Abstract
Allocation of attentional resources to portions of the available sensory input can be regulated by bottom-up processes, i.e. spontaneous orientation towards an oncoming stimulus (stimulus-driven attention), and by top-down processes, i.e. intentionally and driven by knowledge, expectation and goals. The present study aimed at advancing the understanding of brain networks mediating bottom-up and top-down control of visuospatial attention by employing a paradigm that parametrically varied demands on these two processes. Spatial predictability of peripheral targets was parametrically varied by centrally cueing one, two, three or four of four possible locations. Reaction time decreased linearly with more precise valid cueing of the target location and increased with more precise invalid cueing. Event-related functional magnetic resonance imaging (fMRI) enabled measurement of blood oxygenation level-dependent (BOLD) responses to cues and to targets. A mostly left-hemispheric network consisting of left intraparietal sulcus, inferior and superior parietal lobule, bilateral precuneus, middle frontal gyri including superior frontal sulci, and middle occipital gyri displayed BOLD responses to cues that increased linearly with more precise spatial cueing, indicating engagement by top-down spatial selective attention. In contrast, bilateral temporoparietal junction, cingulate gyrus, right precentral gyrus and anterior and posterior insula, bilateral fusiform gyri, lingual gyri and cuneus displayed BOLD responses to targets that increased with their spatial unpredictability, indicating engagement by stimulus-driven orienting. The results suggest two largely dissociated neural networks mediating top-down and bottom-up control of visuospatial selective attention.
Introduction
Visual information available at any one moment in time usually exceeds the input limitations of the visual processing system. Selective attention, regulated by specialized brain systems (Posner and Petersen 1990), enables focusing on those portions of sensory input that are relevant for behavioral organization. Attentional resources can be selectively allocated to specific stimulus features and/or spatial locations. Such allocation is thought to be controlled by “bottom-up” and “top-down” processes. Bottom-up or exogenous attentional control is stimulus-driven, i.e. attention is spontaneously oriented towards an oncoming stimulus. Top-down or endogenous attentional control, by contrast, is intentional and cognitively driven, i.e. directed by knowledge, expectation and current goals (Desimone and Duncan, 1995; Egeth and Yantis, 1997).
Most neuroimaging studies of visuospatial attention have employed covert orienting paradigms where participants respond to targets presented in the left or right periphery of a fixation point. Central informative cues enable endogenous orienting to the expected target location, while peripheral uninformative cues instigate reflexive shifts of attention toward them. With the exception of two reports of frontal activation that showed selectivity for endogenous orienting (Corbetta et al., 1993; Rosen et al., 1999), studies that did not differentiate between cue- and target-induced activity identified largely overlapping activation maps under both conditions, suggesting a common neural network for endogenous and exogenous orienting of attention in space (Nobre et al., 1997; Rosen et al., 1999; Kim et al., 1999; Peelen et al., 2004). However, the difficulty dissociating neural substrates may be due to the interdependent nature of endogenous and exogenous attention and insufficient behavioral dissociation. As such, endogenous orienting towards a cued location may weaken but never abolishes stimulus-driven orienting towards the target when it is presented at that location (Egeth and Yantis, 1997). Conversely, after attention is captured by an uninformative peripheral cue, attention is reoriented endogenously back to the center. Subjects may also employ endogenous control processes to prevent involuntary orienting to such cues. All trials thus encompass both endogenous and exogenous control processes, and alleged dissociations are based on assumptions about their relative contributions.
Several fMRI studies have been designed to dissociate neural responses to cue versus target presentation, enabling separate measurement of activity related to endogenous orienting and to target detection, processing and response. In the expectation period following cue and preceding target onset, activation was seen mostly in intraparietal sulcus (IPS), inferior and superior parietal lobule (IPL, SPL), as well as superior and middle frontal gyri, and frontal eye fields (FEF) (Kastner et al., 1999; Corbetta et al., 2000, 2002; Hopfinger et al., 2000). A recent study (Kincade et al., 2005) compared BOLD responses to central informative and peripheral uninformative cues separately in the expectation and target period. Activations in IPS and FEF were larger in response to endogenous than exogenous or neutral cues. However, no region except fusiform and inferior temporal gyri displayed differential activation by exogenous cues. Regions activated by target presentation generally differed between studies, possibly related to diverging response requirements and the fact that the target phase represents diverse sensory, cognitive and motor processes. Demands on stimulus-driven orienting in the target phase are diminished by cue-induced preparatory shifts of attention, but regional involvement in exogenous orienting has been deduced from stronger responses to targets in invalid than valid trials. The temporoparietal junction, precuneus, FEF, anterior insula and supplementary motor area displayed this pattern (Corbetta et al., 2000; Kincade et al., 2005), but it is unclear to what extent this reflects particular demands on stimulus-driven reorienting or on other processes such as error detection or disengagement of attention from invalidly cued locations.
Parametric manipulation of exogenous and endogenous attentional demands against an identical task background would allow more effective cognitive dissociation. This design avoids comparing qualitatively different task conditions, such as trials with informative central vs. uninformative peripheral cues or valid vs. invalid cues, which do not tax top-down or bottom-up attentional processes selectively, differ in more than just these processes, and thus do not allow clear differentiation. The present study employed a novel attention task that systematically varied the relative contribution of each of the two processes to performance and was able to identify two largely independent networks responsible for bottom-up and top-down processes of spatial selective attention.
Materials and Methods
Participants
Twenty-three right-handed healthy individuals (14 females) participated in the study; one female subject was excluded due to excessive eye-movement (see below). The remaining 22 subjects were aged 19-44 years (mean±stdev 27.5±7.55 years). All participants were non-smokers. Subjects were recruited from the general population through newspaper advertising, flyers and referrals and gave informed consent for a protocol approved by the NIDA-IRP Institutional Review Board. Subjects were screened for major medical illnesses, claustrophobia, history of neurological or psychiatric disorders and drug abuse, pregnancy and appropriateness for MRI. A urine sample was collected and assessed for common drugs of abuse (TRIAGE®).
Procedure
The protocol required three separate visits. During the first visit, participants gave informed consent and were trained on two cognitive tasks (one reported elsewhere), initially on a bench computer and then in a mock scanner that mimicked all properties of the MRI scanner. Training in the mock scanner was equal in length to when the tasks were performed in the real scanner. During performance of the current task, subjects were repeatedly reminded to keep their eyes focused on the central fixation cross.
Sessions 2 and 3 were identical and served as time controls for a pharmacological experiment not reported here. Prior to both MR scans, participants were tested for recent drug use (TRIAGE®) and for alcohol intake or smoking via breath analysis; a pregnancy test was given to female participants. Subjects then received a 10 min reminder task training on a bench computer, during which their eye-position was recorded. Subsequent MR scans started with a brief central executive task (Ross et al., 2005). Eight blocks of the Spatial Attentional Resource Allocation Task were then performed, separated by one-minute rest periods, followed by anatomical scans.
The Spatial Attentional Resource Allocation Task (SARAT)
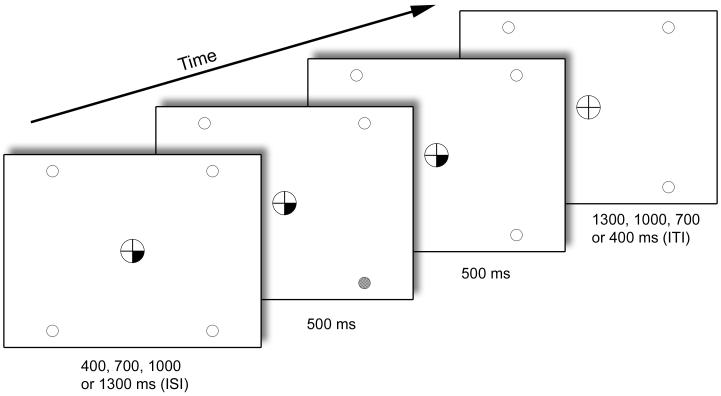
Participants were required to keep their eyes fixated on a central circle containing a fixation cross and to detect a target signal (500 ms) that could occur at any of four peripheral locations marked by empty circles (Figure 1). The central circle and the target circles remained on display throughout runs. With eyes pointed at the center of the fixation cross, the outer edge of the central circle was positioned at 1.3-1.5° and the target locations at 10-12.5° of visual angle. Targets consisted of a peripheral circle filling with a checkerboard of grey and white squares of 3×3 pixels each. Two different target intensities (high: grey squares were 80% grey; low: grey squares were 20% grey) were tested. Upon occurrence of a target, subjects were instructed to press a button with their right index finger as quickly as possible.
Figure 1.
Components of a single target trial in the SARAT.
Onset of a central cue preceded target onset by a variable SOA of 400, 700, 1000 or 1300 ms. The target was presented for 500 ms in the continuing presence of the cue, which remained on display until 500 ms after target offset. Only screen background was then presented for an intertrial interval (ITI) that varied in length such that total trial duration was always 2700 ms. No-target trials differed only in that no target occurred. One, two, three or all four target locations could be cued at the same time, thus varying the predictability of the target.
Cues were displayed in the central circle for a variable stimulus-onset-asynchrony (SOA) of 400, 700, 1000 or 1300 ms prior to target onset and remained on display until 500 ms after target termination. The temporal unpredictability of targets required subjects to continuously allocate attention to cued locations in anticipation of a target. For all analyses reported in the main body of the paper, trials were averaged across SOAs. Cues consisted of quarters of the fixation circle turning black with their location (12-3, 3-6, 6-9 or 9-12 o’clock) indicating the probable location of the peripheral target. One, two, three or four quarters could turn black simultaneously. Thus, predictability of the target location varied across trials. Fewer cued locations equaled more precise advance information about the target location, while more cued locations made the target location more unpredictable. In trials with two and three cued locations, all possible location pairings were presented with roughly equal probability in each run. The cue provided invalid information in 20% of trials with one, two or three cued locations.
The information conveyed by the central cue was expected to induce endogenous orienting of attention. There are reports that non-predictive central cues that directly indicate a spatial direction, such as arrows, can also induce some degree of reflexive orienting (e.g. Ristic and Kingstone, in press), which would be a potential confound. In the current paradigm, the cues themselves have no spatial direction. They are located only minimally closer to cued than uncued target locations, being part of the same central fixation circle, and thus are unlikely to trigger any automatic orienting, although this has not been tested experimentally.
In some trials, the cue was not followed by a target (“no-target trials”). Valid trials with high-intensity targets, valid trials with low-intensity targets and no-target trials were presented with equal frequency. All trial types were randomized within runs. Since no-target trials occurred unpredictably, attention would be allocated to cued locations in anticipation of a target, allowing separate analysis of typical BOLD responses in the expectation period following cue- and preceding target-onset. In no-target trials, the cue assumed the same temporal parameters as in target trials, including the variable SOA and the continuous display for another 1000 ms, i.e. until 500 ms after the target would have disappeared. Subjects may have been able to estimate the time intervals to some extent and may have stopped expecting a target after a period noticeably exceeding the longest SOA. To test for possible confounds of the BOLD data related to premature cessation of attentional resource allocation, all Regions Of Interest (ROIs) identified by analysis of no-target trials (see below) were analyzed for effects of SOA as detailed in the Supplementary materials. These analyses indicated that the length of time that attention was being cued did not modulate the cue effect in no-target trials.
To create sufficient temporal jitter for event-related analysis, no-event trials where no cues or targets were presented were randomly interspersed throughout the task. Trial duration was always 2.7 s (Figure 1). There were 81 trials in each of the eight runs; 32 valid target trials, 16 no-target trials, 6 invalid target trials and 27 no-event trials, resulting in run duration of 3:39 min.
The basic analysis assumption was that more precise advance information about the target location would intensify top-down processes of spatial attentional selection in preparation for target detection. Brain regions involved in the endogenous regulation of visuospatial selective attention were thus expected to display incremental activation with fewer cued locations in no-target trials that reflect only cue-induced processes. By contrast, bottom-up processes are engaged when attention is drawn to a previously unattended location by the occurrence of a stimulus. Increased spatial unpredictability conferred by more cued locations augments demands on the spontaneous orienting to targets upon their onset. Thus, regional activation related to stimulus-driven processes of attention was assumed to increase with more cued locations in target trials. Since such effect would reflect modulation of target-induced activity by the cue, it would be expected only in target trials, not in no-target trials.
Magnetic resonance imaging
Scanning was performed on a 3 Tesla Siemens Allegra scanner (Erlangen, Germany). Whole brain functional EPI images were acquired for measurement of T2*-weighted BOLD effects (4mm sagital slices, 64×64 matrix, FOV=22×22cm, TR=2.7s, TE=27ms, FA=85°). In each scanning session, a whole-brain sagital T1-weighted structural image (MPRAGE) was acquired for anatomical reference (1mm3 isotropic voxels, TR=2.5s, TE=4.38 ms, FA=8°).
Eye-tracking
Eye-position on the screen was recorded using a remote eye-tracking system (IVIEW, Sensomotoric Instruments Inc, Needham, Massachusetts) during performance of the 10 min refresher training that preceded each MR scan. The purpose was to verify that the task was not performed by re-focusing the gaze to where the target was expected. This could have resulted in eye-movement related brain activation changing as a function of the number of cued locations and confounding the contrast of interest. The eye-tracker consisted of a video camera and infrared light source pointed at the subject’s left eye. The percentage of time spent fixating within a central circle of twice the radius of the fixation circle (visual angle less than 3° from the center of fixation into any direction) was calculated. Eye-tracking was not performed in eight subjects due to equipment unavailability.
Analysis of behavioral data
Data from the two scan sessions were analyzed. Target trials with a reaction time below 200 ms or above 1400 ms were considered outliers and excluded from analyses. Reaction times were expressed as averages for each stimulus condition. Omission errors were expressed as the percentage of target trials in each stimulus condition where no response was recorded.
Reaction time and omission errors were analyzed separately for valid and invalid trials by 3-factor ANOVA with cue (1, 2, 3, 4 validly cued locations; 1, 2, 3 invalidly cued locations), target intensity (high, low) and scan session (1, 2) as within-subject factors followed by Bonferroni-adjusted paired t-tests. An additional 2-factor ANOVA was performed on reaction time data with cue (1, 2, 3) and validity (valid, invalid) as within-subject factors.
Analysis of fMRI data
Data were processed using the AFNI software package version 2.55j (Cox, 1996). Motion correction was performed by volume registering each 3D volume to a base volume. The time series was then analyzed by voxel-wise multiple regression; regressors were convolved with a model hemodynamic response function and its temporal derivative. Trials were always modeled in their entirety. Regressors corresponded to 18 different trial types (1/2/3/4 validly cued locations * high/low/no target + 1/2/3 invalidly cued locations * high/low target intensity) and to the six motion parameters as nuisance regressors to help account for residual motion. If applicable, one additional nuisance regressor accounted for target trials in which no response was registered and trials with reaction times below 200 ms or above 1400 ms that were not analyzed. For each subject and each test session, the voxel-wise average amplitude of signal change (β-value) produced by each trial type was determined relative to baseline. This excluded invalid trials due to insufficient trial numbers. The resulting activation maps were resampled to a higher (1μl) resolution and converted to a standard stereotaxic coordinate system (Talairach and Tournoux, 1988). Subsequently, they were spatially blurred using a Gaussian 4.2 mm FWHM isotropic kernel.
Functional regions of interest (ROIs) were derived by two second-level voxel-wise multiple linear regression analyses across subjects, performed on β-values for each trial type. In both analyses, the number of cued locations was the regressor of interest. Thus, all ROIs reflected clusters of voxels whose activation was significantly linearly dependent on the number of cued locations. One analysis was performed on valid target trials and included scan session and target intensity as additional variables. The second analysis was performed on no-target trials and included scan session as additional variable. A voxel-wise threshold of p<0.005 was applied to the F-test activation maps which, combined with a minimum cluster volume threshold of 224μl, yielded an overall false positive p<0.05 as determined by Monte Carlo simulation. One cluster identified by analysis of target trials encompassed both right anterior insula and precentral gyrus and another cluster both right posterior insula and precentral gyrus. These clusters were artificially separated by removing one (X,Y,Z = 37, 3, 18 mm) and two 1μl voxels (X,Y,Z = 41, 7&8, 4 mm) of functional data, respectively. One cluster identified by analysis of no-target trials was located in white matter with some spread into ventricle and thalamus. Its shape was filamentary, partly connected by single voxels. It was not further analyzed because it was not anatomically interpretable.
Activation values were averaged across voxels within each ROI for each subject, session, number of cued locations and, for target trials, target intensity. These average values were subjected to repeated measures ANOVA with session, number of cued locations and, for target trials, target intensity as within-subject factors. In no-target trials, changes in signal with the number of cued locations unequivocally reflect modulation of processes induced by cue-presentation. By contrast, cue-dependent signal changes in target trials may reflect modulation of processes induced by cue- or by target-presentation. In order to determine whether these activation changes reflected modulation of cue- or target-related activity, β-values in no-target trials were averaged within ROIs derived from regression analysis of target trials. These values were analyzed for an effect of the number of cued locations by 1-factor repeated measures ANOVA.
Results
Eye-tracking data
Fourteen out of 15 participants for whom eye-position was recorded spent 98.2±2.8% of the time that eyes were directed at the screen fixating no further than 3° from its center. One participant in one session spent more than 25% of the time fixating outside the 3° radius, indicating insufficient ability or willingness to maintain fixation throughout runs, and was excluded from further analysis. However, no participant displayed any indication that fixations outside this radius were systematically oriented towards target locations.
Behavioral data
Valid trials
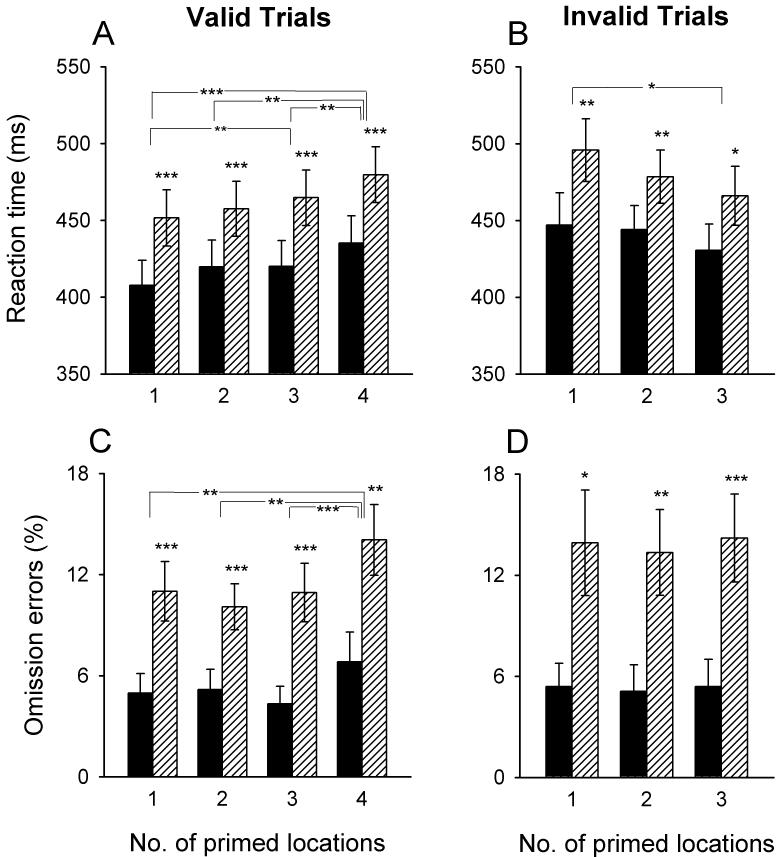
Figure 2A illustrates that reaction time became faster as the number of cued locations decreased, i.e. as the spatial predictability of targets increased. Reaction time was also faster for more intense target signals. This was confirmed by significant main effects for both the number of cued locations [F(3,63)=19.4, P<0.001] and target intensity [F(1,21)=148.0, P<0.001] in 3-way ANOVA with cue, target and scan session as within-subject factors. Neither the session main effect nor any of the interactions were significant.
Figure 2.
Task performance as a function of the number of cued target locations.
Bars represent means (±SEM) of 44 observations (22 subjects, each tested twice). Black bars represent high-intensity targets and striped bars low-intensity targets. Conditions where performance differed significantly between target intensities or between the number of cued locations are marked (*adjusted P<0.05, **adjusted P<0.01, ***adjusted P<0.001; paired t-tests).
For the percentage of omission errors (Figure 2C), main effects were again significant for the number of cued locations [F(3,63)=10.3, P<0.001] and target intensity [F(1,21)=30.8, P<0.001]. Omissions were significantly higher with four cued locations than in any other cue condition and were also higher at the lower target intensity. Neither the session main effect nor any of the interactions were significant.
Invalid trials
Figure 2B illustrates a relationship between the number of cued locations and reaction time opposite to that observed for valid trials, i.e. more precise cueing, when incorrect, resulted in slower reaction time. A significant main effect for cue in 3-factor ANOVA [F(2,42)=3.22, P=0.05] confirmed this. Reaction time was again faster for the high than for the low target intensity [F(1,21)=22.4, P<0.001]. Also the session main effect was significant [F(1,21)=4.96, P<0.05], resulting from reaction time being on average 14 ms faster in session 2 than session 1 (data not shown). None of the interactions were significant. Finally, more omission errors were made in invalid trials with low as compared with high target intensity [F(1,21)=21.4, P<0.001] (Figure 2D). No other significant effects were seen.
Since reaction time in valid and invalid trials was modulated by the number of cued locations in opposite ways, it was desirable to confirm statistically that the cue effect differed depending on validity. A significant cue × validity interaction [F(2,42)=7.11, P<0.01] was obtained in 2-factor ANOVA for repeated measures performed on valid and invalid trials with one, two and three cued locations, averaged across session and target intensity. The analysis also yielded a significant main effect of validity [F(1,21)=43.8, P<0.001], reflecting overall longer reaction times for invalid than valid trials (validity effect). Specifically, paired t-tests identified a validity effect in trials with one [T(21)=5.91, P<0.001] and two [T(21)=2.83, P=0.01] but not in trials with three cued locations [T(21)=1.57, P=0.13].
fMRI data
Bottom-up processes of spatial attentional resource allocation
Linear regression analysis was performed on valid target trials with the aim of identifying brain regions that displayed incremental BOLD responses to targets with more cued locations, i.e. with decreasing predictability of target locations.
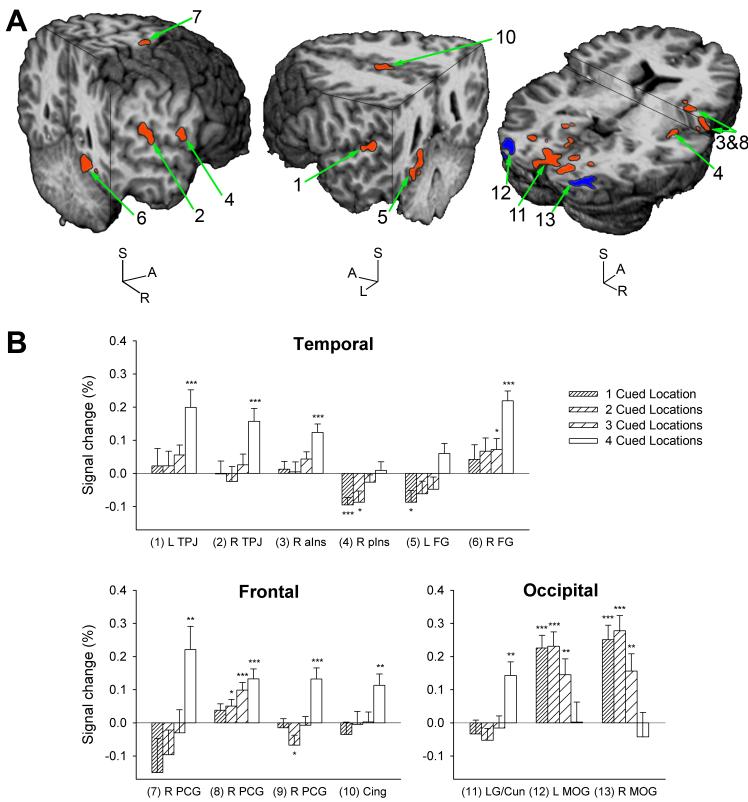
ROIs whose activation in target trials varied as a function of the number of cued locations are shown in Figure 3A and listed in Table 1. ROIs were identified in the left and right superior temporal gyrus (STG), bordering and, in the right hemisphere, extending into the inferior parietal lobule (IPL). These regions will hereafter be referred to as temporoparietal junction (TPJ). Three regions were located in the right precentral gyrus (PCG). Further ROIs were located in the right anterior and posterior insula, the posterior part of the anterior cingulate gyrus (midcingulate gyrus), the left and right fusiform gyrus, and in occipital cortex. Of the three occipital ROIs, two were located in left and right middle occipital gyrus (MOG), and one bilaterally, anterior and medial to the MOG regions. This large but fragmented region spanned lingual gyrus and cuneus in both striate and extrastriate cortex. As can be seen from Figure 3B, most ROIs displayed increasing activation with more cued locations; only left and right MOG decreased in activation. In the right posterior insula and left fusiform gyrus, the increments consisted mostly of sequentially less deactivation. In most other regions, a significant response was evoked only when four locations were cued, i.e. when the target location was completely unpredictable.
Figure 3.
Brain responses to cue precision in target trials: exogenous attentional orienting.
A) Group activation maps are overlaid onto a rendered anatomical scan in Talairach space. In regions drawn in red, BOLD signal increased with incremental spatial unpredictability of targets, a pattern of activation consistent with engagement by stimulus-driven attentional resource allocation. Regions drawn in blue displayed the opposite pattern, i.e. BOLD signal increased with fewer cued locations. Only the middle occipital gyri displayed this pattern in target trials. The numbers correspond to ROIs in Table 1 and graphs in Figure 3B. Cuts are made at 53 mm right, 53 mm posterior and 65 mm superior (left brain), at 54 mm left, 44 mm posterior and 41 mm superior (middle brain), and at 1 mm posterior and 1 and 12 mm superior to the anterior commissure (right brain) in the AC-PC aligned brain.
B) BOLD signal amplitude is shown for ROIs that displayed a linear effect of the number of cued locations in target trials. Bars are means (+SEM) of 22 participants. Conditions where the BOLD signal differed from zero are marked (*P<0.05, **P<0.01, ***P<0.001; one-sample t-tests). (1) L TPJ and (2) R TPJ = left and right temporoparietal junction; (3) R aIns = right anterior insula; (4) R pIns = right posterior insula; (5) L FG and (6) R FG = left and right fusiform gyrus; R PCG = right precentral gyrus, (7) superior region, (8) middle region, (9) inferior region (not shown); (10) Cing = Cingulate gyrus; (11) LG/Cun = lingual gyrus/cuneus; (12) L MOG and (13) R MOG = left and right middle occipital gyrus.
Table 1.
Brain regions identified by analysis of target trials
| Brain Region | Side | Center of Mass (mm) X, Y, Z | Brodmann Area(s) | Volume (μl) | |
|---|---|---|---|---|---|
| 1 | Superior temporal gyrus, temporoparietal junction | L | -54.5, -29, 12.1 | 41, 42 | 539 |
| 2 | Superior temporal gyrus, temporoparietal junction | R | 53.5, -27.2, 16.5 | 40, 41 | 300 |
| 3 & 8 | Anterior insula & precentral gyrus | R | 36.7, 3.2, 17.6 | 13 & 6 | 808 |
| 4 & 9 | Posterior insula & precentral gyrus | R | 46.5, -5.5, 6.3 | 13 & 6 | 582 |
| 5 | Fusiform gyrus, into parahippocampal gyrus | L | -23.5, -45.4, -9.3 | 19, 37 | 680 |
| 6 | Fusiform gyrus | R | 24.1, -53, -9.3 | 19, 37 | 741 |
| 7 | Precentral gyrus | R | 26.3, -13.4, 65.1 | 6 | 240 |
| 10 | Midcingular gyrus | B | -3.3, -7.9, 42 | 24 | 318 |
| 11 | Occipital cortex: lingual gyrus, cuneus | B | 1.5, -78.5, 1.5 | 17, 18 | 9625 |
| 12 | Middle occipital gyrus | L | -31.4, -88.1, 0.7 | 18 | 907 |
| 13 | Middle occipital gyrus | R | 28.8, -88.6, 0.7 | 18 | 1110 |
Regions in Talairach space where the BOLD signal intensity varied linearly with the number of cued locations. All ROIs except the middle occipital gyri increased in activation with less precise spatial cueing, an effect that was dependent upon the presentation of a target signal. L = left, R = right, B = bilateral. Regions 3 and 8 as well as regions 4 and 9 were artificially separated by removing one and two voxels of functional data, respectively.
Three-factor ANOVA for repeated measures confirmed a significant main effect of the number of cued locations for all brain regions (F>6.12, P<0.001). This cue effect interacted with scan session in anterior and posterior insula, one region in precentral gyrus and right fusiform gyrus [F(3,63)>2.81, P<0.05]. However, in 1-factor ANOVA, each of these regions displayed a significant main effect of the number of cued locations and a significant linear contrast in both sessions. The cue effect did not interact with target intensity in any ROI.
Cross-over analysis
To determine if the effect of cue precision in target trials reflected modulation of target-induced activity, as would be expected from stimulus-driven processes of attention, we examined if the effect was dependent on the presence of a target. Thus, activation in no-target trials was analyzed within the same ROIs. A significant main effect of the number of cued locations in 1-factor ANOVA was identified only in the three occipital regions [F(3,63)>3.71, P<0.05 in each case]. As in target trials and contrary to all other ROIs derived from analysis of target trials, the left and right MOG displayed significant activation with one, two and three but not with four cued locations (data not shown). In these two regions, the cue effect in target trials thus probably arose from processes preceding target onset. In the lingual gyrus/cuneus, effects were limited to slight but significant deactivation in trials with two and three cued locations (data not shown). In all other regions, the effect of the number of cued locations in target trials appeared to reflect modulation of target-related processes because it was absent in no-target trials. Direct comparison confirmed that the cue effect differed between target and no-target trials; the effect of the number of cued locations interacted with trial type in all regions [F(3,63)>3.10, P<0.05] except right posterior insula [F(3,63)=2.05, P=0.12] and left and right MOG [F(3,63)<1] in 2-factor ANOVA. Note, however, the potential bias derived from the fact that the comparison was carried out on regions selected for their significant cue effect in target trials.
Top-down processes of spatial attentional resource allocation
Linear regression analysis was performed on no-target trials with the aim of identifying brain regions that displayed a linear increase in BOLD signal in the expectation period with more precise cueing of the target location.
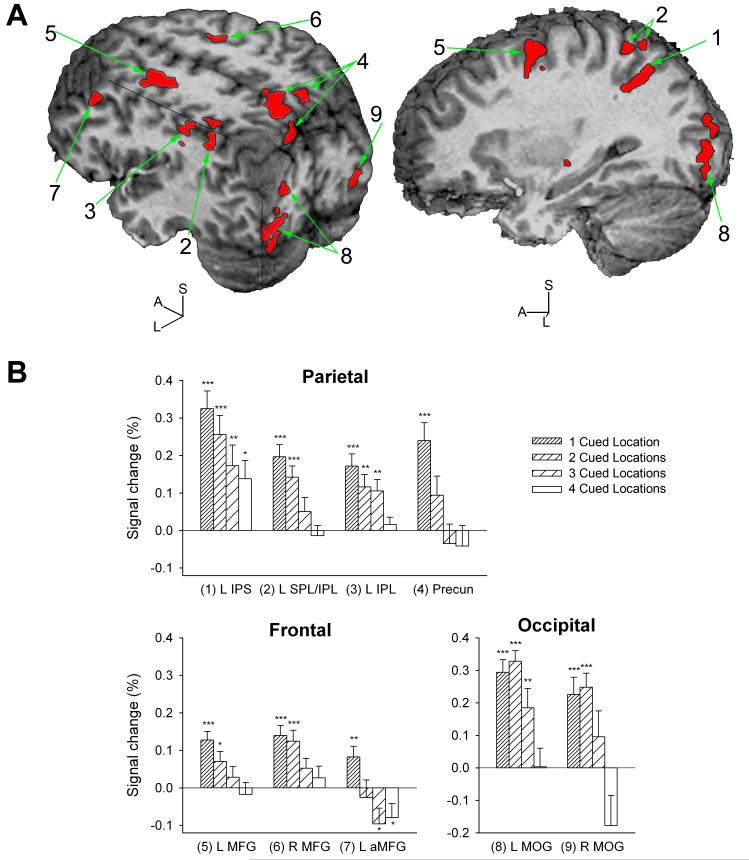
ROIs whose activation varied as a function of the number of cued locations were located in the left and right middle frontal gyrus (MFG) along the superior frontal sulcus, more anterior in left MFG (BA 9), in the left ventral IPS, in left IPL and SPL bridging the IPS dorsally, in the left IPL further anterior in the area of the supramarginal gyrus and BA40, bilateral precuneus, as well as left and right middle/inferior occipital gyri (Table 2, Figure 4A). In all nine regions, BOLD signal intensity was inversely related to the number of cued locations, i.e. highest activation was usually seen when only one location was cued (Figure 4B). In left MFG (BA 9), deactivation was seen with three and four cued locations. The two occipital regions largely overlapped with the left and right MOG regions identified by analysis of target trials that had also displayed increased activation with fewer cued locations.
Table 2.
Brain regions identified by analysis of no-target trials
| Brain Region | Side | Center of Mass (mm) X, Y, Z | Brodmann Area(s) | Volume (μl) | |
|---|---|---|---|---|---|
| 1 | Intraparietal sulcus | L | -29.5, -53.9, 40.7 | 40, 7 | 391 |
| 2 | Superior/inferior parietal lobule | L | -33.6, -51.4, 51.4 | 40, 7 | 1003 |
| 3 | Inferior parietal lobule | L | -42.7, -35.4, 39.6 | 40 | 383 |
| 4 | Precuneus | B | 1.3, -61.9, 46.3 | 7 | 3027 |
| 5 | Middle frontal gyrus, superior frontal sulcus | L | -24.0, -1.6, 50.9 | 6 | 1498 |
| 6 | Middle frontal gyrus, superior frontal sulcus | R | 22.3, -4, 48.7 | 6 | 281 |
| 7 | Middle frontal gyrus | L | -38.1, 23.6, 35.6 | 9 | 387 |
| 8 | Middle/inferior occipital gyrus | L | -31.2, -87.4, 3.6 | 18 | 1546 |
| 9 | Middle/inferior occipital gyrus | R | 26.8, -90.6, -3.7 | 18 | 490 |
In all regions, the BOLD signal displayed a linear increase as the number of cued locations decreased, i.e. as spatial cueing became more precise, in trials where the cue was not followed by a target. L = left, R = right, B = bilateral.
Figure 4.
Brain responses to cue precision in no-target trials: endogenous attentional orienting. A) In all regions (shown as red overlays) responding to cue precision in cue-only trials, BOLD signal increased linearly with fewer cued target locations, i.e. with more precise spatial cueing. This pattern of activation is consistent with engagement by endogenous spatial attentional selection prior to target presentation. The numbers correspond to ROIs in Table 2 and graphs in Figure 4B. Cuts are made at 41 mm left, 86 mm posterior and 49 mm superior (left brain), and at 29 mm left of the anterior commissure (right brain) in the AC-PC aligned brain.
B) BOLD signal amplitude is shown for brain regions that displayed a linear effect of the number of cued locations in no-target trials. Bars are means (+SEM) of 22 participants. Conditions where the BOLD signal differed from zero are marked (*P<0.05, **P<0.01, ***P<0.001; one-sample t-tests). (1) L IPS = left intraparietal sulcus; (2) L SPL/IPL = left superior/inferior parietal lobule, bridging the IPS; (3) L IPL = left inferior parietal lobule; (4) Precun = precuneus; (5) L MFG and (6) R MFG = left and right middle frontal gyrus; (7) L aMFG = anterior ROI in left middle frontal gyrus (BA 9); (8) L MOG and (9) R MOG = left and right middle occipital gyrus.
Two-factor ANOVA for repeated measures confirmed a significant main effect of the number of cued locations in all ROIs [F(3,63)>6.05, P<0.001]. This effect interacted with scan session solely in the left anterior MFG (BA 9) [F(3,63)=3.35, P<0.05]. In this region, the cue effect was significant in both sessions, but significant deactivation with four cued locations was seen only in session 1 and activation with one cued location only in session 2 (data not shown).
Additional analyses detailed in the Supplementary materials investigated whether in these same regions there was any indication that also target-related activity varied with the number of cued locations. Target-related activity was isolated by subtracting no-target trials (i.e. cue-related activity) from target trials (encompassing both cue- and target-related activity) for each cue-condition. Activation in the precuneus, left IPS and adjacent IPL/SPL increased with more cued locations in these subtraction trials, reflecting larger responses to targets as these became less spatially predictable.
Discussion
The present study demonstrated an almost complete dissociation between neuroanatomical substrates of top-down and bottom-up processes of visuospatial selective attention by employing a novel task that parametrically varied demands on these constructs. The design rationale was that narrowing the array of potential target locations would engage endogenous spatial attentional selection prior to target presentation. In contrast, the less predictable the target location, the more demands would be placed on stimulus-driven shifts of attention to this location following target onset.
Performance varied as a function of target predictability in a manner consistent with these assumptions. Faster reaction time with more precise cueing suggested that the cue information was used to orient the attentional focus in space prior to target onset, thus diminishing processing requirements upon target presentation. The linearity of this reduction indicated that the intensity of the spatial focus of attention varied as a function of cue precision. Thus, endogenous selective attention was intensified with fewer cued locations. Reaction time was overall longer in invalid than valid trials, reflecting processing costs at uncued locations when attention was directed to cued locations. Contrary to valid trials, reaction time in invalid trials increased with more precise cueing, giving evidence of an incremental processing deficit at uncued locations with an intensifying attentional focus elsewhere. Taken together, the data support that the SARAT successfully varied the degree to which visuospatial selective attention was engaged. Fewer cued locations created a progressively narrow but more intense attentional focus, at the growing expense of unattended locations.
The validity of the paradigm deserves some further critical consideration. The SARAT was designed to specifically manipulate spatial selective attention. Like traditional covert orienting paradigms based on the Posner task (Posner, 1980), it rests on the assumption that this construct is particularly taxed when a sub-selection of possible target locations is attended, i.e. when the spatial focus is narrowed. The SARAT expanded this concept by adding intermediate steps of a gradually narrowing attentional focus. It could be argued that more cued locations augmented attentional demands in the expectation period due to the increased division of attentional resources across space. Regions mediating such spatial expansion of the attentional focus would activate with more cued locations, but regions involved in spatial selective attention would activate with more precise cueing. All ROIs that displayed linear cue dependency in the expectation period activated with cue precision, indicating engagement by spatial selection.
The SARAT manipulates stimulus-driven attentional orienting to signals that are behaviorally relevant in that they indicate a response requirement, as opposed to cueing paradigms that measure responses to uninformative peripheral cues. As such, it reflects exogenous orienting to stimuli with features that match the current attentional control set, a process known as contingent orienting (Folk et al. 1992). Central mechanisms of exogenous orienting to task-relevant and task-irrelevant stimuli appear to differ (Serences et al., 2005; Kincade et al., 2005); thus, it is important to bear in mind that the SARAT manipulates the former, not the latter.
The rationale behind varying the visual salience of targets was based on reports that directing attention to a spatial location can reduce sensory thresholds at that location and enhance effective stimulus contrast (Reynolds and Chelazzi 2004). This has been shown behaviorally (Bashinski and Bachrach 1980) and electrophysiologically (e.g. Reynolds et al. 2000). Thus, the processing of less intense signals was expected to show greater profit from preparatory orienting than that of signals with higher intensity. However, although target intensity had large effects on behavioral performance, it did not modulate the cue-effect on behavior or BOLD signal. Despite the relatively large sample size (n=22), the study still may have lacked statistical power to detect such fine-grained effects. Furthermore, data were not analyzed as a function of the cued location. Thus, the design was not sensitive to attentional modulation of location-specific activation, which may be where modulating effects of target intensity should have been expected.
In view of previous studies reporting largely overlapping areas of activation in conditions created to tax top-down and bottom-up attentional control (see Introduction), it was perhaps surprising that with the present parametric design there was no overlap between regions derived from whole-brain analyses engaged by each mechanism. Thus, one set of brain areas activated with increasing cue precision in no-target trials, reflecting modulation of cue-induced endogenous orienting, and different regions activated with decreasing target predictability in target trials. None of these latter regions displayed any cue-dependent signal change in no-target trials, i.e. in response to the cue itself, indicating that (1) the observed cue-effect reflected modulation of target-related activity, consistent with stimulus-driven attention, and (2) these regions were not engaged by endogenous attentional control demands. This added further substance to the anatomical dissociation of the two processes. Conversely, of those regions that activated with more precise cueing in no-target trials, only posterior parietal regions also displayed indication of larger responses to the target with increasing spatial unpredictability. No other region showed even a trend of this sort, suggesting selective involvement in top-down spatial selective attention. These results emphasize the neuroanatomical dissociation of endogenous and exogenous processes of visuospatial attentional selection and build upon previous event-related fMRI studies designed to separate responses to cue- and target-presentation (Kastner et al., 1999; Corbetta et al., 2000; Hopfinger et al., 2000).
Most regions that showed a cue relationship in target trials displayed clear activation only with four cued locations, i.e. when no advance orienting of attention prior to target onset was possible whatsoever. This pattern of target-induced activity may suggest that the presence of any preparatory orienting of attention introduces strong limitations to stimulus-driven orienting, which appears to unfold mostly with complete and explicit unpredictability of the target location.
Brain regions that form part of “attention networks” are believed to perform interrelated but different functions that convolve to regulate the selection of information for processing (Posner and Petersen, 1990). In the following, brain regions are discussed with respect to their possible specific contributions to the control of visuospatial attention.
Parietal regions
Left IPS with adjacent IPL and SPL activated proportionally to demands on top-down visuospatial attention, consistent with previous studies reporting activation of IPS, predominantly in the left hemisphere, by cue-induced orienting (Corbetta et al., 2000; Hopfinger et al., 2000). A PET study also reported more activation in left IPS with greater endogenous control demands (Nobre et al., 1997). Accordingly, IPS has been described as part of a dorsal frontoparietal network coding for top-down signals of visual expectancy (Corbetta and Shulman, 2002). But involvement in exogenous orienting and dual function has also been suggested by studies of attentional orienting and visual search (e.g. Corbetta et al., 2000; Nobre et al., 2000; Shulman et al., 2001), and also the present study suggested activation that followed exogenous orienting. Similarly, the precuneus responded, in separate studies, to either endogenous or stimulus-driven attentional shifts (Corbetta et al., 2000; Beauchamp et al., 2001; Makino et al., 2004; Gitelman et al., 1999). A dual function would be consistent with the present response primarily to top-down but upon further probing also to target-induced orienting. Interestingly, involvement in both endogenous and exogenous spatial attentional regulation was also suggested by electrophysiological recordings in monkey lateral intraparietal area (LIP) (Colby et al., 1996; Gottlieb et al., 1998; Goldberg et al., 2002). This parallel between LIP and human IPS/IPL/SPL and precuneus, and knowledge that LIP houses an abstract representation of space (Andersen, 1995), supports hypotheses that certain posterior parietal regions integrate top-down and bottom-up attentional biasing signals to yield a visuospatial priority map (Corbetta and Shulman, 2002).
Frontal regions
The pattern of activation seen in right precentral gyrus was unrelated to motor demands; responses were made with the right hand. Precentral gyrus/sulcus activation was also seen in an fMRI study of covert orienting that did not require manual detection (Corbetta, 1998). Instead, these areas, in particular the ROI in the superior precentral gyrus adjacent to superior frontal sulcus (region 7 in Table 1 and Figure 3), may correspond to human FEF. Areas involved in eye-movement control defined by human cerebral blood flow and electrical stimulation studies are located between and along central and precentral sulci (reviewed by Paus, 1996). There is extensive evidence that covert visuospatial orienting recruits such areas (Corbetta et al., 1998; Beauchamp et al., 2001; Moore and Fallah, 2004). The present finding suggests that areas of FEF may be of particular importance for spontaneous, stimulus-driven shifts of attention in space. FEF has also been implicated in top-down allocation of attention (Corbetta et al., 2002; Kincade et al., 2005), but the focus of activation was anterior to the regions discussed above.
In the present study, frontal ROIs displaying activation patterns consistent with top-down control were located in MFG, tending to be larger in the left hemisphere, and probably outside FEF. Previous reports support selective engagement of MFG, predominantly left hemispheric, by top-down visuospatial attention (Hopfinger et al., 2000; Giesbrecht et al., 2003), possibly reflecting control demands related to task preparation. In particular, the ROI in left anterior MFG (BA 9) was located in an area of dorsolateral prefrontal cortex that has been associated with top-down biasing of task-relevant stimulus processing (Frith and Dolan, 1996; MacDonald et al., 2000). Activation with one cued target location may reflects top-down visuospatial biasing signals, while deactivation in response to three and four cued locations may reflect inhibition when advance information is explicitly absent. This raises the interesting possibility that processes mediated by this region can be actively down-regulated so as to not impede the spontaneous allocation of attention to upcoming target signals.
In accordance with previous studies (Hopfinger et al., 2000; MacDonald et al., 2000), modulation of midcingulate activity (BA 24) was seen only in the presence of target signals, where it increased with spatial unpredictability. This response profile resonates with theories ascribing the anterior cingulate a late evaluative function of detecting uncertainty and conflict, rather than top-down control aimed at advance selection for action. Thus, this region supposedly activates when conflict is still present by the time a response is due (e.g. Botvinick et al., 1999; Carter et al., 2000; Milham et al., 2001). Its role in mobilizing resources to deal with uncertainty may include uncertainties in the input domain such as unpredictability of the target location.
Temporal regions
All temporal regions displayed larger BOLD responses in target trials with decreasing target predictability, i.e. responded to demands on stimulus-driven orienting.
Activation at or near TPJ has been demonstrated repeatedly with visuospatial attention tasks (e.g. Gitelman et al., 1999; Rosen et al., 1999), predominantly in the right hemisphere, following target onset and being stronger in invalid trials that tax stimulus-driven reorienting (Corbetta et al., 2000). The TPJ has thus been suggested to form part of an exogenous orienting system (Corbetta and Shulman 2002). The present results, although not confirming stronger effects in the right hemisphere, support selective engagement by bottom-up processes of attention. Recently, it has been suggested that TPJ is involved in stimulus-driven orienting only to behaviorally relevant signals (Kincade et al., 2005), or to task irrelevant signals that share features with the target stimulus (Serenes et al., 2005). This is in agreement with a lack of TPJ response to uninformative peripheral cues (Rosen et al., 1999; Kim et al., 1999; Peelen et al., 2004; Kincade et al., 2005), and with responsivity to invalidly cued (Corbetta et al., 2000; Kincade et al., 2005) and uncued (present study) targets. The implication is that the TPJ contributes to integrating top-down signals with bottom-up processes of attentional orienting.
Anterior insula, too, has been recruited during previous studies of visuospatial attention (Gitelman et al., 1999; Kim et al., 1999; Nobre et al., 2000), but the current study indicates a specific role in stimulus-driven attention, consistent with a report of stronger responses to invalidly than to validly cued targets (Kincade et al., 2005). While anterior insula was activated by target presentation at unpredictable locations, a small region in posterior insula displayed a complementary pattern of deactivation with greater target predictability. A mechanism by which endogenous control functions inhibit brain regions that promote spontaneous shifts of attention to new sensory input is, as suggested above, an interesting possibility. But clearly, more evidence is needed prior to further speculation.
The fusiform gyri, being part of the ventral processing stream, are critical for shape and higher order object recognition. However, stimulus detection paradigms as simple as the SARAT have evoked their activation (e.g. Corbetta et al., 2000; Shulman et al., 2001). Recent findings are consistent with the present evidence for specific engagement by stimulus-driven attention. Peripheral uninformative cues evoked a differential BOLD response in this structure (Kincade et al. 2005). Furthermore, human intracranial recording identified increased gamma-band oscillation in fusiform gyrus when simple shape stimuli had to be discriminated, but only with the actual stimulus presentation, not with its anticipation (Tallon-Baudry et al., 2005). An area in lateral occipital sulcus that displayed more gamma-oscillation during stimulus anticipation appears to be covered by the current ROIs in MOG that responded as a function of endogenous attentional resource allocation.
Occipital regions
Bilateral MOG displayed a linear increase in BOLD signal with more precise cueing. The ROIs overlapped with extrastriate regions that Yantis et al. (2002) identified as being continuously activated by prolonged allocation of attention to signals in the contralateral visual field, consistent with sensory effector regions, i.e. the substrate of top-down attentional control. The current MOG regions also displayed remarkable similarity with occipital regions identified by Kincade et al. (2005) as displaying stronger activation to endogenous than exogenous or neutral cues. The overlap between studies, despite divergent target locations, suggests that a specific processing area of extrastriate cortex is particularly receptive to top-down spatial biasing signals. Future studies should be designed to interrogate this area more precisely.
A large and diffusely outlined occipital region comprising bilateral lingual gyrus and cuneus in striate and extrastriate cortex displayed signal increases with spatial unpredictability in target trials. In no-target trials, deactivation occurred with two or three cued locations, perhaps reflecting functional inhibition at locations where no target was expected. Such inhibition would be particularly pronounced when information about where the target will not occur is most precise, which is the case in trials with three, followed by two cued locations. Functional inhibition can occur with striate and extrastriate representations of unattended locations at similar eccentricity as in the present study (Slotnick et al., 2003).
Summary
While the present study confirmed previous reports of selective engagement of some brain areas, it suggests that wider and largely distinct networks are involved in bottom-up versus top-down regulation of visuospatial attention. It remains to be established to what extent engagement of these areas generalizes to non-spatial selective attention. There is clear overlap between networks recruited by location- and feature-based selection (Kanwisher and Wojciulik, 2000). Especially for IPS, a more universal role in visual selective attention has been suggested (e.g. Wojciulik and Kanwisher, 1999; Giesbrecht et al., 2003). An important next question is if the same neuroanatomic dissociation between bottom-up and top-down processes of visuospatial attention generalizes to other forms of selective attention.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Drug Abuse.
References
- Andersen RA. Encoding of intention and spatial location in the posterior parietal cortex. Cereb Cortex. 1995;5:457–469. doi: 10.1093/cercor/5.5.457. [DOI] [PubMed] [Google Scholar]
- Bashinski HS, Bacharach VR. Enhancement of perceptual sensitivity as the result of selectively attending to spatial locations. Percept Psychophys. 1980;28:241–248. doi: 10.3758/bf03204380. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage. 2001;14:310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci USA. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annu Rev Psychol. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Frith C, Dolan R. The role of the prefrontal cortex in higher cognitive functions. Brain Res Cogn Brain Res. 1996;5:175–181. doi: 10.1016/s0926-6410(96)00054-7. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. Neuroimage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bisley J, Powell KD, Gottlieb J, Kusunoki M. The role of the lateral intraparietal area of the monkey in the generation of saccades and visuospatial attention. Ann NY Acad Sci. 2002;956:205–215. doi: 10.1111/j.1749-6632.2002.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Wojciulik E. Visual attention: insights from brain imaging. Nat Rev Neurosci. 2000;1:91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kim YH, Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Mesulam MM. The large-scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. Neuroimage. 1999;9:269–277. doi: 10.1006/nimg.1999.0408. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Makino Y, Yokosawa K, Takeda Y, Kumada T. Visual search and memory search engage extensive overlapping cerebral cortices: an fMRI study. Neuroimage. 2004;23:525–533. doi: 10.1016/j.neuroimage.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120:515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996;34:475–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Heslenfeld DJ, Theeuwes J. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. Neuroimage. 2004;22:822–830. doi: 10.1016/j.neuroimage.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Ristic J, Kingstone A. Attention to arrows: Pointing to a new direction. Q J Exp Psychol. doi: 10.1080/17470210500416367. in press. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Rao SM, Caffarra P, Scaglioni A, Bobholz JA, Woodley SJ, Hammeke TA, Cunningham JM, Prieto TE, Binder JR. Neural basis of endogenous and exogenous spatial orienting. A functional MRI study. J Cogn Neurosci. 1999;11:135–152. doi: 10.1162/089892999563283. [DOI] [PubMed] [Google Scholar]
- Ross TJ, Zhang Y, Stein EA. Dissociation of transient executive functions from sustained attentional processes. Neuroimage. 2005 26-S1, Poster No. 1188 TH-PM. [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol Sci. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Linenweber M, Petersen SE, Corbetta M. Multiple neural correlates of detection in the human brain. Proc Natl Acad Sci USA. 2001;98:313–318. doi: 10.1073/pnas.021381198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Schwarzbach J, Yantis S. Attentional inhibition of visual processing in human striate and extrastriate cortex. Neuroimage. 2003;19:1602–1611. doi: 10.1016/s1053-8119(03)00187-3. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Tallon-Baudry C, Bertrand O, Henaff MA, Isnard J, Fischer C. Attention Modulates Gamma-band Oscillations Differently in the Human Lateral Occipital Cortex and Fusiform Gyrus. Cereb Cortex. 2005;15:654–662. doi: 10.1093/cercor/bhh167. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N. The generality of parietal involvement in visual attention. Neuron. 1999;23:747–764. doi: 10.1016/s0896-6273(01)80033-7. [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.