Abstract
Synaptic transmission in hippocampal field CA1 is largely N-methyl-d-aspartate receptor (NMDAR) dependent during the early postnatal period. It becomes increasingly mediated by α-amino-3-hydroxy-5-methylisoxazole-4-proprionate (AMPA) receptors until an adult ratio of AMPA to NMDA receptors is achieved. It is shown here that increases in the AMPA receptor (AMPAR)-mediated field potential response continue over the life span of the F-344 rat at the perforant path–granule cell synapse in the dentate gyrus. In contrast, the NMDAR-dependent component of the response decreases with age between 1 and 27 mo, leading to an increase of AMPAR/NMDAR ratio with age. One possible explanation of this age difference is that the AMPAR/NMDAR ratio can be modified by experience. To test the idea that the changed ratio is caused by the old rats' longer lives, an intensive 10-mo period of enrichment treatment was given to a group of animals, beginning at 3 mo of age. Compared with animals housed in standard cages, the enrichment treatment did not alter the glutamatergic response ratio measured with field potential recording methods. These data provide support for the conclusion that the observed change with age is developmentally regulated rather than experience dependent. Given the role of the NMDAR in synaptic plasticity, these changes suggest a progressive commitment of perforant path synapses to particular weights over the life span. One possible implication of this effect includes preservation of selected memories, ultimately at the expense of a reduced capacity to store new information.
INTRODUCTION
Whole cell recording studies (Durand et al. 1996; Isaac et al. 1995; Liao et al. 1995) indicate that, in the early stages of synaptogenesis, many synapses in the Schaffer collateral input to the hippocampal CA1 region express a functional complement of N-methyl-d-aspartate receptors (NMDAR) but do not express the α-amino-3-hydroxy-5-methylisoxazole-4-proprionate receptors (AMPAR) that come to mediate fast glutamatergic synaptic transmission in the adult (Blake et al. 1988; Davies and Collingridge 1989). At this early stage, functional AMPA receptors can be expressed by induction of long-term potentiation (LTP) (Bliss and Collingridge 1993), which depends on calcium entry through NMDAR channels (Durand et al. 1996; Isaac et al. 1995; Liao et al. 1995). In the Schaffer collateral pathway, the adult strengths of the NMDAR- and AMPAR-mediated components of the compound excitatory postsynaptic potential (EPSP) are achieved by ∼1 mo of age, remain at similar levels in adulthood (9 mo), but show a decline at 26 mo of age. Nonetheless, the ratio of AMPAR- to NMDAR-mediated EPSPs remains virtually constant across the young, adult, and aged groups (Barnes et al. 1997). Thus a progressive increase of the AMPAR-mediated component of transmission at individual synapses during experience cannot be a mechanism of long-term information storage in the CA1 network, unless a compensatory reduction at other synapses occurs. This suggests that if AMPAR currents are increased as a consequence of LTP, as some studies suggest (Bliss and Collingridge 1993; Durand et al. 1996; Foster and McNaughton 1991; Isaac et al. 1995; Kauer et al. 1988; Liao et al. 1995; Lynch et al. 1982; Malenka and Nicoll 1999), this increase must be accompanied by an equal net reduction at other synapses, possibly though a long-term depression (LTD) mechanism. In fact, there is a decrease in the number of AMPAR clustered at synapses following the induction of LTD (Carroll et al. 1999; Shepherd et al. 2006). The induction of LTD, similar to LTP, is usually regulated by NMDAR currents (Christie et al. 1994; Dudek and Bear 1992; Mulkey and Malenka 1992), even though these two types of synaptic plasticity may depend on different subtypes of NMDAR (Liu et al. 2004).
The associative LTP/LTD learning principle is useful for a variety of information storage applications in neural networks (Bear and Malenka 1994; Coussens and Teyler 1996; Lisman and Idiart 1995), but suffers the problem that new information gradually overwrites the old. One way in which a network might function as a relatively long-term repository of information would be to restrict further changes in AMPAR-mediated transmission by reducing NMDAR-mediated currents once a synapse was committed to the storage of specific items. Such a network, however, would require large numbers of synapses and would have to use extremely sparse coding principles (Marr 1971), if its capacity is not to be used up too quickly. The region of the hippocampus that contains by far the largest total number of modifiable synapses (Amaral et al. 1990) and which uses the sparest coding scheme (Jung and McNaughton 1993; Leutgeb et al. 2005) is the dentate gyrus granule cells. Thus the dentate gyrus would be a candidate system for durable information storage.
Whereas the number of axospinous synapses in the CA1 stratum radiatum remains the same in young and old rats (Geinisman et al. 2004), perforant path synapses from the entorhinal cortex to the dentate gyrus do undergo a net loss in numbers over the life span (Geinisman et al. 1992, 1995). Interestingly, the perforant path to granule cell synapses that remain in the dentate gyrus of old rats have previously been found to be functionally more powerful (Foster et al. 1991). Motivated by the foregoing theoretical considerations, the questions addressed in this experiment were whether this net increase in synaptic efficacy in the dentate gyrus reflects an increase of AMPAR-mediated currents in aged rats and whether such changes are accompanied by a net increase or decrease in NMDAR currents. In addition, to address the question of whether changes in NMDAR- and AMPAR-mediated responses over the life span result from a fixed developmental process or are shaped by accumulated experience through life, the effect of experience on glutamatergic responses in hippocampal granule cells was examined in young adult rats.
METHODS
Animals and treatment procedures
In total, 54 male F-344 rats at ages 1, 9, and 27 mo (n = 18 per group) were used to study the effect of age on glutamatergic response ratios. The rats used in the aging experiment were housed singly in standard cages. In the experiment designed to study the effects of experience on glutamatergic response ratios, F-344 rats were raised under three different treatment conditions for 10 mo, beginning at 3 mo of age. In the standard control group (n = 9), rats were housed singly in standard cages, with no specific handling other than the routine weekly weighing and cage cleaning. In the wheel running/social enrichment group (n = 9), on each of 3 consecutive wk, the rat spent 1 wk in a cage in which a running wheel could be accessed freely and the other 2 wk in a cage with another rat from the same treatment group. In the enriched environment group (n = 10), all the rats were housed together in a big cage (∼100 × 100 × 80 cm), scattered with frequently changed toys and tunnels. A running wheel was added into the cage every other week. In addition, the rats were taken to a bigger environment for free exploration for 1 h twice per week. All procedures were in accordance with the University of Arizona's Institutional Animal Care and Use Committee.
Morris swim task
The protocol used for the Morris swim task is described in detail elsewhere (Barnes et al. 1997). In brief, the rats were first given spatial trials for 4 consecutive days, in which a 14-cm platform was hidden in a constant location inside of a 180-cm-diam tank. There were three training blocks each day (two 60-s trials per block, 60-s intertrial interval, 30- to 60-min interblock interval), with the releasing locations changed from trial to trial. One probe trial was given immediately following the last spatial trial on day 4, in which the platform was removed, and the rats were allowed to swim freely for 60 s. The rats were then given six “cued trials,” in which the platform became visible but its location was randomly changed across trials. Another six cued trials were given on day 5. Rats' performance on the swim task was analyzed off-line with in-house software (WMAZE, M. Williams). In the spatial and cued trials, the latency and the path length to escape the water were measured. In the probe task, the search time of the rat in each quadrant and the number of target crossings, that is, how many times the rat swims across the location where the hidden platform used to be, were recorded to measure the rat's memory for the location of the platform.
Brain slices
To study the effect of age on the glutamatergic response ratio, hippocampal slices from different age groups were prepared as described previously (Barnes et al. 1997). Briefly, rats were first anesthetized with methoxyfluorane (Metofane), and the hippocampi were rapidly dissected. Slices (450 μm) were cut parallel to the alvear fibers using a tissue chopper and were transferred to an interface style brain slice chamber perfused (2.0 ml/min) with artificial cerebrospinal fluid (ACSF) at 32 ± 1°C, oxygenated with a 95% O2-5% CO2. For the initial hour, the ACSF consisted of the following (in mM): 124 NaCl, 2 KCl, 1.25 KH2PO4, 2 MgSO4, 26 NaHCO3, 10 dextrose, and 2 CaCl2. The Mg2+ was then reduced to 200 μM to facilitate recording of the NMDAR component of the EPSP. After another 1 h of incubation, a recording pipette and a bipolar stimulating electrode were placed ∼300 μm apart in the stratum moleculare of the dentate gyrus (Fig. 1A). Rats from the three enrichment treatment groups were killed at least 1 wk after being tested on the Morris swim task.
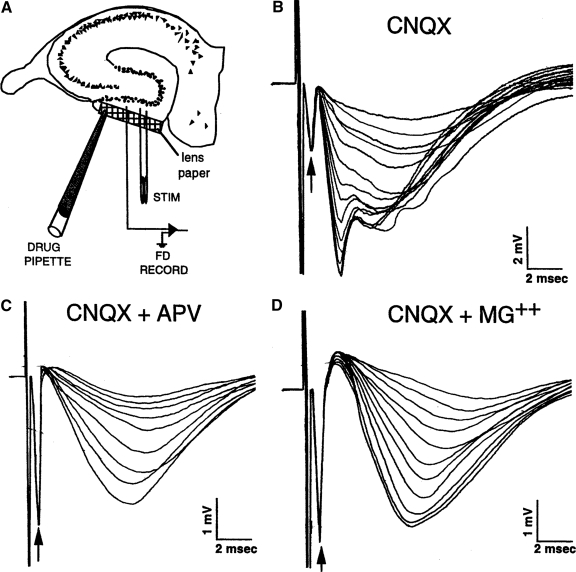
FIG. 1.
α-Amino-3-hydroxy-5-methylisoxazole-4-proprionate receptor (AMPAR)- and N-methyl-d-aspartate receptor (NMDAR)-mediated neurotransmission in hippocampal granule cells. A: configuration of recording and stimulating electrodes. A small strip of Kodak lens paper was placed across the outer molecular layer to serve as a diffusion conduit for drugs applied from a large tipped pipette that was placed in contact with the lens paper. Synaptic field potentials were recorded in the middle of stratum moleculare following constant current electrical stimulation of perforant path axons. B: example of perforant path–evoked excitatory postsynaptic potentials (EPSPs) during the application of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), a competitive inhibitor of the AMPAR. The presynaptic fiber potential (arrows) is a reflection of the number of axons excited by the stimulus. Note the absence of change in the presynaptic fiber potential as the synaptic response declined. The residual synaptic field potential following stabilization of the response was taken as the NMDAR response. Advantage was also taken of the slower time-course of the NMDAR response to maximally segregate the AMPAR and NMDAR components. C: example of the reduction of the residual synaptic response in the presence of CNQX by addition of the NMDAR antagonist d-amino-phosphono-valerate (APV). The residual response was reduced by ∼95% in all age groups. D: example of reduction of the residual response in the presence of CNQX by the additional of 2 mM Mg2+ to the bathing medium. The added Mg2+, which inhibits the opening of NMDAR channels, reduced the residual EPSP by ∼87% in all age groups. Data were excluded if the presynaptic fiber potential changed significantly following drug application.
Field potential recording
Field EPSPs (fEPSPs) and presynaptic fiber potentials were recorded from the lower (detached) blade of the dentate gyrus from rats in the different age groups, and the amplitude was maximized by adjusting the depths of the electrodes into the tissue. Data were acquired and analyzed using Workbench software (DataWave). The responses were characterized by recording stimulus input–response output functions at 12 stimulus intensities using 100-μs biphasic, constant-current stimulus pulses. The synaptic components of these responses are referred to as the AMPAR fEPSPs because they were measured as the voltage difference between the prestimulus baseline and the response at 1.5 ms after stimulus onset. At this latency, there is a negligible contribution from the slower NMDAR-mediated fEPSP, although it is likely that there is some contribution to this response from receptors responsive to kainate.
To compensate for the reduced numbers of synapses with aging, synaptic responses at a given stimulus intensity were expressed as a ratio of the corresponding amplitude of the presynaptic fiber potential. This procedure also minimizes variation caused by electrode location. For each stimulus intensity, the ratio of the synaptic response to the presynaptic fiber potential was computed. Following the measurement of the AMPAR-mediated fEPSP, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), a selective non-NMDA receptor blocker, was applied with a pipette (20 μm tip diam) containing 2 mM CNQX. Diffusion of the CNQX was facilitated by applying via the pipette to the surface of lens paper placed over the outer molecular layer (shown in Fig. 1A). The stimulus input–response output functions were again recorded at 12 stimulus intensities, and the synaptic responses measured 2.5 ms after stimulus onset (referred to as the NMDAR-mediated component) were computed against their respective presynaptic fiber potentials.
The application of CNQX resulted in a large reduction in fEPSP magnitude (Fig. 1B) leaving a residual component that was slower in time course. After measuring the residual fEPSP component, its NMDAR-mediated nature was confirmed by largely abolishing it with a subsequent application of the competitive NMDAR antagonist, d-amino-phosphono-valerate (APV), which was applied by pipette (1 mM) in the same manner as for CNQX application (Fig. 1C). At least one slice in each rat was tested in this manner. At the end of the session, the NMDAR-mediated nature of the residual fEPSP in the last slice recorded from was also confirmed by abolishing it with 2 mM Mg2+ added to the perfusion medium (Fig. 1D). The APV and Mg2+ treatments reduced the residual EPSPs equivalently in both age groups by ∼95 and ∼87%, respectively.
The field potentials of slices from different treatment groups were recorded similarly from the upper (attached) blade of the dentate gyrus. The amplitude of the fEPSPs, however, was measured differently. The amplitude of the AMPAR and NMDAR fEPSPs was measured as the slope of the EPSP before and after the application of CNQX, respectively. The cursors for the slope measurement were set to the initial half of the EPSP so that the measurement performed before the application of CNQX was not contaminated by the NMDA component.
To calculate the presynaptic fiber potential, AMPA and NMDAR-mediated EPSPs, and the AMPAR-to-NMDAR response ratio across different groups, the values collected at the middle three to five stimulus intensities were averaged for each response type in each slice. To avoid of introducing sampling bias, data from different slices of one animal (which varied from 1 to 3) were averaged before the final ratios were calculated, and statistics were performed with ANOVA (i.e., n = number of rats).
Statistical analyses
Statview software was used for statistical analysis. The path length or corrected integrated pathlength (CIPL) in the spatial trials of the Morris swim test was analyzed with repeated-measures ANOVA, with age or environmental treatment as the between-subjects factor and path length across days as the repeated within-subject factor. The cued trials were analyzed similarly. In the probe trial, rats' dwell times in the target quardrant or the number of times they crossed the previous target location were analyzed using one-way ANOVA. Fischer's protected least significant difference analysis was used when a post hoc test was needed.
The presynaptic fiber volley and postsynaptic field potential responses, as well as the ratio of AMPAR- to NMDAR-mediated fEPSPs, were analyzed with one-way ANOVA in both experiments. The Fischer's PLSD test was used for post hoc analysis, and α was set at the 0.05 level.
RESULTS
Changes in NMDAR- and AMPAR-mediated neurotransmission in hippocampal granule cells in young, adult, and old memory-impaired rats
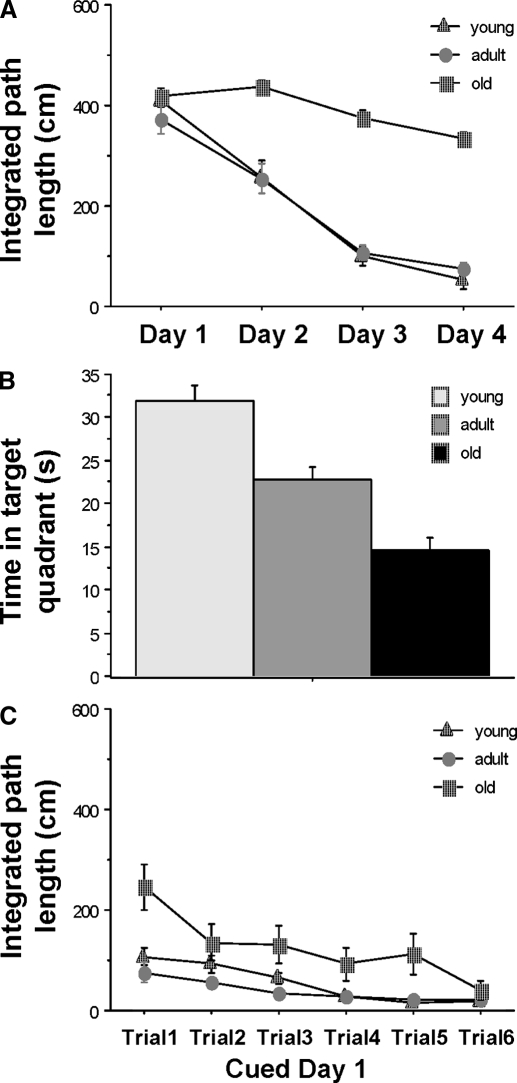
A significant age effect was detected for the spatial trials of the Morris swim task [F(2,51) = 51.36; P < 0.0001]. The young (1 mo) and adult (9 mo) rats performed very similar to each other, and they both did significantly better than did the old (27 mo) rats (P < 0.0001) except on the first day (Fig. 2A). A significant age effect was also detected in the probe trials [F(2,51) = 27.95; P < 0.0001]. Young rats spent significantly more time in the target quadrant than did the adult rats (P < 0.001), and the adult rats spent significantly more time in the target quadrant than did the old rats (P < 0.001; Fig. 2B). Although old rats took significantly longer paths to find the visible platform in the first cued trial compared with the young and adult rats [F(2,51) = 8.66; P < 0.001], there was no significant difference on the performance of the three age groups by the last cued trial [F(2,51) = 1.31; P = 0.28; Fig. 2C], suggesting that there was no impairment in visual discrimination ability in old rats.
FIG. 2.
Effects of age on rats' performance in Morris swim task. A: comparison of young (1 mo), adult (9 mo), and old (27 mo) rats on the spatial version of the Morris swim task. The young and adult rats did not differ from one another, but performed significantly better than did the old rats after the first day of training. B: age comparison of performance on the probe trial that was conducted at the end of the spatial training (A). There was a significant difference between the time spent in the target quadrant between the young and both older age groups and between adult and old animals, consistent with spatial memory changes across age. C: performance on the cued version of the Morris swim task, showing that all groups found the visible platform by the end of only 1 day of training.
Hippocampal slices were prepared from young, adult, and old rats to record synaptic field potentials and presynaptic fiber potentials in the stratum moleculare region of dentate gyrus (Fig. 1A). Measurement of AMPAR-mediated EPSPs and NMDAR-mediated EPSPs were obtained systematically from young, adult, and old rats. In the old rats, the number of perforant path synapses declines (Geinisman et al. 1995). This reduction is accompanied by a corresponding reduction in the magnitude of the presynaptic fiber potential (Foster et al. 1991), presumably reflecting pruning of the perforant path collateral axons arising from the entorhinal cortex. For this reason, and also to reduce variance caused by electrode positioning, the synaptic component of each response was normalized by the magnitude of the corresponding presynaptic fiber potential for analysis (Barnes et al. 1997). In addition, for each slice, the ratio of the AMPAR- to NMDAR- mediated components was computed. These ratios are independent of any normalization factor. Typically, data were collected from several slices in each rat. These “within-rat” data were averaged so that the final n is number of animals.
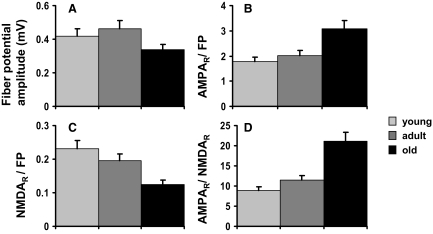
The changes in response characteristics over the life span are summarized in Fig. 3. As found in several previous studies (Barnes et al. 1992), the presynaptic fiber potential was significantly reduced in 27-mo-old animals compared with the adult group (P < 0.05). There was no statistically significant difference between the fiber potential of young and adult rats (Fig. 3A). The AMPAR-mediated EPSP, however, was increased for a given presynaptic fiber potential amplitude (P < 0.05), while the NMDAR EPSP declined in the old rats (P < 0.05). These changes were reflected in an approximately twofold increase in the mean ratio of AMPAR to NMDAR components between 1 and 27 mo of age (P < 0.0001; Fig. 3D).
FIG. 3.
Effects of age on AMPAR- to NMDAR-mediated neurotransmission. A: as observed previously, the magnitude of the presynaptic fiber potential for a given stimulus intensity declined in 27-mo-old rats, as did the total number of perforant path synaptic terminals in the middle molecular layer (Geinisman et al. 1992). B: amplitude of the AMPAR-mediated EPSP per unit fiber potential increased in the old rats. C: amplitude of the NMDAR-mediated component decreased with age. D: for each slice, the ratio of the AMPAR to NMDAR responses was also measured directly. This ratio increased with age.
Effects of experience on spatial memory performance
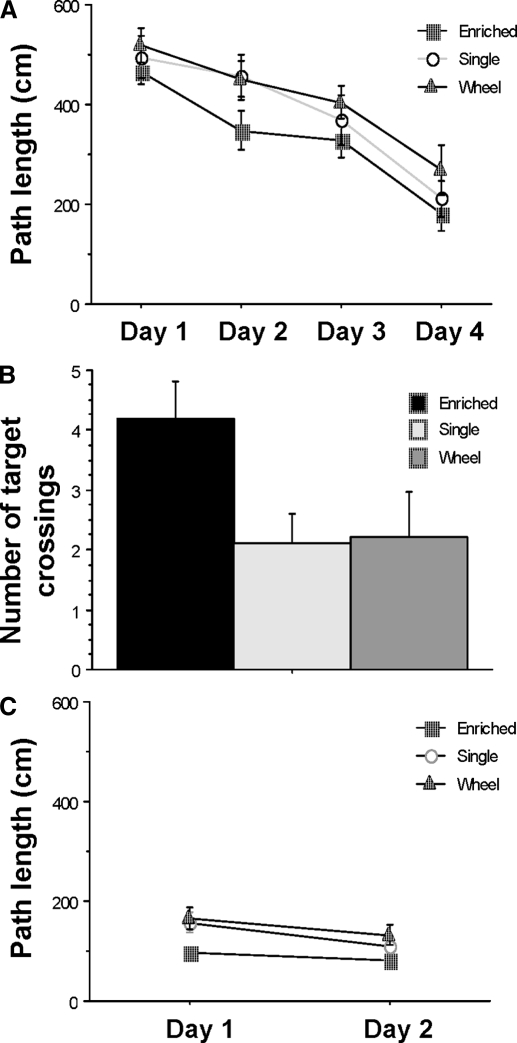
Three treatment conditions were administered to different groups of animals for a 10-mo period, including a standard control group, a wheel running/social enrichment group, and an enriched environment group. Spatial memory was examined following this treatment period using the Morris swim task. A significant treatment effect was detected in the spatial version of the task [F(2,24) = 5.20; P = 0.013]. Rats raised in the enriched environment did significantly better than did rats from the standard control or wheel running/social enrichment groups (P < 0.05), whereas the performance of the latter two groups were similar to each other (Fig. 4A). This result is consistent with previous observations of rats given enriched environment experience (Falkenberg et al. 1992) and suggests that this treatment facilitates the speed of learning in this task. In the probe version of the task, there was also a statistically significant difference between the enriched environment group and the other two control groups in the number of times the rats crossed the location of the escape platform when it was removed from the tank [target crossings, Fig. 4B; F(2,25) = 3.617; P < 0.05].
FIG. 4.
Effects of enrichment experience on rats' performance in Morris swim task. A: mean path length taken by rats in the spatial version of the Morris swim task for each of the 3 treatment groups. The enriched environment group took significantly shorter paths to find the platform on day 2 compared with the other 2 groups. B: mean number of target crossings in the probe task. The enriched environment group crossed the training quadrant platform location significantly more often than did either of the control groups. C: mean and SE of path length on the cued version of the Morris swim task. There is no statistically significant difference among the 3 treatment groups.
In the cued version of the task, although the enriched environment rats seemed to learn the task faster, a repeated-measures ANOVA analysis did not show a statistically significant effect of treatment [F(2,25) = 2.30; P = 0.12], suggesting that all three treatment groups had normal visual discrimination capacity (Fig. 4C).
In all three versions of the task, the performance of the standard control group and the wheel running/social enrichment group were close to each other, suggesting that running plus social contact did not produce improvements in spatial learning and memory.
Effects of experience on NMDAR- and AMPAR-mediated neurotransmission in hippocampal granule cells
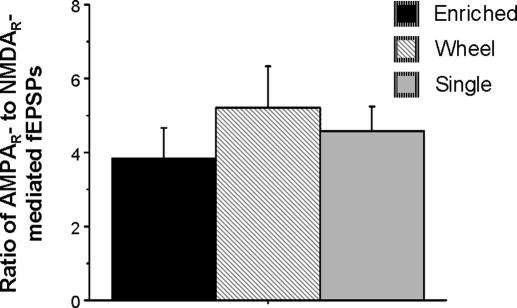
Following the 10 mo of controlled housing and testing in the Morris swim task, the rats were killed, and their hippocampi were used for field potential recording. The amplitude of the presynaptic fiber potential was not significantly affected by treatment conditions (Fig. 5, A and B). For a given presynaptic input amplitude, neither the amplitude of the AMPAR-mediated fEPSPs nor the amplitude of the NMDAR-mediated fEPSPs was significantly different among slices from the enriched environment, the standard control, and the wheel running/social enrichment groups (Fig. 5, C and D). Furthermore, the ratio of AMPAR- to NMDAR-mediated fEPSPs was also not significantly different among the three treatment groups (Fig. 6). These results suggest that substantial enrichment experience does not alter NMDAR- and AMPAR-mediated neurotransmission in the dentate gyrus.
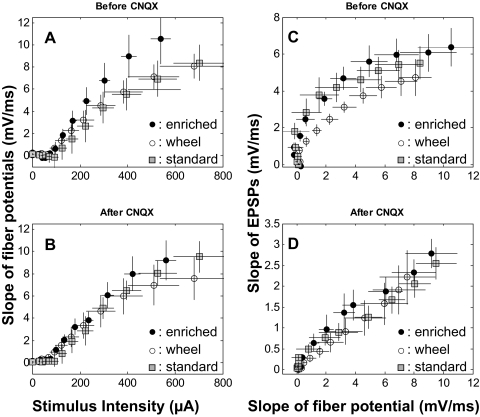
FIG. 5.
AMPAR- and NMDAR-mediated neurotransmission. A: the slope of the fiber potential at different stimulus intensities before the application of CNQX in the 3 treatment groups. B: the slope of the fiber potential at different stimulus intensities after the application of CNQX. C: the slope of the field EPSP (fEPSP) at different fiber potential amplitudes before the application of CNQX. D: the slope of the fEPSP at different fiber potential amplitudes after the application of CNQX. No statistically significant treatment effects were detected between any of the enrichment, wheel/social, or standard caged groups, suggesting that experience did not alter AMPAR- or NMDAR-mediated neurotransmission.
FIG. 6.
Effects of experience on the AMPAR- to NMDAR-response ratio. Effect of experience on the ratio of AMPAR- to NMDAR-mediated fEPSPs. This ratio was not modified by exercise (wheel running/social condition) or enrichment treatment.
DISCUSSION
These data contribute to a growing body of evidence indicating that the process of aging involves a complex, regionally specific pattern of neurobiological changes, not all of which are necessarily deteriorative (Burke and Barnes 2006; Rosenzweig and Barnes 2003; Wilson et al. 2006). There are two main findings in this study. 1) The AMPAR-mediated fEPSP response increased with age in hippocampal granule cells, whereas the NMDAR-mediated fEPSP response decreased. This resulted in an increase of the AMPAR-to-NMDAR response ratio with age. 2) The AMPAR-to-NMDAR response ratio is not altered by extensive experience, suggesting that the observed change in this ratio with aging is a consequence of a fixed developmental process.
Over the past decade, it has become clear that multiple activity-dependent mechanisms cooperate to maintain stability in neural circuit function in response to dynamic changes in synaptic drive (Turrigiano 2007; Turrigiano et al. 1998). The first descriptions of this homeostatic plasticity mechanism came from observations of the phenomenon of denervation supersensitivity in muscle (see Davis 2006 for review). Similar principles, however, also are in play in the brain, where average neural activity levels in networks are continuously adjusted to prevent either excessive excitation or quiescence. Because synapses can change dramatically in response to experience, this mechanism serves to keep cellular excitability levels within a range that effectively “normalizes” total synaptic weights. These mechanisms can be pre- or postsynaptic and can work either at a global network level, a cellular level, or even selectively at individual synapses. Postsynaptic forms of homeostatic plasticity include mechanisms of synaptic “scaling” that adjust synaptic weights up or down to normalize firing rates (O'Brian et al. 1998; Turrigiano et al. 1998). Proportional adjustments of synaptic strengths, for example, can provide a means of preserving relative differences between synapses that have been modified by LTP or LTD, because the weights are modified in proportion to their initial strengths (Liu et al. 2004; Moga et al. 2006; Myme et al. 2003).
In fact, in neocortical areas such as visual or prefrontal cortex, it seems that a constant AMPAR-to-NMDAR response ratio is maintained, even in the face of ongoing changes in synaptic plasticity (Myme et al. 2003; Umemiya et al. 1999; Watt et al. 2000, 2004). In hippocampus, however, there are considerable data that suggest that LTP can result in increased AMPAR-mediated currents (for review, see Malinow et al. 2000) and an altered AMPAR-to-NMDAR ratio (Heynen et al. 2000; Liao et al. 1995; Lu et al. 2001). Although the data on NMDA-LTP are less consistent, the possibility that there is a “lag” in the expression of NMDA-LTP may explain the discrepancies in observations made in the hippocampus. In neocortex, however, it is clear that this receptor ratio is proportionally regulated (Watt et al. 2004), at least in young animals. This study is consistent with the idea that some form of receptor normalization occurs during the course of information processing, because the enriched environment treatment that dramatically increased the “amount of experience” rats received over a 10-mo period did not affect AMPAR-to-NMDAR ratios. Studies designed to examine the magnitude of LTP induction in the hippocampus following enrichment treatment have generated inconsistent results, with one laboratory reporting more LTP induced in animals following the treatment (Duffy et al. 2001) and another suggesting that LTP induction was inhibited following enrichment (Foster et al. 1996). Overall, it seems that there are mechanisms at play that act to dynamically adjust synaptic strengths in circuits, regulating AMPA and NMDA current alterations so that new information can be stored effectively.
Interestingly, the age-related change in the AMPAR-to-NMDAR response ratio is region specific and correlates with differential effects of aging on LTP induction thresholds in different hippocampal subregions. In old rats, there is no effect of age on the maximal expression of LTP at either the Schaffer collateral–pyramidal cell or perforant path–granule cell synapse (Barnes 1979; Diana et al. 1994a,b; Landfield and Lynch 1977; Landfield et al. 1978; Tombaugh et al. 2002) when the stimulation parameters used are well above the LTP induction threshold. When mild stimulation of afferent fibers is paired with intracellularly applied depolarization, however, the threshold for LTP induction is increased with age in the dentate gyrus (Barnes et al. 2000). This is consistent with our observation of an increase in the AMPAR-to-NMDAR response ratio in old rats. Additionally, fewer granule cells express the immediate early gene Arc in old animals compared with young following behavioral exploration (Small et al. 2004), and the expression of Arc is necessary for the maintenance of LTP and the consolidation of spatial memory (Guzowski et al. 2000). In CA1, on the other hand, where the AMPAR-to-NMDAR response ratio does not increase over the life span (Barnes et al. 1997), there is no age-related change in LTP threshold following intracellular peri-threshold stimulation pairings (Barnes et al. 1996) or in the numbers of cells that express Arc (Small et al. 2004). Importantly, these results are unlikely to reflect alterations in the function of the aged cells involving resting membrane potential, input resistance or membrane time constants, because these variables do not change across age (for review, see Barnes 1994).
One possible interpretation of an increment in AMPAR-mediated responses at granule cells synapses of aged rats is that it reflects a mechanism to preserve previously stored information. Granule cells are ideal candidates for information storage sites because of their large numbers and the sparse coding scheme in the dentate gyrus. Additionally, the decreased NMDAR-mediated response in granule cells of aged rats may concurrently prevent stored information from being overwritten by restricting the induction of further plasticity. The observation that administration of the NMDAR antagonist CPP following spatial memory training enhances subsequent memory retention (Villarreal et al. 2002) provides strong support for this hypothesis. Although most memory consolidation theories suggest that the hippocampus is only a temporary repository of information (Squire and Alvarez 1995), an alternative view is that the storage and retrieval of spatial memory may always depend on the hippocampus (Moscovitch et al. 2006). If the hippocampus is involved in such long-term memory maintenance, the dentate gyrus could be a subregion involved in this durable synaptic commitment.
In summary, the AMPAR-to-NMDAR response ratio increases in the dentate gyrus from 1 to 27 mo of age, and this increment seems to be developmentally regulated rather than experience dependent. These results suggest that the dynamics of information storage mechanism in hippocampal granule cells may differ in an important way from other hippocampal subfields. Furthermore, aged rats may preserve the storage of old information by sacrificing the learning of new information (Burke and Mackay 1997; Wilson et al. 2006).
GRANTS
This work was supported by National Institute on Aging Grant R01 AG-03376. This work was also supported by the McKnight Brain Research Foundation.
Acknowledgments
We thank J. Shen, F. P. Houston, and R. White for participation in some of these experiments and M. Carroll for administrative assistance. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Present addresses: Z. Yang, EnVivo Pharmaceuticals, Watertown, MA 02472; M. Krause, Ernest Gallo Clinic and Research Center, Emeryville, CA 94608; G. Rao, Department of Neurobiology and Anatomy, W. M. Keck Center for the Neurobiology of Learning and Memory, University of Texas Medical School at Houston, Houston, TX 77225.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Amaral et al. 1990.Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res 83: 1–11, 1990. [DOI] [PubMed] [Google Scholar]
- Barnes 1979.Barnes CA Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93: 74–104, 1979. [DOI] [PubMed] [Google Scholar]
- Barnes 1994.Barnes CA Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci 17: 13–18, 1994. [DOI] [PubMed] [Google Scholar]
- Barnes et al. 1992.Barnes CA, Rao G, Foster TC, McNaughton BL. Region-specific age effects on AMPA sensitivity: electrophysiological evidence for loss of synaptic contacts in hippocampal field CA1. Hippocampus 2: 457–468, 1992. [DOI] [PubMed] [Google Scholar]
- Barnes et al. 2000.Barnes CA, Rao G, Houston FP. LTP induction threshold change in old rats at the perforant path–granule cell synapse. Neurobiol Aging 21: 613–620, 2000. [DOI] [PubMed] [Google Scholar]
- Barnes et al. 1996.Barnes CA, Rao G, McNaughton BL. Functional integrity of NMDA-dependent LTP induction mechanisms across the lifespan of F-344 rats. Learn Mem 3: 124–137, 1996. [DOI] [PubMed] [Google Scholar]
- Barnes et al. 1997.Barnes CA, Rao G, Shen J. Age-related decrease in the N-methyl-D-aspartateR-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiol Aging 18: 445–452, 1997. [DOI] [PubMed] [Google Scholar]
- Bear and Malenka 1994.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 4: 389–399, 1994. [DOI] [PubMed] [Google Scholar]
- Blake et al. 1988.Blake J, Brown M, Collingridge G. CNQX blocks acidic amino acid induced depolarizations and synaptic components mediated by non-NMDA receptors in rat hippocampal slices. Neurosci Lett 89: 182–186, 1988. [DOI] [PubMed] [Google Scholar]
- Bliss and Collingridge 1993.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993. [DOI] [PubMed] [Google Scholar]
- Burke and Mackay 1997.Burke DM, Mackay DG. Memory, language, and aging. Philos Trans R Soc Lond B Biol Sci 352: 1845–1856, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke and Barnes 2006.Burke SN, Barnes CA. Neural plasticity in the aging brain. Nat Rev Neurosci 7: 30–40, 2006. [DOI] [PubMed] [Google Scholar]
- Carroll et al. 1999.Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci 2: 454–460, 1999. [DOI] [PubMed] [Google Scholar]
- Christie et al. 1994.Christie BR, Kerr DS, Abraham WC. Flip side of synaptic plasticity: long-term depression mechanisms in the hippocampus. Hippocampus 4: 127–135, 1994. [DOI] [PubMed] [Google Scholar]
- Coussens and Teyler 1996.Coussens CM, Teyler TJ. Long-term potentiation induces synaptic plasticity at nontetanized adjacent synapses. Learn Mem 3: 106–114, 1996. [DOI] [PubMed] [Google Scholar]
- Davies and Collingridge 1989.Davies SN, Collingridge GL. Role of excitatory amino acid receptors in synaptic transmission in area CA1 of rat hippocampus. Proc R Soc Lond B Biol Sci 236: 373–384, 1989. [DOI] [PubMed] [Google Scholar]
- Davis 2006.Davis GW Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci 29: 307–323, 2006. [DOI] [PubMed] [Google Scholar]
- Diana et al. 1994a.Diana G, Domenici MR, Loizzo A, Scotti de Carolis A, Sagratella S. Age and strain differences in rat place learning and hippocampal dentate gyrus frequency-potentiation. Neurosci Lett 171: 113–116, 1994a. [DOI] [PubMed] [Google Scholar]
- Diana et al. 1994b.Diana G, Scotti de Carolis A, Frank C, Domenici MR, Sagratella S. Selective reduction of hippocampal dentate frequency potentiation in aged rats with impaired place learning. Brain Res Bull 35: 107–111, 1994b. [DOI] [PubMed] [Google Scholar]
- Dudek and Bear 1992.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA 89: 4363–4367, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy et al. 2001.Duffy SN, Craddock KJ, Abel T, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem 8: 26–34, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand et al. 1996.Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature 381: 71–75, 1996. [DOI] [PubMed] [Google Scholar]
- Falkenberg et al. 1992.Falkenberg T, Mohammed AK, Henriksson B, Persson H, Winblad B, Lindefors N. Increased expression of brain-derived neurotrophic factor mRNA in rat hippocampus is associated with improved spatial memory and enriched environment. Neurosci Lett 138: 153–156, 1992. [DOI] [PubMed] [Google Scholar]
- Foster et al. 1991.Foster TC, Barnes CA, Rao G, McNaughton BL. Increase in perforant path quantal size in aged F-344 rats. Neurobiol Aging 12: 441–448, 1991. [DOI] [PubMed] [Google Scholar]
- Foster et al. 1996.Foster TC, Gagne J, Massicotte G. Mechanism of altered synaptic strength due to experience: relation to long-term potentiation. Brain Res 736: 243–250, 1996. [DOI] [PubMed] [Google Scholar]
- Foster and McNaughton 1991.Foster TC, McNaughton BL. Long-term synaptic enhancement in hippocampal field CA1 is due to increased quantal size, not quantal content. Hippocampus 1: 79–91, 1991. [DOI] [PubMed] [Google Scholar]
- Geinisman et al. 1995.Geinisman Y, de Toledo-Morrell L, Morrell F, Heller RE. Hippocampal markers of age-related memory dysfunction: behavioral, electrophysiological and morphological perspectives. Prog Neurobiol 45: 223–252, 1995. [DOI] [PubMed] [Google Scholar]
- Geinisman et al. 1992.Geinisman Y, de Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus 2: 437–444, 1992. [DOI] [PubMed] [Google Scholar]
- Geinisman et al. 2004.Geinisman Y, Ganeshina O, Yoshida R, Berry RW, Disterhoft JF, Gallagher M. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol Aging 25: 407–416, 2004. [DOI] [PubMed] [Google Scholar]
- Guzowski et al. 2000.Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent Arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci 20: 3993–4001, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen et al. 2000.Heynen AJ, Quinlan EM, Bae DC, Bear MF. Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron 28: 527–536, 2000. [DOI] [PubMed] [Google Scholar]
- Isaac et al. 1995.Isaac JTR, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron 15: 427–434, 1995. [DOI] [PubMed] [Google Scholar]
- Jung and McNaughton 1993.Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus 3: 165–182, 1993. [DOI] [PubMed] [Google Scholar]
- Kauer et al. 1988.Kauer JA, Malenka RC, Nicoll RA. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron 1: 911–917, 1988. [DOI] [PubMed] [Google Scholar]
- Landfield and Lynch 1977.Landfield PW, Lynch G. Impaired monosynaptic potentiation in in vitro hippocampal slices from aged, memory-deficient rats. J Gerontol 32: 523–533, 1977. [DOI] [PubMed] [Google Scholar]
- Landfield et al. 1978.Landfield PW, McGaugh JL, Lynch G. Impaired synaptic potentiation processes in the hippocampus of aged, memory-deficient rats. Brain Res 150: 85–101, 1978. [DOI] [PubMed] [Google Scholar]
- Leutgeb et al. 2005.Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science 309: 619–623, 2005. [DOI] [PubMed] [Google Scholar]
- Liao et al. 1995.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during LTP in CA1 region of hippocampal slice. Nature 375: 400–404, 1995. [DOI] [PubMed] [Google Scholar]
- Lisman and Idiart 1995.Lisman JE, Idiart MAP. Storage of 7 +/− 2 short-term memories, in oscillatory subcycles. Science 267: 1512–1515, 1995. [DOI] [PubMed] [Google Scholar]
- Liu et al. 2004.Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304: 1021–1024, 2004. [DOI] [PubMed] [Google Scholar]
- Lu et al. 2001.Lu W, Man H, Ju W, Trimble W, MacDonald J, Wang Y. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29: 243–254, 2001. [DOI] [PubMed] [Google Scholar]
- Lynch et al. 1982.Lynch G, Halpain S, Baudry M. Effects of high-frequency synaptic stimulation on glumate receptor binding studied with a modified in vitro hippocampal slice preparation. Brain Res 244: 101–111, 1982. [DOI] [PubMed] [Google Scholar]
- Malenka and Nicoll 1999.Malenka RC, Nicoll RA. Long-term potentiation –a decade of progress? Science 285: 1870–1874, 1999. [DOI] [PubMed] [Google Scholar]
- Malinow et al. 2000.Malinow R, Mainen ZF, Hayashi Y. LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol 10: 352–357, 2000. [DOI] [PubMed] [Google Scholar]
- Marr 1971.Marr D Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci 262: 23–81, 1971. [DOI] [PubMed] [Google Scholar]
- Moga et al. 2006.Moga DE, Shapiro ML, Morrison JH. Bidirectional redistribution of AMPA but not NMDA receptors after perforant path simulation in the adult rat hippocampus in vivo. Hippocampus 16: 990–1003, 2006. [DOI] [PubMed] [Google Scholar]
- Moscovitch et al. 2006.Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol 16: 179–190, 2006. [DOI] [PubMed] [Google Scholar]
- Mulkey and Malenka 1992.Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron 9: 967–975, 1992. [DOI] [PubMed] [Google Scholar]
- Myme et al. 2003.Myme CI, Sugino K, Turrigiano GG, Nelson SB. The NMDA-to-AMPA ratio at synapses onto layer 2/3 pyramidal neurons is conserved across prefrontal and visual cortices. J Neurophysiol 90: 771–779, 2003. [DOI] [PubMed] [Google Scholar]
- O'Brien et al. 1998.O'Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron 23: 309–323, 1998. [DOI] [PubMed] [Google Scholar]
- Rosenzweig and Barnes 2003.Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol 69: 143–179, 2003. [DOI] [PubMed] [Google Scholar]
- Shepherd et al. 2006.Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 52: 475–484, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small et al. 2004.Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci USA 101: 7181–7186, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire and Alvarez 1995.Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol 5: 169–177, 1995. [DOI] [PubMed] [Google Scholar]
- Tombaugh et al. 2002.Tombaugh GC, Rowe WB, Chow AR, Michael TH, Rose GM. Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats. J Neurosci 22: 9932–9940, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano 2007.Turrigiano G Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol 17: 318–324, 2007. [DOI] [PubMed] [Google Scholar]
- Turrigiano et al. 1998.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391: 892–896, 1998. [DOI] [PubMed] [Google Scholar]
- Umemiya et al. 1999.Umemiya M, Senda M, Murphy TH. Behaviour of NMDA and AMPA receptor-mediated miniature EPSCs at rat cortical neuron synapses identified by calcium imaging. J Physiol 521: 113–122, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal et al. 2002.Villarreal DM, Do V, Haddad E, Derrick BE. NMDA receptor antagonists sustain LTP and spatial memory: active processes mediate LTP decay. Nat Neurosci 5: 48–52, 2002. [DOI] [PubMed] [Google Scholar]
- Watt et al. 2004.Watt AJ, Sjostrom PJ, Hausser M, Nelson SB, Turrigiano GG. A proportional but slower NMDA potentiation follows AMPA potentiation in LTP. Nat Neurosci 7: 518–524, 2004. [DOI] [PubMed] [Google Scholar]
- Watt et al. 2000.Watt AJ, van Rossum MCW, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron 26: 659–670, 2000. [DOI] [PubMed] [Google Scholar]
- Wilson et al. 2006.Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci 29: 662–670, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]