Abstract
Background
A number of studies have shown strong graded positive relationships between size at birth and grip strength and estimates of muscle mass in older people. However no studies to date have included direct measures of muscle size.
Methods
We studied 313 men and 318 women born in Hertfordshire UK between 1931 and 1939 who were still resident there and had historical records of growth in early life. Information on lifestyle was collected and participants underwent peripheral quantitative computed tomography to directly measure forearm and calf muscle size.
Results
Birth weight was positively related to forearm muscle area in the men (r = 0.24 p < 0.0001) and women (r = 0.17 p =0.003). There were similar but weaker associations between birth weight and calf muscle area in the men (r=0.13, p=0.03) and in the women (r=0.17, p=0.004). These relationships were all attenuated by adjustment for adult size.
Conclusion
We present first evidence that directly measured muscle size in older men and women is associated with size at birth. This may reflect tracking of muscle size and is important because it suggests that benefit may be gained from taking a life course approach both to understanding the aetiology of sarcopenia and to developing effective interventions.
Introduction
Sarcopenia is defined as the loss of muscle mass and strength with age. The loss of muscle function in particular is associated with profound consequences for older people in terms of increased risk of morbidity, disability and mortality yet remains poorly understood. A number of studies have shown strong graded positive relationships between size at birth and grip strength in older people and there is growing interest in the effects of developmental influences on muscle in later life1-4. The underlying mechanisms are unknown but there is some evidence from animal models that prenatal undernutrition is associated with a reduced number of muscle fibres5;6 and it has been proposed that the relationship between low birth weight and impaired adult muscle strength reflects reduced muscle size.
Adult body composition studies involving indirect measures of fat-free or lean mass suggest associations between small size at birth and lower muscle mass7-9. However no studies to date have included direct measures of muscle size. Recent technological advances in imaging allowed us to utilise peripheral quantitative computed tomography (pQCT) to directly measure muscle cross-sectional area in our clinic. We addressed the hypothesis that low birth weight was associated with smaller muscle size in older men and women participating in the Hertfordshire Cohort Study.
Methods
Study population
In the late 1990s, 3000 men and women aged 59 - 72 years were recruited to take part in the Hertfordshire Cohort Study which was designed to investigate the relationship between developmental, genetic and adult lifestyle influences on long term health, ageing and disease10. These individuals had historical records of early growth and had been traced through the National Health Service Central Registry. They participated in a baseline study which included a home interview at which trained nurses collected information including self-reported walking speed (response options: unable to walk; very slow; stroll at an easy pace; normal speed; fairly brisk; fast) as a marker of physical activity11 and social class. Men and women who were willing, subsequently attended a clinic for a number of investigations including measurement of grip strength12. A subgroup of 498 men and 468 women who were resident in East Hertfordshire also underwent dual x-ray absorptiometry (DXA) scans for assessment of bone mineral content and density13 .
Study group
In 2004-5, a follow-up study was performed in East Hertfordshire. The family doctors of participants in the baseline survey were contacted to ask if we could approach their patients again. Of the original 498 men and 468 women who had undergone a DXA scan, 8 had died, 6 had moved away, we were unable to obtain GP permission to approach 4 people, 47 were no longer on family doctor lists, and 17 were unavailable. Hence, we were able to invite 437 men and 447 women to take part in the follow-up study. Of these, 322 men (74%) and 320 women (72%) agreed to attend a follow-up clinic, and 313 (97%) of the men, and 318 (99%) of the women, also underwent a pQCT scan.
Follow-up clinic visit
At the follow-up clinic visit, a detailed health and lifestyle questionnaire was again administered to update the medical and social histories. Information was specifically collected on current smoking status and alcohol consumption. Anthropometry included measurement of height to the nearest 0.1cm using a Harpenden pocket stadiometer (Chasmors Ltd, London, UK) and weight to the nearest 0.1kg on a SECA floor scale (Chasmors Ltd, London, UK).
Determination of muscle size
Peripheral quantitative computed tomography (pQCT) was performed to determine the muscle cross-sectional area (CSA) of the non-dominant forearm and lower leg using a Stratec XCT-2000 instrument (Stratec, Pforzheim, Germany). Data presented here were derived from 2.3-mm-thick transverse scans obtained at a standard position 66% along the length of the humerus and tibia14 15. Previous studies have shown that this is the region with the largest outer diameter and little variability across individuals16. The total dose of radiation administered to the participants was 0.03 mSv (below that of a standard hand x-ray) and reproducibility, expressed as a coefficient of variation, has been reported as 1.93% for muscle cross-sectional area17. The muscle area and bone cortical area were separated by a built-in software algorithm18.
Ethics approval
The East and North Hertfordshire Ethics Committee granted ethics approval for the study and all participants provided written informed consent.
Statistical analysis
Weight was positively skewed and loge transformed to a normal distribution. Social class was coded from most recent full time occupation according to the 1990 OPCS standard occupational classification scheme for occupation and social class. Social class for ever-married women was coded from the husband’s most recent full time occupation. Variables were summarised using means and standard deviations (SD’s) or frequency and percentage distributions. Geometric means and standard deviations were calculated for weight. Relationships between potential adult determinants of forearm and calf muscle cross-sectional area (CSA) were explored using Pearson correlation coefficients and one-way analysis of variance (ANOVA). Mutually adjusted relationships were subsequently explored using linear regression. Height and weight were strongly correlated (r=0.39, p<0.0001 for men; r=0.40, p<0.0001 for women); to avoid multi-collinearity problems these variables were included as predictors in regression models in turn.
Pearson’s pairwise and partial correlation coefficients were used to describe the relationships between muscle CSA and birth weight without, and with, adjustment for the adult determinants of muscle CSA. For presentational purposes, means and confidence intervals of muscle CSA were derived according to quintiles of birth weight. However, statistical tests of association with muscle CSA were based on the continuously distributed birth weight variable throughout.
All analyses were carried out for men and women separately, using the Stata statistical software package, release 8.0.
Results
Participant characteristics
The characteristics of the study group are shown in Table 1.
Table 1. Participant characteristics.
| Mean (SD) | MEN (n=313) | WOMEN (n=318) |
|---|---|---|
| pQCT Forearm muscle cross-sectional area (mm2) | 4033 (518) | 2555 (370) |
| pQCT Calf muscle area cross-sectional (mm2) | 8035 (1204) | 6212 (981) |
| Birth weight (kg) | 3.5 (0.5) | 3.4 (0.5) |
| Age at pQCT scan (years) | 69.2 (2.5) | 69.5 (2.6) |
| Height (cm) | 173.7 (6.5) | 160.5 (6.1) |
| Weight (kg)* | 81.1 (1.2) | 70.5 (1.2) |
| Percentage | ||
| Walking speed Very slow | 3.5 | 3.8 |
| Stroll at an easy pace | 19.5 | 19.2 |
| Normal speed | 39.9 | 45.9 |
| Fairly brisk | 32.6 | 25.2 |
| Fast | 4.5 | 6.0 |
| Social class I-IIINM | 40.9 | 42.8 |
| IIIM-V | 54.0 | 57.2 |
| Unclassified | 5.1 | 0.0 |
| Alcohol ≤21/≤14 units per week men/women | 83.7 | 96.8 |
| >21/>14 units per week men/women | 16.3 | 3.2 |
| Smoker status Never | 38.3 | 63.2 |
| Ex | 53.4 | 31.4 |
| Current | 8.3 | 5.4 |
SD: Standard deviation
I-IIINM represents social classes one to three non-manual of the 1990 OPCS standard occupational classification scheme for occupation and social class. IIIM-V represents classes three manual to five.
Geometric mean and standard deviation
Adult determinants of muscle cross-sectional area
Univariate analyses showed that older age was associated with lower forearm muscle cross-sectional area (CSA) in men and women; associations between age and calf muscle area were similar, but not statistically significant. Adult size was strongly related to muscle CSA. Taller height and heavier weight were both significantly associated with increased forearm and calf muscle CSA in men and women. There were no associations between forearm or calf muscle CSA and walking speed or alcohol intake in men or women. Average forearm muscle CSA was higher among men of manual (4097mm2) compared with non-manual social class (3946mm2, p=0.05); there were no other associations between muscle CSA and social class in men or women. Men and women who were current smokers had higher forearm muscle CSA in comparison with those who were ex- or never-smokers (p=0.03 for men, p=0.02 for men). Current smoking was also associated with increased calf muscle area in women (p=0.05) but not men (p=0.73). These results are summarised in Table 2.
Table 2. Adult determinants of pQCT forearm and calf muscle cross-sectional area (CSA).
| Forearm muscle CSA | Calf muscle CSA | |||
|---|---|---|---|---|
| Correlation coefficient, p-value* | MEN | WOMEN | MEN | WOMEN |
| Age at pQCT scan (years) | -0.17 p=0.002 |
-0.14 p=0.01 |
-0.10 p=0.09 |
-0.09 p=0.12 |
| Height (cm) | 0.17 p=0.002 |
0.25 p<0.0001 |
0.17 p=0.004 |
0.25 p<0.0001 |
| Weight (kg) | 0.59 p<0.0001 |
0.52 p<0.0001 |
0.60 p<0.0001 |
0.57 p<0.0001 |
| Mean (SD), p-value** | ||||
| Walking speed Very slow | 4255 (535) | 2507 (435) | 8061 (1332) | 5885 (858) |
| Stroll at an easy pace | 4072 (570) | 2658 (392) | 8222 (1269) | 6422 (966) |
| Normal | 4011 (526) | 2516 (345) | 7898 (1163) | 6190 (957) |
| Fairly brisk | 4020 (484) | 2524 (356) | 8103 (1250) | 6085 (923) |
| Fast | 4001 (441) p=0.64 |
2691 (448) p=0.05 |
7992 (850) p=0.53 |
6431 (1385) p=0.23 |
| Social class I-IIINM | 3946 (492) | 2540 (410) | 7956 (1255) | 6132 (1022) |
| IIIM-V | 4097 (538) | 2566 (337) | 8078 (1160) | 6275 (946) |
| Unclassified | 4068 (410) p=0.05 |
- p=0.54 |
8196 (1280) p=0.61 |
- p=0.21 |
| Alcohol ≤21/≤14 units per week men/women | 4033 (535) | 2555 (372) | 8019 (1217) | 6201 (977) |
| >21/>14 units per week men/women | 4033 (421) p=0.99 |
2538 (342) p=0.89 |
8113 (1144) p=0.62 |
6367 (1052) p=0.60 |
| Smoker status Never | 3936 (507) | 2516 (360) | 8053 (1225) | 6121 (940) |
| Ex | 4082 (514) | 2589 (360) | 8052 (1179) | 6313 (1015) |
| Current | 4160 (539) p=0.03 |
2747 (445) p=0.02 |
7853 (1288) p=0.73 |
6655 (1136) p=0.05 |
Pearson correlation coefficient for each adult variable vs muscle cross-sectional area.
P-value from oneway ANOVA for muscle cross-sectional area vs adult variable
I-IIINM represents social classes one to three non-manual of the 1990 OPCS standard occupational classification scheme for occupation and social class. IIIM-V represents classes three manual to five.
Age, height or weight, social class and smoking were subsequently included as predictors of muscle CSA in mutually adjusted regression models; all of the significant univariate relationships described above remained significant after mutual adjustment. Hence, age, height or weight, social class and smoking were taken forward as adjustment variables for the analyses of birth weight in relation to muscle CSA. Adult height and weight were the strongest influences on muscle CSA so their individual effects on the relationship between birth weight and muscle CSA were ascertained prior to a multivariate model determining the effect of all the adult influences.
Relationship between birth weight and adult muscle cross-sectional area
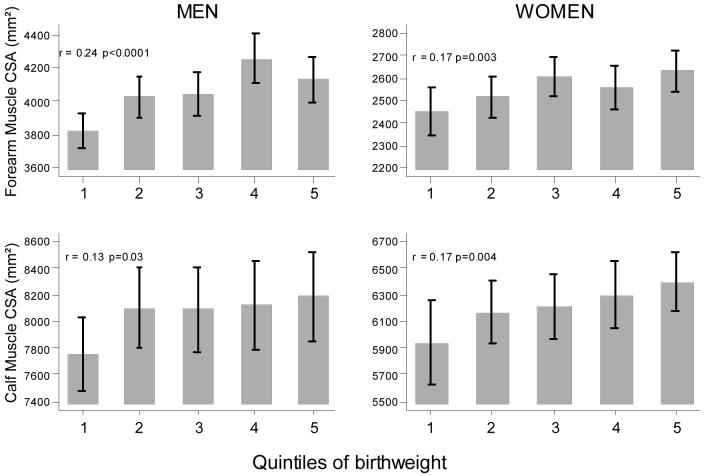
Lower birth weight was associated with reduced forearm muscle area in the men (r = 0.24 p < 0.0001, Figure 1) and women (r = 0.17 p =0.003, Figure 1). These associations were attenuated but remained significant after adjustment for height alone, or collectively for height, age, social class and smoking status, in the men but not the women (Table 3). There were similar but weaker associations between calf muscle area and birth weight in the men (r=0.13, p=0.03, Figure 1) and in the women (r=0.17, p=0.004, Figure 1) but these did not remain significant after adjustment for height, or for height, age, social class and smoking status (Table 3). Adjustment for adult weight alone fully explained the associations between lower birth weight and reduced muscle area at the forearm for women, and the calf for men and women (Table 3); the relationship between birth weight and calf muscle area in men was also substantially attenuated although remained statistically significant. Adjustment for age, social class and smoking status in addition to weight had little further impact on the results.
Figure 1. Relationships between birth weight and adult muscle cross-sectional area (CSA).
Foot note: Muscle cross-sectional area presented according to quintiles of birthweight; correlation coefficients (r) and p-values based on continuously distributed variables
Table 3. Relationships between birth weight and adult muscle cross-sectional area (CSA).
| Forearm muscle CSA | Calf muscle CSA | |||
|---|---|---|---|---|
| Correlation coefficient, p-value | MEN | WOMEN | MEN | WOMEN |
| Unadjusted | 0.24 p<0.0001 |
0.17 p=0.003 |
0.13 p=0.03 |
0.17 p=0.004 |
| Adjusted for adult size: | ||||
| Adjusted for height | 0.22 p<0.001 |
0.11 p=0.06 |
0.11 p=0.06 |
0.11 p=0.07 |
| Adjusted for weight | 0.17 p=0.003 |
0.07 p=0.25 |
0.02 p=0.72 |
0.05 p=0.42 |
| Adjusted for adult size and other determinants: | ||||
| Adjusted for height, age, social class and smoking | 0.19 p=0.001 |
0.08 p=0.14 |
0.10 p=0.09 |
0.08 p=0.16 |
| Adjusted for weight, age, social class and smoking | 0.14 p=0.01 |
0.05 p=0.40 |
0.03 p=0.67 |
0.03 p=0.63 |
Discussion
We have shown that directly measured muscle size in older men and women is positively associated with size at birth. This is the first study investigating developmental influences on sarcopenia to utilise direct imaging of muscle with pQCT. However the findings are consistent with previous studies showing relationships between poor early growth and lower lean or non-fat mass as estimated by urinary creatinine excretion, anthropometry and dual x-ray absorptiometry7-9. There was evidence of regional variation in the strength of the associations with a stronger correlation between muscle size and birth weight at the forearm than the calf. This might reflect the greater contribution of adult influences such as voluntary activity to muscle size in the lower limb.
There may be a number of explanations for the relationship between birth weight and adult muscle size. It could represent a chance finding although the consistency in findings across studies using different methodologies to characterise muscle suggests a true association. The association was largely explained by measures of adult size, particularly adult weight, and tracking of muscle size and weight from early life could underlie a causal association. Support for this comes from a number of studies linking early growth to muscle size in children and young people19-23 and recognition that the ageing muscle phenotype reflects not only loss of muscle in later life but also the peak reached in early adulthood24.
Studies in a wide range of animal models have shown that early environmental influences, such as prenatal and postnatal nutrition, are important determinants of early muscle growth and development5;6;25-32. It appears that muscle fibre number is largely complete by the time of birth suggesting that prenatal influences may be particularly important for long-term muscle quantity and possibly quality33. There has been one human metabolic study linking small size at birth with alteration in adult muscle fibre composition in young adults34 and these findings now need to be replicated in older men and women.
Our study has a number of potential limitations. There have been losses to follow up both in the tracing process and through gaining consent to take part in the study. However we have been able to characterize those who did not take part in a number of ways10. There were no substantial differences in birth weight or weight at one year between those who were traced and eligible to take part and those who chose not to. There were also no differences in terms of early life measurements between those who attend the home visit but chose not to attend a clinic appointment. Furthermore there were no statistical differences in social class distribution in the home interviewed participants who did not attend clinic. However there was evidence for a healthy participant bias in those who attended clinic. For example they were less likely to smoke or drink. However, our comparisons are internal; unless the relationship between early size, growth, and muscle size differed between those who did and did not take part in the study, no bias should have been introduced.
We present first evidence that directly measured muscle size in older men and women is associated with size at birth. This may reflect tracking of muscle size and is consistent with the aging muscle phenotype representing peak muscle attained as well as subsequent loss. This is important because it suggests that benefit may be gained from taking a life course approach both to understanding the aetiology of sarcopenia and to developing effective interventions.
Acknowledgements
This work was supported by the Medical Research Council and the University of Southampton UK.
References
- 1.Sayer AA, Cooper C, Evans JR, Rauf A, Wormald RP, Osmond C, et al. Are rates of ageing determined in utero? Age Ageing. 1998;27:579–83. doi: 10.1093/ageing/27.5.579. [DOI] [PubMed] [Google Scholar]
- 2.Kuh D, Bassey J, Hardy R, Aihie Sayer A, Wadsworth M, Cooper C. Birth weight, childhood size, and muscle strength in adult life: evidence from a birth cohort study. Am J Epidemiol. 2002;156:627–33. doi: 10.1093/aje/kwf099. [DOI] [PubMed] [Google Scholar]
- 3.Sayer AA, Syddall HE, Gilbody HJ, Dennison EM, Cooper C. Does sarcopenia originate in early life? Findings from the Hertfordshire Cohort Study. J Gerontol. 2004;59A:930–4. doi: 10.1093/gerona/59.9.m930. [DOI] [PubMed] [Google Scholar]
- 4.Yliharsila H, Kajantie E, Osmond C, Forsen T, Barker DJ, Eriksson JG. Birth size, adult body composition and muscle strength in later life. Int J Obes.(Lond) 2007 doi: 10.1038/sj.ijo.0803612. In press. [DOI] [PubMed] [Google Scholar]
- 5.Maltin CA, Delday MI, Sinclair KD, Steven J, Sneddon AA. Impact of manipulations of myogenesis in utero on the performance of adult skeletal muscle. Reproduction. 2001;122:359–74. doi: 10.1530/rep.0.1220359. [DOI] [PubMed] [Google Scholar]
- 6.Costello P, Rowlerson A, Braddick L, Burrage D, Cooper C, Hanson MA, Aihie Sayer A, Green LR. Fetal skeletal muscle fibre number and type are altered by early and late gestation maternal undernutrition in sheep. Proc Physiol Soc. 2006;3:C116. [Google Scholar]
- 7.Phillips DIW. Relation of fetal growth to adult muscle mass and glucose tolerance. Diab Med. 1995;12:686–90. doi: 10.1111/j.1464-5491.1995.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 8.Gale CR, Martyn CN, Kellingray S, Eastell R, Cooper C. Intrauterine programming of adult body composition. J Clin Endocrinol Metab. 2001;86:267–72. doi: 10.1210/jcem.86.1.7155. [DOI] [PubMed] [Google Scholar]
- 9.Sayer AA, Syddall HE, Dennison EM, Gilbody HJ, Duggleby SL, Cooper C, et al. Birth weight, weight at one year and body composition in older men: findings from the Hertfordshire Cohort Study. Am J Clin Nutr. 2004;80:199–203. doi: 10.1093/ajcn/80.1.199. [DOI] [PubMed] [Google Scholar]
- 10.Syddall HE, Sayer AA, Dennison EM, Martin HJ, Barker DJ, Cooper C. Cohort Profile: The Hertfordshire Cohort Study. Int.J Epidemiol. 2005 doi: 10.1093/ije/dyi127. [DOI] [PubMed] [Google Scholar]
- 11.Bendall MJ, Bassey EJ, Pearson MB. Factors affecting walking speed of elderly people. Age Ageing. 1989;18:327–32. doi: 10.1093/ageing/18.5.327. [DOI] [PubMed] [Google Scholar]
- 12.Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch.Phys.Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- 13.Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatr.Res. 2005;57:582–6. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- 14.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl.Physiol. 2003;95:1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr.Soc. 2007;55:400–6. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rittweger J, Beller G, Ehrig J, Jung C, Koch U, Ramolla J, et al. Bone-muscle strength indices for the human lower leg. Bone. 2000;27:319–26. doi: 10.1016/s8756-3282(00)00327-6. [DOI] [PubMed] [Google Scholar]
- 17.Neu CM, Rauch F, Rittweger J, Manz F, Schoenau E. Influence of puberty on muscle development at the forearm. Am J Physiol Endocrinol.Metab. 2002;283:E103–E107. doi: 10.1152/ajpendo.00445.2001. [DOI] [PubMed] [Google Scholar]
- 18.Schoenau E, Neu CM, Mokov E, Wassmer G, Manz F. Influence of puberty on muscle area and cortical bone area of the forearm in boys and girls. J Clin Endocrinol Metab. 2000;85:1095–8. doi: 10.1210/jcem.85.3.6451. [DOI] [PubMed] [Google Scholar]
- 19.Ford GW, Kitchen WH, Doyle LW. Muscular strength at 5 years of children with a birthweight under 1500 g. Aust.Paediatr.J. 1988;24:295–6. doi: 10.1111/j.1440-1754.1988.tb01366.x. [DOI] [PubMed] [Google Scholar]
- 20.Rogers M, Fay TB, Whitfield MF, Tomlinson J, Grunau RE. Aerobic capacity, strength, flexibility, and activity level in unimpaired extremely low birth weight (<or=800 g) survivors at 17 years of age compared with term-born control subjects. Pediatrics. 2005;116:e58–e65. doi: 10.1542/peds.2004-1603. [DOI] [PubMed] [Google Scholar]
- 21.Martorell R, Ramakrishnan U, Schroeder DG, Melgar P, Neufeld L. Intrauterine growth retardation, body size, body composition and physical performance in adolescence. Eur.J Clin Nutr. 1998;52(Suppl 1):S43–S52. [PubMed] [Google Scholar]
- 22.Sachdev HS, Fall CH, Osmond C, Lakshmy R, Dey Biswas SK, Leary SD, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–66. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 23.Inskip HM, Godfrey KM, Martin HJ, Simmonds SJ, Cooper C, Aihie Sayer A. Size at birth and its relation to muscle strength in young adult women. J Int Med. 2007;262:368–374. doi: 10.1111/j.1365-2796.2007.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aihie Sayer A, Cooper C. Aging, sarcopenia and the life course. Rev Clin Gerontol. 2007 In press. [Google Scholar]
- 25.Zhu MJ, Ford SP, Means WJ, Hess BW, Nathanielsz PW, Du M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol. 2006;575:241–50. doi: 10.1113/jphysiol.2006.112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quigley SP, Kleemann DO, Kakar MA, Owens JA, Nattrass GS, Maddocks S, et al. Myogenesis in sheep is altered by maternal feed intake during the peri-conception period. Anim Reprod.Sci. 2005;87:241–51. doi: 10.1016/j.anireprosci.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Fahey AJ, Brameld JM, Parr T, Buttery PJ. The effect of maternal undernutrition before muscle differentiation on the muscle fiber development of the newborn lamb. J Anim Sci. 2005;83:2564–71. doi: 10.2527/2005.83112564x. [DOI] [PubMed] [Google Scholar]
- 28.Bayol S, Jones D, Goldspink G, Stickland NC. The influence of undernutrition during gestation on skeletal muscle cellularity and on the expression of genes that control muscle growth. Br.J Nutr. 2004;91:331–9. doi: 10.1079/BJN20031070. [DOI] [PubMed] [Google Scholar]
- 29.Lefaucheur L, Ecolan P, Barzic YM, Marion J, Le Dividich J. Early postnatal food intake alters myofiber maturation in pig skeletal muscle. J Nutr. 2003;133:140–7. doi: 10.1093/jn/133.1.140. [DOI] [PubMed] [Google Scholar]
- 30.Greenwood PL, Hunt AS, Hermanson JW, Bell AW. Effects of birth weight and postnatal nutrition on neonatal sheep: II. Skeletal muscle growth and development. J Anim Sci. 2000;78:50–61. doi: 10.2527/2000.78150x. [DOI] [PubMed] [Google Scholar]
- 31.Dwyer CM, Stickland NC, Fletcher JM. The influence of maternal nutrition on muscle fiber number development in the porcine fetus and on subsequent postnatal growth. J Anim Sci. 1994;72:911–7. doi: 10.2527/1994.724911x. [DOI] [PubMed] [Google Scholar]
- 32.Ward SS, Stickland NC. The effect of undernutrition in the early postnatal period on skeletal muscle tissue. Br J Nutr. 1993;69:141–50. doi: 10.1079/bjn19930016. [DOI] [PubMed] [Google Scholar]
- 33.Dauncey MJ, Gilmour RS. Regulatory factors in the control of muscle development. Proc Nutr Soc. 1996;55:543–59. doi: 10.1079/pns19960047. [DOI] [PubMed] [Google Scholar]
- 34.Jensen CB, Storgaard H, Madsbad S, Richter EA, Vaag AA. Altered skeletal muscle fiber composition and size precede whole-body insulin resistance in young men with low birth weight. J Clin Endocrinol Metab. 2007;92:1530–4. doi: 10.1210/jc.2006-2360. [DOI] [PubMed] [Google Scholar]