Abstract
The lectin-like ox-LDL receptor 1 (LOX-1) expressed on vascular cells plays a major role in atherogenesis by internalizing and degrading oxidized LDL. LOX-1 can be cleaved from the cell surface and released as soluble LOX-1 (sLOX-1), and elevated sLOX-1 levels may be indicative of atherosclerotic plaque instability. We examined associations between the LOX-1 3′UTR-C/T and G501C polymorphisms and plasma sLOX-1 levels in 97 healthy older men and women. The frequencies for the 3′UTR-T and 501C alleles were 46% and 10%, respectively. Plasma sLOX-1 levels were significantly higher in the 3′UTR CC genotype group compared to both the CT (p=0.02) and TT (p=0.002) genotype groups. Plasma sLOX-1 were also significantly higher in the 501GC genotype group compared to the GG genotype group (p=0.004). In univariate analyses, sLOX-1 levels were significantly associated with both the 3′UTR-C/T and G501 C polymorphisms. These associations remained significant after adjusting for age, gender, race, and BMI. In conclusion, variation in the LOX-1 gene is associated with plasma sLOX-1 levels in older men and women.
Keywords: receptor, cardiovascular, gene expression
Introduction
The oxidative modification of LDL is a key step in the initiation and progression of atherosclerosis (Witztum & Steinberg, 1991). Ox-LDL is taken up by macrophages and vascular smooth muscle cells through a variety of scavenger receptors, including SR-A, SR-BI, and CD36 (Witztum & Steinberg, 1991;Chen et al., 2002). Vascular endothelial cells can also internalize and degrade ox-LDL and this occurs primarily via the lectin-like ox-LDL receptor 1 (LOX-1). LOX-1 is the major receptor for ox-LDL on endothelial cells and mediates many of the atherogenic effects of ox-LDL in the vascular wall (Sawamura et al., 1997;Chen et al., 2002). Ox-LDL-LOX-1 interactions stimulate several intracellular signaling pathways that regulate the expression of atherosclerosis-related genes (Mehta et al., 2006). LOX-1 expression is upregulated in hypercholesterolemia, hypertension, diabetes, and myocardial ischemia (Mehta et al., 2006). In addition, LOX-1 is prominently expressed by endothelial cells covering early atherosclerotic lesions, and by intimal smooth muscle cells and macrophages in advanced atherosclerotic plaques (Kataoka et al., 1999).
Like many cell-surface receptors with a single transmembrane domain, LOX-1 can be cleaved at the membrane proximal extracellular domain by serine proteases and released in a soluble form (Murase et al., 2000;Kume & Kita, 2001). Elevated levels of soluble membrane proteins in plasma may reflect increased expression of membrane-bound proteins and disease activities. Although the pathophysiological roles of sLOX-1 remain unclear, circulating sLOX-1 levels are elevated in acute coronary syndromes, but not in general inflammatory diseases, thereby differentiating sLOX-1 from inflammatory markers such as C-reactive protein (Hayashida et al., 2005). While increased protease activities in patients with vulnerable plaques may contribute to increased sLOX-1 levels, the biological and/or genetic factors contributing to the formation of sLOX-1 are still largely unknown.
Several polymorphisms have been identified in the LOX-1 gene, including a C-to-T substitution in the 3′-untranslated region (3′UTR-C/T; rs1050283) (Mango et al., 2003). This polymorphism affects the binding of nuclear proteins, and individuals with the 3′UTR/T allele may have an increased risk for coronary artery disease (CAD) and myocardial infarction (Mango et al., 2003;Chen et al., 2003;Sentinelli et al., 2006;Novelli et al., 2007). In addition, the 3′UTR polymorphism is in complete linkage disequilibrium with intronic polymorphisms that regulate the splicing of exon 5 (Mango et al., 2003;Mango et al., 2005). A second functional polymorphism has been identified in exon 4 which involves a G-to-C substitution at nucleotide 501 (G501C; rs11053646), leading to an amino acid change in codon 167 (Tatsuguchi et al., 2003). Due to its location in the ligand-binding domain, this polymorphism may affect the binding and uptake of ox-LDL and subsequent LOX-1 activation (Chen et al., 2001;Tatsuguchi et al., 2003;Ohmori et al., 2004). Some studies support a protective role for the 501C allele, while others report a positive association with cardiovascular disease, or no association at all (Mango et al., 2003;Tatsuguchi et al., 2003;Ohmori et al., 2004;Hattori et al., 2006;Trabetti et al., 2006). Given the potential effect of these two functional polymorphisms on LOX-1 expression and/or activity, it is likely that sLOX-1 levels are influenced by LOX-1 gene variation. Thus, the purpose of this study was to determine whether the LOX-1 3′UTR-C/T and G501C polymorphisms are associated with plasma sLOX-1 levels.
Methods
Subjects and screening
This study included 50–75 yr-old men and women who were recruited to participate in an exercise training study. All subjects were sedentary, non-smokers, non-diabetic, and free of heart, liver, kidney, and lung disease. Subjects were either normotensive with at least one National Cholesterol Education Program lipid abnormality (total cholesterol > 200 mg/dL, LDL-cholesterol > 130 mg/dL, HDL-cholesterol < 40 mg/dL, or triglycerides > 200 mg/dL) or hypertensive (blood pressure < 160/90 mmHg), but controlled by medications not affecting lipid metabolism. All females were postmenopausal (absence of menses > 2 years). Individuals with a body mass index (BMI) > 37 kg/m2, fasting triglycerides > 400 mg/dL, fasting glucose > 126 mg/dL, or postprandial glucose > 200 mg/dL were excluded from the study. Individuals with a maximal graded exercise test indicating signs or symptoms of cardiovascular disease or other chronic diseases that would preclude exercise testing or training were also excluded from the study (American College of Sports Medicine, 2000). Subjects were informed of the study requirements and provided written consent. This study complies with the Declaration of Helsinki and was approved by the University of Maryland at College Park Institutional Review Board.
Dietary stabilization
Subjects were stabilized for 6 weeks on an American Heart Association Step I diet, which consisted of ≤ 30% of daily calories from fat, ~55% from carbohydrates, and 15% from protein, with cholesterol intake limited to < 300 mg/day. Before all laboratory measurements, subjects completed a 7-day food record to assess compliance with the prescribed diet. Food records were analyzed by a registered dietitian using Computrition software (Computrition Inc., Chatsworth, CA).
Laboratory measures
Blood was drawn in the morning after a 12-hour overnight fast and frozen plasma aliquots were stored at −80°C until further analysis. Fasted samples were taken on at least 2 separate days and averaged to calculate baseline lipid levels. Plasma total cholesterol, triglycerides, HDL-cholesterol, and LDL-cholesterol levels were determined using conventional methods (Friedewald et al., 1972;Allain et al., 1974;Sampson et al., 1975;Warnick et al., 1982). Total body fat was measured by dual energy x-ray absorptiometry (DEXA), and intra-abdominal fat was quantified using computerized tomography (CT), as described previously (Mazess et al., 1990;Nicklas et al., 1996). Due to technical reasons, 12 subjects were missing lipid data, 8 were missing DEXA data, and 20 were missing CT data. Plasma sLOX-1 levels were measured by a sandwich chemiluminescent ELISA using two different human LOX-1-specific monoclonal antibodies with a recombinant human LOX-1 extracellular domain as an assay standard, which was modified from the previously described sandwich ELISA (Hayashida et al., 2005). Monoclonal antibodies directed to human LOX-1 were established by standard hybridoma techniques after immunizing mice with a recombinant protein corresponding to the extracellular domain of human LOX-1. Intra-assay and inter-assay coefficients of variation were 1.8%–6.4% and 4.4%–10.7%, respectively.
Genotyping
To identify the LOX-1 variants, DNA was isolated from peripheral lymphocytes and genotyped using fluorescence polarization. Primers for the 3′UTR polymorphism were: forward, 5′-AGCTATTCTTTGTCACTTGGGTG-3′; reverse, 5′-CTGAGTTCAGAGGGTTTTCAAGC-3′; and internal reverse, 5′-GGGAAGCTTGGGACAAG CTAGGTGAAATAATACAG-3′. Primers for the G501C polymorphism were: forward, 5′-CAGCTCCTTGTCCGCAAGACTGGAT-3′; reverse, 5′-GAACACTCACCAGATCAGCTGT GCT-3′; and internal reverse, 5′-CTTGGCATCCAAGACAAGCACTTCTCTTGGCT-3′. DNA was amplified using PCR, followed by purification of the PCR products and single base extension. The fluorescence polarization measurement and genotype assignments were performed as described previously (Chen et al., 1999).
Statistical analyses
All statistical analyses were performed using SAS version 9.1. Plasma sLOX-1 levels were log transformed to achieve a normal distribution. One-way analysis of variance was used to compare differences between genotype groups. Linear regression was used to examine the association of sLOX-1 levels with LOX-1 polymorphisms and to determine the relationship between LOX-1 polymorphisms and plasma sLOX-1 levels after controlling for demographic variables and BMI. A value of p ≤ 0.05 was considered statistically significant.
Results
The study population consisted of 53% Whites, 38% Blacks, and 9% Other. Given the racial heterogeneity of the study population, we tested for an interactive effect of race and LOX-1 genotype on sLOX-1 levels and did not find a significant interaction for either polymorphism. In addition, we found that the direction of the association between LOX-1 genotype and sLOX-1 levels were similar in Whites and Blacks. Thus, all races were combined for the analyses. The mean age was 58.4 ± 0.6 yrs and the mean BMI was 28.7 ± 0.4 kg m−2. Forty-seven percent of the population was female, and of those, 37% were on HRT. The frequencies for the 3′UTR CC, CT and TT genotypes were 33%, 42% and 25%, respectively (Table 1). The frequencies for the 501 GG and GC genotypes were 79% and 21%, respectively. There were no individuals with the 501CC genotype in this study. The allele and genotype frequencies for both polymorphisms were in Hardy-Weinberg equilibrium. In addition, the two polymorphisms were in complete linkage disequilibrium (D′=1.0, R2=0.097)
Table 1.
LOX-1 allele and genotype frequencies
| Polymorphism | Genotype Frequencies | Allele Frequencies | |||
|---|---|---|---|---|---|
| 3′UTR-C/T | CC | CT | TT | C | T |
| N | 32 | 41 | 24 | ||
| % | 32.99 | 42.27 | 24.74 | 0.54 | 0.46 |
| G501C | GG | GC | CC | G | C |
| N | 77 | 20 | 0 | ||
| % | 79.38 | 20.62 | 0 | 0.90 | 0.10 |
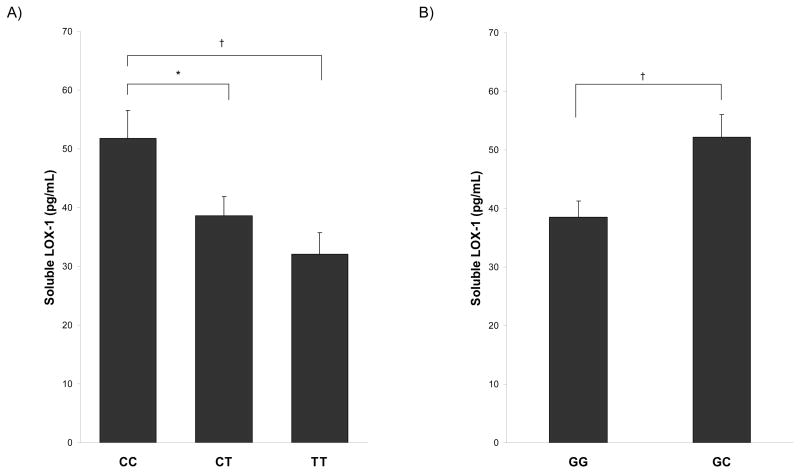
There were no significant differences in age, weight, BMI, body composition, or plasma lipid levels between LOX-1 genotype groups for both polymorphisms (Table 2). Plasma sLOX-1 levels were significantly different among the 3′UTR-C/T genotype groups (p=0.006), as shown in panel A of the Figure. Plasma levels were significantly higher in the 3′UTR CC genotype group compared to both the CT genotype group (p=0.02) and the TT genotype group (p=0.002). There was no difference in sLOX-l levels between the CT and TT genotype groups. Similarly, plasma sLOX-1 levels were significantly higher in the 501GC genotype group compared to the GG genotype group (p=0.004) (Figure, panel B).
Table 2.
Subject characteristics by LOX-1 genotype
| LOX-1 polymorphism |
|||||
|---|---|---|---|---|---|
| 3′UTR-C/T |
G501C |
||||
| CC (n=23–32) | CT (n=36–41) | TT (n=23–24) | GG (n=67–77) | GC (n=15–20) | |
| Age (yrs) | 58.6 ± 1.0 | 57.8 ± 0.9 | 59.4 ± 1.2 | 58.6 ± 0.6 | 57.7 ± 1.3 |
| Weight (kg) | 84.0 ± 2.5 | 85.8 ± 2.2 | 81.8 ± 2.8 | 84.0 ± 1.6 | 85.2 ± 3.1 |
| BMI (kg m−2) | 29.2 ± 0.7 | 28.8 ± 0.6 | 27.6 ± 0.7 | 28.5 ± 0.4 | 29.0 ± 0.8 |
| Total body fat (%) | 39.2 ± 1.7 | 35.2 ± 1.4 | 33.1 ± 1.9 | 35.4 ± 1.1 | 38.0 ± 2.1 |
| Intra-abdominal fat (cm2) | 117.9 ± 11.0 | 133.8 ± 9.1 | 142.2 ± 11.3 | 132.9 ± 6.6 | 124.6 ± 14.7 |
| Total cholesterol (mg dl−1) | 196.3 ± 6.8 | 190.7 ± 5.5 | 185.1 ± 6.8 | 190.7 ± 4.0 | 190.5 ± 8.5 |
| HDL-cholesterol (mg dL−1) | 52.8 ± 3.5 | 48.4 ± 2.8 | 43.3 ± 3.5 | 48.5 ± 2.1 | 46.9 ± 4.4 |
| LDL-cholesterol (mg dl−1) | 120.7 ± 6.0 | 115.4 ± 4.8 | 115.3 ± 6.0 | 117.0 ± 3.5 | 116.3 ± 7.4 |
| Triglycerides (mg dl−1) | 106.4 ± 11.2 | 121.9 ± 9.0 | 122.7 ± 11.2 | 119.4 ± 6.6 | 110.7 ± 13.9 |
Table values are means ± SEM. BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Figure.
Plasma sLOX-1 levels by the LOX-1 3′UTR-C/T polymorphism (A) and the G501C polymorphism (B), *p<0.05, †p<0.01.
In univariate analysis, sLOX-1 levels were significantly associated with both the 3′UTR-C/T and G501C polymorphisms (p=0.002 and p=0.004, respectively). Both polymorphisms remained associated with sLOX-1 after adjustment for age, gender, race and BMI (3′UTR-C/T, p=0.02; G501C, p=0.009).
Discussion
The present study is the first to report the relationship between LOX-1 gene polymorphisms and circulating sLOX-1 levels. We found that the 3′UTR-T allele was associated with lower sLOX-1 levels. These findings were surprising given that this allele has been positively associated with cardiovascular disease (Mango et al., 2003;Chen et al., 2003). While elevated sLOX-1 levels have been reported in patients with acute coronary syndromes (Hayashida et al., 2005), the physiological significance of sLOX-1 is not known. Thus, the reasons for the discrepancy in these findings is not apparent, but may be related to the study population. We also found that the LOX-1 501GG genotype was associated with lower sLOX-1 levels. The association between the G501C polymorphism and cardiovascular disease is less clear, as published results have been largely inconsistent (Mango et al., 2003;Tatsuguchi et al., 2003;Ohmori et al., 2004;Hattori et al., 2006;Trabetti et al., 2006). As such, determining the influence of LOX-1 polymorphisms on sLOX-1 levels may help to further elucidate the biological effects of LOX-1 gene variation on cardiovascular disease.
Both the 3′UTR and G501C polymorphisms may affect LOX-1 expression. Chen et al. reported that the 3′UTR-T allele has a 3-fold lower binding affinity for regulatory proteins compared with the C allele and proposed that the 3′UTR polymorphism may directly affect LOX-1 mRNA stability and/or translation (Chen et al., 2003). Moreover, Mango et al. found that the 3′UTR polymorphism and five intronic polymorphisms comprise a linkage disequilibrium block that regulates the relative expression of two LOX-1 isoforms (Mango et al., 2005). On the other hand, it has been suggested that the G501C polymorphism may alter gene expression by affecting LOX-1 binding activity (Tatsuguchi et al., 2003). This polymorphism results in a non-conservative amino acid change (Lysine to Asparagine) in codon 167, which is located in the ligand-binding domain. Electrostatic interactions between basic residues in this domain and negatively charged residues in ox-LDL are critical for LOX-1 activity, and substitution of these residues may cause reduced ox-LDL binding and internalization (Chen et al., 2001). Although more functional studies are needed to understand the impact of LOX-1 gene variation on the expression and activity of this receptor, our results suggest that common genetic variants in LOX-1 may affect plasma concentrations of sLOX-1.
As mentioned previously, Hayashida et al. found that serum sLOX-1 levels were significantly elevated in patients with acute coronary syndromes compared to individuals with normal coronary arteries and patients with stable CAD and non-cardiovascular diseases (Hayashida et al., 2005). In patients with acute coronary syndromes, median sLOX-1 levels were 2,910 pg ml−1 (range, <500 to 170,000 pg ml−1) compared to all other patient groups and controls, whose levels ranged from <500 to 14,000 pg ml−1. It was concluded that sLOX-1 levels do not reflect general inflammation or lesion size, but rather the instability of atherosclerotic plaques, which is indicative of more prominent LOX-1 expression and enhanced protease activities. In vulnerable plaques, an increase in protease activities may serve to cleave LOX-1 from the cell surface, thereby increasing the circulating sLOX-1 levels. In our population of clinically healthy individuals, plasma sLOX-1 levels were substantially lower (median, 36.23 pg ml−1; interquartile range, 24.63 to 54.15 pg ml−1). Thus, the significance of elevated sLOX-1 levels in a disease-free population remains to be elucidated.
The main limitation in this study was the small sample size. With a 3′UTR/T-allele frequency of 46%, we had sufficient statistical power (~83%) to detect differences in sLOX-1 levels between the CC and TT genotype groups. However, given a 501C allele frequency of 10%, we had limited power to detect differences in sLOX-1 levels between the 501GG and GC genotype groups. Nevertheless, this is the first study to investigate the effects of the LOX-1 3′UTR-C/T and G501C polymorphisms on plasma sLOX-1 levels. Our findings demonstrate that LOX-1 gene variation may be important in the regulation of sLOX-1 levels in plasma. More studies are needed to clarify the significance of sLOX-1 in normal and pathological settings and the effect of genetic and behavioral factors on circulating sLOX-1 levels.
Acknowledgments
This research was supported by NIH grants AG-17474, AG-15389, AG-00268, DK-46204, AG-18408, the Geriatric Research, Education, and Clinical Center and Medical Research Service of the Department of Veterans Affairs (Merit Review APG), and the University of Maryland Claude D. Pepper OAIC P60 AG12583.
References
- Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 6. Lippincott Williams & Wilkins; Baltimore: 2000. [Google Scholar]
- Chen M, Inoue K, Narumiya S, Masaki T, Sawamura T. Requirements of basic amino acid residues within the lectin-like domain of LOX-1 for the binding of oxidized low-density lipoprotein. FEBS Lett. 2001;499:215–219. doi: 10.1016/s0014-5793(01)02557-1. [DOI] [PubMed] [Google Scholar]
- Chen M, Masaki T, Sawamura T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther. 2002;95:89–100. doi: 10.1016/s0163-7258(02)00236-x. [DOI] [PubMed] [Google Scholar]
- Chen Q, Reis SE, Kammerer C, Craig WY, LaPierre SE, Zimmer EL, McNamara DM, Pauly DF, Sharaf B, Holubkov R, Bairey Merz CN, Sopko G, Bontempo F, Kamboh MI. Genetic variation in lectin-like oxidized low-density lipoprotein receptor 1 (LOX1) gene and the risk of coronary artery disease. Circulation. 2003;107:3146–3151. doi: 10.1161/01.CIR.0000074207.85796.36. [DOI] [PubMed] [Google Scholar]
- Chen X, Levine L, Kwok PY. Fluorescence polarization in homogeneous nucleic acid analysis. Genome Res. 1999;9:492–498. [PMC free article] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Hattori H, Sonoda A, Sato H, Ito D, Tanahashi N, Murata M, Saito I, Watanabe K, Suzuki N. G501C polymorphism of oxidized LDL receptor gene (OLR1) and ischemic stroke. Brain Res. 2006;1121:246–249. doi: 10.1016/j.brainres.2006.08.091. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Kume N, Murase T, Minami M, Nakagawa D, Inada T, Tanaka M, Ueda A, Kominami G, Kambara H, Kimura T, Kita T. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: a novel marker for early diagnosis. Circulation. 2005;112:812–818. doi: 10.1161/CIRCULATIONAHA.104.468397. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Kume N, Miyamoto S, Minami M, Moriwaki H, Murase T, Sawamura T, Masaki T, Hashimoto N, Kita T. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–3117. doi: 10.1161/01.cir.99.24.3110. [DOI] [PubMed] [Google Scholar]
- Kume N, Kita T. Roles of lectin-like oxidized LDL receptor-1 and its soluble forms in atherogenesis. Curr Opin Lipidol. 2001;12:419–423. doi: 10.1097/00041433-200108000-00008. [DOI] [PubMed] [Google Scholar]
- Mango R, Biocca S, del VF, Clementi F, Sangiuolo F, Amati F, Filareto A, Grelli S, Spitalieri P, Filesi I, Favalli C, Lauro R, Mehta JL, Romeo F, Novelli G. In vivo and in vitro studies support that a new splicing isoform of OLR1 gene is protective against acute myocardial infarction. Circ Res. 2005;97:152–158. doi: 10.1161/01.RES.0000174563.62625.8e. [DOI] [PubMed] [Google Scholar]
- Mango R, Clementi F, Borgiani P, Forleo GB, Federici M, Contino G, Giardina E, Garza L, Fahdi IE, Lauro R, Mehta JL, Novelli G, Romeo F. Association of single nucleotide polymorphisms in the oxidised LDL receptor 1 (OLR1) gene in patients with acute myocardial infarction. J Med Genet. 2003;40:933–936. doi: 10.1136/jmg.40.12.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Murase T, Kume N, Kataoka H, Minami M, Sawamura T, Masaki T, Kita T. Identification of soluble forms of lectin-like oxidized LDL receptor-1. Arterioscler Thromb Vasc Biol. 2000;20:715–720. doi: 10.1161/01.atv.20.3.715. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Rogus EM, Colman EG, Goldberg AP. Visceral adiposity, increased adipocyte lipolysis, and metabolic dysfunction in obese postmenopausal women. Am J Physiol. 1996;270:E72–E78. doi: 10.1152/ajpendo.1996.270.1.E72. [DOI] [PubMed] [Google Scholar]
- Novelli G, Borgiani P, Mango R, Lauro R, Romeo F. Further evidence that polymorphisms of the OLR1 gene are associated with susceptibility to coronary artery disease and myocardial infarction. Nutr Metab Cardiovasc Dis. 2007;17:e7–e8. doi: 10.1016/j.numecd.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Ohmori R, Momiyama Y, Nagano M, Taniguchi H, Egashira T, Yonemura A, Nakamura H, Kondo K, Ohsuzu F. An oxidized low-density lipoprotein receptor gene variant is inversely associated with the severity of coronary artery disease. Clin Cardiol. 2004;27:641–644. doi: 10.1002/clc.4960271112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson EJ, Demers LM, Krieg AF. Faster enzymatic procedure for serum triglycerides. Clin Chem. 1975;21:1983–1985. [PubMed] [Google Scholar]
- Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- Sentinelli F, Filippi E, Fallarino M, Romeo S, Fanelli M, Buzzetti R, Berni A, Baroni MG. The 3′-UTR C>T polymorphism of the oxidized LDL-receptor 1 (OLR1) gene does not associate with coronary artery disease in Italian CAD patients or with the severity of coronary disease. Nutr Metab Cardiovasc Dis. 2006;16:345–352. doi: 10.1016/j.numecd.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Tatsuguchi M, Furutani M, Hinagata J, Tanaka T, Furutani Y, Imamura S, Kawana M, Masaki T, Kasanuki H, Sawamura T, Matsuoka R. Oxidized LDL receptor gene (OLR1) is associated with the risk of myocardial infarction. Biochem Biophys Res Commun. 2003;303:247–250. doi: 10.1016/s0006-291x(03)00326-7. [DOI] [PubMed] [Google Scholar]
- Trabetti E, Biscuola M, Cavallari U, Malerba G, Girelli D, Olivieri O, Martinelli N, Corrocher R, Pignatti PF. On the association of the oxidised LDL receptor 1 (OLR1) gene in patients with acute myocardial infarction or coronary artery disease. Eur J Hum Genet. 2006;14:127–130. doi: 10.1038/sj.ejhg.5201513. [DOI] [PubMed] [Google Scholar]
- Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]