests and are two-sided.
Survival curves were constructed with the Kaplan-Meier estimation method and compared with the log-rank test. For analyses involving adjustments for confounding factors, we used the Cox proportional hazards method to estimate survival. Patients were stratified by surgical and nonsurgical treatment in all survival analyses. All analyses were conducted using SAS, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Resection
Table 1 reports that dually eligible patients were younger, were more likely to be African American, female, and to have more comorbid conditions relative to the Medicare sample. Dually eligible patients were also more likely than their Medicare counterparts to reside in census tracts with low median income. Cancer stage and histology was comparable between the two insurance groups.
Table 1.
Sample Characteristics and Adjusted Likelihood of Surgical Resection for Michigan Patients With Lung Cancer (N = 2,626)
| Characteristic | Medicare
|
Dually Eligible
|
P* | Surgical Resection
|
|||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | OR | 95% CI† | ||
| Medicare | 2,353 | NA | 1.0 | Referent | |||
| Dually eligible | NA | 273 | 0.50 | 0.38 to 0.67‡ | |||
| Age, years | < .001 | ||||||
| 66-69 | 494 | 20.99 | 87 | 31.87 | 1.0 | Referent | |
| 70-74 | 800 | 34.00 | 87 | 31.87 | 0.90 | 0.72 to 1.14 | |
| 75-79 | 610 | 25.92 | 59 | 21.61 | 0.65 | 0.51 to 0.83‡ | |
| ≥ 80 | 449 | 19.08 | 40 | 14.65 | 0.29 | 0.22 to 0.38‡ | |
| Race | < .001 | ||||||
| White | 2,159 | 91.76 | 208 | 76.19 | 1.0 | Referent | |
| African American | 194 | 8.24 | 65 | 23.81 | 0.88 | 0.65 to 1.19 | |
| Sex | < .001 | ||||||
| Male | 1,326 | 56.35 | 111 | 40.66 | 1.0 | Referent | |
| Female | 1,027 | 43.65 | 162 | 59.34 | 0.99 | 0.83 to 1.17 | |
| Comorbidity | < .001 | ||||||
| 0 | 1,287 | 54.70 | 114 | 41.76 | 1.0 | Referent | |
| 1 | 636 | 27.03 | 89 | 32.60 | 0.97 | 0.80 to 1.18 | |
| 2+ | 430 | 18.27 | 70 | 25.64 | 0.64 | 0.51 to 0.80‡ | |
| Census tract median annual income, $ | < .001 | ||||||
| < 35,000 | 1,345 | 57.16 | 222 | 81.32 | 0.80 | 0.67 to 0.98‡ | |
| ≥ 35,001 | 903 | 38.38 | 37 | 13.55 | 1.0 | Referent | |
| Missing | 105 | 4.46 | 14 | 5.13 | NA | ||
| Urban/rural | .4554 | ||||||
| Metropolitan | 1,816 | 77.18 | 196 | 71.79 | 1.0 | Referent | |
| Rural, adjacent to metropolitan | 11 | 0.47 | 1 | 0.37 | 1.18 | 0.35 to 3.99 | |
| Isolated rural | 42 | 1.78 | 5 | 1.83 | 1.79 | 0.91 to 3.53 | |
| Urban, not adjacent to metropolitan | 200 | 8.50 | 32 | 11.72 | 1.31 | 0.96 to 1.79 | |
| Urban, adjacent to metropolitan | 189 | 8.03 | 27 | 9.89 | 1.34 | 0.96 to 1.84 | |
| Missing | 95 | 4.04 | 12 | 4.40 | NA | ||
| Cancer stage | .1669 | ||||||
| I | 770 | 32.74 | 88 | 32.23 | 6.12 | 4.56 to 8.22‡ | |
| II | 1,264 | 53.72 | 143 | 52.38 | 3.29 | 2.49 to 4.34‡ | |
| IIIA | 319 | 13.56 | 42 | 15.38 | 1.0 | Referent | |
| Histology | .0949 | ||||||
| Adenocarcinomas | 928 | 39.44 | 112 | 10.58 | 1.0 | Referent | |
| Large-cell carcinomas | 172 | 7.31 | 90 | 8.84 | 0.48 | 0.34 to 0.67‡ | |
| Others/unknown | 306 | 13.00 | 25 | 12.69 | 0.25 | 0.19 to 0.33‡ | |
| Squamous cell carcinomas | 947 | 40.25 | 46 | 13.07 | 0.61 | 0.50 to 0.74‡ | |
| Resection | 1,238 | 52.61 | 98 | 35.90 | < .001 | ||
Abbreviations: OR, odds ratio; NA, not applicable.
Statistical significance determined by the two-sided χ2 test.
Statistical significance is determined by dividing the maximum likelihood coefficient by its standard error (Z) with two-tailed, statistical significance level set at P > Z.
Statistically significant at P < .05.
More than half (53%) of the Medicare sample underwent resection, whereas only 36% of the dually eligible sample underwent resection. (The adjusted likelihood of a resection is listed in the last column of Table 1.) Dually eligible patients were half as likely as Medicare patients to undergo resection (OR = .50; 95% CI, 0.38 to 0.67). Other variables statistically significant and negatively associated with resection were age (75 years and older v 66 to 69 years), two or more comorbid conditions, and residing in a census tract where the median household income is less than $35,000. Patients with early-stage cancer were more likely to undergo resection than patients with stage IIIA. Histologies other than adenocarcinomas were negatively associated with the likelihood of resection.
Chemotherapy and Radiation
Table 2 lists descriptive characteristics of the chemotherapy and radiation sample and the adjusted likelihood of initiating chemotherapy and radiation. The sample characteristics mirrored the characteristics reported in Table 1. In the adjusted analysis, dual eligibility was not statistically significantly associated with chemotherapy but was positively associated with radiation (OR = 1.46; 95% CI, 1.09 to 1.95). Older patients were statistically significantly less likely to receive chemotherapy but more likely to receive radiation relative to their younger counterparts. African American patients were less likely than white patients to be treated with chemotherapy (OR = 0.70; 95% CI, 0.50 to 0.97) or radiation (OR = 0.58; 95% CI, 0.43 to 0.80). Patients with two or more comorbidities were also more likely to receive radiation than patients without comorbidities. Patients with stage I and II disease were statistically significantly less likely to receive chemotherapy or radiation relative to patients with stage IIIB disease, whereas patients with stage IIIA cancer were more likely to receive radiation relative to patients with stage IIIB cancer (OR = 1.89; 95% CI, 1.29 to 2.77). Patients with large-cell or squamous cell carcinomas were more likely than patients with adenocarcinomas to be treated with chemotherapy, and patients with large-cell or squamous cell or other carcinomas were more likely than patients with adenocarcinomas to be treated with radiation.
Table 2.
Sample Characteristics and Adjusted Likelihood of Chemotherapy and Radiation for Michigan Patients With Lung Cancer (N = 2,348)
| Characteristic | Medicare
|
Dually Eligible
|
P* | Chemotherapy
|
Radiation
|
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | OR | 95% CI† | OR | 95% CI† | ||
| Medicare | 2,087 | NA | 1.0 | Referent | 1.0 | Referent | |||
| Dually eligible | NA | 261 | 0.98 | 0.71 to 1.36 | 1.46 | 1.09 to 1.95‡ | |||
| Age, years | .0027 | ||||||||
| 66-69 | 481 | 23.05 | 82 | 31.42 | 1.0 | Referent | 1.0 | Referent | |
| 70-74 | 729 | 34.93 | 97 | 37.16 | 0.97 | 0.75 to 1.26 | 1.19 | 0.94 to 1.52 | |
| 75-79 | 512 | 24.53 | 59 | 21.61 | 0.87 | 0.66 to 1.16 | 1.46 | 1.12 to 1.89‡ | |
| ≥ 80 | 365 | 17.49 | 40 | 14.65 | 0.53 | 0.38 to 0.73‡ | 2.03 | 1.53 to 2.70‡ | |
| Race | < .001 | ||||||||
| White | 1,893 | 90.70 | 194 | 74.33 | 1.0 | Referent | 1.0 | Referent | |
| African American | 194 | 9.30 | 67 | 25.67 | 0.70 | 0.49 to 0.97‡ | 0.58 | 0.43 to 0.80‡ | |
| Sex | < .001 | ||||||||
| Male | 1,156 | 55.39 | 99 | 37.93 | 1.0 | Referent | 1.0 | Referent | |
| Female | 931 | 44.61 | 162 | 62.07 | 0.97 | 0.79 to 1.19 | 0.94 | 0.78 to 1.13 | |
| Comorbidity | < .001 | ||||||||
| 0 | 1,182 | 56.64 | 116 | 44.44 | 1.0 | Referent | 1.0 | Referent | |
| 1 | 558 | 26.74 | 86 | 32.95 | 0.95 | 0.75 to 1.20 | 1.02 | 0.82 to 1.25 | |
| 2+ | 347 | 16.63 | 59 | 22.61 | 0.86 | 0.65 to 1.14 | 1.34 | 1.05 to 1.72‡ | |
| Census tract median annual income, $ | < .001 | ||||||||
| < 35,000 | 1,182 | 56.64 | 209 | 80.08 | 1.09 | 0.87 to 1.37 | 0.97 | 0.79 to 1.19 | |
| ≥ 35,001 | 817 | 39.15 | 37 | 14.18 | 1.0 | Referent | 1.0 | Referent | |
| Missing | 88 | 4.22 | 15 | 5.75 | NA | NA | |||
| Urban/rural | .2107 | ||||||||
| Metropolitan | 1,625 | 77.86 | 188 | 72.03 | 1.0 | Referent | 1.0 | Referent | |
| Rural, adjacent to metropolitan | 8 | 0.38 | 0 | 0.00 | 0.93 | 0.18 to 4.88 | 0.81 | 0.41 to 1.62 | |
| Isolated rural | 38 | 1.82 | 5 | 1.92 | 0.46 | 0.20 to 1.04 | NA | ||
| Urban, not adjacent to metropolitan | 183 | 8.77 | 28 | 10.73 | 0.78 | 0.53 to 1.14 | 1.03 | 0.74 to 1.44 | |
| Urban, adjacent to metropolitan | 152 | 7.28 | 27 | 10.34 | 0.79 | 0.53 to 1.14 | 0.91 | 0.64 to 1.29 | |
| Missing | 81 | 3.88 | 13 | 4.98 | NA | NA | |||
| Cancer stage | .2869 | ||||||||
| I | 626 | 30.00 | 75 | 27.47 | 0.10 | 0.07 to 0.14‡ | 0.21 | 0.15 to 0.28‡ | |
| II | 1,012 | 48.49 | 118 | 45.21 | 0.24 | 0.18 to 0.32‡ | 0.42 | 0.32 to 0.56‡ | |
| IIIA | 218 | 10.45 | 29 | 11.11 | 1.39 | 0.96 to 1.52 | 1.89 | 1.29 to 2.77‡ | |
| IIIB | 231 | 11.07 | 39 | 14.94 | 1.0 | Referent | 1.0 | Referent | |
| Histology | .0915 | ||||||||
| Adenocarcinomas | 835 | 40.01 | 86 | 32.95 | 1.0 | Referent | 1.0 | Referent | |
| Large-cell carcinomas | 172 | 7.31 | 22 | 8.43 | 1.59 | 1.09 to 2.32 | 2.37 | 1.68 to 3.34‡ | |
| Others/unknown | 263 | 12.60 | 44 | 16.86 | 1.20 | 0.87 to 1.65 | 2.67 | 2.01 to 3.55‡ | |
| Squamous cell carcinomas | 825 | 39.53 | 109 | 41.76 | 1.10 | 0.87 to 1.40 | 2.10 | 1.71 to 2.59‡ | |
| Chemotherapy | 569 | 27.26 | 74 | 28.35 | .7109 | NA | NA | ||
| Radiation | 827 | 39.63 | 123 | 47.13 | .0207 | NA | NA | ||
Abbreviations: OR, odds ratio; NA, not applicable.
Rural, adjacent to a metropolitan area, and rural isolated were combined because few patients resided in a rural isolated area.
Statistical significance is determined by dividing the maximum likelihood coefficient by its standard error (Z) with two-tailed, statistical significance level set at P > Z.
Statistically significant at P < .05.
Survival
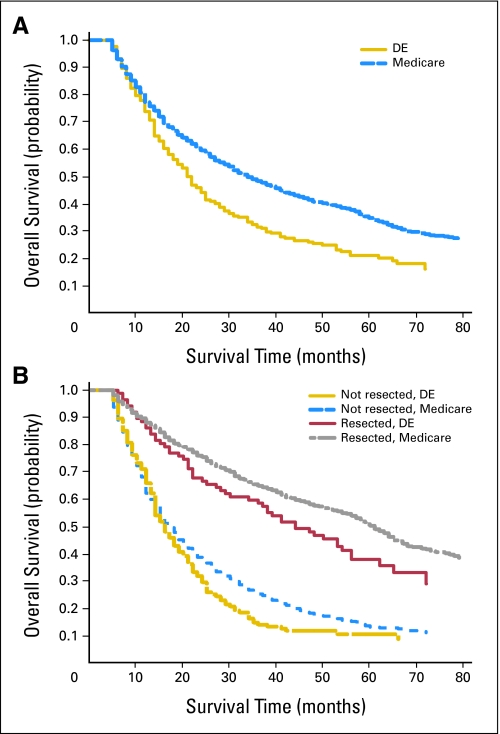
Overall median survival was longer for Medicare-insured patients relative to dually eligible patients. When patients were stratified by whether they underwent resection, survival was equivalent among Medicare and dually eligible patients who did not undergo resection (Fig 1). The survival differences approach statistical significance (P = .08) between dually eligible and Medicare patients who underwent resection (Fig 1). Survival for surgically treated patients, regardless of health insurance coverage, was superior to that of patients who did not have surgery.
Fig 1.
Survival curves by Medicaid coverage and resection. (A) Overall survival (all-cause mortality) was statistically significantly better for Medicare patients compared with Medicaid patients (P < .05). (B) Overall survival (all-cause mortality) was not significantly different for Medicare and Medicaid patients when the sample was stratified by surgical resection, although statistical significance was approached for patients who underwent resection (P = .08). Survival curves were constructed with the Kaplan-Meier estimation method and compared with the log-rank test. DE, dually eligible.
Table 3 lists the adjusted hazard ratios for longer-term survival and reports ORs for perioperative survival. The sample is stratified by resection status. In the first column, dually eligible patients who underwent resection had a statistically significantly higher likelihood of dying than did Medicare patients who underwent resection (hazard ratio = 1.42, 95% CI, 1.09 to 1.86). The likelihood of survival was statistically equivalent for patients who did not undergo resection. The likelihood of perioperative mortality was statistically similar for dually eligible and Medicare patients.
Table 3.
Adjusted Risk of Death for Michigan Patients With Lung Cancer
| Explanatory Variable | Resection (n = 1,690)
|
No Surgery (n = 2,612)
|
Perioperative Mortality (n = 1,690)
|
|||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | OR | 95% CI | |
| Medicare | 1.0 | Referent | 1.0 | Referent | 1.0 | Referent |
| Dually eligible | 1.42 | 1.09 to 1.87* | 1.14 | 0.96 to 1.37 | 1.67 | 0.54 to 5.17 |
NOTE. Models predicting survival for those who underwent resection and those who were not surgically treated include all controls shown in Table 1. Coefficients for these variables are not reported. The model predicting perioperative mortality includes all controls shown in Table 1, with the exception of urban and rural residence. Few patients within these geographic designations died.
Abbreviations: HR, hazard ratio; OR, odds ratio.
Statistically significant at P < .05.
DISCUSSION
We examined the role of dual eligibility in treatment and survival in older patients with NSCLC. Without sample stratification, Medicare-insured patients with NSCLC had superior survival overall. Once we adjusted for surgical treatment, dually eligible patients who underwent resection had a greater likelihood of death than Medicare patients who underwent resection, but they had a much lower likelihood of death than patients who did not undergo resection. Survival for patients who did not undergo resection was similar, regardless of insurance coverage. The key finding with regard to treatment was that dually eligible patients were half as likely to undergo resection as Medicare patients. This finding was statistically significant despite controls for age, socioeconomic status, comorbid conditions, and disease stage and the exclusion of nursing home patients from the sample. However, a resection tended to narrow, but not close, the survival gap between dually eligible and Medicare patients.
It is possible that dually eligible patients, despite comprehensive insurance coverage, may have difficulty accessing experienced thoracic surgeons. Alternatively, surgeons may not offer surgery to dually eligible patients or these patients may be inclined to refuse surgery or to have conditions that are counter-indications for surgery.
Other noteworthy findings include the following. First, dually eligible patients were as likely to initiate chemotherapy as compared with Medicare patients and were more likely to initiate radiation than Medicare patients. Second, patients residing in low-income census tracts were less likely to undergo resection, suggesting that these patients may have difficulty accessing appropriate health care. Third, African American patients were less likely to initiate chemotherapy or radiation than white patients. Finally, older age was negatively associated with resection and chemotherapy but was positively associated with radiation.
The study has some limitations. First, the study is specific to Michigan, and as such, it may not be generalized to other states or regions. However, the only way to identify Medicaid-insured patients, at this time, is at the state level. The state buy-in variable, which is in the Medicare denominator file, does not adequately identify Medicaid patients. Second, published estimates indicate that only half of older Medicare beneficiaries with incomes at or below poverty enroll in Medicaid.23 The inclusion of older patients who qualify for but who are not enrolled in Medicaid would diminish the relationship between Medicaid and the outcomes we study. Third, unmeasured differences in comorbidity status may exist between the two insurance groups. We chose the Deyo et al19 and Klablunde et al20 modifications of the Charlson Comorbidity Index because they are conducive to assessing comorbidity burden with administrative claims data. However, in older patients with cancer, the Charlson Comorbidity score does not adequately reflect functional ability or predict tolerance to treatment.24,25 Fourth, we lacked data on patient preferences, counter-indications for surgery, and smoking history. Finally, the study is specific to patients age 66 and older and excluded patients enrolled in a managed care organization; these patients may have different patterns of care.
The reasons why dually eligible patients were less likely to undergo resection are unclear and worthy of further investigation. Among patients treated surgically, there is a substantial survival advantage. However, surgically treated dually eligible patients still have shorter survival times than Medicare patients, suggesting that other factors place the dually eligible at a disadvantage. Patients become eligible for Medicaid because they are either medically needy, which means that they have few financial resources to allocate for medical expenses, or they are disabled. Disabled dually eligible patients may have counter-indications for surgery, implying that surgical and survival rates may not ever be equivalent between dually eligible and Medicare patients. Nevertheless, survival advantages may be realized if treatment differences attributable to socioeconomic status can be reduced.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Cathy J. Bradley, Bassam Dahman, Charles W. Given
Financial support: Cathy J. Bradley
Administrative support: Cathy J. Bradley
Collection and assembly of data: Cathy J. Bradley
Data analysis and interpretation: Cathy J. Bradley, Bassam Dahman, Charles W. Given
Manuscript writing: Cathy J. Bradley, Bassam Dahman, Charles W. Given
Final approval of manuscript: Cathy J. Bradley, Bassam Dahman, Charles W. Given
published online ahead of print at www.jco.org on September 15, 2008
Supported by National Cancer Institute Grant No. R01-CA101835-01, In-Depth Examination of Disparities in Cancer Outcomes (C.J.B., principal investigator).
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Edwards B, Brown M, Wingo P, et al: Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst 97:1407-1427, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bradley C, Given C, Luo Z, et al: Cancer incidence in elderly Medicare and dual eligible beneficiaries. Health Serv Res [epub ahead of print on May 12, 2008] [DOI] [PMC free article] [PubMed]
- 3.Sullivan V, Tran T, Holmstrom A, et al: Advanced age does not exclude lobectomy for non-small cell lung carcinoma. Chest 128:2671-2676, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Winton T, Livingston R, Johnson D, et al: Vinorelbine plus cisplatin vs. observation in resected non-small cell lung cancer. N Engl J Med 352:2589-2597, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Arriagada R, Bergman B, Dunant A, et al: Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small cell lung cancer. N Engl J Med 350:351-360, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Visbal A, Leigh N, Feld R, et al: Adjuvant chemotherapy for early-stage non-small cell lung cancer. Chest 128:2933-2943, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Birim O, Kappetein P, Goorden T, et al: Proper treatment selection may improve survival in patients with clinical early-stage nonsmall cell lung cancer. Ann Thorac Surg 80:1021-1026, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Wisnivesky J, Bonomi M, Henschke C, et al: Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest 128:1461-1467, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Earle C, Neumann P, Gelber R, et al: Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol 20:1786-1792, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Bach P, Cramer L, Warren J, et al: Racial differences in the treatment of early-stage lung cancer. N Engl J Med 341:1198-1205, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Lathan C, Neville B, Earle C: The effect of race on invasive staging and surgery in non-small cell lung cancer. J Clin Oncol 24:413-418, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Earle C, Venditti L, Neumann P, et al: Who gets chemotherapy for metastatic lung cancer? Chest 117:1239-1246, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Potosky A, Saxman S, Wallace R, et al: Population variations in the initial treatment of non-small-cell lung cancer. J Clin Oncol 22:3261-3268, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Medicare Payment Advisory Commission (MedPAC): Analysis of Medicare Current Beneficiary Survey (MCBS), 2001. http://www.medpac.gov/publications/congressional_reports/Jun04DatabookSec2.pdf
- 15.Murray L, Shatto A: Dually eligible Medicare beneficiaries. Health Care Financ Rev 20:131-140, 1998 [PMC free article] [PubMed] [Google Scholar]
- 16.Department of Health and Human Services, Centers for Medicare and Medicaid Services, Center for Medicaid and State Operations: Medicaid-at-a-glance 2005. http://www.cms.hhs.gov/MedicaidEligibility/Downloads/MedicaidataGlance05.pdf
- 17.Bradley C, Given C, Luo Z, et al: Medicaid, Medicare, and the Michigan Tumor Registry: A Linkage Strategy. Med Decis Making 27:352-363, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Allison PD: Missing Data. Thousand Oaks, CA, Sage Publications, 2001
- 19.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613-619, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CE, Potosky AL, Legler JM, et al: Development of a comorbidity index using physician claims data. J Clin Epidemiol 53:1258-1267, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40:373-383, 1987 [DOI] [PubMed] [Google Scholar]
- 22.Baldwin LM, Klabunde CE, Green P, et al: In search of the perfect comorbidity measure for use with administrative claim data: Does it exist? Med Care 44:745-753, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pezzin LE, Kasper JD: Medicaid enrollment among elderly Medicare beneficiaries: Individual determinants, effects of state policy, and impact on service use. Health Serv Res 37:827-847, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Froehner M, Koch R, Litz R, et al: Comparison of the American Society of Anesthesiologists physical status classification with the Charlson score as predictors of survival after radical prostatectomy. Urology 62:698-701, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Extermann M: Measuring comorbidity in older cancer patients. Eur J Cancer 36:453-471, 2000 [DOI] [PubMed] [Google Scholar]