Abstract
Generation of long-term antibody-mediated immunity depends on the germinal centre (GC) reaction, which requires cooperation between antigen-specific T and B lymphocytes. In the human X-linked lymphoproliferative disease and its gene-targeted mouse model, loss-of-function mutations in signalling lymphocyte activation molecule-associated protein (SAP, encoded by SH2D1a) cause a profound defect in GC formation by an as yet unknown mechanism. Using two-photon intravital imaging, here we show that SAP deficiency selectively impairs the ability of CD4+ T cells to stably interact with cognate B cells but not antigen-presenting dendritic cells. This selective defect results in a failure of antigen-specific B cells to receive adequate levels of contact-dependent T cell help to expand normally, despite sap−/− T cells exhibiting the known characteristics of otherwise competent helper T cells. Furthermore, lack of stable interactions with B cells renders sap−/− T cells unable to be efficiently recruited to and retained in a nascent GC to sustain the GC reaction. These results offer a compelling explanation for the GC defect due to SAP deficiency and provide novel insights into the bi-directional communication between cognate T and B cells in vivo.
The germinal centre (GC) reaction, which supports antibody affinity maturation and the generation of B cell memory1–3, requires activation of CD4+ T cells by antigen-bearing dendritic cells (DCs), followed by differentiation of these T cells and their physical interactions with antigen-activated B cells4. The activated T cells promote the survival, proliferation, and differentiation of the B cells by delivering contact-dependent helper signals in an antigen-specific fashion5–8. Follicular helper T (TFH) cells, a subset of activated CD4+ T cells that highly express CXCR5, CD40L, ICOS, and signalling lymphocyte activation molecule-associated protein (SAP), are particularly important for a sustained GC response9.
In human X-linked lymphoproliferative disease (XLP) and its gene-targeted mouse model, lack of functional SAP protein in T cells causes a profound defect in GC formation and humoral immunity10–17, but how SAP regulates the GC-promoting T helper cell response is unknown. An intracellular adaptor protein, SAP binds to signalling lymphocyte activation molecule (SLAM) and other transmembrane molecules of the SLAM family18,19. It modulates T cell antigen receptor signalling and promotes type 2 T helper (TH2) cell differentiation13,14,20. However, impaired TH2 differentiation alone is not an adequate explanation for the profound GC defect seen in SAP-deficient animals17,21,22. To better understand how SAP deficiency results in impaired GC formation, we took a combined approach of classical cellular immunology and intravital 2-photon imaging, which allowed us to examine not only the activation phenotype of T cells but also the dynamics of their critical interactions with DCs and B cells in vivo23.
Sap−/− T cells undergo normal activation by DCs
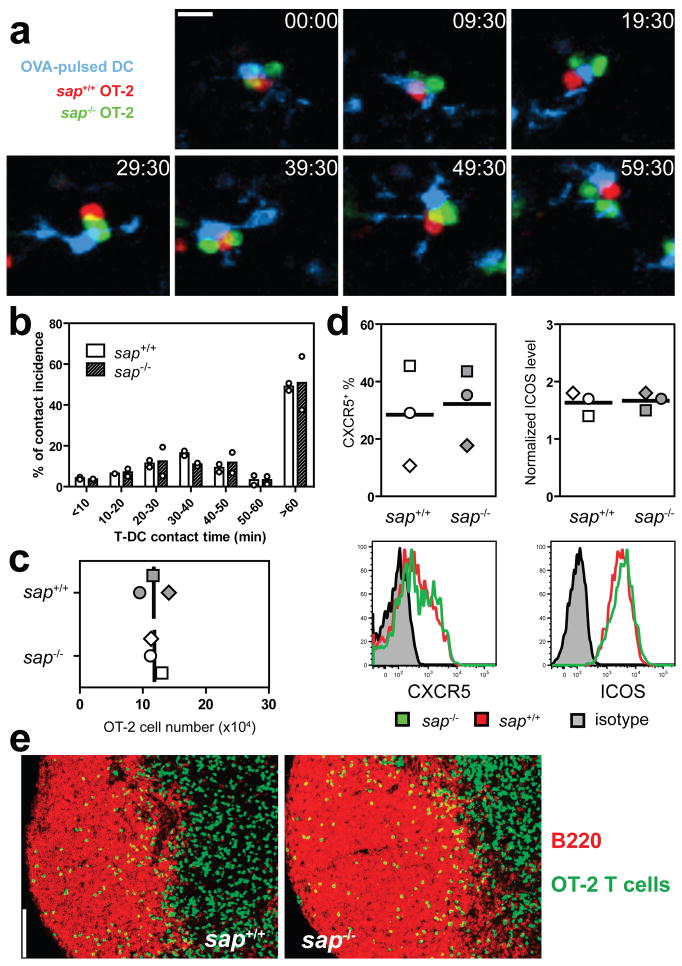
Because optimal T cell activation and differentiation in vivo require long-lasting T-DC conjugation24–27, we first examined the impact of SAP deficiency on T-DC interactions using OT-2 TCR transgenic T cells specific to I-Ab complexed with an ovalbumin-derived peptide (OVA323). Behaviours of sap−/− and sap+/+ T cells in the same draining lymph node (LN) were compared following co-transfer into mice previously injected s.c. with OVA323-pulsed DCs. Sap−/− and sap+/+ OT-2 T cells showed equivalent contact times with DCs presenting OVA323 (Figs. 1a and 1b, Supplementary Movie 1). Consistent with this result, sap−/− and sap+/+ OT-2 T cells proliferated and accumulated to a similar extent (Fig. 1c). Within the expanded pool of activated T cells of either genotype, similar frequencies of CXCR5+ICOS+ TFH precursors were observed and both groups of T cells migrated into the B cell follicular areas of the LN (Fig. 1d and 1e). These data indicate SAP deficiency does not grossly alter initial T cell interaction with or activation by antigen-presenting DCs in vivo.
Figure 1. Sap−/− T cells normally interact with and are activated by DCs in vivo.
a, Time-lapse images of sap+/+ and sap−/− OT-2 T cells interacting with OVA323-pulsed DCs in vivo (see also Supplementary Movie 1). Scale bar=10 μm. b, Distribution of T-DC contact durations (circles: individual experiments; bars: means). A total of 232 and 224 contacts were scored for sap+/+ and sap−/− T cells, respectively. c, d, e, T cell activation phenotypes 96 hours after transfer into recipients of OVA323-pulsed DCs. Absolute numbers of OT-2 cells (c) and typical patterns of CXCR5 and ICOS expression (d). Individual symbols: mean of 3–4 mice/group/experiment; Lines: mean of the 3 experiments. (e) The distribution of activated T cells in B cell follicles, representative of three experiments. Scale bar=100 μm.
Sap−/− T cells exhibit a selective defect in stable interactions with B cells
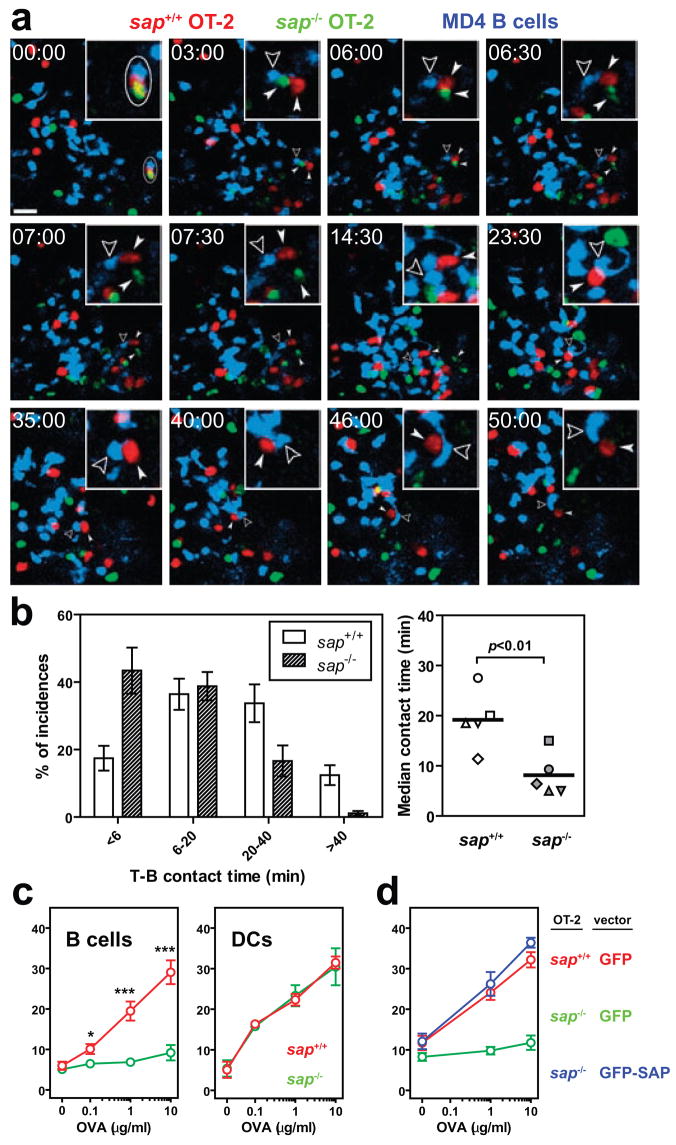
During a T-dependent B cell response, helper T cells activated by DCs must subsequently engage antigen-triggered B cells based on class II major histocompatibility complex (MHC)-restricted antigen recognition7,28. This process is characterized by long-lasting, mobile conjugate pairs formed between cognate T and B cells29. Based on these prior observations, we used intravital imaging to assess whether SAP deficiency affects antigen-dependent T-B interactions in vivo. Sap−/− and sap+/+ OT-2 T cells were co-transferred into mice together with wild-type B cells expressing the MD4 transgenic B cell receptor, which recognizes hen egg lysozyme (HEL). As antigen, cross-linked conjugates of HEL and OVA (HEL-OVA) were used to allow activation of both T and B transgenic cells and to permit MHC class II-dependent antigen presentation to the OT-2 T cells by the MD4 B cells. Following immunization, sap+/+ OT-2 T cells engaged migrating MD4 B cells and formed long-lasting mobile conjugate pairs, while sap−/− OT-2 T cells showed predominantly brief contacts with the same cohort of MD4 B cells (Figs. 2a and 2b; Supplementary Movies 2 and 3; mean±SEM of the median T-B contact time by sap+/+ vs. sap−/− T cells: 19.2±2.6 vs. 8.2±1.9 minutes, 5 experiments). Thus, are in striking contrast to the lack of significant effects on the duration of T-DC interactions (Fig. 1a and 1b), the absence of SAP expression in T cells severely reduces the longevity of T-B interactions in vivo.
Figure 2. Sap−/− T cells are defective in adhesion to cognate B cells.
a, Time-lapse images of sap+/+ and sap−/− T cells interacting with MD4 B cells 24–36 hours following HEL-OVA immunization. Circle: a cell cluster composed of one sap+/+ and one sap−/− T cell in contact with the same MD4 B cell at time zero. Solid arrowheads follow the two T cells, and open arrowheads highlight the B cell. Inserts: the MD4 cell and its immediate surrounding (see also Supplementary Movie 2). Scale bar=20 μm. b, Left, distribution of contact durations between T cells and MD4 B cells (mean±SEM of 5 experiments; 190 and 173 contacts scored for sap+/+ and sap−/− T cells, respectively). Right, the median durations of T-B contacts measured in individual experiments. Line: mean of the 5 medians. c, Conjugation efficiency of sap+/+ or sap−/− OT-2 T cells with OVA323-pulsed B cells or DCs, expressed as mean±SEM frequencies of CD4+CD19+ or CD4+CD11c+ conjugates in total CD4+ events (5 experiments, * p<0.05, *** p<0.001; see representative cytometry plots in Supplementary Fig. 1). . d, T-B conjugation assays were conducted using OT-2 cells transiently transfected with DNA constructs expressing either GFP or GFP-tagged SAP as indicated. Frequencies measured in 6 independent experiments are presented as mean±SEM (also see Supplementary Fig. 1).
To examine more quantitatively the differential effect of SAP deficiency on T-APC interactions, a flow cytometry-based in vitro assay was utilized in which shear stress was imposed on unfixed conjugates between activated OT-2 T cell blasts and OVA323-pulsed DCs or activated B cells. While a dose-dependent increase in T-B conjugate efficiency was observed with sap+/+ OT-2 T cells, conjugate formation by sap−/− T cells was low at all peptide concentrations tested (Fig. 2c). In contrast, within the same non-saturating peptide dose range, sap−/− T cells were as efficient as wild-type T cells in forming conjugates with DCs (Fig. 2d). To determine whether the defective adhesion to B cells by activated sap−/− T cells might have been programmed during their priming interactions with DCs and whether SAP expression is specifically required during T-B interactions, DNA constructs encoding either green fluorescent protein (GFP) or a GFP-SAP fusion protein were independently transfected into sap−/− T cell blasts. Complementation with GFP-SAP but not GFP alone fully rescued T-B conjugate formation by transfected sap−/−T cell blasts (Fig. 2e; also see Supplementary Fig. 1). Thus, the requirement for SAP expression in T cells for optimal adhesion to B cells is contemporaneous with T-B interaction. SAP facilitates the recruitment of Fyn kinase to cytoplasmic tails of SLAM-related proteins, which is important for SAP regulation of Th2 cytokine production20,30 but less so for SAP-controlled GC development17,31. Consistent with these latter results, we found that SAPR78A, a mutant SAP capable of binding SLAM-related molecules but severely impaired in binding Fyn kinase20,30, rescued T-B conjugate formation in vitro (Supplementary Fig. 2). Taken together, our analyses of T cell-APC conjugation establish that SAP critically regulates the contact duration and the adhesive strength of cognate T-B but not antigen-driven T-DC interactions.
B cells fail to acquire sufficient help from sap−/− T cells
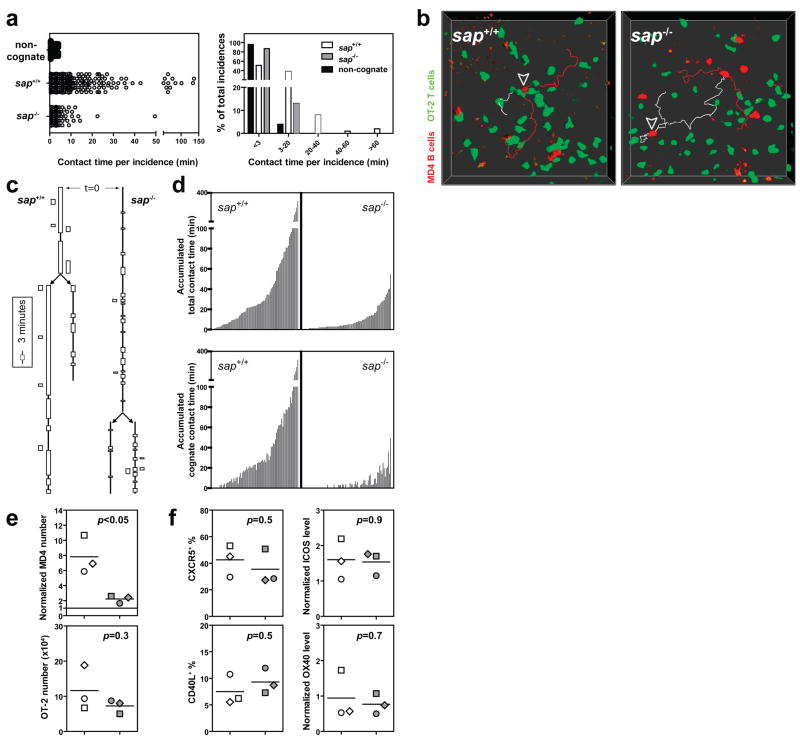
The cell numbers used in the preceding experiments can result in competition among T cells for antigen on presenting cells in vivo32, raising the question of whether sap−/− T cells would, if present alone, form more stable contacts with antigen-specific B cells or provide B cells with a summed contact time from sequential brief interactions comparable to the total interaction time with wild-type T cells. To examine these issues, a small number (6×104) of sap+/+ or sap−/− OT-2 T cells were separately transferred into recipient B6 mice that concomitantly received 3×105 MD4 B cells. Cumulative interactions between B and T cells were assessed by continuous intravital imaging over a 3-hour period of time between 60 to 72 hours post- immunization, when substantial clonal expansion has begun and physical co-localization of activated T and B cells can be observed 8,29,33,34. Under these modified conditions, sap−/− OT-2 cells still demonstrated abbreviated contacts with activated MD4 B cells following immunization with HEL-OVA (2.1±0.2 minutes [mean±SEM] vs. 8.9±0.8 minutes for sap+/+ T cells, p<0.0001; Fig. 3a, Supplementary Movie 4). While many contacts involving sap−/− T cells were brief and similar to non-cognate T-B contacts in duration, on the population level they lasted for a statistically longer period of time (2.1±0.2 vs. 1.3±0.1 minutes for non-cognate interactions, p=0.0014), indicating that antigen-specific interactions occurred between sap−/− OT-2 cells and MD4 B cells but were not sustained. Individual B cells were also tracked for their interactions with different T cells. MD4 cells often accumulated >1 hour of interactions with sap+/+ OT-2 T cells, while they were rarely able to accumulate more than 20 minutes of contact with sap−/− OT-2 T cells over the same period (Figs. 3b–d; Supplementary Movies 4 and 5). B cells remained equally trackable under the two conditions and interacted with similar numbers of sap−/− or sap+/+ T cells (Supplementary Fig. 3). The severe reduction in cumulative time of contact with sap−/− OT-2 T cells correlated with a pronounced impairment of MD4 B cell clonal expansion (Fig. 3e). Nonetheless, sap−/− OT-2 T cells expanded comparably to their sap+/+ counterparts and expressed similar levels of CD40L, ICOS, OX40, and CXCR5 (Fig. 3f; Supplementary Fig. 4) at this time. The latter is a phenotype considered characteristic of activated T cells capable of effectively promoting humoral responses and with features of TFH precursor cells as commonly defined9,35. In concert with the previous data, these findings suggest that it is not an intrinsic inability of SAP-deficient T cells to express key molecular signals, but rather a failure to exchange these signals during sufficiently long periods of contact with B cells that accounts for their markedly reduced capacity to promote B cell expansion and subsequent GC development.
Figure 3. B cells fail to receive contact-dependent help from SAP-deficient T cells.
a, Individual T-B contact durations (left) and their distribution (right). The non-cognate condition involves sap+/+ OT-2 cells non-specifically interacting with MD4 B cells following immunization with HEL-BSA mixed with OVA protein (137 measurements from 2 experiments). The cognate condition involves specific interactions between sap+/+ or sap−/− OT-2 T cells and MD4 B cells following immunization with HEL-OVA (380 and 390 measurements from 3 experiments). b, c, d, Individual MD4 B cells were tracked for their cumulative interactions with OT-2 T cells over 3 hours. b, Examples of tracked MD4 cells (arrowheads). The MD4 cells represented by white tracks divided during the imaging period, giving rise to two daughter cells represented by red tracks. c, Interaction histories of the MD4 cells in (b) are plotted in the line-box graphs. Vertical lines indicate the periods during which the B cells were present in the imaged volume. Arrows indicate cell divisions giving rise to daughter cells. Each box represents one contact with a T cell, and its height indicates the contact duration (see also Supplementary Movie 5). d, A total of 82 MD4 cells in each condition were tracked in 3 experiments. Each MD4 B cell is plotted as one bar. The bar height is the sum of all T cell contacts (top) or only those contacts that were not shorter than 3 minutes (bottom), a cut-off differentiating cognate from non-cognate T-B interactions with a 95% confidence as determined in (a). e, f, MD4 B cells (7-AAD−CD19+IgMa+CD4−GFP− singlet events) and OT-2 T cells (7-AAD−CD19−CD4+GFP+ singlet events) were analyzed by FACS in 3 experiments at 96 hours post HEL-OVA immunization. Individual symbols: mean of 3–4 mice/group/experiment; Lines: mean of the 3 experiments. e, Cell expansion. Top, numbers of MD4 cells recovered after co-transfer of sap+/+ or sap−/− OT-2 T cells were normalized against the number obtained where no exogenous OT-2 cells were co-transferred (dotted line at unit 1). Bottom, absolute numbers of recovered OT-2 cells under the condition of co-transfer with B cells. f, Surface expression of CXCR5, CD40L, ICOS, and OX40 by the two types of OT-2 T cells. See Online Methods for details of quantitative analysis and Supplementary Fig. 4 for representative cytometry plots.
Sap−/− T cells are not efficiently recruited into and retained in forming GCs
Recruitment of helper T cells to a forming GC is necessary for maintaining the GC reaction36, and GC-localized TFH cells are required for effective immunoglobulin class-switching and antibody affinity maturation9. To explore whether SAP deficiency also impairs T cell recruitment to and retention within nascent GCs, we transferred equal numbers (3×104) of CFP-expressing sap+/+ OT-2 cells and GFP-expressing sap−/− OT-2 cells into B6 mice together with 3×105 non-fluorescent MD4 cells. Six to 8 days after HEL-OVA immunization, GCs developed within the draining LN, seen as GL7+ areas that largely excluded IgD+ naïve B cells (Supplementary Fig. 5). While the IgD+ follicular mantle zone was populated by both sap+/+ and sap−/− OT-2 cells, the GC area predominantly contained sap+/+ OT-2 cells, consistent with a failure of GC recruitment and/or retention of sap−/− T cells.
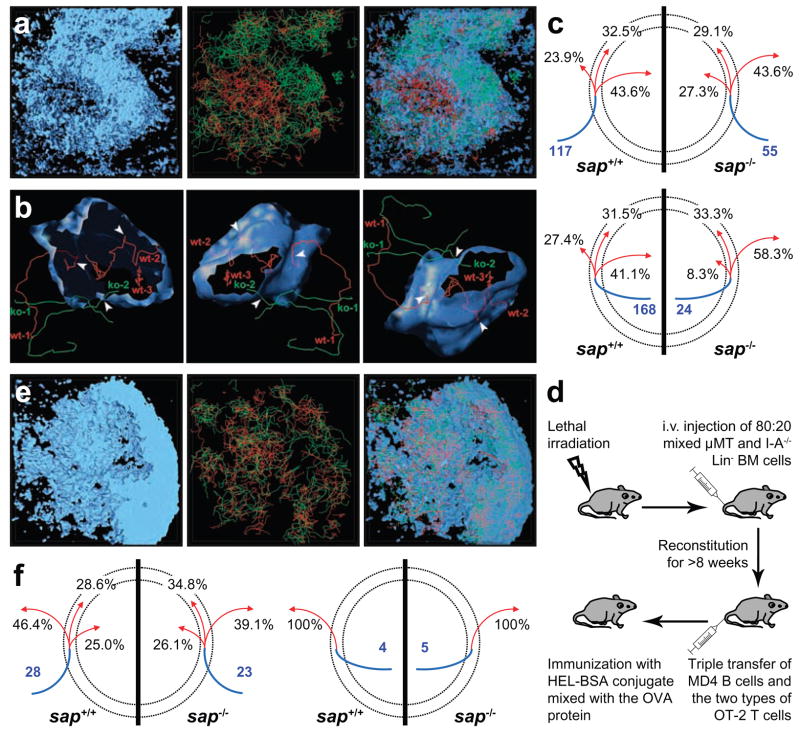
To examine this issue dynamically, recipient mice were also given dye-labelled naïve B cells 24 to 48 hours before intravital imaging to differentiate follicular mantle and GC areas. Although individual naïve B cells migrate in and out of GCs37, as a population they remain substantially excluded from GCs (Supplementary Fig. 5), allowing simultaneous identification of the follicular area and approximation of the GC-mantle border using time-averaged images (see Supplementary Fig. 6 for details). Sap+/+ and sap−/− OT-2 T cells exhibited strikingly different dynamic patterns within the follicle (Supplementary Movie 6 and Fig. 4a). While sap+/+ OT-2 T cells migrated freely into and accumulated within the GCs, sap−/− OT-2 T cells mainly swarmed in the follicular mantle. To quantify migratory behaviours of the two types of T cells around GCs, a tessellation algorithm was used to retrieve the GC surface (Supplementary Fig. 6) and define a virtual mantle-GC interface zone was then defined as encompassing spatial coordinates within 10 μm of the GC outer surface. T cell tracks were subsequently classified according to their interactions with this interface zone (Fig. 4b and 4c). When reaching the interface from the follicular mantle, sap+/+ OT-2 T cells were more likely to continue into the GC than to return to the mantle. Sap−/− T cells exhibited the opposite behaviour, being more likely to turn back than to cross into the GC. Conversely, when reaching the interface zone from within the GC, sap+/+ T cells were more likely to return to the GC than to escape into the mantle, whereas sap−/− T cells were much more likely to escape than to return. For those tracks that started from the interface zone, sap−/− T cells exhibited a preference for migrating into the mantle as compared to moving into the GC, while for sap+/+ T cells the movement in these two directions was comparable. Consistent with these distinct migration patterns, sap−/− T cells also exhibited significantly shorter GC retention times (Supplementary Fig. 7). Therefore, in the absence of SAP, T cells are not efficiently recruited into or retained within a nascent GC. These data suggest SAP-deficient T cells cannot act as effective GC-associated TFH cells to sustain the GC reaction, a defect that would contribute to the profound impairment of GC responses in SAP-deficient hosts.
Figure 4. Defective GC recruitment and retention of sap−/− T cells due to lack of efficient cognate interactions with B cells.
a, Typical migration patterns of sap+/+ (red) and sap−/− (green) OT-2 T cells in follicles containing cognate GCs. Left: the naïve B cell-dominated follicular mantle zone and its encased GC area (see Supplementary Fig. 6 for details of this 3-D rendering); Middle: the distribution of migration tracks of the two types of T cells; Right: an overlay with the mantle zone rendered as semi-transparent (see also Supplementary Movie 6). b, The GC surface retrieved from the dataset in (a) is rendered semi-transparent in 3 different orientations together with examples of 5 T cell tracks that differentially interacted with this surface. Tracks are labelled at their temporal beginnings. Arrowheads highlight the positions at which tracks cross the GC surface. c, Migration patterns of sap+/+ and sap−/− T cells that arrived at a 10 μm-wide virtual mantle-GC interface zone. The area between the two dotted circles represents the 10 μm-wide interface zone; the inner circle represents the GC surface, while area outside of the outer circle is the follicular mantle. The blue line segment indicates the region in which a track began, i.e. the mantle zone (top) or the GC (bottom). The number in blue is the total number of tracks of the indicated type analyzed. The three red arrows denote tracks that subsequently moved into the mantle zone, stayed within the interface zone, or moved into the GC area, respectively. Corresponding numbers denote percentages of tracks of each genotype that exhibited the indicated behaviour. Data are pooled from 3 experiments. d, e, f, Migration of sap+/+ and sap−/− OT-2 T cells in follicles containing non-cognate GCs. d, A schematic diagram for the protocol used to generate GCs that are non-cognate to the OT-2 cells activated in the same LN. e, In the same configuration as in (a), typical migration patterns of the two types of T cells (see also Supplementary Movie 7). f, T cells tracks that came within 10 μm from non-cognate GCs were analyzed for their subsequent migrations using the same method as used in (c). Data from 2 experiments are pooled.
To address whether the inefficient GC recruitment and retention of sap−/− T cells resulted from their reduced antigen-specific interactions with B cells, radiation bone-marrow chimeras were constructed so that endogenous B cells were deficient in class II MHC expression and thus unable to engage in antigen-specific interactions with T cells, while T cells could still interact with and be activated by non-B antigen presenting cells including DCs (Supplementary Fig. 8; see a methodological diagram in Fig. 4d). Following co-transfer of both sap+/+ and sap−/− OT-2 T cells together with MD4 B cells into these chimeric animals, they were immunized with a mixture of HEL-BSA and intact OVA proteins. In this setting, MD4 B cells cannot engage transferred OT-2 T cells as cognate partners but can still form GCs (Supplementary Fig. 9a) by utilizing cognate help from endogenous CD4+ T cells. OT-2 cells in these mice can be normally activated by OVA-presenting DCs, but are deprived of cognate interactions with B cells, which are either transferred MD4 cells that do not present OVA or endogenous B cells that are derived from class II MHC-deficient bone marrow progenitors. While both sap+/+ and sap−/− OT-2 T cells upregulated CXCR5 and ICOS expression in this experimental system (Supplementary Fig. 9b), now they both failed to be efficiently recruited into and retained within GCs (Figs. 4e and 4f, Supplementary Fig. 7, Supplementary Movies 7 and 8). When the same chimeric mice were immunized with HEL-OVA, a condition in which OT-2 T cells could engage MD4 B cells as cognate partners, sap+/+ OT-2 T cells were recruited to the GC, while sap−/− OT-2 T cells were not (data not shown). These findings indicate that T cells require cognate interactions with B cells to be recruited into and retained within GCs and suggest that, by failing to engage productively in such cell-cell interactions, SAP-deficient T cells are unable to physically localize to the GC to effectively sustain the GC reaction.
Conclusions
This study demonstrates that SAP expression in T cells is critical for stable antigen-dependent T-B adhesion but dispensable for T-DC interactions, revealing that distinct molecular rules govern the stable interactions of CD4+ T cells with two major antigen presenting cell types in vivo. Stabilization of T-B interactions may rely on a T cell-autonomous mechanism orchestrated by SAP and its associated SLAM family molecules at the cell-cell interface, while stable T-DC association might be actively promoted via DC-mediated mechanisms, such as those involving cytoskeletal and membrane mechanics38,39. In accord with this notion, B cells but not DCs express high levels of SLAM, CD84, Ly9, and Ly108 molecules (Supplementary Fig. 10), which are also highly expressed by T cells activated in vitro and by TFH cells in vivo (ref. 40 and data not shown). The differential SAP regulation of T-DC and T-B interactions correlates with our observations that CD4+ T cell activation in vivo is grossly normal irrespective of SAP expression, while otherwise competent effector T cells fail to efficiently deliver contact-dependent help to cognate B cells in the absence of SAP. At the cellular level, these results provide a likely explanation for why SAP deficiency specifically impairs T-dependent humoral immunity but leaves intact or even exaggerates cell-mediated immune responses that also require CD4+ T cell activity13–15,41,42. While additional factors may also contribute to the overall defects of humoral immunity seen in SAP-deficient hosts43, our results strongly suggest the predominant mechanism is the disruption of antigen-specific T-B adhesion. Future studies are necessary to determine the molecular basis for how SAP selectively controls cognate T-B association, to identify SAP-associated SLAM family proteins involved in this process, and to elucidate the cascade of intercellular signal exchange that transpires across a stable T-B synapse.
In conclusion, our findings offer a compelling explanation for the profound impairment of the GC response in SAP-deficient mice and suggest a mechanism contributing to the B cell-centric pathologies associated with the XLP syndrome. They provide novel insights into the operating principles of intercellular communication between T cells and diverse antigen presenting cells and serve as a striking example of the importance of a direct visualization approach in understanding immune system function in vivo.
METHODS SUMMARY
Cell conjugation assay in vitro
OT-2 T cells (5×105/well) were incubated for 30 min at 37°C in 96-well U-bottom plates with DCs (106/well) or LPS-activated B cells (2×106 per well) that had been pulsed with antigen. Conjugates were enumerated by flow cytometry after the cell mixture was stained at 4°C for CD4, CD11c, and CD19 and repeatedly washed.
Adoptive transfer, cell phenotyping, and intravital imaging
To visualize T-DC interactions (Fig. 1), OVA323-pulsed DCs were injected subcutaneously at 2×106 per mouse 24 hours prior to intravenous transfer of sap+/+ and sap−/− T cells (3×106 each). Imaging was conducted 12 to 24 hours later. To examine activation phenotypes, 106 DCs and 2×105 GFP-expressing T cells per mouse were used. To visualize interactions between B cells and sap+/+ and sap−/− T cells in the same LN (Fig. 2), 3×106 OT-2 T cells of each genotype were co-transferred into mice together with 5×106 wild-type B cells. Immunization was done 12 hours prior to cell transfer, and imaging was conducted 24 to 36 hours thereafter. To visualize T-B interactions under non-competitive conditions and to assay T and B cell expansion (Fig. 3), 6×104 GFP-expressing OT-2 T cells were co-transferred together with 3×105 CFP-expressing B cells 24 hours prior to immunization. Imaging and cytometric analyses were conducted 60–72 and 96 hours later, respectively. To visualize GC recruitment and retention of T cells (Fig. 4), 3×104 CFP-expressing sap+/+ and 3×104 GFP-expressing sap−/− OT-2 T cells were co-transferred with 3×105 non-fluorescent MD4 B cells. Imaging was conducted 6 to 8 days post immunization. Dye-labelled naïve B cells (2–4×107) were given 1 day before imaging to provide follicle/GC landmarks. The imaging set-up was essentially as previously described44. For imaging sessions longer than 2 hours, the animal’s hydration was maintained by lactated Ringer’s solution given via a catheter. The typical X-Y-Z dimension was 0.5–1.1×0.5–1.1×3 μm, and the time resolution was 30–45s. For experiments involving co-transfer of two types of dye-labelled T cells, the cells were always reciprocally labelled to control for potential dye-induced behavioural differences.
Statistical analysis
The Mann-Whitney rank sum test was used to calculate p values for highly skewed distributions. For Gaussian-like distributions, two-tailed t tests were used.
Supplementary data
This file contains Supplementary Figures 1-10 and Supplementary movie legends.
Acknowledgments
H.Q. is in debt to Y. Hong for support, encouragement, and inspiration. This work was funded by the intramural research programs of the NIAID and NHGRI, NIH, U.S.A.
Footnotes
Reprints and permission information is available online at www.nature.com/reprints. The authors declare no competing financial interests.
References
- 1.Coico RF, Bhogal BS, Thorbecke GJ. Relationship of germinal centers in lymphoid tissue to immunologic memory. VI Transfer of B cell memory with lymph node cells fractionated according to their receptors for peanut agglutinin. J Immunol. 1983;131:2254–2257. [PubMed] [Google Scholar]
- 2.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 3.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 4.MacLennan IC, et al. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. 1997;156:53–66. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 5.Raff MC. Role of thymus-derived lymphocytes in the secondary humoral immune response in mice. Nature. 1970;226:1257–1258. doi: 10.1038/2261257a0. [DOI] [PubMed] [Google Scholar]
- 6.Mitchison NA. The carrier effect in the secondary response to hapten-protein conjugates. II Cellular cooperation. Eur J Immunol. 1971;1:18–27. doi: 10.1002/eji.1830010104. [DOI] [PubMed] [Google Scholar]
- 7.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 8.Garside P, et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 9.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 10.Purtilo DT, Cassel CK, Yang JP, Harper R. X-linked recessive progressive combined variable immunodeficiency (Duncan’s disease) Lancet. 1975;1:935–940. doi: 10.1016/s0140-6736(75)92004-8. [DOI] [PubMed] [Google Scholar]
- 11.Grierson HL, et al. Immunoglobulin class and subclass deficiencies prior to Epstein-Barr virus infection in males with X-linked lymphoproliferative disease. Am J Med Genet. 1991;40:294–297. doi: 10.1002/ajmg.1320400309. [DOI] [PubMed] [Google Scholar]
- 12.Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol. 2007;25:337–379. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- 13.Wu C, et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat Immunol. 2001;2:410–414. doi: 10.1038/87713. [DOI] [PubMed] [Google Scholar]
- 14.Czar MJ, et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci U S A. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crotty S, et al. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 16.Morra M, et al. Defective B cell responses in the absence of SH2D1A. Proc Natl Acad Sci U S A. 2005;102:4819–4823. doi: 10.1073/pnas.0408681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannons JL, et al. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffey AJ, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 19.Sayos J, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 20.Cannons JL, et al. SAP regulates TH2 differentiation and PKC-θ-mediated activation of NF-κB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Dent AL, Hu-Li J, Paul WE, Staudt LM. T helper type 2 inflammatory disease in the absence of interleukin 4 and transcription factor STAT6. Proc Natl Acad Sci U S A. 1998;95:13823–13828. doi: 10.1073/pnas.95.23.13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andoh A, et al. Absence of interleukin-4 enhances germinal center reaction in secondary immune response. Immunol Lett. 2000;73:35–41. doi: 10.1016/s0165-2478(00)00202-9. [DOI] [PubMed] [Google Scholar]
- 23.Huang AY, Qi H, Germain RN. Illuminating the landscape of in vivo immunity: insights from dynamic in situ imaging of secondary lymphoid tissues. Immunity. 2004;21:331–339. doi: 10.1016/j.immuni.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 25.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 26.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celli S, Lemaitre F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Abbas AK, Haber S, Rock KL. Antigen presentation by hapten-specific B lymphocytes. II Specificity and properties of antigen-presenting B lymphocytes, and function of immunoglobulin receptors. J Immunol. 1985;135:1661–1667. [PubMed] [Google Scholar]
- 29.Okada T, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson D, et al. Genetic evidence linking SAP, the X-linked lymphoproliferative gene product, to Src-related kinase FynT in TH2 cytokine regulation. Immunity. 2004;21:707–717. doi: 10.1016/j.immuni.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 31.McCausland MM, et al. SAP regulation of follicular helper CD4 T cell development and humoral immunity is independent of SLAM and Fyn kinase. J Immunol. 2007;178:817–828. doi: 10.4049/jimmunol.178.2.817. [DOI] [PubMed] [Google Scholar]
- 32.Garcia Z, et al. Competition for antigen determines the stability of T cell-dendritic cell interactions during clonal expansion. Proc Natl Acad Sci U S A. 2007;104:4553–4558. doi: 10.1073/pnas.0610019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelsoe G, Zheng B. Sites of B-cell activation in vivo. Curr Opin Immunol. 1993;5:418–422. doi: 10.1016/0952-7915(93)90062-w. [DOI] [PubMed] [Google Scholar]
- 34.Liu YJ, et al. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 35.Moser B, Schaerli P, Loetscher P. CXCR5+ T cells: follicular homing takes center stage in T-helper-cell responses. Trends Immunol. 2002;23:250–254. doi: 10.1016/s1471-4906(02)02218-4. [DOI] [PubMed] [Google Scholar]
- 36.de Vinuesa CG, et al. Germinal centers without T cells. J Exp Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwickert TA, et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 38.Al-Alwan MM, Rowden G, Lee TD, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452–1456. doi: 10.4049/jimmunol.166.3.1452. [DOI] [PubMed] [Google Scholar]
- 39.Benvenuti F, et al. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- 40.Chtanova T, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 41.Hron JD, et al. SH2D1A regulates T-dependent humoral autoimmunity. J Exp Med. 2004;200:261–266. doi: 10.1084/jem.20040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crotty S, et al. Hypogammaglobulinemia and exacerbated CD8 T-cell-mediated immunopathology in SAP-deficient mice with chronic LCMV infection mimics human XLP disease. Blood. 2006;108:3085–3093. doi: 10.1182/blood-2006-04-018929. [DOI] [PubMed] [Google Scholar]
- 43.Ma CS, et al. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest. 2005;115:1049–1059. doi: 10.1172/JCI23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary Figures 1-10 and Supplementary movie legends.