Abstract
Smooth muscle cell (SMC) contraction is controlled by the Ca2+ and Rho kinase signalling pathways. While the SMC Rho kinase system seems to be reasonably constant, there is enormous variation with regard to the mechanisms responsible for generating Ca2+ signals. One way of dealing with this diversity is to consider how this system has been adapted to control different SMC functions. Phasic SMCs (vas deferens, uterus and bladder) rely on membrane depolarization to drive Ca2+ influx across the plasma membrane. This depolarization can be induced by neurotransmitters or through the operation of a membrane oscillator. Many tonic SMCs (vascular, airway and corpus cavernosum) are driven by a cytosolic Ca2+ oscillator that generates periodic pulses of Ca2+. A similar oscillator is present in pacemaker cells such as the interstitial cells of Cajal (ICCs) and atypical SMCs that control other tonic SMCs (gastrointestinal, urethra, ureter). The changes in membrane potential induced by these cytosolic oscillators does not drive contraction directly but it functions to couple together individual oscillators to provide the synchronization that is a characteristic feature of many tonic SMCs.
Smooth muscle cells (SMCs) are characterized by their phenotypic plasticity and diversity. The activation mechanisms that control contraction display a similar diversity in that each SMC type has a signalling system that is uniquely adapted to control its particular function. The aim of this perspective is to confront this diversity by considering how the activation mechanisms for generating Ca2+ signals have been adapted to control different SMC functions.
Classification of smooth muscle Ca2+ activation mechanisms
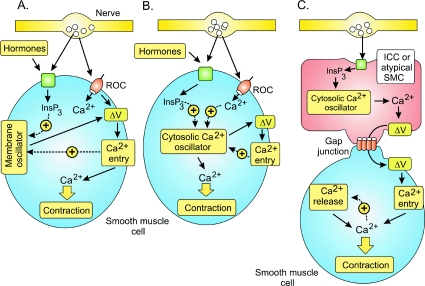
Excitation–contraction coupling in SMCs occurs through two main mechanisms. Many SMCs are activated by Ca2+ signalling cascades (Haddock & Hill, 2005; Wray et al. 2005). In addition, there is a Rho/Rho kinase signalling pathway that acts by altering the Ca2+ sensitivity of the contractile system (Somlyo & Somlyo, 2003). Since the latter appears to have more of a modulatory function, most attention will focus on how Ca2+ signalling is activated. Since membrane depolarization is a key element for the activation of many SMCs, much attention will focus on the mechanisms responsible for depolarizing the membrane. However, there are other SMCs where activation depends on the periodic release of Ca2+ from internal stores. These different Ca2+ activation mechanisms fall into the following three main groups (Fig. 1).
Figure 1. The three main mechanisms responsible for generating the Ca2+ transients that trigger smooth muscle cell (SMC) contraction.
A, receptor-operated channels (ROCs) or a membrane oscillator induces the membrane depolarization (ΔV) that triggers Ca2+ entry and contraction. B, a cytosolic Ca2+ oscillator induces the Ca2+ signal that drives contraction. C, a cytosolic Ca2+ oscillator in interstitial cells of Cajal (ICCs) or atypical SMCs induces the membrane depolarization that spreads through the gap junctions to activate neighbouring SMCs. Reproduced from Berridge (2008), with permission.
Mechanism A
Many SMCs are activated by membrane depolarization (ΔV) that opens L-type voltage-operated channels (VOCs) allowing external Ca2+ to flood into the cell to trigger contraction. This depolarization is induced either by ionotropic receptors (vas deferens) or a membrane oscillator (bladder and uterus). The membrane oscillator, which resides in the plasma membrane, generates the periodic pacemaker depolarizations responsible for the action potentials that drive contraction. The depolarizing signal that activates gastrointestinal, urethral and ureter SMCs is described in mechanism C.
Mechanism B
The rhythmical contractions of vascular, lymphatic, airway and corpus cavernosum SMCs depend on an endogenous pacemaker driven by a cytosolic Ca2+ oscillator that is responsible for the periodic release of Ca2+ from the endoplasmic reticulum. The periodic pulses of Ca2+ often cause membrane depolarization, but this is not part of the primary activation mechanism but has a secondary role to synchronize and amplify the oscillatory mechanism. Neurotransmitters and hormones act by modulating the frequency of the cytosolic oscillator.
Mechanism C
A number of SMCs are activated by pacemaker cells such as the interstitial cells of Cajal (ICCs) (gastrointestinal and urethral SMCs) or atypical SMCs (ureter). These pacemaker cells have a cytosolic oscillator that generates the repetitive Ca2+ transients that activate inward currents that spread through the gap junctions to provide the depolarizing signal (ΔV) that triggers contraction through mechanism A.
In the following sections, some selected SMC types will illustrate how these signalling mechanisms have been adapted to control different contractile functions with particular emphasis on how Ca2+ signals are activated.
Vas deferens
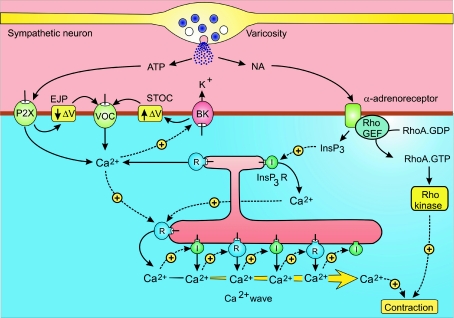
Sympathetic neurons activate the rapid peristaltic contractions of the vas deferens responsible for inducing sperm transfer during the brief period of ejaculation. The ATP and noradrenaline (NA) released during each burst of neural activity induce rapid contractions using different signalling mechanisms (Mulryan et al. 2000). ATP acting on P2X1 receptors stimulates an influx of Ca2+ to produce the excitatory junctional potentials (EJPs) that sum to depolarize the membrane sufficiently to activate the VOCs (Brain et al. 2002) (Fig. 2). The entry of Ca2+ then activates ryanodine receptors (RYRs) to release Ca2+ from the internal store. The initial release occurs close to the plasma membrane and then spreads into the cell through the regenerative release of Ca2+ by the RYRs and/or the inositol 1,4,5-trisphosphate (InsP3) receptors in the form of an intracellular Ca2+ wave (Brain et al. 2003). The Ca2+ near the membrane activates large-conductance Ca2+-sensitive K+ (BK) channels and the resulting spontaneous transient outward currents (STOCs) hyperpolarize the membrane and help to terminate the activation process (Ohi et al. 2001; White & McGeown, 2003).
Figure 2. Neural activation of vas deferens smooth muscle cells.
Sympathetic neurons have numerous varicosities that release ATP and noradrenaline (NA) that activate contraction through separate signalling pathways. See text for further details. BK, large conductance Ca2+-sensitive K+ channels; EJP, excitatory junction potential; STOC, spontaneous transient outward currents; I, inositol 1,4,5-trisphosphate (InsP3) receptor; R, ryanodine receptor. Reproduced from Berridge (2008), with permission.
Noradrenaline (NA) acts by stimulating α1-adrenoreceptors to produce InsP3, which then releases Ca2+ that may induce an intracellular Ca2+ wave similar to that triggered by the ATP-dependent entry of external Ca2+. In addition, the α1-adrenoreceptors also activate the smooth muscle Rho/Rho kinase signalling pathway that serves to increase the Ca2+ sensitivity of the contractile machinery.
Detrusor smooth muscle cells
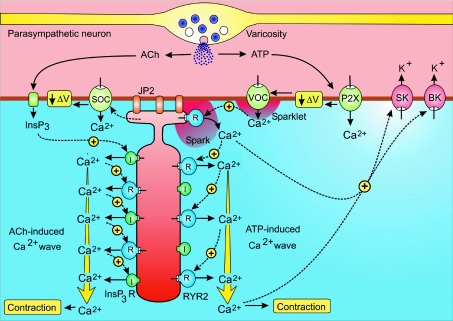
The bladder, which functions to store and expel urine, is surrounded by layers of detrusor SMCs. The latter have two operational modes: during bladder filling they remain relaxed but contract vigorously to expel urine during micturition. The switch from relaxation to contraction, which is triggered by neurotransmitters released from parasympathetic nerves, depends on the acceleration of an endogenous membrane oscillator that produces the repetitive trains of action potentials (mechanism A in Fig. 1) that drive contraction (Hashitani et al. 2004).
The main components of the membrane oscillator are the Ca2+ and K+ channels that sequentially depolarize and hyperpolarize the membrane, respectively. This oscillator generates the periodic pacemaker depolarizations that trigger each action potential (Fig. 3). The resulting Ca2+ signal lags behind the action potential because it spreads into the cell as a slower Ca2+ wave (Morimura et al. 2005) mediated by the type 2 RYRs (Hotta et al. 2007). About 10 ms after the depolarization, Ca2+ appears in the form of sparks near the membrane. These sparks then develop into waves that spread into the cell to produce a global signal after about 200 ms. Not all depolarization-induced Ca2+ sparks trigger a regenerative wave and this ‘loose coupling’ implies that the signalling mechanism has a low pass filter capable of integrating information from high-frequency electrical signals (Collier et al. 2000). If the RYR2s are blocked by 100 μm ryanodine, the Ca2+ signal is restricted to a smaller elevation immediately below the membrane, which might represent Ca2+ sparklets resulting from the opening of the VOCs (Fig. 3). The L-type VOC, which is responsible for the upstroke of the action potential, is terminated by Ca2+-induced desensitization of the VOCs and by the activation of the BK and SK channels that hyperpolarize the membrane (Hashitani & Brading, 2003a,b; Meredith et al. 2004). As Ca2+ is pumped out of the cell, the hyperpolarizing influence of these channels declines and this contributes to the gradual pacemaker depolarization. Oscillator frequency is acutely sensitive to changes in membrane potential being accelerated by depolarization and slowed or stopped when the membrane is hyperpolarized.
Figure 3. Bladder smooth muscle cells (SMCs) have a membrane oscillator that generates the periodic action potentials that initiate the process of excitation–contraction coupling.
Neurotransmitters such as ATP and acetylcholine (ACh), which are released from parasympathetic axonal varicosities that innervate the bladder, activate or accelerate the oscillator by inducing membrane depolarization (ΔV) through two separate pathways. Reproduced from Berridge (2008), with permission.
During the resting phase when the bladder is filling, electrical activity generated by this membrane oscillator is restricted to small areas of the bladder. During bladder emptying, neural stimulation induces action potentials throughout the bladder causing most of the bladder cells to contract in unison. The parasympathetic neurons that innervate the bladder release both acetylcholine (ACh) and ATP, which then induce a more widespread activation of this membrane oscillator by increasing membrane depolarization using different mechanisms. ATP acts through a P2X receptor to increase the entry of Ca2+, which produces an excitatory junction potential (EJP) and this depolarization (ΔV) then activates the VOCs (Fig. 3) (Hashitani et al. 2000,2004; Heppner et al. 2005). ACh stimulates an increase in InsP3 that acts through the InsP3 receptors (I, shown in green) to release Ca2+ that can also accelerate the membrane oscillator through a mechanism that remains to be determined. One possibility is that InsP3 depletes stores near the membrane to activate store-operated channels (SOCs) to provide the inward current necessary to depolarize the membrane.
The depolarizing drive provided by NA and ACh induces all the detrusor SMCs to enter an excitable state such that a spontaneous action potential in one cell spreads rapidly through the gap junctions at a rate of 40 mm s−1 to provide the near-synchronous contraction of the bladder (Hashitani et al. 2001,2004).
Uterine smooth muscle
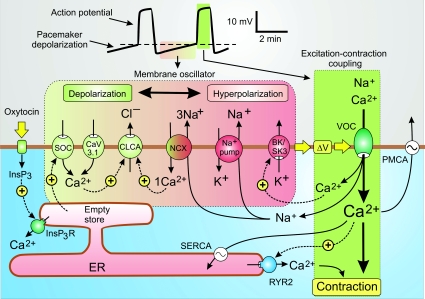
During early pregnancy, the uterus is relatively quiescent. There are a few weak twitches resulting from localized SMC contractions. With the onset of labour, the uterus begins to develop stronger and more frequent contractions that appear first in the fundus and then spread rapidly through the body of the uterus (Young, 2007; Wray & Noble, 2008). This remarkable switch from quiescence to oscillatory cycles of strong contractions is an example of phenotypic plasticity that depends on cells being able to remodel their signalling systems (Berridge et al. 2003). In the case of the uterus, this remodelling results in the emergence of a membrane oscillator, which is an endogenous pacemaker mechanism located in the plasma membrane that generates the repetitive action potentials responsible for driving contractions during labour (Young, 2007).
The uterine contractions that occur during labour are driven by periodic action potentials that are preceded by a characteristic pacemaker depolarization (Fig. 4). Synchronous uterine contractions depend on action potentials spreading through the smooth muscle cell layer with conduction velocities of approximately 4 cm s−1. Even though all the SMCs are capable of generating a rhythm, those in the fundus are inherently more rhythmical and thus initiate each contraction wave. Since there appear to be no ICCs and there is little neural innervation, the uterine myometrium is driven by an endogenous membrane oscillator (horizontal box in Fig. 4) that generates a pacemaker depolarization (ΔV) to trigger the excitation–contraction coupling mechanism (vertical green box). The Ca2+ that enters through the VOCs then activates contraction. There appears to be little direct role for Ca2+ release from the internal stores since contractions continue when these stores have been depleted (Kupittayanant et al. 2002; Wray & Shmygol, 2007). However, as part of the remodelling process during the end of pregnancy, there is an increased expression of RYR2s and these may open in response to the entry of external Ca2+ to provide an additional boost of Ca2+ to increase the force of contraction.
Figure 4. A model for the activation mechanism of human uterus contractility.
Contractions of the uterus during labour are driven by an endogenous membrane oscillator (horizontal green to pink box) that induces the slow pacemaker depolarization responsible for triggering the excitation–contraction coupling mechanism (vertical green box). The oscillator depends on an interaction between ion channels, pumps and exchangers that are colour coordinated to indicate their contribution to either depolarization (green) or hyperpolarization (red). When the pacemaker depolarization (ΔV) reaches the threshold for the activation of the L-type voltage-operated channels (VOCs) (dashed line in the membrane potential trace at the top), excitation–contraction coupling is triggered and the muscle contracts. See text for further details. Reproduced from Berridge (2008), with permission.
While the nature of this excitation–contraction coupling mechanism is fairly well established (Shmygol et al. 2007), there is less information on the nature of the membrane oscillator that generates the pacemaker depolarization that triggers the periodic action potentials. The following membrane oscillator model attempts to explain how the slow pacemaker depolarization is generated (Fig. 4). At any moment in time, membrane potential depends upon the coordinated activity of ion channels, pumps and exchangers that can either depolarize or hyperpolarize the membrane. At the end of an action potential, the hyperpolarizing components (shown in red) are dominant but as their influence wanes the depolarizing components (shown in green) begin to take over to produce the pacemaker depolarization. A number of candidates have been implicated in this periodic switching from hyperpolarization to depolarization.
Hyperpolarizing components BK and SK channel
The Ca2+-sensitive BK and SK channels are activated during the repolarizing phase of the action potential and thus contribute to the membrane hyperpolarization at the start of the pacemaker phase. As Ca2+ is pumped out of the cell, their hyperpolarizing influence will wane and this will contribute to the gradual pacemaker depolarization. Inhibition of the SK3 channel with apamin results in an acceleration of the oscillator. Conversely, if SK3 is overexpressed in rat uterus, there is a drastic reduction in oscillator frequency (Brown et al. 2007).
In addition to contributing to the membrane oscillator during labour, the BK channel maintains the quiescent state of the uterus during pregnancy. These BK channels are concentrated in caveolae, which are rich in cholesterol that enhances BK channel activity to reduce membrane excitability and thus contributes to the quiescent state. The high cholesterol levels that occur in obesity may increase the risk of complications in pregnancy by reducing uterine contractility during labour (Wray, 2007).
Na+–K+-ATPase pump
During the course of the action potential, opening of the L-type VOC results in Na+ entering the cell together with Ca2+. A hyperpolarization is generated when the electrogenic Na+ pump extrudes 2Na+ for 3K+. The pump will be activated immediately after an action potential to extrude Na+ and this will contribute to the early hyperpolarizing phase. As the Na+ concentration returns to resting levels, the resulting decline in this hyperpolarizing effect will contribute to the depolarizing pacemaker.
Na+/Ca2+ exchanger (NCX)
The Na+/Ca2+ exchanger (NCX) has a somewhat ambiguous role because it can operate in both forward and reverse modes and thus can contribute to either depolarization or hyperpolarization. When functioning in its forward mode to extrude Ca2+ it will hyperpolarize the membrane. When the uterus begins to oscillate, the resting membrane potential is approximately −50 mV, which is probably more positive than the equilibrium potential for Na+, so it is likely that the exchanger will be operating in its reverse mode during which 1Ca2+ is exchanged for 3Na+ to induce depolarization as indicated in Fig. 4.
Depolarizing components
Less is known about the channels responsible for providing inward current during the pacemaker depolarization.
Store-operated Ca2+ channel (SOC)
SMCs are known to express SOCs that may contribute inward current. The ability of oxytocin to accelerate the membrane oscillator in the human myometrium (Nakao et al. 1997) may be explained by activation of such a SOC mechanism. The suggestion is that oxytocin stimulates the formation of InsP3 that then empties part of the endoplasmic reticulum (ER) store that lies near the membrane to promote the opening of the SOCs and this increase in inward current will steepen the pacemaker depolarization to accelerate the oscillator. The Ca2+ that enters the cell may also switch on channels such as the Ca2+-sensitive chloride channels (CLCA) to induce the efflux of Cl− and membrane depolarization (Fig. 4). Ca2+ may also activate TRPM4 or TRPM5 channels that gate inward monovalent currents, which have been implicated in the operation of other membrane oscillators.
CaV3 T-type channels
Uterine smooth muscle cells express CaV3.1 (Blanks et al. 2007), which is one of the T-type channel isoforms that have been implicated in the pacemaker activity of other excitable cells. At the low membrane potential of activated uterine SMCs, these T-type channels are probably inactivated. However, a small population may still contribute to a pacemaker depolarization by gating a small inward flux of Ca2+. Such a role for the T-type channel is consistent with the observation that low concentrations of Ni2+ slow the oscillator (Blanks et al. 2007). The entry of Ca2+ through these T-type channels may also contribute to the depolarization by activating other inward currents such as those gated by TRPM4 or TRPM5 mentioned above.
In summary, uterine contraction is driven by a membrane oscillator that provides the pacemaker depolarization to generate the periodic action potentials that trigger contractions. Many of the hormones that modulate contractility are likely to influence some of these oscillatory components. For example, the model outlined in Fig. 4 suggests that oxytocin may enhance contractions by stimulating the formation of InsP3 which then switches on a SOC to provide an inward Ca2+ current resulting in a depolarizing signal. Oxytocin may have additional actions because it can increase the force of contraction in muscle cells where the internal stores have been depleted of Ca2+ following treatment with cyclopiazonic acid (CPA) (Kupittayanant et al. 2002). In this case, it seems likely that oxytocin may switch on the smooth muscle Rho/Rho kinase signalling pathway to enhance the sensitivity of the contractile mechanism.
Vascular, lymphatic and airway smooth muscle cells
Vascular, lymphatic and airway smooth muscle, which generate rhythmical contractions over an extended period of time, have an endogenous pacemaker mechanism driven by a cytosolic Ca2+ oscillator. In addition, these SMCs also respond to neurotransmitters released from the neural innervation. In the case of mesenteric arteries, the perivascular nerves release both ATP and noradrenaline (NA). The ATP acts first to produce a small initial contraction that is then followed by a much larger contraction when NA initiates a series of Ca2+ transients (Lamont et al. 2003). Such agonist-induced Ca2+ oscillations are a characteristic feature of the activation mechanisms of vascular (Iino et al. 1994; Lee et al. 2001; Peng et al. 2001; Perez & Sanderson, 2005b; Shaw et al. 2004) and airway SMCs (Kuo et al. 2003; Perez & Sanderson, 2005a; Sanderson et al. 2008). In some blood vessels, a specific tone is maintained by the spatial averaging of asynchronous oscillations. However, there are some vessels where the oscillations in groups of cells are synchronized resulting in the pulsatile contractions known as vasomotion (Mauban et al. 2001; Peng et al. 2001; Lamboley et al. 2003; Haddock & Hill, 2005). Such vasomotion is also a feature of lymphatic vessels (Imtiaz et al. 2007). Another feature of this oscillatory activity is that variations in transmitter concentration are translated into a change in contractile tone through a mechanism of frequency modulation (Iino et al. 1994; Kuo et al. 2003; Perez & Sanderson, 2005a,b). Frequency modulation is one of the mechanisms used for encoding and decoding signalling information through Ca2+ oscillations (Berridge, 2007).
The periodic pulses of Ca2+ that drive these rhythmical SMCs are derived from the internal stores through the operation of a cytosolic Ca2+ oscillator (Haddock & Hill, 2005; Imtiaz et al. 2007; Sanderson et al. 2008). The following general model, which applies to vascular, lymphatic, airway and perhaps also to corpus cavernosum SMCs, attempts to describe the nature of this oscillator and how it can be induced or modulated by neurotransmitters. A luminal loading Ca2+ oscillation mechanism (Berridge & Dupont, 1994; Berridge, 2007) forms the basis of this cytosolic oscillator model that depends upon the following sequential series of events (Fig. 5).
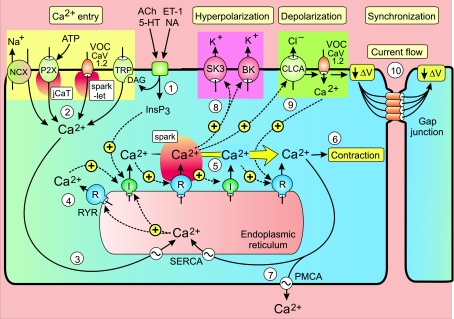
Figure 5. Vascular or airway SMCs are driven by a cytosolic oscillator that generates a periodic release of Ca2+ from the endoplasmic reticulum that usually appears as a propagating Ca2+ wave.
The oscillator is induced/modulated by neurotransmitters such as acetylcholine (ACh), 5-hydroxytryptamine (5-HT), noradrenaline (NA) and endothelin-1 (ET-1), which act through inositol 1,4,5-trisphosphate (InsP3) that initiates the oscillatory mechanism. The sequence of steps 1–9 is described in the text. Reproduced from Berridge (2008), with permission.
(1) The initiation and/or modulation of this oscillator depends upon the action of transmitters and hormones such as ACh, 5-HT, NA and endothelin-1 (ET-1) that increase the formation of InsP3 and diacylglycerol (DAG), both of which promote oscillatory activity.
(2) The oscillator is very dependent on Ca2+ entry to provide the Ca2+ necessary to charge up the stores for each oscillatory cycle. The nature of these entry mechanisms (yellow panel in Fig. 5) vary between cell types and are still being characterized. Some of the main contenders are the Na+/Ca2+ exchanger (NCX) operating in its reverse mode (Lee et al. 2001; Dai et al. 2006; Rebolledo et al. 2006), the ATP-sensitive P2X receptor responsible for generating junctional Ca2+ transients (jCaTs) (Lamont & Wier, 2002) or the L-type CaV1.2 channel operating as clusters in ‘a high open probability mode’ to produce persistent Ca2+ sparklets (Amberg et al. 2007). In the case of the latter, entry of Ca2+ through these L-type channels does not activate RYRs directly to produce sparks as occurs in cardiac myocytes. Rather, the persistent sparklets provide much of the background entry of Ca2+ necessary to charge up the ER to sensitize the RYRs (Essin et al. 2007). Other possibilities are the various isoforms of the transient receptor potential (TRP) ion channel family (Saleh et al. 2008). For example, TRPC3 and TRPC7 are of interest because they are sensitive to DAG that is formed together with InsP3 during the action of the transmitters that drive the cytosolic oscillator (Peppiatt-Wildman et al. 2007). Members of the CaV3 family of T-type channels have also been implicated as an entry mechanism in airway SMCs (Perez & Sanderson, 2005a). The ability of hormones and neurotransmitters to accelerate oscillator frequency may depend on their ability to increase some of these Ca2+ influx mechanisms.
(3) The entry of external Ca2+ charges up the ER to sensitize the RYRs and InsP3 receptors prior to the next phase of release. An important determinant of this sensitivity is the luminal concentration of Ca2+ and as this builds up the release channels become sensitive to Ca2+ and can participate in the process of Ca2+-induced Ca2+ release (CICR), which is responsible for orchestrating the regenerative release of Ca2+ from the ER. The proposed role of cyclic ADP-ribose (cADPR) in airway SMCs (Deshpande et al. 2005) is consistent with this aspect of the model on the basis of its proposed action of stimulating the SERCA pump to enhance store loading (Berridge et al. 2003) and such a mechanism has been described in colonic SMCs (Bradley et al. 2003).
(4) The mechanism responsible for initiating Ca2+ release may depend either on the RYRs or the InsP3 receptors (I). RYR channels are sensitive to store loading and the InsP3 receptors will be sensitized by the agonist-dependent formation of InsP3.
(5) This initial release of Ca2+ is then amplified by regenerative Ca2+ release by either the RYRs or InsP3 receptors, depending on the cell type (Dai et al. 2006; Sanderson et al. 2008). The resulting global Ca2+ signal often appears as a wave travelling down the length of the cell (Iino et al. 1994; Shaw et al. 2004).
(6) The global Ca2+ signal then activates contraction.
(7) The recovery phase depends on the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA), that pumps some of the Ca2+ back into the ER, and the plasma membrane Ca2+-ATPase (PMCA), that pumps Ca2+ out of the cell.
(8) One of the effects of the released Ca2+ is to stimulate Ca2+-sensitive K+ channels such as the BK and SK channels that will lead to membrane hyperpolarization. The BK channels are activated by Ca2+ sparks resulting from the opening of RYRs.
(9) Another action of Ca2+ is to stimulate Ca2+-sensitive chloride channels (CLCA) (Liu & Farley, 1996; Haddock & Hill, 2002), which result in membrane depolarization to activate the CaV1.2 channels that introduce Ca2+ into the cell resulting in further membrane depolarization (ΔV). The entry of further Ca2+ completes a positive feedback loop that helps to speed up and complete the release of internal Ca2+.
(10) This depolarization can spread to neighbouring cells by current flow through the gap junctions to provide a synchronization mechanism in those cases where the oscillators are coupled together to provide vasomotion (Haddock & Hill, 2002,2005; Imtiaz et al. 2007). The membrane depolarization will activate CaV1.2 channels in neighbouring cells to provide a Ca2+ pulse that will help to initiate their Ca2+ transients thus bringing them into phase with each other. This synchronization mechanism is explored more fully later in a section on the slow waves generated by the ICCs.
Corpus cavernosum smooth muscle cells
The corpora cavernosum, which are two elongated bodies that lie on either side of the penis, fill up with blood during an erection. The sponge-like cavernous spaces are connected to the penile arteries that are lined with SMCs, which regulate the flow of blood into the corpora cavernosum. The penis is flaccid when these muscles are contracted, but becomes rigid when these muscles relax to allow blood to flow into the cavernous spaces. Contraction is activated by a cytosolic Ca2+ oscillator similar to that found in vascular and airway SMCs (Fig. 5). This oscillator generates pulses of Ca2+ that spread through the muscle cells as fast waves travelling at about 800 μm s−1. This wave may spread to neighbouring cells by current flow through the gap junctions to synchronize the oscillators of neighbouring cells as described for the ICC phase waves (see below).
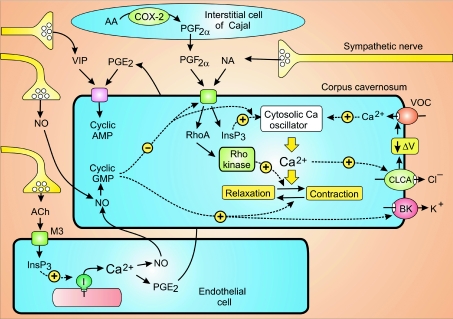
A large number of neural stimuli control the switch between muscle contraction and relaxation (Fig. 6). One of the primary regulators of contraction is the NA released from sympathetic nerves. The ICCs that have been described in the corpus cavernosum do not appear to play a direct role in regulating contraction but they express large amounts of cyclooxygenase-2 (COX-2) that functions in the synthesis of prostaglandin F2α (PGF2α), which diffuses across to activate the corpus cavernosum SMCs (Hashitani, 2006). NA and PGF2α have two main actions. Firstly, they generate InsP3, which activates the cytosolic Ca2+ oscillator. Secondly, they can activate the Rho/Rho kinase signalling pathway to increase the Ca2+ sensitivity of the contractile machinery (Wang et al. 2002). The Ca2+ transients also activate Ca2+-sensitive chloride channels (CLCAs) that result in membrane depolarization to activate voltage-operated channels (VOCs) (Craven et al. 2004; Hashitani et al. 2005), which not only introduce Ca2+ to modulate the oscillator but also create a flow of current to entrain the oscillatory activity of neighbouring cells to account for the way these corpora cavernosa cells contract in near-unison with each other.
Figure 6. The corpus cavernosum SMCs, which are poised between contraction and relaxation, are controlled by multiple stimuli released from neurons, endothelial cells and the interstitial cells of Cajal.
Some of the stimuli such as noradrenaline (NA) and prostaglandin F2α (PGF2α) drive contraction, whereas nitric oxide (NO), prostaglandin E2 (PGE2) and vasoactive intestinal peptide (VIP) control relaxation. See text for details of their signalling pathways. Reproduced from Berridge (2008), with permission.
A different set of signalling systems control the relaxation responsible for penile erection. One of the major stimuli is nitric oxide (NO) derived from non-adrenergic non-cholinergic (NANC) neurons. NO is also synthesized by the endothelial cells following stimulation by ACh released from the parasympathetic neurons. The ACh acts through muscarinic M3 receptors to stimulate InsP3 formation and the release of Ca2+, which then activates endothelial nitric oxide synthase (eNOS) to produce NO. The NO causes relaxation by increasing the formation of cyclic GMP, which acts on various targets such as the BK channels and the Rho/Rho kinase systems, and it may also reduce the formation and action of InsP3 to slow down the cytosolic oscillator. Prostaglandin E2 (PGE2), which is also synthesized in the endothelial cells in response to cholinergic stimulation and vasoactive intestinal peptide (VIP) released from neurons act by increasing the formation of cyclic AMP that can also promote relaxation, but the mechanisms are less well understood than those mediated by cyclic GMP.
Gastrointestinal and urethral smooth muscle cells
Gastrointestinal and urethral SMCs share a common activation mechanism in that they are driven by a pacemaker signal received from the ICCs (see mechanism C in Fig. 1) (Sergeant et al. 2000; Hirst & Ward, 2003; McHale et al. 2006; Sanders et al. 2006). These ICCs are long thin cells connected together through gap junctions to form a network (Komuro, 2006) that coordinates the activity of large populations of SMCs to form the peristaltic waves of contraction. In the urethra, the contractions close off the sphincter that prevents urine leaving the bladder during urine storage, but they relax when urine is voided. The neural control of these two functions depends on the ability of transmitters to modulate the pacemaker activity of the ICCs (Ward & Sanders, 2006). The ICCs are thus key regulators of gastrointestinal and urethral SMC activation.
ICC cytosolic Ca2+ oscillator model
The ICCs generate pulses of Ca2+, which are responsible for the periodic depolarizations known as slow waves that activate the inward currents that spread through the gap junctions providing the depolarization that activates the SMCs (see mechanism C in Fig. 1). Since the ICCs do not produce action potentials, excitation is propagated through the network using a potential-dependent mechanism to synchronize the individual oscillators. This ICC oscillator resembles that found in various SMCs (Fig. 5) but there are enough differences for them to be described separately. These differences are related to how the ICCs function to activate the SMCs. The endogenous muscle oscillator of the vascular and airway SMCs provides the internal Ca2+ signal that triggers contraction (Fig. 5), whereas the ICC oscillator generates the depolarizing signal that activates the local population of muscle cells (Fig. 7). The following hypothesis describes how the ICC pacemaker mechanism might be generated and synchronized (Fig. 7).
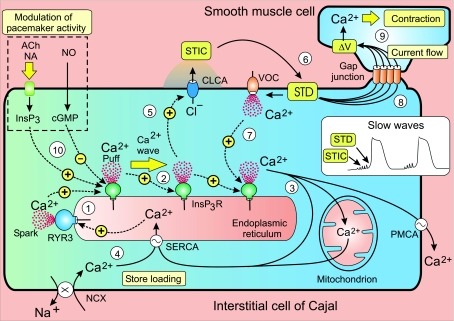
Figure 7. The cytosolic Ca2+ oscillator responsible for pacemaker activity in interstitial cells of Cajal releases periodic pulses of Ca2+ that form a Ca2+ wave.
The increase in Ca2+ activates Cl− channels (CLCA) to give the spontaneous transient inward currents (STICs) that sum to form the spontaneous transient depolarizations (STD) resulting in the slow waves of membrane depolarization (see inset). Current flow through gap junctions allows these waves to spread into neighbouring smooth muscle cells to activate contraction. See text for a description of the oscillator that drives this activation process. Reproduced from Berridge (2008), with permission.
(1) The onset of each transient is probably a stochastic process that is primed by loading up the store with Ca2+ that then sensitizes the release channels, for example the ryanodine receptor 3 (RYR3), which is strongly expressed in ICCs (Aoyama et al. 2004), may also initiate transients. This early release appears as elementary events such as the Ca2+ puffs and sparks that activate the spontaneous transient inward currents (STICs) that occur during the pacemaker phase preceding each transient (Sergeant et al. 2006a).
(2) Once the elementary events reach a particular amplitude and/or frequency, they initiate a Ca2+ wave that spreads along the cell through the process of Ca2+-induced Ca2+ release (CICR) to form a Ca2+ wave. The wave terminates when release stops, perhaps because the decrease in Ca2+ concentration within the store inactivates the release channels.
(3) During the recovery period, Ca2+ is removed from the cytoplasm by three processes: it is extruded from the cell by the plasma membrane Ca2+-ATPase (PMCA); it is pumped back into the ER by the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) to begin reloading the store; and it is rapidly taken up by the mitochondria. The ICCs are tightly packed with mitochondria that are closely associated with the ER to form ‘pacemaker units’ that are a central feature of an alternative ICC oscillator model (for details see Sanders et al. 2006). In the model depicted in Fig. 7, the mitochondria have a more passive role as a temporary store of Ca2+, which is taken up rapidly during the recovery period and is then released slowly during the interburst period to help reload the store.
(4) Such store reloading is facilitated by entry of external Ca2+ and this accounts for why ICC Ca2+ oscillations are critically dependent on the external concentration of Ca2+ (Johnstone et al. 2005; Hashitani & Suzuki, 2007). When the concentration of external Ca2+ was either elevated or lowered, the baseline level of Ca2+ increased or decreased, respectively. Such marked changes in the resting level of Ca2+ are indicative of a high resting level of Ca2+ entry, which is essential for maintaining oscillatory activity. The nature of the ICC Ca2+ entry mechanisms is still being determined. In the case of the urethral ICCs, entry might be maintained by the Na+/Ca2+ exchanger (NCX) operating in its reverse mode (Bradley et al. 2006; McHale et al. 2006), but there may be other entry mechanisms such as T-type channels (Kito et al. 2005).
(5) The Ca2+ that is released during each wave activates Ca2+-sensitive chloride channels that set up the slow waves of membrane depolarization (see inset in Fig. 7). The early Ca2+ puffs generate the STICs that occur during the pacemaker depolarization (McHale et al. 2006).
(6) Summation of these STICs then results in the spontaneous transient depolarization (STD) that initiates each slow wave.
(7) In many cases, this initial pacemaker depolarization provided by the STD is enhanced by the opening of a VOC, which accounts for the rapid rising phase observed for many slow waves. Opening of the VOCs is not essential for the operation of this ICC oscillator, but the additional depolarization and entry of external Ca2+ have two important functions. Firstly, entry of Ca2+ helps release Ca2+ from the internal stores distributed down the length of the long thin ICC. If the recruitment of all the stores depended solely on CICR, the rate of rise of the slow wave would be much slower. This is evident when the opening of the VOCs is prevented by raising the external concentration of potassium to depolarize the membrane (Kito & Suzuki, 2003). The slow rate of rise can be explained by the fact that it takes time for the Ca2+ wave to travel down the long thin ICC cell when the VOCs are disabled. The Ca2+ coming from the VOCs thus serves as an entrainment signal to sharpen the wave front by speeding up the recruitment of the intracellular release channels located down the length of the long ER store. The second role for this rapid depolarization is to drive the phase wave that synchronizes the oscillatory activity of individual cells throughout large regions of the ICC network (see next section).
(8) The ICC pacemaker slow wave provides the depolarizing stimulus to activate the SMCs. Transfer of the activation stimulus from ICC to SMC is achieved through a passive flow of current through the gap junctions (Fig. 7). During the pacemaker slow wave, therefore, the inward current flow that discharges the membrane of the ICC simultaneously draws current from the SMCs that also begin to depolarize.
(9) SMC contraction begins when this depolarization reaches the activation threshold for the L-type Ca2+ channels that open to initiate excitation–contraction coupling as occurs in colonic SMCs (McCarron et al. 2004). There is a close relationship between the Ca2+ transient in the ICC, which initiates the slow wave, and the resulting Ca2+ transient in the SMC (Yamazawa & Iino, 2002; Hashitani & Suzuki, 2007). However, there is a striking difference in the time course of the two responses. The ICC Ca2+ responses last very much longer than the brief Ca2+ transient in the SMCs. The functional significance of this temporal difference may relate to the way in which the ICCs transmit their pacemaker signal to the SMCs. Since the ICC network does not produce action potentials, it has to transmit its message through a passive electrotonic mechanism that decays rapidly as it spreads through the gap junctions into the large population of SMCs. By generating a prolonged Ca2+ transient, the ICC maximizes its chances of spreading a meaningful signal to the SMCs. Another way of maximizing pacemaker activity is for all the ICCs within the network to operate together and this synchronization of oscillatory activity creates a phase wave as described below.
(10) Agonists such as ACh, NA and nitric oxide (NO) modulate ICC pacemaker activity (van Helden & Imtiaz, 2003). For example, ACh and NA accelerate the oscillator by increasing the formation of InsP3 that then enhances the sensitivity of the InsP3 receptor to the stimulatory action of Ca2+ (step 10 in Fig. 7). Conversely, inhibitory nerves that release NO have the opposite effect of reducing slow wave amplitude and frequency. This action of NO, which reduces the pacemaking drive, is mediated through cyclic GMP that acts through cyclic GMP-dependent protein kinase Iβ (cGKIβ) that phosphorylates the InsP3 receptors to reduce their Ca2+ sensitivity (Sergeant et al. 2006b).
ICC phase waves
This ICC network coordinates the contractile activity of large groups of smooth muscle cells by synchronizing its individual oscillators (van Helden & Imtiaz, 2003; Park et al. 2006). This synchronicity, which has been referred to as a phase wave, is achieved by coupling oscillators together through a depolarizing signal spreading through the gap junctions (Kim et al. 2002; van Helden & Imtiaz, 2003; Kito et al. 2005; Imtiaz et al. 2006; Sanders et al. 2006). If slow waves are recorded from two cells widely separated from each other by distances of 4.5 mm, the regular waves of membrane depolarization occur almost exactly in phase with each other (van Helden & Imtiaz, 2003). In reality, there are small phase shifts that result from the fact that excitation spreads quickly down the network at 5–40 mm s−1 to account for the waves of peristalsis. Phase waves can also be observed when the Ca2+ transients responsible for these slow waves are simultaneously recorded from separate ICCs (Park et al. 2006). This phase locking of ICC Ca2+ oscillators depends on a signal being passed through the gap junctions.
The phase wave spreads too fast to be explained by an intercellular Ca2+ wave, which has an upper propagation rate of about 100 μm s−1. Most attention is now focused on the idea that the oscillatory activities of neighbouring cells are coupled together by a depolarization signal that spreads through the gap junctions. Since this oscillatory activity depends upon the spontaneous activation of InsP3 receptors (Fig. 7), depolarization may entrain oscillations by enhancing the local supply of either the Ca2+ or InsP3 necessary to trigger release. It seems most likely that the coupling mechanism is the depolarization-induced influx of Ca2+, which can rapidly initiate the release of Ca2+ from primed InsP3 receptors. Since all the cells within the network have Ca2+ oscillators that pulse at similar frequencies, the cell that generates a Ca2+ signal first will provide the depolarization to entrain its neighbours. The importance of this depolarization is that it spreads electronically very rapidly to provide an influx of external Ca2+, which entrains neighbouring cells to release their pulse of Ca2+ in phase with everyone else.
The critical feature of this mechanism is that all the oscillators in the network are coupled together through a voltage-dependent mechanism. At any moment in time, an individual cell is either functioning as a pacemaker to initiate a Ca2+ transient or it is responding to the depolarization emanating from a neighbouring pacemaker cell. It seems that these two functions are not fixed, because cells can switch roles on a beat by beat basis (van Helden & Imtiaz, 2003). This system of coupled oscillators is highly dynamic in that each cell is not only capable of initiating each wave but it is also finely tuned to respond to an entrainment signal coming from any of its neighbours and this ensures that each beat is synchronous within a large population of cells.
Ureter smooth muscle cell
Transfer of urine from the kidney to the bladder is carried out by the pelviureteric system that has two components, the renal pelvis and the ureter. Spontaneous action potentials that begin in the proximal pelvis propel urine through the distal pelvis and ureter. Within the renal pelvis, the propagating action potentials are associated with a wave of Ca2+ that sweeps through the typical SMCs at a rate of approximately 1 mm s−1 (Lang et al. 2007). Activation of ureter SMC contraction resembles most closely mechanism C in Fig. 1. The pelviureteric pacemaker mechanism responsible for triggering the periodic action potentials seems to be located in the atypical SMCs, which are long thin cells that form a loose network lying on top of the smooth muscle layer.
The pacemaker mechanism in these atypical SMCs is similar to that found in the ICCs (Fig. 7). They have a cytosolic Ca2+ oscillator that produces spontaneous transient depolarizations (STDs) to provide the depolarizing drive to trigger the action potentials that spread to the layer of typical SMCs (Lang et al. 2007). One important difference is that the oscillations in cytosolic Ca2+ activate an inward Na+ current carried by channels that remain to be determined rather than the Ca2+-sensitive Cl− channels found in many ICCs. Like the ICCs, the L-type Ca2+ channels synchronize the STDs into large enough pacemaker events capable of triggering the action potentials. Despite the fact that the atypical smooth muscle cells generate STDs at a high frequency (10–40 min−1), the action potentials that they initiate in the typical SMCs occur at a much lower frequency (6–15 min−1) and this difference is caused by a prolonged refractory period induced by the Ca2+ that enters the cell during the action potential. During the recovery period, much of the Ca2+ released during the action potential is sucked up by the ER and this loading of the store sensitizes the RYR2s, which immediately begin to generate Ca2+ sparks (Burdyga & Wray, 2005). These sparks then activate BK channels and the resulting hyperpolarization inhibits action potentials thus resulting in a refractory period. Once the Ca2+ level in the stores returns to normal, the sparks decline and the membrane can now respond to the depolarizing drive coming from the atypical smooth muscle cells to initiate another action potential. This action potential spreads down the ureter to drive the peristaltic waves of contraction that propel urine into the bladder.
Conclusion
SMCs employ a great variety of Ca2+ signalling systems that are adapted to control their different contractile functions. Those that have to respond quickly use transmitters to induce the membrane depolarization and rapid influx of Ca2+ as occurs in the vas deferens. A membrane oscillator provides the periodic depolarizations necessary to drive contractions in bladder and uterine SMCs. A model for the uterus oscillator attempts to provide a mechanism to account for the pacemaker depolarization and how it might be modulated by oxytocin. Tonic SMCs, which are less reliant on membrane depolarization to initiate Ca2+ signals, are driven by a cytosolic Ca2+ oscillator. In some cases (vascular, airways and corpus cavernosum), this oscillator is located within the SMC whereas in others (gastrointestinal, urethra) it is located in specialized pacemaker cells such as the interstitial cells of Cajal (ICCs) that are connected to the SMCs through gap junctions. A luminal loading Ca2+ oscillator model can explain this spontaneous release of stored Ca2+ and how such oscillations can be accelerated by neurotransmitters and hormones. The periodic pulses of Ca2+ not only drive contraction but they also induce the membrane depolarization that couples the individual oscillators together to provide the characteristic synchronization of many tonic SMCs.
References
- Amberg GC, Navedo MF, Nieves-Cintrón M, Molkentin JD, Santana LF. Calcium sparklets regulate local and global calcium in murine arterial smooth muscle. J Physiol. 2007;579:187–201. doi: 10.1113/jphysiol.2006.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, Hotta S, Ito Y, Matsubara T, Shimokata K, Chen SRW, Imaizumi Y, Nakayama S. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sci. 2004;117:2813–2825. doi: 10.1242/jcs.01136. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium oscillations. Biochem Soc Symp. 2007;74:1–7. doi: 10.1042/BSS0740001. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Cell Signalling Biology. Portland Press Limited; 2008. http://www.cellsignallingbiology.org. [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Dupont G. Spatial and temporal signalling by calcium. Curr Opin Cell Biol. 1994;6:267–274. doi: 10.1016/0955-0674(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Blanks AM, Zhao Z-H, Shmygol A, Bru-Mercier G, Astle S, Thornton S. Characterization of the molecular and electrophysiological properties of the T-type calcium channel in human myometrium. J Physiol. 2007;581:915–926. doi: 10.1113/jphysiol.2007.132126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KN, Currie S, MacMillan D, Muir TC, McCarron JG. Cyclic ADP-ribose inceases Ca2+ removal in smooth muscle. J Cell Sci. 2003;116:4291–4306. doi: 10.1242/jcs.00713. [DOI] [PubMed] [Google Scholar]
- Bradley E, Hollywood MA, Johnston L, Large RJ, Matsuda T, Baba A, McHale NG, Thornbury KD, Sergeant GP. Contribution of reverse Na+–Ca2+ exchange to spontaneous activity in interstitial cells of Cajal in the rabbit urethra. J Physiol. 2006;574:651–661. doi: 10.1113/jphysiol.2006.110932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain KL, Cuprian AM, Williams DJ, Cunnane TC. The sources and sequestration of Ca2+ transients in the mouse vas deferens. J Physiol. 2003;553:627–635. doi: 10.1113/jphysiol.2003.049734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain KL, Jackson MJ, Trout SJ, Cunnane TC. Intermittent ATP release from nerve terminals elicits focal smooth muscle Ca2+ transients in mouse vas deferens. J Physiol. 2002;541:849–862. doi: 10.1113/jphysiol.2002.019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Cornwell T, Korniyenko I, Solodushko V, Bond CT, Adelman JP, Taylor MS. Myometrial expression of small conductance Ca2+-activated K+ channels depresses phasic uterine contractions. Am J Physiol Cell Physiol. 2007;292:C832–C840. doi: 10.1152/ajpcell.00268.2006. [DOI] [PubMed] [Google Scholar]
- Burdyga T, Wray S. Action potential refractory period in ureter smooth muscle is set by Ca sparks and BK channels. Nature. 2005;436:559–562. doi: 10.1038/nature03834. [DOI] [PubMed] [Google Scholar]
- Collier ML, Ji G, Wang Y-X, Kotlikoff MI. Calcium-induced calcium release in smooth muscle. Loose coupling between the action potential and calcium release. J Gen Physiol. 2000;115:653–662. doi: 10.1085/jgp.115.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven M, Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Modulation of spontaneous Ca2+-activated Cl− currents in the rabbit corpus cavernosum by the nitric oxide-cGMP pathway. J Physiol. 2004;556:495–506. doi: 10.1113/jphysiol.2003.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai JM, Kuo K-H, Leo JM, van Breemen C, Lee C-H. Mechanism of ACh-induced asynchronous calcium waves and tonic contraction in porcine tracheal muscle bundle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L459–L469. doi: 10.1152/ajplung.00092.2005. [DOI] [PubMed] [Google Scholar]
- Deshpande DA, White TA, Dogan S, Walseth TF, Panettieri RA, Kanna MS. CD38/cyclic ADP-ribose signalling: role in the regulation of calcium homeostasis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;288:L733–L788. doi: 10.1152/ajplung.00217.2004. [DOI] [PubMed] [Google Scholar]
- Essin K, Welling A, Hofmann F, Luft FC, Gollasch M, Moosmang S. Indirect coupling between CaV1.2 channels and ryanodine receptors to generate Ca2+ sparks in murine arterial smooth muscle cells. J Physiol. 2007;584:205–219. doi: 10.1113/jphysiol.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock RE, Hill CE. Differential activation of ion channels by inositol 1,4,5-trisphosphate (IP3)- and ryanodine-sensitive calcium stores in rat basilar artery vasomotion. J Physiol. 2002;545:615–627. doi: 10.1113/jphysiol.2002.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock RE, Hill CE. Rhythmicity in arterial smooth muscle. J Physiol. 2005;566:645–656. doi: 10.1113/jphysiol.2005.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Fukuta H, Takano H, Klemm MF, Suzuki H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol. 2001;530:273–286. doi: 10.1111/j.1469-7793.2001.0273l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H. Interaction between interstitial cells and smooth muscles in the lower urinary tract and penis. J Physiol. 2006;576:707–714. doi: 10.1113/jphysiol.2006.116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol. 2003a;140:146–158. doi: 10.1038/sj.bjp.0705319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003b;140:159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity of detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004;141:183–193. doi: 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Bramich NJ, Hirst GDS. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol. 2000;524:565–579. doi: 10.1111/j.1469-7793.2000.t01-2-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Suzuki H. Properties of spontaneous Ca2+ transients recorded from interstitial cells of Cajal-like cells of the rabbit urethra in situ. J Physiol. 2007;583:505–519. doi: 10.1113/jphysiol.2007.136697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Shirasawa N, Soji T, Tomita A, Kohri K, Suzuki H. Interaction between spontaneous and neurally mediated regulation of smooth muscle cell tone in the rabbit corpus cavernosum. J Physiol. 2005;569:723–735. doi: 10.1113/jphysiol.2005.099309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. Elementary purinergic Ca2+ transients evoked by nerve stimulation in rat urinary bladder smooth muscle. J Physiol. 2005;564:201–212. doi: 10.1113/jphysiol.2004.077826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta S, Morimura K, Ohya S, Muraki K, Takeshima H, Imaizumi Y. Ryanodine receptor type 2 deficiency changes excitation–contraction coupling and membrane potential in urinary bladder smooth muscle. J Physiol. 2007;582:489–506. doi: 10.1113/jphysiol.2007.130302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M, Kasai H, Yamazawa T. Visualization of neural control of intracellular Ca2+ concentration in single vascular smooth muscle cells in situ. EMBO J. 1994;13:5026–5031. doi: 10.1002/j.1460-2075.1994.tb06831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz MS, Katnik CP, Smith DW, van Helden DF. Role of voltage-dependent modulation of store Ca2+ release in synchronization of Ca2+ oscillations. Biophys J. 2006;90:1–23. doi: 10.1529/biophysj.104.058743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz MS, Zhao J, Hosaka K, von der Weid P-Y, Crowe M, van Helden DF. Pacemaking through Ca2+ stores interacting as coupled oscillators via membrane depolarization. Biophys J. 2007;92:3843–3861. doi: 10.1529/biophysj.106.095687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone L, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG. Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol. 2005;565:449–461. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Koh SD, Sanders KM. Voltage-dependent inward currents of intestinal cells of Cajal from murine colon and small intestine. J Physiol. 2002;541:797–810. doi: 10.1113/jphysiol.2002.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553:803–818. doi: 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Ward SM, Sanders KM. Pacemaker potentials generated by interstitial cells of Cajal in the murine intestine. Am J Physiol Cell Physiol. 2005;288:C710–C720. doi: 10.1152/ajpcell.00361.2004. [DOI] [PubMed] [Google Scholar]
- Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576:653–658. doi: 10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo K-H, Dai J, Seow CY, Lee C-H, van Breemen C. Relationship between asynchronous Ca2+ waves and force development in intact smooth muscle bundles of the porcine trachea. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1345–L1353. doi: 10.1152/ajplung.00043.2003. [DOI] [PubMed] [Google Scholar]
- Kupittayanant S, Luckas MJM, Wray S. Effect of inhibiting the sarcoplasmic reticulum on spontaneous and oxytocin-induced contractions of human myometrium. Br J Obstet Gynaec. 2002;109:289–296. doi: 10.1111/j.1471-0528.2002.01110.x. [DOI] [PubMed] [Google Scholar]
- Lamboley M, Schuster A, Bény J-L, Meister J-J. Recruitment of smooth muscle cells and arterial vasomotion. Am J Physiol Heart Circ Physiol. 2003;285:H562–H569. doi: 10.1152/ajpheart.00526.2002. [DOI] [PubMed] [Google Scholar]
- Lamont C, Vainorius E, Wier WG. Purinergic and adrenergic Ca2+ transients during neurogenic contractions of rat mesenteric small arteries. J Physiol. 2003;549:801–808. doi: 10.1113/jphysiol.2003.043380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont C, Wier WG. Evoked and spontaneous purinergic junctional Ca2+ transients (jCaTs) in rat small arteries. Circ Res. 2002;91:454–456. doi: 10.1161/01.res.0000035060.98415.4b. [DOI] [PubMed] [Google Scholar]
- Lang RJ, Hashitani H, Tonta MA, Parkington HC, Suzuki H. Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis. J Physiol. 2007;583:1049–1068. doi: 10.1113/jphysiol.2007.137034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-H, Poburko D, Sahota P, Sandhu J, Ruehlmann DO, van Breemen C. The mechanism of phenylephrine-mediated [Ca2+]i oscillations underlying tonic contraction in the rabbit inferior vena cava. J Physiol. 2001;534:641–650. doi: 10.1111/j.1469-7793.2001.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Farley JM. Acetylcholine-induced chloride current oscillations in swine tracheal smooth muscle cells. J Pharmacol Exp Ther. 1996;276:178–186. [PubMed] [Google Scholar]
- McCarron JG, MacMillan D, Bradley KN, Chalmers S, Muir TC. Origin and mechanisms of Ca2+ waves in smooth muscle as revealed by localized photolysis of caged inositol 1,4,5-trisphosphate. J Biol Chem. 2004;279:8417–8427. doi: 10.1074/jbc.M311797200. [DOI] [PubMed] [Google Scholar]
- McHale NG, Hollywood MA, Sergeant GP, Shafei M, Thornbury KT, Ward SM. Organization and function of ICC in the urinary tract. J Physiol. 2006;576:689–694. doi: 10.1113/jphysiol.2006.116657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauban JRH, Lamont C, Balke CW, Wier WG. Adrenergic stimulation of rat resistance arteries affects Ca2+ sparks, Ca2+ waves, and Ca2+ oscillations. Am J Physiol Heart Circ Physiol. 2001;280:H2399–H2405. doi: 10.1152/ajpheart.2001.280.5.H2399. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- Morimura K, Ohi Y, Yamamura H, Ohya S, Muraki K, Imaizumi Y. Two-step Ca2+ intracellular release underlies excitation-contraction coupling in mouse urinary bladder myocytes. Am J Physiol Cell Physiol. 2005;290:C388–C403. doi: 10.1152/ajpcell.00409.2005. [DOI] [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, Evans RJ. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- Nakao K, Inoue Y, Okabe K, Kawarabayashi T, Kitamura K. Oxytocin enhances action potentials in pregnant human myometrium – a study with microelectrodes. Am J Obstet Gynecol. 1997;177:222–228. doi: 10.1016/s0002-9378(97)70465-4. [DOI] [PubMed] [Google Scholar]
- Ohi Y, Yamamura H, Nagano N, Ohya S, Muraki K, Watanabe M, Imaizumi Y. Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J Physiol. 2001;534:313–326. doi: 10.1111/j.1469-7793.2001.t01-3-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KJ, Hennig GW, Lee H-T, Spencer NJ, Ward SM, Sith TK, Sanders KM. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol. 2006;290:C1411–C1427. doi: 10.1152/ajpcell.00447.2005. [DOI] [PubMed] [Google Scholar]
- Peng H, Matchkov V, Ivarsen A, Aalkjaer C, Nilsson H. Hypothesis for the initiation of vasomotion. Circ Res. 2001;88:810–815. doi: 10.1161/hh0801.089603. [DOI] [PubMed] [Google Scholar]
- Peppiatt-Wildman CM, Albert AP, Saleh SN, Large WA. Endothelin-1 activates a Ca2+-permeable cation channel with TRPC3 and TRPC7 properties in rabbit coronary artery myocytes. J Physiol. 2007;580:755–764. doi: 10.1113/jphysiol.2006.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol. 2005a;125:535–553. doi: 10.1085/jgp.200409216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JF, Sanderson MJ. The contraction of smooth muscle cells of intrapulmonary arterioles is determined by the frequency of Ca2+ oscillations induced by 5-HT and KCl. J Gen Physiol. 2005b;125:555–567. doi: 10.1085/jgp.200409217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebolledo A, Speroni F, Raingo J, Salemme SV, Tanzi F, Munin V, Añón MC, Milesi V. The Na+/Ca2+ exchanger is active and working in the reverse mode in human umbilical artery smooth muscle. Biochem Biophys Res Commun. 2006;339:840–845. doi: 10.1016/j.bbrc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- Saleh SN, Albert AP, Peppiatt-Wildman CM, Large WA. Diverse properties of store-operated TRPC channels activated by protein kinase C in vascular myocytes. J Physiol. 2008;586:2463–2476. doi: 10.1113/jphysiol.2008.152157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Koy SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway SMC contractility by Ca2+ signaling and sensitivity. Proc Am Thorac Soc. 2008;5:23–31. doi: 10.1513/pats.200704-050VS. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Ca2+ signalling in urethral interstitial cells of Cajal. J Physiol. 2006a;576:715–720. doi: 10.1113/jphysiol.2006.115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Johnston L, McHale NG, Thornbury KD, Hollywood MA. Activation of the cGMP/PKG pathway inhibits electrical activity in rabbit urethral interstitial cells of Cajal by reducing the spatial spread of Ca2+ waves. J Physiol. 2006b;574:167–181. doi: 10.1113/jphysiol.2006.108621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L, O'Neill S, Jones CJP, Austin C, Taggart MJ. Comparison of U46619-, endothelin-1- or phenylephrine-induced changes in cellular Ca2+ profiles and Ca2+ sensitization of constriction of pressurised rat resistance arteries. Br J Pharm. 2004;141:678–688. doi: 10.1038/sj.bjp.0705647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmygol A, Blanks AM, Bru-Mercier G, Gullam JE, Thornton S. Control of uterine Ca2+ by membrane voltage: Toward understanding the excitation-contraction coupling in human myometrium. Ann N Y Acad Sci. 2007;1101:97–109. doi: 10.1196/annals.1389.031. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- van Helden DF, Imtiaz MS. Ca2+ phase waves: a basis for cellular pacemaking and long-range synchronicity in the guinea-pig gastric pylorus. J Physiol. 2003;548:271–296. doi: 10.1113/jphysiol.2002.033720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Eto M, Steers WD, Somlyo AP, Somlyo AV. RhoA-mediated Ca2+ sensitization in erectile function. J Biol Chem. 2002;277:30614–30621. doi: 10.1074/jbc.M204262200. [DOI] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576:675–682. doi: 10.1113/jphysiol.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, McGeown JG. Inositol 1,4,5-trisphosphate receptors modulate Ca2+ sparks and Ca2+ store content in vas deferens myocytes. Am J Physiol Cell Physiol. 2003;285:C195–C204. doi: 10.1152/ajpcell.00374.2002. [DOI] [PubMed] [Google Scholar]
- Wray S. Insights into the uterus. Exp Physiol. 2007;92:621–631. doi: 10.1113/expphysiol.2007.038125. [DOI] [PubMed] [Google Scholar]
- Wray S, Burdyga T, Noble K. Calcium signalling in smooth muscle. Cell Calcium. 2005;38:397–407. doi: 10.1016/j.ceca.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wray S, Noble K. Sex hormones and excitation-contraction coupling in the uterus: The effects of oestrus and hormones. J Neuroendocrinol. 2008;20:451–461. doi: 10.1111/j.1365-2826.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- Wray S, Shmygol A. Role of the calcium store in uterine contractility. Semin Cell Dev Biol. 2007;18:315–320. doi: 10.1016/j.semcdb.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Yamazawa T, Iino M. Simultaneous imaging of Ca2+ signals in interstitial cells of Cajal and longitudinal smooth muscle cells during rhythmic activity in mouse ileum. J Physiol. 2002;538:823–835. doi: 10.1113/jphysiol.2001.013045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC. Myocytes, myometrium, and uterine contraction. Ann N Y Acad Sci. 2007;1101:72–84. doi: 10.1196/annals.1389.038. [DOI] [PubMed] [Google Scholar]