Abstract
Patients with inflammatory bowel disease often suffer from gastrointestinal motility and sensitivity disorders. The aim of the current study was to investigate the role of transient receptor potential of the vanilloid type 1 (TRPV1) receptors in the pathophysiology of colitis-induced pelvic afferent nerve sensitization. Trinitrobenzene sulphate (TNBS) colitis (7.5 mg, 30% ethanol) was induced in Wistar rats 72 h prior to the experiment. Single-fibre recordings were made from pelvic nerve afferents in the decentralized S1 dorsal root. Fibres responding to colorectal distension (CRD) were identified in controls and rats with TNBS colitis. The effect of the TRPV1 antagonist N-(4-tertiarybutylphenyl)-4-(3-chlorophyridin-2-yl)tetrahydropyrazine-1(2H)carboxamide (BCTC; 0.25–5 mg kg−1) or its vehicle (hydroxypropyl-β-cyclodextrin) was tested on the afferent response to repetitive distensions (60 mmHg). Immunocytochemical staining of TRPV1 and NF200, a marker for A-fibre neurons, was performed in the dorsal root ganglia L6–S1. TNBS colitis significantly increased the response to colorectal distension of pelvic afferent C-fibres. BCTC did not significantly affect the C-fibre response in controls, but normalized the sensitized response in rats with colitis. TNBS colitis increased the spontaneous activity of C-fibres, an effect which was insensitive to administration of BCTC. TNBS colitis had no effect on Aδ-fibres, nor was their activity modulated by BCTC. TNBS colitis caused an immunocytochemical up-regulation of TRPV1 receptors in the cell bodies of pelvic afferent NF200 negative neurons. TRPV1 signalling mediates the colitis-induced sensitization of pelvic afferent C-fibres to CRD, while Aδ-fibres are neither sensitized by colitis nor affected by TRPV1 inhibition.
Patients with inflammatory bowel disease (IBD) often suffer from abnormalities of gastrointestinal motor and sensory function (De Schepper et al. 2008a). These neurogastroenterological manifestations can have a profound effect on the patient's quality of life, both during inflammatory episodes and in periods of remission (Pezzone & Wald, 2002; Farrokhyar et al. 2006). The mechanisms underlying these inflammation-induced alterations of neuromuscular function remain largely unknown but a role for afferent neurons was suggested. Extrinsic afferent nerves consist of polymodal unmyelinated C-fibres and thinly myelinated Aδ-fibres which respond to distension, temperature and chemical mediators (Blackshaw & Gebhart, 2002). Sensitization of these fibres by inflammation leads to an increase in neuronal spontaneous activity and/or hyperresponsiveness to stimulation (Cervero & Laird, 1999; McMahon, 2004). The pathophysiology of afferent nerve sensitization is very complex and involves a variety of inflammatory mediators (Costigan & Woolf, 2000). One of the key modulators is the transient receptor potential of the vanilloid type 1 (TRPV1) receptor. The latter is a non-selective cation channel which is activated by the pungent capsaicin, protons (pH 6–5), heat (42–53°C), mechanical stimuli, the endocannabinoid anandamide and a number of lipoxygenase metabolites (Geppetti & Trevisani, 2004). TRPV1 receptors are readily cross-sensitized by the activation of sensory neuron receptors for a number of inflammatory mediators and thereby act as downstream integrators of the sensitizing effects of an inflammatory soup (Geppetti & Trevisani, 2004). As such, TRPV1 is highly involved in the pathophysiology of sensitization of afferent nerves, leading to visceral hypersensitivity (Di Marzo et al. 2002) and the occurrence of abnormal reflex behaviour, as demonstrated by the phenomenon of cologastric gastroparesis (De Schepper et al. 2007). TRPV1 receptor antagonists may therefore represent an attractive target in the development of pharmaceutical therapeutics for visceral pain and inflammation-induced motility disorders. Still, the role of TRPV1 receptors in in vivo colitis-induced afferent nerve sensitization has never been fully explored.
Current knowledge about the pathophysiology of gastrointestinal inflammation-induced afferent sensitization is on the one hand based on in vitro investigations of intestinal loops or flattened preparations with the innervating nerve attached (Lynn & Blackshaw, 1999; Jones et al. 2005; Wang et al. 2005), or on studies of isolated and cultured neurons (Xu & Huang, 2002). On the other hand, a number of studies reported in vivo gastrointestinal afferent sensitization in experimental models of instant and transient colonic inflammation induced by short exposure of the colon to mustard oil or zymosan (Gebhart, 2000; Su et al. 2001). We previously demonstrated that TNBS-induced colitis, an established animal model for IBD, influences the afferent pelvic nerve and increases its TRPV1 expression (De Schepper et al. 2008b). Therefore, the aim of the current study was to document the effect of experimental TNBS-induced colitis in rats on pelvic afferent nerve activity and to investigate the role of TRPV1 receptors in colitis-induced neuromodulation of afferent neurons.
Methods
Ethical approval
All procedures were approved by the Committee for Medical Ethics and the use of Experimental Animals at the University of Antwerp. All anaesthesia was induced using pentobarbital (45 mg kg−1i.p.). Rats were killed at the end of the experiment using overdosage of pentobarbital (100 mg kg−1i.v.). In total, we studied 48 control rats and 44 rats with TNBS colitis.
Animal model
Distal colitis was induced in female Wistar rats (200–225 g). Rats were fasted for 24 h. After pentobarbital anaesthesia (45 mg kg−1i.p.) an enema of 0.5 ml of saline (controls) or 0.5 ml of a trinitrobenzene sulphate solution (7.5 mg TNBS dissolved in 30% ethanol) was administered in the colorectum. As the rats showed no pain behaviour after recovery from the anaesthesia, no postprocedure analgesia was administered. All further experiments were performed 72 h after induction of colitis. The extent of inflammation was verified by macroscopic evaluation, using a standardized scoring system ranging from 0 (no inflammation) to 10 (severe damage extending over > 5 cm) (Wallace & Keenan, 1990). Additionally, histological assessment of the affected region using standard haematoxylin eosin (HE) staining, and quantification of myeloperoxidase (MPO) content, a biochemical marker for neutrophilic infiltration, were performed (Moreels et al. 2001). For MPO quantification, full-thickness tissue samples were taken from the distal colon. Samples were blotted dry and placed in a potassium phosphate buffer pH 6.0 containing 0.5% hexadecyltrimethylammonium bromide (5 g tissue per 100 ml buffer). The samples were placed on ice, homogenized for 30 s and subjected to two sonication and freeze-thawing cycles. The suspension was centrifuged at 15 000 g for 15 min at 4°C. Aliquots (0.1 ml) of the supernatant were added to 2.9 ml of an o-dianisidine solution (16.7 mg of o-dianisidine in 1 ml methanol, 98 ml 50 mmol l−1 potassium phosphate buffer pH 6.0 and 1 ml of a 0.05% H2O2 solution as a substrate for the MPO enzyme). The change in absorbency was read at 460 nm over 60 s using a Spectronic Genesys 5 spectrophotometer (Milton Roy, Rochester, NY, USA). One unit of MPO activity was defined as the quantity able to convert 1 μmol H2O2 to H2O per min at 25°C and was expressed in units per gram tissue.
Electrophysiological studies: preparation
The protocol for measurement of pelvic afferent nerve activity was adapted from Sengupta & Gebhart (1994). Rats were anaesthetized with a bolus of pentobarbital (45 mg kg−1i.p.). Body temperature was continuously monitored and maintained at 37°C. The trachea was cannulated and exposed to room air. The jugular vein was cannulated for administration of anaesthetic throughout the experiment (pentobarbital 7.5 mg kg−1 h−1i.v.). The carotid artery was cannulated and connected to a pressure transducer for continuous measurement of arterial blood pressure (ABP). The latter was maintained with supplemental doses of 1 ml of 5% dextrose in saline (i.v.) if ABP dropped below 80 mmHg. A laparotomy was performed. The urinary bladder was catheterized and drained. The femoral nerve, the sciatic nerve and the pudendal nerve were isolated and transected. The pelvic nerve was identified and carefully freed from surrounding tissues. A pair of Teflon coated silver wires was connected to the nerve and covered in non-reactive silicone gel (Wacker Chemie, Munich, Germany). The lumbosacral spinal cord was exposed by laminectomy (vertebral laminae T12–S1). The skin of the incision was attached to a suspended metal ring and the cavity thus formed was filled with highly liquid paraffin (37°C). The dura mater was carefully removed exposing the lumbosacral dorsal rootlets. Finally, a latex balloon (5–6 cm length) was inserted into the colorectum and connected to a pressure-controlled distension device allowing for graded colorectal distension (CRD). To determine whether TNBS-induced colitis increased colonic wall stiffness, a compliance curve was constructed by measuring colonic pressure at different intraballoon volumes (0.25–1.5 ml, 10 s).
Electrophysiological studies: recording
The S1 dorsal rootlet was identified and cut close to its entry into the spinal cord. The rootlet was placed on a platform immersed in the paraffin bath and split into very fine strands. These were draped over a platinum bipolar electrode for recording of action potential frequency or spike rate. Nerve activity was recorded with a Neurolog headstage (NL104AK, Digitimer, Hertfordshire, UK), amplified (NL104) and filtered (NL 125, bandpass 500–5000 Hz). The signal was continuously monitored on a storage oscilloscope (Tektronix), digitized (CED1902, Cambridge Electronic Design, Cambridge, UK), stored on a PC and analysed off-line using spike2 software (Cambridge Electronic Design) to identify single fibre activity. Single fibre action potentials (spikes) reacting to both pelvic nerve stimulation and CRD were then counted and the spike firing rate (Hz) was determined.
Pelvic nerve input originating from the colon was identified using three criteria: a reproducible response to electrical stimulation of the pelvic nerve (S44, Grass Technologies/Astro-Med Inc., West Warwick, RI, USA; 0.5 ms square wave pulse at 1–8 V), a reproducible response to CRD (40 mmHg) and no response to tactile stimulation of the S1 dermatome (perineum and tail base). If a nerve action potential response met these criteria, its spontaneous activity was registered during 1 min and a distension protocol was applied. To determine the actual response of the fibre to CRD, the spontaneous activity was subtracted from the spike rate during distention. Fibres were categorized as having a low (< 30 mmHg) or a high (≥ 30 mmHg) threshold of response. We used 30 mmHg since it has been repeatedly shown that high threshold fibres only start firing at intracolonic pressures over 25–28 mmHg (Sengupta & Gebhart, 1994; Wynn et al. 2003). Coincidentally, this threshold value concurs with the pain response threshold to CRD (Ness & Gebhart, 1988). The conduction velocity of the fibre under study was determined based upon the time lag between application of the pelvic nerve stimulus and recording of the resulting evoked action potential at the registration electrode (conduction velocity CV = distance between stimulation and registration electrodes (m)/lag (s)). If the CV was less than 2.5 m s−1, the fibre was considered an unmyelinated C-fibre, if CV was more than 2.5 m s−1, the fibre was considered a thinly myelinated Aδ-fibre (Sengupta & Gebhart, 1994).
Electrophysiological studies: protocols
Phasic colorectal distensions provide a reproducible, well used and easily quantifiable stimulus which evokes afferent responses similar in magnitude to the responses elicited by slow ramp distension (Sengupta & Gebhart, 1994; Joshi et al. 2000). Therefore, we preferred to use phasic CRD in all our experiments.
In the first part of this study, a graded CRD protocol was applied (20–30–40–60–80 mmHg, 20 s, 4 min interval) generating pressure response curves commonly termed stimulus response functions (SRFs). These SRF curves were compared in controls and in rats with TNBS-induced colitis. The analysis was repeated with respect to the threshold of response of the afferent neurons (low versus high) and with respect to their CV (C versus Aδ).
In a second part of the study, we investigated the effect of the TRPV1 receptor antagonist N-(4-tertiarybutylphenyl)-4-(3-chlorophyridin-2-yl)tetrahydropyrazine-1(2H)carboxamide (BCTC; Valenzano et al. 2003) on the response of individual pelvic afferent neurons to consecutive phasic colorectal balloon distensions (60 mmHg, 20 s, 4 min interval) (Sengupta et al. 2002). To exclude the possibility of mechanical desensitization of the afferent nerve fibres by the consecutive nociceptive distensions, we first investigated the reproducibility of the neuronal spike rate response to seven consecutive colorectal distensions in controls and rats with colitis. Consequently, an intravenous solution (100 μl (100 g)−1) of 25% hydroxypropyl-β-cyclodextrin (vehicle) or BCTC (0.25, 0.5, 1, 2 and 5 mg kg−1) was administered 210 s before the onset of distension to construct cumulative dose–response functions. We analysed the effect of BCTC on spontaneous activity and the response to CRD.
Immunocytochemistry
In the morphological part of this study, we determined whether the up-regulation of TRPV1 receptors on afferent neuronal cell bodies we observed previously (De Schepper et al. 2008b) was related to the fibre type of the neuron under study (C- compared to Aδ-fibres). To characterize colon-derived Fast Blue-traced spinal neurons, a double-labelling experiment was conducted, locating TRPV1 receptor immunoreactivity and phosphorylated neurofilament 200 (NF200), a marker for medium-to-large sized neurons associated with A-type, myelinated fibres (Lawson & Waddell, 1991; Ma et al. 2001; Priestley et al. 2002; Amaya et al. 2003; Kobayashi et al. 2005).
Rats were anaesthetized with pentobarbital (60 mg kg−1i.p.). A midline laparotomy was performed and the tracer dye Fast Blue (2% in 10% DMSO) was injected in the colonic wall at eight sites (4 injections at two levels) under aseptic conditions. The animals were allowed to recover for 14 days before induction of TNBS colitis. Three days later the rats were anaesthetized, a lumbar laminectomy was performed and the DRG L6 and S1 were harvested. The specimens were immediately immersed in 4% paraformaldehyde (PF) in 0.1 m phosphate buffer (pH 7.0) at room temperature. After a 10 min fixation period, the DRG were processed for cryosectioning according to published methods (De Jonge et al. 2003). To assess the presence of TRPV1 and NF200 in DRG neurons, cryostat sections were immunocytochemically stained using a rabbit antibody directed against TRPV1 (AB5370; Chemicon International Inc., Temecula, CA, USA; diluted 1 : 400), and a mouse antibody directed against phosphorylated NF200 (RT 97) (Lawson & Waddell, 1991; Perry et al. 1991) (MAB5262; Chemicon; 1 : 500). Briefly, all incubations were performed at room temperature. Sections were immersed for 30 min in 0.1 m phosphate-buffered saline (PBS, pH 7.4) containing 0.05% thimerosal (PBS*), 5% normal horse serum (NHS; Jackson Immunoresearch Laboratories, West Grove, PA, USA), and 1% Triton X-100, prior to incubation for 18 h with the primary antibodies diluted in PBS* containing 5% NHS and 0.1% Triton X-100. After rinsing in 0.01 m PBS, they were incubated for 1 h with a FITC-conjugated goat anti-rabbit IgG (Jackson Immunoresearch Laboratories; diluted 1 : 200), and a Cy3-conjugated goat anti-mouse IgG (Jackson Immunoresearch Laboratories; diluted 1 : 400) diluted in PBS* containing 1% NHS. After washing, the cryosections were mounted in Citifluor. Negative and interference controls were performed by omitting one or both of the primary or secondary antibodies.
For quantification, the number of neurons expressing TRPV1, NF200 and/or Fast Blue tracing was counted on 10 slides per ganglion. The number of colonic afferent neurons showing TRPV1 immunoreactivity was expressed as a percentage of the total pool of Fast Blue-traced DRG neurons. This population was then further subdivided into those expressing NF200 (Aδ-type neurons) and those not immunostained for NF200 (C-type neurons).
Solutions and drugs
BCTC was purchased from Biomol International, Exeter, UK. Hexadecyltrimethyl-ammonium bromide and o-dianisidine dihydrochloride were purchased from Sigma-Aldrich Inc., St Louis, MO, USA. Hydrogen peroxide and highly liquid paraffin were purchased from Merck, Darmstadt, Germany. TNBS was obtained from Fluka, Neu Ulm, Germany. Pentobarbital (Nembutal®) was purchased from Ceva, Brussels, Belgium.
Presentation of results and statistical analysis
All parametric data are presented as means ±s.e.m. for n the number of fibres under study, non-parametric data as medians ± 25/75 quartiles. For statistical analysis of parametric values, Student's t test for unpaired data or (repeated measures) two-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls post hoc test was performed. Non-parametric frequency distributions to compare fractions of the neuronal populations were analysed using Fisher's exact test. Non-parametric scores of inflammation were analysed using a Mann–Whitney U test. P < 0.05 was considered as statistically significant. Data were analysed using SPSS 11.5 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.00 (GraphPad Software Inc., San Diego, CA, USA).
Results
Inflammation of the colon
Three days after TNBS instillation in the distal colon, rats developed overt transmural colorectal inflammation characterized macroscopically by hyperaemia, oedema, ulceration and limited necrosis. This resulted in a median macroscopic score of 5 (4–7) compared to 0 (0–0) in controls (P < 0.05, n= 32–36). Microscopic evaluation of the inflamed region in TNBS colitis showed oedema of the submucosa, transmural infiltration of immunocytes, and localized ulceration. Inflammation of the colonic wall was confirmed biochemically by quantification of colonic MPO content, which increased from 6.4 ± 2.1 U g−1 in controls to 29.9 ± 7.7 U g−1 in TNBS rats (P < 0.05, n= 4).
As inflammation of the colonic wall could modify its elastic properties, hence influencing basal activity and responsiveness to distension, compliance curves were established. TNBS-induced colitis had no significant effect on colonic wall compliance compared to controls (Fig. 1).
Figure 1. The effect of TNBS-induced colitis on the compliance of the colorectal wall.
Results are expressed as intracolonic pressure (mmHg). *P < 0.05, significantly different from controls, unpaired Student's t test.
Electrophysiological study: general
We identified 68 neurons in 60 rats responding to pelvic nerve stimulation and to graded CRD but not to somatic dermatome stimulation. Of these, 36 neurons were obtained in 32 control rats and 32 neurons in 28 rats with TNBS-induced colitis. All fibres were classified in subgroups based upon their conduction velocity (C or Aδ) and the pressure threshold of their response (low or high). Fifty-eight per cent of the afferent fibres identified in controls were of the unmyelinated, slow conducting C-type (mean velocity 1.48 ± 0.15 m s−1), while thinly myelinated Aδ-fibres accounted for 42% (mean velocity 5.43 ± 0.91 m s−1). A similar distribution was identified in rats with TNBS-induced colitis, with 60% C-fibres (1.72 ± 0.13 m s−1) and 40% Aδ-fibres (5.59 ± 0.71 m s−1). TNBS-induced colitis had no significant influence on the conduction velocity of either C- or Aδ-fibres. In control rats, 64% of the fibres had a low threshold of response, while 36% only responded to higher distension pressures. The distribution according to threshold was comparable in rats with TNBS-induced colitis compared to control rats (66% low threshold, 34% high threshold). In this control population, more C-fibres had a low threshold of response (76% in controls, 67% in colitis) while more Aδ-fibres had a high threshold of response (53% in control, 57% in colitis) suggesting the latter are more suited to register noxious stimuli.
Electrophysiological study: effect of TNBS-induced colitis
In the absence of balloon distension, 62% of the afferent fibres under study displayed spontaneous activity in controls. In contrast, almost all fibres identified in TNBS rats had ongoing activity at rest (93%, P < 0.01). In addition, this activity was significantly higher in rats with TNBS colitis compared to controls (P < 0.01) (Table 1). Subgroup analysis revealed that this increase was significant in both low and high threshold afferent fibres. Spontaneous activity was enhanced in unmyelinated C-fibres but not in thinly myelinated Aδ-fibres (P < 0.05, Table 1).
Table 1.
Effect of TNBS-induced colitis on the spontaneous activity of pelvic afferent neurons responding to colorectal distension
| All | LT | HT | C-fibres | Aδ-fibres | |
|---|---|---|---|---|---|
| Control | 0.31 ± 0.07 | 0.34 ± 0.09 | 0.13 ± 0.05 | 0.31 ± 0.08 | 0.27 ± 0.11 |
| TNBS | 0.87 ± 0.22* | 0.90 ± 0.23* | 0.50 ± 0.18* | 1.33 ± 0.31* | 0.53 ± 0.25 |
Spontaneous activity is expressed as frequency (Hz) and presented as means ±s.e.m.
P < 0.05, significantly different from controls, Student's unpaired t test or two-way ANOVA with post hoc Student–Newman–Keuls test. LT, low threshold; HT, high threshold.
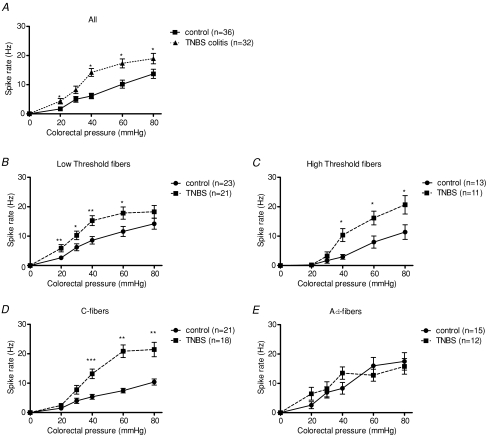
All neurons showed a pressure-dependent action potential frequency response to CRD. Without distinguishing fibres according to threshold or conduction velocity, pelvic primary afferent neurons from rats with TNBS-induced colitis showed a higher response rate to graded CRD compared to controls, suggesting significant pelvic nerve sensitization occurred (P < 0.05, Fig. 2A). Subsequently, the pelvic afferent neurons were subdivided according to their threshold of response. We found that low threshold fibres from rats with TNBS-induced colitis showed a significantly higher response rate to almost every colorectal pressure compared to low threshold fibres from control rats (P < 0.05, Fig. 2B). TNBS-induced colitis also increased the response rate of high threshold fibres (P < 0.05, Fig. 2C). Thus, colitis-induced sensitization of pelvic afferent fibres occurred irrespective of the threshold of response of the neuron under study.
Figure 2. Effect of TNBS-induced colitis on the response to colorectal distension.
Effect of TNBS-induced colitis on the response to CRD of all single pelvic afferent neurons identified (A), on pelvic afferent neurons with a low threshold response (B) or a high threshold response (C), and with low conduction velocity (C-fibres, D) or high conduction velocity (Aδ-fibres, E). Results are expressed as spike rate (Hz). *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from controls, two-way ANOVA with post hoc Student–Newman–Keuls test.
Examination of the effect of TNBS-induced colitis according to the conduction velocity of the nerve fibre revealed that colitis sensitized unmyelinated C-fibre reactivity to CRD, and more so at higher pressures of distension (P < 0.01, Fig. 2D). In contrast to this pronounced effect in C-fibres, the afferent response to CRD was not affected by inflammation in thinly myelinated Aδ-fibres (Fig. 2E). This observation is in agreement with the effect of TNBS colitis on the spontaneous activity of pelvic afferent neurons, which was equally different in C- and Aδ-fibres (Table 1).
Electrophysiological study: pharmacology
For the pharmacological part of our study, we identified a total of 24 pelvic afferent nerve fibres in the S1 dorsal root that responded to CRD. Twelve of these fibres were found in controls and 12 in rats with TNBS-induced colitis. In both controls and TNBS rats, seven unmyelinated C-fibres and five thinly myelinated Aδ-fibres were included.
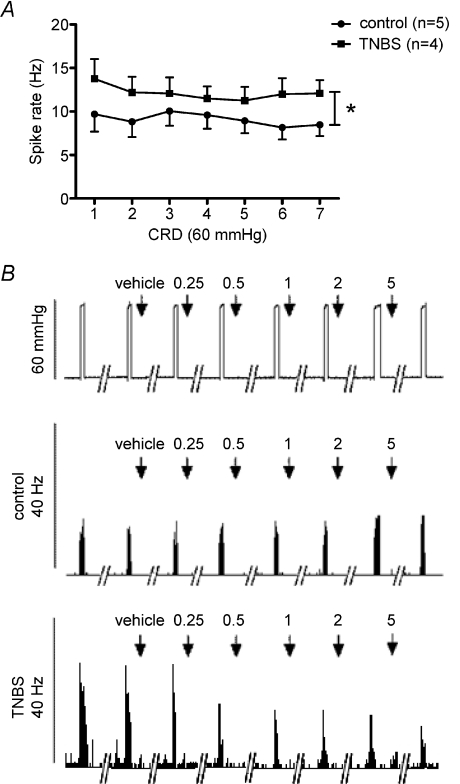
In the absence of pharmacological interventions, the afferent neuronal response to CRD (60 mmHg) did not change significantly over time in healthy controls and in rats with TNBS-induced colitis (Fig. 3A). The latter showed a sensitized or increased spike rate response to CRD for every consecutive distension applied (Fig. 3B), confirming our earlier findings. This experiment shows that repeated distension results in reproducible responses and is not subject to mechanical desensitization.
Figure 3. Pelvic afferent nerve response to repeated colorectal distension.
A, reproducibility of the pelvic afferent nerve response to repeated colorectal distension (CRD; 60 mmHg, 20 s, 4 min interval between consecutive distensions) in healthy controls and in rats with TNBS-induced colitis. Two-way ANOVA only showed significance for the effect of TNBS colitis (*P < 0.05, n= 6). B, typical tracings showing the effect of the specific TRPV1 receptor antagonist BCTC (0.25, 0.5, 1, 2 and 5 mg kg−1i.v.) or its vehicle (25% hydroxypropyl-β-cyclodextrin) on the frequency response of a pelvic afferent neuron to repeated CRD (60 mmHg, 20 s, 4 min interval) in a control rat and in a rat with colitis. Both tracings are of unmyelinated C-fibres.
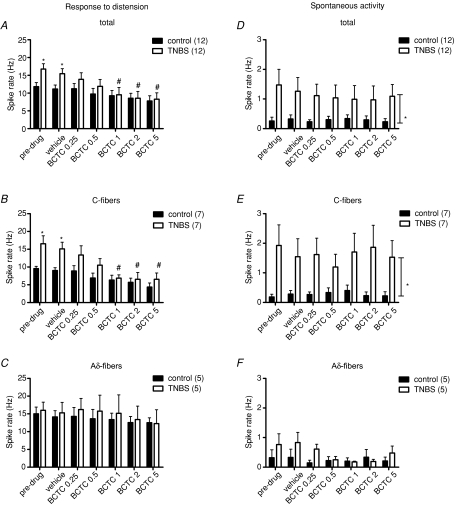
TNBS-induced colitis caused a significant increase in the pelvic afferent response to noxious CRD (Fig. 4A–C). Intravenous injection of 25% hydroxypropyl-β-cyclodextrin (the vehicle of BCTC) had no significant effect on the spike rate response of pelvic afferent neurons to CRD in controls or in rats with TNBS-induced colitis (Fig. 4A–C).
Figure 4. Effect of BCTC on the frequency response to repeated colorectal distension and spontaneous activity of pelvic afferent neurons.
Effect of BCTC (0.25, 0.5, 1, 2 and 5 mg kg−1i.v.) or its vehicle (25% hydroxypropyl-β-cyclodextrin) on the frequency response to repeated colorectal distension (60 mmHg, 20 s, 4 min interval) (A–C) and the spontaneous activity (D–F) of pelvic afferent neurons in controls and in rats with TNBS-induced colitis. Results are shown for all neurons (A and D), for C-fibres only (B and E) and for Aδ-fibres only (C and F). *P < 0.05, significantly different from controls; #P < 0.05, significantly different from vehicle treatment, repeated measures two-way ANOVA with post hoc Student–Newman–Keuls analysis.
Cumulative administration of the specific TRPV1 receptor antagonist BCTC had no statistically significant effect in controls, but dose-dependently decreased the pelvic afferent response to CRD in rats with TNBS colitis (Fig. 4A). At a dose of 1 mg kg−1 BCTC, this reduction was significant compared to the initial control distension and compared to vehicle treatment, and returned the sensitized afferent spike rate response to control values. When studying C-fibres only, the response to CRD was clearly sensitized before and after administration of vehicle. Injection of increasing doses of BCTC dose-dependently reduced the response to CRD in TNBS rats reaching significance at 1 mg kg−1 (Fig. 4B). At this dose, the afferent spike rate response to CRD was identical in rats with TNBS-induced colitis and in controls. In Aδ-type neurons, TNBS colitis did not influence the spike rate response to CRD. In agreement with this resistance to the sensitizing effects of the inflammatory environment, BCTC had no additional effects on the Aδ-fibre response to CRD (Fig. 4C).
TNBS-induced colitis not only increased the pelvic afferent response to CRD, but also affected the spontaneous activity of these neurons (4D). Again this effect was only significant in C-fibres (Fig. 4E), while the spontaneous activity of Aδ-fibres was not affected (Fig. 4F). BCTC had no effect on the spontaneous activity of pelvic afferent neurons in control animals. In contrast to its effect on the sensitized response to CRD, BCTC did not reduce the increased spontaneous activity of pelvic afferent neurons (Fig. 4D). Even when considering C- and Aδ-fibres separately, BCTC had no statistically significant effect on spontaneous activity (Fig. 4E and F).
Morphological study
In this part of the study, we looked at the fibre type specificity of the effect of TNBS colitis on TRPV1 receptor expression in pelvic afferent cell bodies (DRG L6–S1). To this end, we used neurofilament 200 (NF200) as a marker for Aδ-type neurons (Fig. 5). The proportion of colon-derived pelvic afferent neurons expressing NF200 was not significantly altered by colitis (47.4 ± 4.8% in controls and 38.8 ± 4.7% in colitis, respectively). The amount of colonic pelvic sensory neurons expressing TRPV1 was higher in rats with TNBS-induced colitis compared to controls (Table 2). If only the neurofilament (NF)-positive Aδ-type neurons were considered, this increase was no longer present (Table 2). Subgroup analysis showed that the rise in TRPV1 expression was entirely due to a significant increase of expression in unmyelinated afferent NF-negative C-type neurons (Table 2).
Figure 5. Immunocytochemical detection of NF200 and TRPV1 receptors.
A–C, immunocytochemical detection of NF200 (red, A) and TRPV1 receptors (green, B) on cryosections of the Fast Blue-traced (blue, C) dorsal root ganglion (DRG). D is the merged picture of A, B and C. This representative example of the triple stain was obtained in a control rat. Arrowheads indicate colon-derived C-fibres.
Table 2.
Effect of TNBS-induced colitis on the expression of TRPV1 receptors in pelvic afferent nerve cell bodies innervating the rat colon (DRG L6-S1)
| TRPV1+ neurons | TRPV1+/NF− neurons | TRPV1+/NF+ neurons | |
|---|---|---|---|
| Control | 36.1 ± 2.6 | 22.6 ± 2.8 | 13.5 ± 2.3 |
| TNBS | 46.6 ± 2.4* | 34.4 ± 3.2* | 12.3 ± 1.7 |
Results are expressed as percentage of Fast Blue positive nerve cell bodies and are presented as means ±s.e.m.
P < 0.05,
P < 0.01, significantly different from controls, Student's unpaired t test or two-way ANOVA with post hoc Student–Newman–Keuls test. NF, neurofilament.
Discussion
In this study, we showed that TNBS-induced colitis sensitized pelvic afferent neurons, as evidenced by an increase in the spontaneous activity of the neurons and as an increase in the afferent discharge response to CRD. We also showed that this sensitization to mechanical stimulation was restricted to unmyelinated C-fibres and did not affect the thinly myelinated Aδ-fibres. In a second part of the study, we focused on the pathophysiology of pelvic afferent neuron sensitization. Using pharmacological and immunocytochemical methods we demonstrated that the sensitization of pelvic afferent C-fibres is mediated by activation of TRPV1 receptors. As our electrophysiological experiments were performed on decentralized primary afferent neurons, we conclude that the TRPV1-mediated sensitization is peripheral rather than central in nature.
Our in vivo data are in agreement with the in vitro findings of Wynn et al. (2004) who equally found an increase in pelvic nerve spontaneous activity and an enhancement of the action potential frequency response to CRD. However, they did not examine the fibre-type specificity of their observations. Using a similar electrophysiological setup as ours, Sengupta et al. (1999) found that 4–5 days after induction, TNBS colitis induced a significant increase in pelvic afferent spontaneous activity, while the afferent response to CRD was not affected. Still, the rats did develop hyperalgesia as evidenced by an increase in the visceromotor response to CRD, suggesting that primary afferent nerve and subsequent central sensitization had occurred in their setup. This primary effect can possibly only be detected when subgroup analysis is performed.
TNBS-induced colitis sensitized both low and high threshold afferent neuronal responses to CRD. This sensitization was most pronounced at higher or noxious pressures of distension next to a more moderate hypersensitivity to pressures within the physiological range (0–30 mmHg). Functionally, this implies that the visceral responses to low-grade distension such as visceral perception are heightened, but that the responses to noxious mechanical stimuli, i.e. visceral pain, are intensified even more (hyperalgesia). The latter can be seen as a defensive ‘alarm’ function of the gut to protect the fragile and inflamed colon from overly high-pressure stimulation.
A number of studies have provided clues on the pathophysiology of afferent sensitization and the multiple mediators involved. A promising modulator in this context is the TRPV1 receptor. TRPV1 receptors belong to the superfamily of transient receptor potential (TRP) receptors, and are involved in thermosensation (42–53°C) and inflammation-induced sensitization of sensory neurons (Geppetti & Trevisani, 2004). Their role in healthy circumstances is not entirely understood however. TRPV1 receptor knock-out mice were shown to be significantly less sensitive to CRD as measured by the visceromotor response and direct in vitro measurements of colon afferent mechanosensitivity (Jones et al. 2005). This knock-out effect was mimicked by pretreating wild-type mice with the TRPV1 receptor antagonist capsazepine. This suggests that TRPV1 receptors contribute to normal visceral mechanosensation, a statement which is supported by in vitro measurements of jejunal afferent mechanosensitivity in TRPV1 receptor knock-out mice (Rong et al. 2004). In our experiments with rats, selective TRPV1 receptor inhibition using BCTC did not significantly affect the afferent response to CRD in controls, although a tendency was apparent at higher doses. Further experiments using different specific TRPV1 receptor antagonists in different in vitro and in vivo animal models are needed to conclusively determine whether and how vanilloid signalling is involved in the mechanosensitivity of visceral afferent neurons in physiological conditions.
TNBS-induced colitis sensitized the pelvic afferent response to CRD in our in vivo electrophysiological assay. Specific inhibition of TRPV1 receptors using the antagonist BCTC resulted in complete normalization of the pelvic afferent response in rats with colitis. This finding suggests that TRPV1 receptors are indeed crucial modulators of afferent nerve sensitization. This TRPV1 receptor antagonist effect was already demonstrated in vivo using the visceromotor response to colorectal distension as a marker for visceral perception (Miranda et al. 2007). Our results now indicate for the first time that a TRPV1 antagonist effectively inhibits colitis-induced primary afferent nerve sensitization in an in vivo situation. The mechanisms by which TRPV1 receptors mediate inflammation-induced sensitization are not completely understood, but are most likely related to a G-protein/protein kinase effect on TRPV1 by binding of inflammatory mediators on their cognate receptors (Geppetti & Trevisani, 2004). Alternatively, G-protein mediated activation of phospholipase C (PLC) may result in degradation of phosphatidyl inositol diphosphate (PIP2), a constitutive inhibitor of TRPV1 activity (Geppetti & Trevisani, 2004). These mechanistic observations imply that TRPV1 receptors are integrators of the individual effects of different inflammatory stimuli, suggesting that TRPV1 receptor inhibition might suffice to normalize inflammation-induced hypersensitivity, as we have shown in our experiments with BCTC.
Subgroup analysis revealed that the electrophysiological sensitization of mechanosensation in rats with TNBS-induced colitis was only due to hyperactivation of unmyelinated C-fibres rather than thinly myelinated Aδ-fibres. BCTC significantly reduced the response to CRD of C-fibres to control values. It did not affect the mechanosensation or the spontaneous activity of Aδ-fibres. These pharmacological data were reinforced by immunocytochemical experiments on DRG L6 and S1, where the cell bodies of pelvic afferent neurons are found: we showed that TNBS-induced colitis increased the proportion of pelvic sensory neurons that express TRPV1. This is in agreement with previous reports in oesophagitis and neonatal insult-induced visceral hypersensitivity (Banerjee et al. 2007; Winston et al. 2007). Subsequently, by analysing results based on the presence or absence of myelination using NF200 as a marker for medium-sized neurons with Aδ-fibres (Priestley et al. 2002; Kobayashi et al. 2005), we showed that a minority of the Aδ-fibre neurons in controls expressed TRPV1 receptor immunoreactivity, confirming recent observations in rat DRG L4 and L5 (Kobayashi et al. 2005). In agreement, in vitro electrophysiological experiments on dissociated DRG neurons in culture suggested that small-diameter, and thus most likely C-type, neurons are highly sensitive to the TRPV1 agonist capsaicin, while Aδ-type neurons are less sensitive or do not respond at all (Heyman & Rang, 1985; Gold et al. 1996). The immunohistochemical finding that only a minority of Aδ-fibres express TRPV1 receptors probably accounts for the lack of effect of BCTC on this fibre type, both in controls and in rats with TNBS-induced colitis.
The observation that the colitis-induced rise in TRPV1 expression was restricted to unmyelinated C-fibres could explain why the BCTC-sensitive electrophysiological sensitization of pelvic afferent nerve fibres only affected this population of neurons. To our knowledge, this is the first study providing both electrophysiological and morphological evidence that acute inflammation-induced TRPV1 receptor up-regulation in pelvic visceral afferent neurons is confined to unmyelinated C-fibres rather than thinly myelinated Aδ-fibres.
Only few studies addressed the inflammation-induced differential expression of TRPV1 receptors in the afferent literature. Amaya et al. (2003) showed that while in controls TRPV1 expression was limited to NF200-negative C-type DRG neurons, these receptors started to appear on the NF200-positive Aδ-type neurons after intraplantar injection of Freund's complete adjuvant, a local irritant. Still, this rise was modest compared to the pronounced TRPV1 up-regulation in C-fibres. On the other hand, TRPV1 receptor up-regulation induced by somatic inflammation was influenced by p38, a MAPK (mitogen-activated protein kinase) which is activated by cellular stress, cytokines and nerve growth factor (Ji et al. 2002). The authors showed that inflammation-associated p38 up-regulation was restricted to NF200-negative, unmyelinated C-fibres, providing additional evidence for the notion that these neurons are particularly equipped to perceive inflammatory changes and to mediate inflammation-induced changes of neuronal excitability.
We used female rats in our studies, and are aware that female hormones may affect visceral sensitivity (Sapsed-Byrne et al. 1996), although it is not clear whether this effect is situated at the primary afferent level, where our measurements are made, or in the central nervous system. We did not screen specifically for oestrus cycle status. However, the variability of the spike rate responses we obtained was modest and allowed interpretation of significant differences between controls and rats with colitis.
TNBS-induced colitis increased both the response to CRD and the spontaneous activity of pelvic afferent C-fibres. Surprisingly, however, the rise in spontaneous firing rate was not affected by intravenous administration of BCTC. This observation suggests that afferent spontaneous hyperactivity during colitis is not modulated by TRPV1 receptors, and that the effect of inflammation on pelvic afferent neurons is mechanistically different for the response to distension compared to the immunomodulation of spontaneous activity. This discrepancy also stresses the importance of TRPV1 receptors in visceral mechanosensation, i.e. the response to distension, especially in pathological states such as colitis. The mechanism behind the colitis-induced increase in spontaneous activity remains to be identified, but purinergic receptors might be involved, as the P2X receptor antagonist PPADS was able to reduce the TNBS-colitis induced increase in afferent nerve spontaneous activity as measured using an isolated colon-pelvic nerve electrophysiological set-up (Wynn et al. 2004).
In conclusion, we showed that TNBS-induced colitis in rats sensitizes both the spontaneous activity and the response to noxious CRD of unmyelinated C-fibres but not Aδ-fibres. The increase in mechanosensation was clearly TRPV1 dependent, while the increase in spontaneous activity was not. The C-fibre specific effect of colitis and TRPV1 was confirmed using immunocytochemical techniques. Our results suggest that TRPV1 receptors are a very attractive target for the development of new therapeutic agents for IBD-associated sensorimotor disturbances and for functional gastrointestinal disorders in general.
Acknowledgments
H. De Schepper is an aspirant of the Fund for Scientific Research (FWO), Flanders. This work was supported financially by the Interuniversity Poles of Attraction (IUPA) program P5/20 and by the FWO (grant no. G.0200.05).
References
- Amaya F, Oh-hashi K, Naruse Y, Iijima N, Ueda M, Shimosato G, Tominaga M, Tanaka Y, Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963:190–196. doi: 10.1016/s0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- Banerjee B, Medda BK, Lazarova Z, Bansal N, Shaker R, Sengupta JN. Effect of reflux-induced inflammation on transient receptor potential vanilloid one (TRPV1) expression in primary sensory neurons innervating the oesophagus of rats. Neurogastroenterol Motil. 2007;19:681–691. doi: 10.1111/j.1365-2982.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Gebhart GF. The pharmacology of gastrointestinal nociceptive pathways. Curr Opin Pharmacol. 2002;2:642–649. doi: 10.1016/s1471-4892(02)00211-4. [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JM. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- Costigan M, Woolf CJ. Pain: Molecular mechanisms. J Pain. 2000;1:35–44. doi: 10.1054/jpai.2000.9818. [DOI] [PubMed] [Google Scholar]
- De Jonge F, Van Nassauw L, Adriaensen D, Van Meir F, Miller HR, Van Marck E, Timmermans JP. Effect of intestinal inflammation on capsaicin-sensitive afferents in the ileum of Schistosoma mansoni-infected mice. Histochem Cell Biol. 2003;119:477–484. doi: 10.1007/s00418-003-0532-5. [DOI] [PubMed] [Google Scholar]
- De Schepper HU, De Man JG, Moreels TG, Pelckmans PA, De Winter BY. Review article: gastrointestinal sensory and motor disturbances in inflammatory bowel disease – clinical relevance and pathophysiological mechanisms. Aliment Pharmacol Ther. 2008a;27:621–637. doi: 10.1111/j.1365-2036.2008.03624.x. [DOI] [PubMed] [Google Scholar]
- De Schepper HU, De Man JG, Ruyssers NE, Deiteren A, Van Nassauw L, Timmermans JP, Martinet W, Herman AG, Pelckmans PA, De Winter BY. TRPV1 receptor signaling mediates afferent nerve sensitization during colitis-induced motility disorders in rats. Am J Physiol Gastrointest Liver Physiol. 2008b;294:G245–G253. doi: 10.1152/ajpgi.00351.2007. [DOI] [PubMed] [Google Scholar]
- De Schepper HU, De Man JG, Van Nassauw L, Timmermans JP, Herman AG, Pelckmans PA, De Winter BY. Acute distal colitis impairs gastric emptying in rats via an extrinsic neuronal reflex pathway involving the pelvic nerve. Gut. 2007;56:195–202. doi: 10.1136/gut.2006.104745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Blumberg PM, Szallasi A. Endovanilloid signaling in pain. Curr Opin Neurobiol. 2002;12:372–379. doi: 10.1016/s0959-4388(02)00340-9. [DOI] [PubMed] [Google Scholar]
- Farrokhyar F, Marshall JK, Easterbrook B, Irvine EJ. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: prevalence and impact on health. Inflamm Bowel Dis. 2006;12:38–46. doi: 10.1097/01.mib.0000195391.49762.89. [DOI] [PubMed] [Google Scholar]
- Gebhart GF. Visceral pain – peripheral sensitisation. Gut. 2000;47:iv54–iv55. doi: 10.1136/gut.47.suppl_4.iv54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppetti P, Trevisani M. Activation and sensitisation of the vanilloid receptor: role in gastrointestinal inflammation and function. Br J Pharmacol. 2004;141:1313–1320. doi: 10.1038/sj.bjp.0705768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Dastmalchi S, Levine JD. Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience. 1996;71:265–275. doi: 10.1016/0306-4522(95)00433-5. [DOI] [PubMed] [Google Scholar]
- Heyman I, Rang HP. Depolarizing responses to capsaicin in a subpopulation of rat dorsal root ganglion cells. Neurosci Lett. 1985;56:69–75. doi: 10.1016/0304-3940(85)90442-2. [DOI] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SK, Su X, Porreca F, Gebhart GF. κ-Opioid receptor agonists modulate visceral nociception at a novel, peripheral site of action. J Neurosci. 2000;20:5874–5879. doi: 10.1523/JNEUROSCI.20-15-05874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with Ad/C-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn PA, Blackshaw LA. In vitro recordings of afferent fibres with receptive fields in the serosa, muscle and mucosa of rat colon. J Physiol. 1999;518:271–282. doi: 10.1111/j.1469-7793.1999.0271r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QP, Hill R, Sirinathsinghji D. Colocalization of CGRP with 5-HT1B/1D receptors and substance P in trigeminal ganglion neurons in rats. Eur J Neurosci. 2001;13:2099–2104. doi: 10.1046/j.0953-816x.2001.01586.x. [DOI] [PubMed] [Google Scholar]
- McMahon SB. Sensitisation of gastrointestinal tract afferents. Gut. 2004;53:ii13–ii15. doi: 10.1136/gut.2003.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience. 2007;148:1021–1032. doi: 10.1016/j.neuroscience.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreels TG, De Man JG, De Winter BY, Timmermans JP, Herman AG, Pelckmans PA. Effect of 2,4,6-trinitrobenzenesulphonic acid (TNBS)-induced ileitis on the motor function of non-inflamed rat gastric fundus. Neurogastroenterol Motil. 2001;13:339–352. doi: 10.1046/j.1365-2982.2001.00273.x. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Perry MJ, Lawson SN, Robertson J. Neurofilament immunoreactivity in populations of rat primary afferent neurons – A quantitative study of phosphorylated and nonphosphorylated subunits. J Neurocytol. 1991;20:746–758. doi: 10.1007/BF01187848. [DOI] [PubMed] [Google Scholar]
- Pezzone MA, Wald A. Functional bowel disorders in inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:347–357. doi: 10.1016/s0889-8553(01)00021-8. [DOI] [PubMed] [Google Scholar]
- Priestley JV, Michael GJ, Averill S, Liu M, Willmott N. Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can J Physiol Pharmacol. 2002;80:495–505. doi: 10.1139/y02-034. [DOI] [PubMed] [Google Scholar]
- Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapsed-Byrne S, Ma DQ, Ridout D, Holdcroft A. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res. 1996;742:10–16. doi: 10.1016/s0006-8993(96)00989-4. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol. 1994;71:2046–2060. doi: 10.1152/jn.1994.71.6.2046. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Medda BK, Shaker R. Effect of GABA-B receptor agonist on distension-sensitive pelvic nerve afferent fibers innervating rat colon. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1343–G1351. doi: 10.1152/ajpgi.00124.2002. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Snider A, Su X, Gebhart GF. Effects of kappa opioids in the inflamed rat colon. Pain. 1999;79:175–185. doi: 10.1016/s0304-3959(98)00175-4. [DOI] [PubMed] [Google Scholar]
- Su X, Burton MB, Gebhart GF. Effects of octreotide on responses to colorectal distension in the rat. Gut. 2001;48:676–682. doi: 10.1136/gut.48.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano KJ, Grant ER, Wu G, Hachicha M, Schmid L, Tafesse L, Sun Q, Rotshteyn Y, Francis J, Limberis J, Malik S, Whittemore ER, Hodges D. N-(4-Tertiarybutylphenyl)-4-(3-chloropyridin-2-yl) tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties. I. In vitro characterization and pharmacokinetic properties. J Pharmacol Exp Ther. 2003;306:377–386. doi: 10.1124/jpet.102.045674. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Keenan CM. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol. 1990;258:G527–G534. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
- Wang B, Glatzle J, Mueller MH, Kreis M, Enck P, Grundy D. Lipopolysaccharide-induced changes in mesenteric afferent sensitivity of rat jejunum in vitro: role of prostaglandins. Am J Physiol Gastrointest Liver Physiol. 2005;289:G254–G260. doi: 10.1152/ajpgi.00329.2004. [DOI] [PubMed] [Google Scholar]
- Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Wynn G, Ma B, Ruan HZ, Burnstock G. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G647–G657. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- Wynn G, Rong W, Xiang Z, Burnstock G. Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology. 2003;125:1398–1409. doi: 10.1016/j.gastro.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Xu GY, Huang LYM. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci. 2002;22:93–102. doi: 10.1523/JNEUROSCI.22-01-00093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]