Abstract
In all sensory systems, information is processed along several parallel streams. In the vibrissa-to-barrel cortex system, these include the lemniscal system and the lesser-known paralemniscal system. The posterior medial nucleus (POm) is the thalamic structure associated with the latter pathway. Previous studies suggested that POm response latencies are positively correlated with stimulation frequency and negatively correlated with response duration, providing a basis for a phase locked loop-temporal decoding of stimulus frequency. We tested this hypothesis by analyzing response latencies of POm neurons, in both awake and anesthetized rats, to vibrissae deflections at frequencies between 0.3 and 11 Hz. We found no significant, systematic correlation between stimulation frequency and the latency or duration of POm responses. We obtained similar findings from recording in awake rats, in rats under different anesthetics, and in anesthetized rats in which the reticular activating system was stimulated. These findings suggest that stimulus frequency is not reliably reflected in response latency of POm neurons. We also tested the hypothesis that POm neurons respond preferentially to sensor motion, that is, they respond to whisking in air, without contacts. We recorded from awake, head-restrained rats while monitoring vibrissae movements. All POm neurons responded to passive whisker deflections, but none responded to noncontact whisking. Thus like their counterparts in the trigeminal ganglion, POm neurons may not reliably encode whisking kinematics. These observations suggest that POm neurons might not faithfully encode vibrissae inputs to provide reliable information on vibrissae movements or contacts.
INTRODUCTION
Sensory information in any given modality is processed along multiple parallel streams. For example, at each level of the somatosensory system, there are several groups of spatially segregated neurons that share physiological and anatomical properties and that relay information upstream to similarly segregated neuronal populations (Dykes 1983). Parallel processing of somatosensory information has been studied extensively in the rodent trigeminal vibrissae-to-cortex system, where the anatomical correlates of neuronal groupings are most evident (reviewed by Woolsey 1996). Here, stimuli are transduced by mechanoreceptors associated with the vibrissae and relayed along trigemino-tectal and trigemino-thalamic pathways (Cohen and Castro-Alamancos 2007; Hemelt and Keller 2007).
Most studies have focused on the trigemino-thalamic pathway and its role in relaying vibrissal inputs to the neocortex. Vibrissa-related information is relayed directly to the vibrissa representation in the somatosensory cortex—the “barrel cortex” (Woolsey and Van der Loos 1970)—through two major trigemino-thalamic nuclei: 1) the lemniscal ventroposterior medial (VPM) nucleus that receives vibrissae inputs primarily from the principal trigeminal nucleus (PrV) and 2) the paralemniscal posterior medial nucleus (POm) whose vibrissae responses originate both from ascending inputs from the trigeminal nucleus interpolaris (SpVi) and from descending inputs from the barrel cortex (Bureau et al. 2006; Deschenes et al. 2005; Diamond et al. 1992a; Pierret et al. 2000; Trageser and Keller 2004; Yu et al. 2006).
Convergent evidence from physiological, anatomical, behavioral, and computational studies supports the conclusion that VPM and the lemniscal system are involved in high-resolution encoding of contact and texture information relayed from the vibrissae (reviewed by Kleinfeld et al. 2006). The role of POm and the paralemniscal system is more obscure. In a series of seminal studies, Ahissar and collaborators provided evidence to support the hypothesis that this pathway functions as a phase locked loop (PLL), which translates the frequency of vibrissae deflections into a rate code (Ahissar 1998; Ahissar et al. 2000). A key prediction of the PLL model is that the latency of POm responses increases systematically as the frequency of vibrissae stimuli increases, a prediction supported by data recorded from anesthetized rats (Ahissar et al. 2000). However, we recently presented preliminary findings suggesting that POm responses are unaffected by changes in the frequency of vibrissae stimuli (Masri et al. 2006). Here, we rigorously test the relationship between vibrissae stimulation parameters and POm responses recorded under different anesthetics and—for the first time—in awake animals. Our results are consistent with our preliminary reports and suggest that POm neurons are unreliable encoders of vibrissae contacts.
It has also been suggested that POm neurons encode sensor motion signals; that is, they are preferentially responsive during voluntary vibrissae movements (whisking; Yu et al. 2006). We directly test this hypothesis by recording from POm neurons in rats and report that whisking in air, without vibrissae contacts, fails to evoke significant activity in POm neurons.
METHODS
We used female Sprague-Dawley rats weighing 250–350 g for awake (7 rats) or anesthetized (22 rats) in vivo recordings. All procedures adhered strictly to institutional and federal guidelines.
Experiments in anesthetized animals
URETHANE ANESTHESIA.
Rats were anesthetized with an intraperitoneal injection of urethane (1.5 g/kg), and supplemental injections (150 mg/kg) were administered as needed. We monitored electrocorticograms (ECoGs) to assess the stage of anesthesia and maintained the rats at stages III/3–4 (Friedberg et al. 1999).
FENTANYL ANALGESIA.
We anesthetized the animals initially with halothane (3%) and inserted a venous catheter in the jugular vein for drug delivery and a second catheter in the femoral artery for monitoring blood pressure and heart rate. Following the insertion of catheters, we discontinued the administration of halothane and infused the rats intravenously with fentanyl (10 μg/kg/h) for the rest of the experiment. We immobilized the rats with pancuronium bromide (1.5 mg/kg/h), and they were artificially respired with a positive pressure respirator at 90 breaths/min. We monitored blood pressure and heart rate throughout the experiment to ensure that the animal was in no pain or distress.
SURGICAL PROCEDURES AND ELECTROPHYSIOLOGY.
Anesthetized rats were placed in a stereotaxic device for the duration of the experiments. All incision sites were infused with local anesthetics. We maintained body temperature at 37°C with a servo-controlled heating blanket. We advanced electrodes in the right hemisphere based on stereotaxic coordinates (Paxinos and Watson 1998) and obtained extracellular unit recordings with quartz-insulated platinum electrodes (2–4 MΩ) from POm neurons. We digitized waveforms (40 kHz) recorded from well-isolated units through a Plexon (Dallas, TX) data acquisition system and sorted units off-line with Plexon's Off-line Sorter, using dual thresholds and principal component analyses. We generated autocorrelograms with Neuroexplorer software (Littleton, MA) to confirm that we obtained recordings from single units.
We marked recording sites with electrolytic lesions (5 μA for 10 s) at the end of the experiment and deeply anesthetized the rats with pentobarbital sodium (60 mg/kg) and perfused them transcardially with buffered saline followed by 4% buffered paraformaldehyde. We obtained coronal brain sections (70 μm thick) and Nissl stained the sections to identify recording sites.
Laterodorsal tegmentum and pedunculopontine tegmentum stimulation
As in our previous study (Masri et al. 2006), we targeted a concentric bipolar stimulating electrode (250 μm diam; Frederick Haer, Bowdoinham, ME) to the laterodorsal tegmentum (LDT) and the pedunculopontine tegmentum (PPT) nuclei, based on stereotaxic coordinates (AP, 8–9.0; ML, 0.5–1.5; 5–6.0 mm deep). Electrical stimulation (200 μA) of LDT/PPT consisted of 200-μs pulses delivered at 100 Hz for 1 s.
Experiments in awake animals
SURGERY.
Seven female rats were handled daily for 1 mo before surgery. Under halothane anesthesia (1–3%) and aseptic surgical conditions, a small incision was made in the skin overlying the masseter muscle, and a pair of dipolar EMG electrodes (“0.003”, Teflon-coated stainless steel wire) were tunneled subcutaneously into the deep intrinsic musculature of the vibrissae pad. The ends of the wires were run to the top of the head and soldered to pin connectors. Correct placement of the wires was verified using microstimulation to evoke vibrissae movements. The animals were pretreated with dexamethasone (2 mg/kg) to minimize brain swelling and atropine (0.1 mg/kg) to minimize secretions.
The rats were placed in a stereotaxic frame, a scalp incision was made above the midline, and the periosteum was reflected. All incision sites were infused with local anesthetics, and body temperature was maintained at 37°C with a servo-controlled heating blanket. The skull was cleaned and dried, and 15–20 skull screws (TX00-2-C, Small Parts) were inserted (4 screws anterior to Lambda, 3 in between Bregma and Lambda, 4 to 6 behind Bregma, 3 in the occipital bone, and 3 in each temporal bone) to provide support for a dental cement platform that served to hold the head mounting bolts, EMG connectors, and ECoG recording connector in place. We performed a craniotomy (bilaterally in 1 animal, unilaterally in the others) directly above the thalamus and cemented a pin above Bregma to be used as a reference point for stereotaxic coordinates. The craniotomy site was covered with a thin layer of dental cement. At the end of surgery, the skin was sutured, and the animals received an injection of the analgesic buprenorphine hydrochloride (0.3 mg/kg).
MONITORING VIBRISSAE MOVEMENTS.
In addition to monitoring vibrissae movements with EMG recordings (Cramer and Keller 2006), we modified the optoelectronic method of Zeigler and collaborators (Bermejo et al. 1998) to optically track vibrissae movements. We used the analog output from a high-resolution charge-coupled detector (CCD) device (MetraLight, Sunnyvale, CA) to monitor vibrissa position as a function of time. We attached a marker of self-adhesive insulating foam to the vibrissa (Bermejo et al. 1998). The vibrissa crossed the detectors ∼15 mm from the face. CCD analog signals were digitized at 10 kHz through the Plexon data acquisition system.
ELECTROPHYSIOLOGY.
At least 1 wk following the surgery, rats were placed in a custom made box and head restrained (Friedman et al. 2006). Quartz-insulated platinum electrodes (2–4 MΩ) were dipped into DiI before recording to facilitate postmortem visualization of the electrode tracts as described by DiCarlo et al. (1996). Before electrode insertion, we removed the dental cement covering the craniotomy and advanced the electrodes to target POm or VPM neurons. Recordings were performed 5 d/wk for an average of 2 h/session for a period ranging from 1 to 2 mo. After each recording session, the craniotomy site was covered with a thin layer of dental cement.
To identify recording sites, at the end of the experiments, we made electrolytic lesions (5 μA for 10 s) at different coordinates and deeply anesthetized the rats with pentobarbital sodium (60 mg/kg). The rats were perfused transcardially with buffered saline followed by 4% buffered paraformaldehyde. We obtained coronal brain sections (70 μm thick) and Nissl stained them with Sytox Green. The sections were examined under fluorescent microscopy to identify recording tracts and lesion sites.
Vibrissae stimulation
Following the isolation of vibrissae sensitive neurons, we stimulated the vibrissae with air puffs delivered through a tube (0.5 mm diam) by a computer-controlled Picospritzer (General Valve, Fairfiled, NJ). We delivered 50-ms air-puffs at 2, 5, 8, or 11 Hz in the anesthetized experiments and 0.3, 1, or 5 Hz in the awake experiments. The pressure was set at 60 psi, resulting in vibrissae deflections of ∼30°. We regularly calibrated the output from the Picospritzer using a piezoelectric device to correct for temporal delay between the trigger to the Picospritzer and the vibrissae deflection and to ascertain that the kinematics of the pulses were reproducible. At the stimulus frequencies we used, air-puffs were delivered with a temporal precision ≤0.5 ms, well below the latency differences reported by Ahissar and collaborators (17, 24, 36, and 43 ms for 2, 5, 8, and 11 Hz, respectively; Sosnik et al. 2001).
We determined receptive field properties—identity of vibrissae evoking responses and the preferred direction for eliciting responses—by manually deflecting the vibrissae using a thin cylindrical probe and monitoring the audio conversion of the amplified activity signal. Once the receptive field was mapped, we deflected vibrissae in their preferred direction, i.e., the direction that elicited the highest magnitude response. We deflected at least four vibrissae simultaneously to evoke responses in POm neurons. We chose the vibrissae stimulation parameters described above to replicate the parameters reported by Ahissar and collaborators (Ahissar 1998; Ahissar et al. 2000; Sosnik et al. 2001). To confirm that our stimuli were quantitatively indistinguishable from those used in the earlier studies, in a separate set of experiments, we recorded vibrissae responses from spinotrigeminal interpolaris (SPVi) neurons, the primary brain stem input to POm (Veinante et al. 2000). In agreement with the report by Sosnik et al. (2001), we found that neither the onset nor the offset latencies of the responses of SPVi neurons were affected by deflecting the vibrissae at different frequencies (2, 5, 8, or 11 Hz; Fig. 1).
FIG. 1.
Response magnitude and latency of neurons in the spinal trigeminal interpolaris (SPVi) nucleus are not affected by changes in stimulation frequency. Rasters (top) with peristimulus time histograms (PSTHs; bottom) constructed from recordings of a single SPVi neuron in response to a 50-ms stimulus delivered to the vibrissae at t = 0. Air puffs were delivered at 2, 5, 8, or 11 Hz. Recordings obtained under urethane anesthesia.
Data analysis
We isolated single units off-line with Off-line Sorter, as described above. We exported time stamps of well-isolated units and of stimulus triggers to Matlab (MathWorks, Natick, MA) for analyses using custom-written algorithms. We constructed peristimulus time histograms (PSTHs; 1-ms bins) and defined significant stimulus-evoked responses as PSTH bins whose response magnitude significantly exceeded [99% confidence interval (CI)] spontaneous activity levels, computed from a 200-ms period preceding the stimuli.
We defined response onset as the first two consecutive bins (poststimulus) displaying significant responses (defined as above) and defined response offset as three consecutive bins in which response magnitude fell below the 99% CI. Steady-state onset latency (T0) was calculated from the PSTHs by eliminating the first eight trials and determining the latency to the first significant response. Using PSTHs, we also calculated the latency to the half-maximal response (T50; Sosnik et al. 2001).
We defined response magnitude as the total number of spikes per stimulus occurring between response onset and offset. We performed statistical analyses in SPSS (SPSS, Chicago, IL) and assessed, in individual neurons, changes in latency using the Kruskal-Wallis test; P < 0.05 was considered significant. To determine the sample size required to test hypothetical differences, for each of the statistical comparisons, we performed a power analysis using α = 0.05 and a power = 0.85.
EMG recordings were digitized (10 kHz) through the Plexon data acquisition system. The onset of individual whisks was identified in the EMG records using a custom-written Matlab algorithim, where a whisking burst was defined as significant increases in activity lasting ≥50 ms and preceded by ≥100 ms of no activity (Friedman et al. 2006). We collected the time stamps of these events and used them as a reference to construct PSTHs (1-ms bins) of each neuron's activity. We also used custom-written Matlab routines to construct spike-triggered averages (STAs) from rectified EMG using spikes of individual cells. We defined significant activity in the STA as activity that exceeded the 95% CI, relative to the mean baseline (200-ms pretrigger period). STAs were also constructed from CCD movement signals in a similar manner (without rectification).
RESULTS
POm responses in anesthetized animals
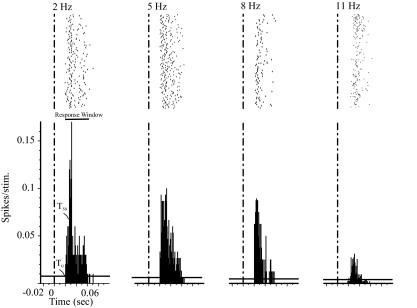
We tested the frequency dependency of POm responses under either urethane anesthesia (to replicate the approach of Ahissar and collaborators) or under fentanyl analgesia, a paradigm reported to produce neuronal responses that closely resemble responses recorded from awake rats (Simons et al. 1992). The vibrissae responses of a POm neuron, recorded under urethane anesthesia, are shown in the rasters and PSTHs of Fig. 2. As we and others described previously (Diamond et al. 1992b; Lavallee et al. 2005; Masri et al. 2006; Trageser and Keller 2004), in response to low-frequency stimuli (2 Hz), this neuron responded at relatively long latency (20 ms) and low magnitude (0.58 spikes/stimulus), and its responses diminished appreciatively as we increased stimulation frequency. We recorded qualitatively similar responses from 42 neurons, one half of which were recorded under urethane and the other half under fentanyl. The neuron depicted in Fig. 2 was unusual in that it produced statistically significant responses to stimuli delivered at 2, 5, 8, and 11 Hz; most neurons (38/42; 90%) failed to respond to stimuli ≥11 Hz.
FIG. 2.
Increasing stimulus frequency has no significant effect on response latency. Response magnitude is negatively correlated with stimulus frequency. Rasters (top) with PSTHs (bottom) constructed from recordings of 1 posterior medial nucleus (POm) neuron in response to a 50-ms stimulus delivered to the vibrissae at t = 0 (dashed vertical line). Stimuli were delivered at 2, 5, 8, or 11 Hz. Recordings obtained under urethane anesthesia. Horizontal line represents 99% CI of spontaneous activity rates. T0, latency to the first significant spike; T50, latency to half-maximum peak. Horizontal bar labeled “Response Window” depicts the significant response period.
POPULATION ANALYSES.
A prediction of the PLL model is that response latencies of POm neurons increase with increasing stimulus frequencies (see Introduction). To test this, we computed the latency to the first significant bin in the PSTH (T0, Fig. 2; see methods). The neuron depicted in Fig. 2 responded with identical latencies to stimuli delivered at all frequencies tested (P > 0.05, Kruskal-Wallis test). We obtained similar results from all 21 neurons recorded from urethane-anesthetized rats: the mean onset latency (T0) was not significantly different across the frequencies tested (P = 0.7, Kruskal-Wallis test; in milliseconds: 2 Hz = 17 ± 12, 5 Hz = 20 ± 21, 8 Hz = 14 ± 11; Fig. 3). Similar to the results obtained under urethane, the onset latency of POm neurons recorded under fentanyl (n = 21) was not significantly different across the frequencies tested (P = 0.12; in milliseconds: 2 Hz = 23 ± 14, 5 Hz = 39 ± 22, 8 Hz = 34 ± 18; Fig. 3A). There were no significant differences in onset latency of responses at any frequency between neurons recorded under urethane anesthesia and fentanyl analgesia (P = 0.8, Mann-Whitney test).
FIG. 3.
A: population analysis of POm neurons: onset latency does not change with increased stimulation frequency. Group data of onset latency of POm neurons recorded under urethane anesthesia (top) or fentanyl analgesia (bottom) at different stimulation frequencies. Box plots show mean (dashed lines), median (solid lines), and interquartile range (box). Whiskers represent data within 1.5 times the range from the 1st to 3rd quartile. P values were computed with a Kruskal-Wallis test. B: population analysis showing that stimulation of the reticular activating system [laterodorsal tegmentum and the pedunculopontine tegmentum (LDT/PPT)] does not significantly affect onset latencies to stimuli at different frequencies. P value was computed with a Kruskal-Wallis test.
To further test the prediction that response latency is affected by stimulus frequency, we used the metric for latency used by Ahissar and collaborators (Sosnik et al. 2001): the latency to the PSTH half-peak (T50; Fig. 2). This analysis also showed no statistically significant differences in latencies of responses to different frequencies of stimuli (P = 0.9; in milliseconds: 2 Hz = 25 ± 12, 5 Hz = 40 ± 25, 8 Hz = 33 ± 17; n = 42).
The highly variable stimulus-evoked activity of individual POm neurons (Diamond et al. 1992b; Lavallee et al. 2005; Masri et al. 2006; Trageser and Keller 2004) renders them poorly suited for coding stimulus features. It is possible, however, that the integrated output of local populations of POm neurons may be considerably less variable, because of signal averaging inherent to a population code. To test this, we combined recordings of single and multiunits obtained through a single electrode into local populations (n = 11 populations, 3.5 ± 0.21 units per population) as described in Sosnik et al. (2001). We constructed PSTHs from these local populations and calculated onset latencies (T0) as described above. Similar to single units, in these local populations, there was no significant relationship between response latency and stimulus frequency (P = 0.49, in milliseconds: 2 Hz = 35.4 ± 4.4; 5 Hz = 47.6 ± 11.5; 8 Hz = 49.0 ± 22.3).
Both latency metrics (T0 and T50) allowed us to compare changes in the mean latency in POm neurons as a group and as local populations, and analyses using either of these metrics failed to support the prediction that stimulus frequency significantly affects response latency.
We previously reported that responses of POm neurons to vibrissae stimuli at all frequencies are enhanced by cholinergic inputs. POm responses were enhanced following application of carbachol to POm neurons or stimulation of the LDT/PPT nuclei (Masri et al. 2006). Here, we compared responses of POm neurons (n = 10), recorded under urethane, before and after stimulation of LDT/PPT. High-amplitude slow oscillations were evident in the cortical ECoG before LDT-PPT stimulation, and following stimulation, the ECoG transitioned to low-amplitude fast oscillations, consistent with previous reports (Castro-Alamancos and Oldford 2002; Steriade 2003). Following LDT-PPT stimulation, response magnitudes for all frequencies significantly increased, whereas response duration and spontaneous activity rates were not significantly affected (see Fig. 2 in Masri et al. 2006). Here, we compared the steady-state response latency (T0) at each stimulation frequency recorded during control conditions with that recorded after LDT/PPT stimulation. Analysis of individual neurons showed that LDT-PPT stimulation had no significant effect on response latency of any individual neuron (P > 0.10). We obtained similar results when group data were analyzed: under control conditions, group analysis showed no significant differences in latencies across frequencies (Fig. 3B).
WITHIN NEURON ANALYSIS.
We reasoned that the relatively large population variance in response latency might obscure frequency-dependent latency shifts in individual neurons. To test whether individual POm neurons encode variable temporal inputs, we calculated separately, for each of the POm neurons recorded, the mean and variance of response latencies (T0) from PSTHs constructed from trains of 10 stimuli repeated 10 times with 10 s inter-train interval. Only 2 of 42 neurons (4.8%) showed significant increases in onset latency when stimulation frequency was increased from 2 to 5 Hz (71 ± 15% increase; P = 0.0003, Kruskal-Wallis test). These two cells failed to respond to frequencies >5 Hz. In the remaining 40 neurons (95.2%), there was no significant relationship between stimulus frequency and response latency.
FIRST SPIKE LATENCY.
Analyses of response latencies from PSTHs are common and convenient, but they rely on an important assumption: that the neural system performs an analysis similar to that used by us to compute the PSTH. That is, the system averages and normalizes responses to discriminate spikes evoked by a stimulus, from spikes occurring because of other causes, such as “spontaneous” activity. An alternative, and more liberal assumption is that any spike occurring after a stimulus be considered an evoked response. This approach takes into account the variability in spike timing as a potential contributor to the “code” (Foffani et al. 2004; Oram et al. 2002). We used this approach by computing the mean latency to the very first spike (first spike latency) that occurred within the significant response period in the PSTH (Fig. 2).
Figure 4A shows, for each of the neurons tested, the mean and SD of the first spike latency, across the stimulation frequencies tested. Sixteen of 42 neurons (38.1%) showed no significant difference in first spike latencies evoked by stimulation at different frequencies (P > 0.05, Kruskal-Wallis test). Seventeen of 42 neurons (40.5%) showed a significant (P ≤ 0.05) increase in first spike latency, as stimulation frequency increased from 2 to 5 Hz, but no significant differences between responses to other frequency pairs. In eight neurons (19%), response latency increased significantly between 5 and 8 Hz or 5 and 11 Hz. Most significantly, none of the neurons showed a systematic increase in first spike latency across the four frequencies tested, in contrast to the prediction of the PLL model.
FIG. 4.
POm neurons do not systematically and reliably encode stimulus frequency. A: latency to the 1st spike (within the significant response window; see Fig. 2) of individual neurons recorded under urethane or fentanyl. Each neuron was tested at 2, 5, 8 and 11 Hz, and the mean response latency to each frequency is indicated by a different symbol (see legend). Error bars here and in the following plots represent SD. If a neuron responded to any pair of frequencies at significantly different latencies (P ≤ 0.05, Kruskal-Wallis test), those stimulation frequencies and their relation are indicated above each column. For example, (2 < 5) means that a neuron responds to 2-Hz stimulation at a significantly shorter latency than to 5-Hz stimulation. Only 40.5% of the neurons showed a significant increase in 1st spike latency as stimulation frequency increased from 2 to 5 Hz, but none of these showed significant differences between responses to other frequency pairs. Most significantly, none of the neurons showed a systematic increase in 1st spike latency across the 4 frequencies tested. B: increasing stimulation frequency results in significant increases in failure rates. P value was computed with ANOVA, followed by the Scheffe multiple comparison test. C: scatterplot depicting the effects of varying stimulation frequencies (2–5, 2–8, or 5–8 Hz) on the percent change in 1st spike latency vs. the percent change in failure rate. Positive correlation was significant using the Spearman correlation test. D: mean response duration of POm neurons is unaffected by stimulation frequency. P value was computed using ANOVA.
RESPONSE FAILURES.
For POm neurons to reliably encode response latency, or any other response variable, they should respond reliably to the physiologically relevant stimulus frequencies used here—5–11 Hz—frequencies at which whisking occurs in behaving animals (Brecht et al. 1997; Carvell et al. 1991; Harvey et al. 2001; Hattox et al. 2003). We tested this by computing the mean failure rates at each stimulus frequency. We calculated the mean number of trials (out of 100) where a cell failed to produce a spike after the stimulus during the significant response period. Figure 4B shows that the failure rates of POm neurons are high (e.g., 60.2% at 5 Hz, 84.4% at 11 Hz) and that failure rates increase significantly with increased stimulation frequency (P < 0.0001, ANOVA). Indeed, the increases in failure rates are significantly and positively correlated with changes in first spike latencies (r = 0.55, P = 0.007, Spearman correlation test; Fig. 4C). Thus even in the minority of neurons in which there was an increase in first spike latency between a pair of stimulus frequencies, this increase was associated with a significant increase in response failure.
RESPONSE DURATION.
The PLL model states that frequency-dependent changes in response latencies are transformed to a rate code, because the response duration of POm neurons shortens with increased input frequency (Sosnik et al. 2001). This is because onset latencies are reported to increase and offset latencies remain constant, such that the latency increments are translated into a rate code: increasing onset latencies lead to lower spike counts (Ahissar et al. 2000). We explicitly tested the prediction that response duration increases with stimulus frequency and found that, in our hands, this was not the case. Response duration of POm neurons remained constant across the frequencies tested (Fig. 4D; P = 0.7, ANOVA).
POm responses in the awake rat
A critical test of any model of neuronal processing scheme is its application to awake, unanesthetized animals. We therefore recorded from well-isolated POm neurons in awake, head-restrained rats (n = 7), and applied similar stimulus, recording, and analyses procedures described above for experiments with anesthetized rats. The only difference, apart from the absence of anesthesia or analgesia, was that we applied air-puff stimuli at lower frequencies (0.3, 1, and 5 Hz), because some animals appeared agitated when we used higher-frequency trains. We monitored ECoG activity throughout the recordings and found that it consistently had dominant activity in the beta or higher frequency (>12 Hz) bandwidth. Furthermore, in these head-restrained animals, we periodically stimulated the vibrissae. This strongly implies that the animals were, indeed, alert.
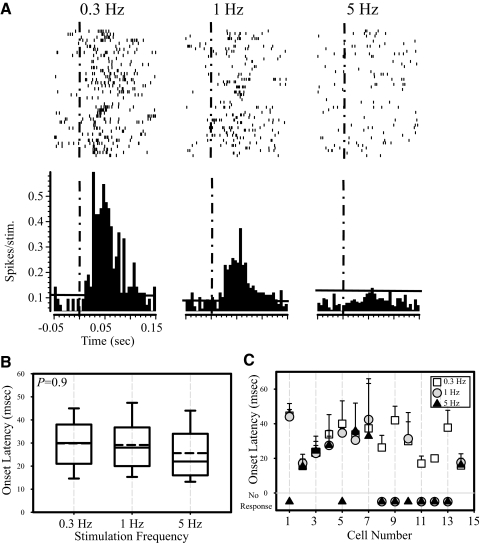
We acclimated the animals to the apparatus over a period of several weeks to suppress their tendency to whisk in response to passive deflections of the vibrissae. We analyzed responses to passive vibrissae deflections only during periods when no active whisking took place, as determined from analyses of EMG or optical movement records. Figure 5A shows the responses of a representative POm neuron to air-puff stimulation at the three frequencies tested. Similar to the anesthetized preparation, POm responses were labile, and the failure rate increased with increased stimulation frequency. As a group (n = 14), POm neurons failed to show significant changes in onset latency with increased stimulus frequencies (P = 0.9, Kruskal-Wallis test; Fig. 5B). Onset latency calculated from trains of 10 stimuli repeated 10 times, for each of the POm neurons, are shown in Fig. 5C. None of the cells showed significant shifts in latency with increased stimulus frequencies. These findings suggest that, like in the anesthetized rat, stimulus frequency is not reliably reflected in the response latency of POm neurons in the awake rat.
FIG. 5.
Responses of POm neurons in alert, head-restrained animals. A: rasters (top) with PSTHs (bottom) constructed from recordings of a single POm neuron in response to a 50-ms stimulus delivered to the vibrissae at t = 0 (dashed vertical line). Stimuli were delivered at 0.3, 1, or 5 Hz. Horizontal line represents 99% CI of spontaneous activity rates. B: onset latency does not change with increased stimulation frequency. Group data of onset latency in response to different stimulation frequencies. P values were computed with a Kruskal-Wallis test. C: onset latency of individual neurons recorded in alert head-restrained animals. Onset latency was calculated from PSTHs constructed from trains of 10 stimuli repeated 10 times for each of the POm neurons. In none of the neurons did onset latency change with increased stimulation frequency.
In marked contrast to spontaneous firing rates in anesthetized animals (in spikes/s: range, 0.4–3.5; mean = 1.1 ± 1.7; median = 0.5), the spontaneous activity of POm neurons recorded in awake rats was relatively high (in spikes/s: range, 2.5–30; mean = 15 ± 14; median = 7.9).
POm neurons do not convey vibrissae motion signals
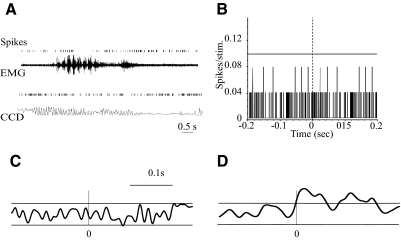
As discussed in the Introduction, it has also been suggested that POm neurons may encode sensor motion signals, that is, they are preferentially responsive during whisking that does not generate vibrissae contacts (whisking; Yu et al. 2006). We tested this prediction by recording from well-isolated POm neurons (n = 23) in awake, head-restrained rats (n = 7), while monitoring voluntary vibrissae movements using EMG or a CCD device (see methods). We included in the analyses only vibrissae-related neurons, that is, cells that had significant repsonses to air-puff stimuli applied to the vibrissae. Responses to active whisking and passive stimuli were recorded in the same electrode penetrations. The rats were positioned such that their moving vibrissae did not contact any objects. Figure 6A shows two examples of POm responses during such “whisking in air.” Visual inspection of these spike rasters and the associated movement record failed to show temporal correlations between these events.
FIG. 6.
POm activity is not correlated with whisking kinematics. A: 2 representative examples of POm activity during whisking in air. Shown are spike rasters and raw EMG recording (top) or movements monitored using a CCD device (bottom). B: a representative PSTH constructed from POm neuron activity, using the onset of individual whisks in the EMG record as a reference (t = 0; dashed vertical line). Horizontal line represents 95% CI. Although all POm neurons recorded responded to passive deflections of the vibrissae, none showed a significant response time locked to the onset of movement. C: spike-triggered average (STA) of rectified EMG triggered by spikes recorded from a POm cell. A total of 2,251 spikes were used to compute this STA. Horizontal lines represent 95% CI. None of the POm neurons recorded produced a significant STA. D: STA of rectified EMG triggered by spikes recorded from a ventroposterior medial (VPM) cell. A total of 2,590 spikes were used to compute this STA. Portions of the trace above the 95% confidence line represent significant correlation between VPM activity and whisker movement.
We quantitatively tested for correlations between POm activity and whisking in two ways. First, we identified the onset of individual whisks in the EMG records and collected the time stamps of these events (see methods). We used these time stamps as the reference to construct PSTHs (1-ms bins) of each neuron's activity (Fig. 6B). None of the computed PSTHs showed significant, time-locked increases in POm activity (n = 23 neurons).
In a second analysis, we used time stamps of POm spikes to compute STAs of rectified EMG or raw CCD records of whisking movements (see methods for details). We defined activity in the STA as significant if it exceeded the 95% CI. Figure 6C shows an example of an STA computed in this way for a POm neuron, showing the absence of a significant correlation between neuronal firing and movements. None of the POm neurons tested (n = 23) showed a significant correlation with whisker movements.
As a positive control, we performed similar analyses on spike trains recorded from vibrissa-responsive VPM neurons. An example of an STA of EMG acitivty is shown in Fig. 6D, where the averaged EMG significanly exceeds the CI at and immediately after the occurence of the VPM spike. In contrast to POm neurons, 8 of 18 (44%) VPM neurons showed significant correlations with vibrissae movements.
Not only were there no significant time-locked correlations between POm activity and vibrissae movements, we also found no difference (P = 0.35, Mann-Whitney test) in the mean firing rate of POm neurons during whisking (16 ± 13 Hz) compared with nonwhisking periods (15 ± 14 Hz). Similarly, when analyzed individually, most neurons (14 of 23, 60.9%) had similar firing rates at rest and during whisking epochs. A smaller percentage of neurons (7 of 23, 30.4%) exhibited increased firing rates during whisking epochs (15–95% increase, P < 0.04, t-test), and two neurons showed significant reductions in firing.
In conclusion, whisking in air does not seem to evoke significant, reproducible firing in POm neurons.
DISCUSSION
Our goal was to test the ability of POm neurons to encode movements of and contacts with the mystacial vibrissae. For this purpose, we recorded vibrissae responses from POm neurons in awake rats, in rats under analgesia or anesthesia, and in rats following LDT/PPT stimulation. We found that, under all conditions, POm responses have low magnitude and high failure rates. This is the case for responses analyzed from the activity of individual neurons, local neuronal ensembles, or the entire neuronal population. Our findings are in agreement with previous reports on POm responses recorded under a variety of experimental conditions (Diamond et al. 1992b; Lavallee et al. 2005; Masri et al. 2006; Trageser and Keller 2004). These observations suggest that POm neurons do not faithfully encode vibrissae inputs and therefore might not provide reliable information on temporal features of vibrissae movements or contacts.
POm and the PLL model
It has been suggested that POm participates in PLLs that translate the frequency of vibrissae deflections into a rate code (Ahissar 1998; Ahissar et al. 2000). The PLL model proposes that, as the frequency of vibrissae deflections increases, the response latency of POm neurons increases, and their spike count decreases. These input transformations are proposed to involve interactions in POm between ascending trigeminal inputs and descending cortical influences (Ahissar and Kleinfeld 2003), as well as strong modulation by inputs from the reticular nucleus, mediated by GABAB receptors (Golomb et al. 2006).
In this study, we replicated the experimental procedures reported in those studies and found that neither the onset latency nor the response period of POm neurons consistently reflect features of vibrissal inputs. Specifically, analyses of PSTHs computed from the activity of individual POm neurons, local neuronal ensembles, or pooled data from the entire population showed no significant differences between these parameters and stimulus frequency (Fig. 3). This was the case whether response latency was defined as the first significant bin in the PSTH or as the latency to the PSTH's half-max. Even when we applied a very liberal definition of response latency—the very first spike within the significant response period—we found that none of the cells displayed a systematic relationship between stimulation frequency and response latency or duration (Fig. 4). We obtained similar findings under urethane anesthesia, fentanyl analgesia, following LDT/PPT stimulation, and, most significantly, in awake rats (Fig. 5). Thus our findings are not consistent with the hypothesis that POm neurons reliably encode stimulation frequency.
Our anesthetic (urethane), surgical, and recording procedures and analytical approaches closely resemble the ones used in previous studies (Ahissar et al. 2000; Sosnik et al. 2001). The only difference was that we stimulated the vibrissae in the neurons' preferred direction, whereas in the previous studies, the vibrissae were deflected in the protracted direction. We consider it unlikely that this would account for the different results, because a majority of our neuronal population has protraction as its preferred direction. Nevertheless, we cannot exclude the possibility that the PLL model applies only to a very restricted stimulus space, such as weak stimuli at a specific direction.
POm responses to whisking in air
As discussed in the Introduction, sensory inputs in the vibrissa-to-barrel cortex system are processed along several parallel streams: a lemniscal pathway, represented in the thalamus by VPM, a paralemniscal pathway that includes POm, an extralemniscal pathway involving the ventrolateral segment of VPM (VPMvl), and a newly discovered pathway involving the lateral dorsal nucleus of the thalamus (Bezdudnaya and Keller 2008; Pierret et al. 2000). It was recently suggested that each of the pathways conveys to the neocortex different qualities of vibrissa-related inputs: the extralemniscal pathway (VPMvl) responds preferentially to vibrissae contacts, the paralemniscal system (POm) is activated almost exclusively by contact-less whisking in air (sensor motion), and the lemniscal system responds to combined whisking-touch signals (Yu et al. 2006). Support for the postulated role of POm in encoding sensor motion comes from a report that in response to fictive whisking—vibrissae movements evoked by electrical stimulation of the facial nerve in anesthetized animals—POm neurons respond preferentially to whisking in air, when the vibrissae make no contact with an external object (Yu et al. 2006).
We tested the hypothesis that POm neurons encode sensor motion by recording from awake, alert rats during whisking in air. To our knowledge, this is the first report of recordings from POm neurons in unanesthetized, behaving rats. None of the cells responded during whisking in air, although all responded to vibrissae contact (Fig. 6, B and D). These findings are inconsistent with the proposed sensor motion role of the paralemniscal system.
Our approach differs significantly from that used by Yu et al. (2006) in that we recorded responses to naturally ocurring, voluntary whisking, whereas they recorded responses to fictive whisking, evoked by electrical stimulation of the facial nerve. It is possible that electrical stimulation of the nerve causes inadvertent activation of other fibers, such as small diameter nociceptive afferents. Indeed, facial nerve stimulation in humans commonly produces unbearable pain (Stoddart and Cooper 1999). It is therefore possible that some of the POm responses recorded by Yu et al. (2006) represent noxious responses. Below we discuss the important role of POm in processing pain responses.
Nevertheless, in agreement with the hypothesis by Yu et al. (2006), we found that some VPM neurons relay both vibrissae contact and movement signals (Fig. 6D). This finding suggests that our failure to detect movement-related responses in POm is not caused by the experimental or analytical procedures used.
Our finding that POm neurons do not respond during whisking in air is consistent with reports that the responses of most trigeminal ganglion neurons during natural whisking behaviors are of low magnitude and high variability (Khatri et al. 2007; Leiser and Moxon 2007). Thus the sluggish responses of POm neurons may be related, at least in part, to the languid responses to whisking in air of primary afferents in this system.
Function of POm
Our findings indicate that POm neurons are unlikely to encode the frequency of vibrissae contacts or sensor motion. It is conceivable that these neurons respond preferentially to a stimulus parameter space yet to be explored. There is evidence that POm may be preferentially involved in processing of nociceptive inputs (Dostrovsky and Guilbaud 1990; Poggio and Mountcastle 1960). Indeed, we have recently found that POm activity is dramatically increased in an animal model of central pain (Masri and Keller, unpublished observations). We are now exploring the hypothesis that POm is specifically and preferentially involved in encoding noxious stimuli.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-051799 and NS-31078 to A. Keller and Fellowship F31 NS-046123 to J. C. Trageser.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Ahissar 1998.Ahissar E Temporal-code to rate-code conversion by neuronal phase-locked loops. Neural Comput 10: 597–650, 1998. [DOI] [PubMed] [Google Scholar]
- Ahissar and Kleinfeld 2003.Ahissar E, Kleinfeld D. Closed-loop neuronal computations: focus on vibrissa somatosensation in rat. Cereb Cortex 13: 53–62, 2003. [DOI] [PubMed] [Google Scholar]
- Ahissar et al. 2000.Ahissar E, Sosnik R, Haidarliu S. Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature 406: 302–306, 2000. [DOI] [PubMed] [Google Scholar]
- Bermejo et al. 1998.Bermejo R, Houben D, Zeigler HP. Optoelectronic monitoring of individual whisker movements in rats. J Neurosci Methods 83: 89–96, 1998. [DOI] [PubMed] [Google Scholar]
- Bezdudnaya and Keller 1997.Bezdudnaya T, Keller A. The laterodorsal nucleus of the thalamus: a processor of somatosensory inputs. J Comp Neurol 507: 1979–1989, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht et al. 1997.Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res 84: 81–97, 1997. [DOI] [PubMed] [Google Scholar]
- Bureau et al. 2006.Bureau I, von Saint Paul F, Svoboda K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol 4: E382, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvell et al. 1991.Carvell GE, Simons DJ, Lichtenstein SH, Bryant P. Electromyographic activity of mystacial pad musculature during whisking behavior in the rat. Somatosens Mot Res 8: 159–164, 1991. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos and Oldford 2002.Castro-Alamancos MA, Oldford E. Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. J Physiol 541: 319–331, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen and Castro-Alamancos 2007.Cohen JD, Castro-Alamancos MA. Early sensory pathways for detection of fearful conditioned stimuli: tectal and thalamic relays. J Neurosci 27: 7762–7776, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer and Keller 2006.Cramer NP, Keller A. Cortical control of a whisking central pattern generator. J Neurophysiol 96: 209–217, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes et al. 2005.Deschenes M, Timofeeva E, Lavallee P, Dufresne C. The vibrissal system as a model of thalamic operations. Prog Brain Res 149: 31–40, 2005. [DOI] [PubMed] [Google Scholar]
- Diamond et al. 1992a.Diamond ME, Armstrong-James M, Budway MJ, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus: dependence on the barrel field cortex. J Comp Neurol 319: 66–84, 1992a. [DOI] [PubMed] [Google Scholar]
- Diamond et al. 1992b.Diamond ME, Armstrong-James M, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J Comp Neurol 318: 462–476, 1992b. [DOI] [PubMed] [Google Scholar]
- DiCarlo et al. 1996.DiCarlo JJ, Lane JW, Hsiao SS, Johnson KO. Marking microelectrode penetrations with fluorescent dyes. J Neurosci Methods 64: 75–81, 1996. [DOI] [PubMed] [Google Scholar]
- Dostrovsky and Guilbaud 1990.Dostrovsky JO, Guilbaud G. Nociceptive responses in medial thalamus of the normal and arthritic rat. Pain 40: 93–104, 1990. [DOI] [PubMed] [Google Scholar]
- Dykes 1983.Dykes RW Parallel processing of somatosensory information: a theory. Brain Res Rev 6: 47–115, 1983. [DOI] [PubMed] [Google Scholar]
- Foffani et al. 2004.Foffani G, Tutunculer B, Moxon KA. Role of spike timing in the forelimb somatosensory cortex of the rat. J Neurosci 24: 7266–7271, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg et al. 1999.Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol 81: 2243–2252, 1999. [DOI] [PubMed] [Google Scholar]
- Friedman et al. 2006.Friedman WA, Jones LM, Cramer NP, Kwegyir-Afful EE, Zeigler HP, Keller A. Anticipatory activity of motor cortex in relation to rhythmic whisking. J Neurophysiol 95: 1274–1277, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb et al. 2006.Golomb D, Ahissar E, Kleinfeld D. Coding of stimulus frequency by latency in thalamic networks through the interplay of GABAB-mediated feedback and stimulus shape. J Neurophysiol 95: 1735–1750, 2006. [DOI] [PubMed] [Google Scholar]
- Harvey et al. 2001.Harvey M, Bermejo R, Zeigler HP. Optoelectronic monitoring of discriminative whisking in the head-fixed rat. Somatosens Mot Res 18: 211–222, 2001. [DOI] [PubMed] [Google Scholar]
- Hattox et al. 2003.Hattox AM, Li Y, Keller A. Serotonin regulates rhythmic whisking. Neuron 39: 342–352, 2003. [DOI] [PubMed] [Google Scholar]
- Hemelt and Keller 2007.Hemelt ME, Keller A. Superior sensation: superior colliculus participation in rat vibrissa system. BMC Neurosci 8: 12, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri et al. 2007.Khatri V, Bermejo R, Brumberg JC, Keller A, Zeigler HP. Encoding of whisker movement kinematics by trigeminal ganglion neurons in awake, actively whisking rats. Soc Neurosci Abstr 403.24, 2007. [Google Scholar]
- Kleinfeld et al. 2006.Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol 16: 435–444, 2006. [DOI] [PubMed] [Google Scholar]
- Lavallee et al. 2005.Lavallee P, Urbain N, Dufresne C, Bokor H, Acsady L, Deschenes M. Feedforward inhibitory control of sensory information in higher-order thalamic nuclei. J Neurosci 25: 7489–7498, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser and Moxon 2007.Leiser SC, Moxon KA. Responses of trigeminal ganglion neurons during natural whisking behaviors in the awake rat. Neuron 53: 117–133, 2007. [DOI] [PubMed] [Google Scholar]
- Masri et al. 2006.Masri RM, Trageser JC, Bezdudnaya T, Li Y, Keller A. Cholinergic regulation of the posterior medial thalamic nucleus. J Neurophysiol 96: 2265–2273, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram et al. 2002.Oram MW, Xiao D, Dritschel B, Payne KR. The temporal resolution of neural codes: does response latency have a unique role? Philos Trans R Soc Lond B Biol Sci 357: 987–1001, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos and Watson 1998.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, 1998.
- Pierret et al. 2000.Pierret T, Lavallee P, Deschenes M. Parallel streams for the relay of vibrissal information through thalamic barreloids. J Neurosci 20: 7455–7462, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggio and Mountcastle 1960.Poggio GF, Mountcastle VB. A study of the functional contributions of the lemniscal and spinothalamic systems to somatic sensibility. Central nervous mechanisms in pain. Bull Johns Hopkins Hosp 106: 266–316, 1960. [PubMed] [Google Scholar]
- Simons and Carvell 1992.Simons DJ, Carvell GE, Hershy AE, Bryant DP. Responses of barrel cortex neurons in awake rats and effects of urethane anesthesia. Exp Brain Res 91: 259–272, 1992. [DOI] [PubMed] [Google Scholar]
- Sosnik et al. 2001.Sosnik R, Haidarliu S, Ahissar E. Temporal frequency of whisker movement. I. Representations in brain stem and thalamus. J Neurophysiol 86: 339–353, 2001. [DOI] [PubMed] [Google Scholar]
- Steriade 2003.Steriade M The corticothalamic system in sleep. Front Biosci 8: D878–D899, 2003. [DOI] [PubMed] [Google Scholar]
- Stoddart et al. 1999.Stoddart RL, Cooper HR. Electrode complications in 100 adults with multichannel cochlear implants. J Laryngol Otol Suppl 24: 18–20, 1999. [PubMed] [Google Scholar]
- Trageser and Keller 2004.Trageser JC, Keller A. Reducing the uncertainty: gating of peripheral inputs by zona incerta. J Neurosci 24: 8911–8915, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante et al. 2000.Veinante P, Jacquin MF, Deschenes M. Thalamic projections from the whisker-sensitive regions of the spinal trigeminal complex in the rat. J Comp Neurol 420: 233–243, 2000. [DOI] [PubMed] [Google Scholar]
- Woolsey 1996.Woolsey TA Barrels: 25 years later. Somatosens Mot Res 13: 181–186, 1996. [DOI] [PubMed] [Google Scholar]
- Woolsey and Van der Loos 1970.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. Brain Res 17: 205–242, 1970. [DOI] [PubMed] [Google Scholar]
- Yu et al. 2006.Yu C, Derdikman D, Haidarliu S, Ahissar E. Parallel thalamic pathways for whisking and touch signals in the rat. PLoS Biol 4: E124, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]