Abstract
Short-interval intracortical inhibition (SICI), intracortical facilitation (ICF) and short-interval intracortical facilitation (SICF) were assessed in the cortical motor area of the first dorsal interosseous muscle (FDI) of 16 healthy subjects. Paired-pulse TMS was delivered to the left hemisphere at the following interstimulus intervals (ISIs): 2 and 3 ms for SICI, 10 and 15 ms for ICF and 1–5 ms for SICF. Motor-evoked potentials were recorded from the resting and active right FDI. The effects exerted on SICI and ICF by four intensities (60–90% of active motor threshold, AMT) of the conditioning stimulus (S1) and by three levels of muscle contraction (10%, 25%, 50% of maximal voluntary contraction, MVC) were evaluated. The effects exerted on SICF were evaluated with two intensities (90% and 70% of AMT) of the test stimulus (S2) and with the same levels of muscle contraction. Results showed that: (i) during 10% MVC, maximum SICI was observed with S1 = 70% AMT; (ii) the amount of SICI obtained with S1 = 70% AMT was the same at rest as during 10% MVC, but decreased at higher contraction levels; (iii) ICF was observed only at rest with S1 = 90% AMT; (iv) SICF was facilitated at 10% and 25% MVC, but not at 50% MVC. We conclude that during muscle activation, intracortical excitability reflects a balance between activation of SICI and SICF systems. Part of the reduction in SICI during contraction is due to superimposed recruitment of SICF. Low intensity (70% AMT) conditioning stimuli can test SICI independently of effects on SICF at low contraction levels.
The paired-pulse transcranial magnetic stimulation protocol is a useful tool to explore inhibitory and facilitatory circuitry of the human motor cortex (Kobayashi & Pascual-Leone, 2003). When a subthreshold conditioning pulse (S1) and a suprathreshold test pulse (S2) are applied to the motor cortex through the same coil, the test response is inhibited at interstimulus intervals (ISIs) between 1 and 5 ms (short-interval intracortical inhibition, SICI) while it is facilitated at ISIs of 7–20 ms (intracortical facilitation, ICF) (Kujirai et al. 1993). A large number of studies have characterized the properties of SICI in healthy subjects. Thus it has been shown that the interaction between conditioning and test pulses occurs at cortical level (Kujirai et al. 1993; Nakamura et al. 1997; Di Lazzaro et al. 1998b; Hanajima et al. 1998) through the activation of an intracortical inhibitory GABAergic circuit (Ziemann et al. 1996b; Di Lazzaro et al. 2000; Ilic et al. 2002) that particularly affects the late I3 wave (Di Lazzaro et al. 1998a,b; Hanajima et al. 1998; Ilic et al. 2002).
In 1995, Ridding et al. showed that during a slight contraction of the target muscle, SICI is strongly reduced in comparison with the resting condition. Although this finding has been confirmed by several authors (Fisher et al. 2002; Roshan et al. 2003; Jaberzadeh et al. 2007), it has never been fully clarified whether it represents a reduction in cortical GABAergic inhibition, or superimposition of a concurrent facilitation that is recruited during muscle contraction. One potential source of such facilitation was explored by Hanajima and colleagues who found that during muscle contraction, the excitability of spinal motoneurons increases so that they become responsive to the first (I1 wave) descending volley induced by TMS that is usually not affected by SICI (Hanajima et al. 1998). This makes the conditioned MEPs larger than they were when relaxed, reducing the apparent amount of SICI.
A second potential facilitatory input that overlaps in time with SICI is short-interval intracortical facilitation (SICF). This phenomenon is usually studied with a paired-pulse protocol consisting of an S1 and S2 which are both near the motor threshold (Tokimura et al. 1996) or a suprathreshold S1 followed by a subthreshold S2 (Ziemann et al. 1998b). SICF consists of three peaks of MEP facilitation occurring at discrete interstimulus intervals of about 1.1–1.5 ms, 2.3–2.9 ms and 4.1–4.4 ms. Since cortical neurons are likely to be refractory at ISI = 1.1–1.5 ms, it is currently believed that the first peak of SICF reflects an S2-induced direct excitation of the initial segments of excitatory glutamatergic intracortical interneurons, which had been previously depolarized by the S1-induced EPSPs (Hanajima et al. 2002). Later peaks may represent conventional summation of synaptic inputs at postsynaptic membranes. SICI and SICF are commonly considered to be two independent, antagonistic systems (Tokimura et al. 1996; Ziemann et al. 1998b; Chen & Garg, 2000) and that the balance between them determines the final outcome of SICI or SICF protocols (Ziemann et al. 1998a,c; Awiszus et al. 1999; Schwenkreis et al. 1999, 2000; Ilic et al. 2002; Fisher et al. 2002; Roshan et al. 2003). However, it is not known in detail how SICF is modulated by contraction, and whether this could account for the reduced SICI that is observed.
It is important to clarify the mechanism of reduced SICI during muscle activation. In many papers there is a tendency to focus on the possibility that it is due to selective down-regulation of inhibitory neurons, which project onto corticomotoneuronal cells involved in the intended movement to allow the execution of a specific muscle task (Ridding et al. 1995; Reynolds & Ashby, 1999; Zoghi et al. 2003; Zoghi & Nordstrom, 2007). However, if the reduction in SICI is due to superimposed facilitation then this interpretation is incorrect, and greater weight would have to be given to facilitatory mechanisms. The aim of this work was to study the effects of volitional contraction on both SICI and SICF systems and to evaluate whether and how these two systems interact with a range of stimulus parameters.
Methods
Subjects
Sixteen healthy subjects (10 males and 6 females, aged 26–52 years (mean ±s.e.m.; 32.7 ± 1.7 years)) participated in this study. All of them gave their written informed consent and the procedure, approved by the local ethics committee, was in accordance with the ethical standards established in the Declaration of Helsinki. No side-effects were noted in any of the individuals tested.
EMG recordings
Motor-evoked potentials (MEPs) were recorded from the right first dorsal interosseous muscle (FDI) using 9 mm diameter Ag–AgCl surface cup electrodes. The active electrode was placed over the muscle belly, the reference electrode was placed over the metacarpophalangeal joint of the index finger and the earth electrode was over the forearm. Participants were seated in a comfortable chair, with their head and neck supported. Unrectified and rectified FDI EMG activities were recorded (Digitimer D360 amplifier, Digitimer Ltd, Welwyn Garden City, Herts, UK) in both resting and active conditions. The level of EMG activity during muscle contraction was determined offline by analysing the rectified EMG in a 50 ms time window preceding the stimulus. Signals were amplified (× 1000), filtered (bandwidth 3 Hz to 3 kHz) and sampled (5 kHz per channel) from 50 ms before to 50 ms after stimulus delivery, using a 1401 plus A/D converter (Cambridge Electronic Design, Cambridge, UK) and Signal 3.06 software on a computer. Rectified and filtered EMG activity of the right FDI, was shown on an oscilloscope to assist subjects to keep the muscle completely relaxed or to exert a steady level of muscular contraction in the different experiments. Auditory feedback of EMG activity was also provided. Unless specified otherwise the standard level of muscle contraction was 10% of maximal voluntary contraction (MVC).
TMS stimulation
Transcranial magnetic stimulation (TMS) was performed using a figure-of-eight-shaped coil with external loop diameter of 9 cm connected to two Magstim 200 stimulators through a Y connector or Bistim module (Magstim Co., Whitland, Dyfed, UK). All the experiments were performed with the coil held tangentially to the scalp overlying the left-hand motor cortex and orientated with the handle pointing backwards and laterally approximately perpendicular to the assumed line of the central sulcus (around 30 deg away from the midline). The optimal spot to elicit MEPs in the right FDI was carefully searched in each subject and the optimal coil position able to evoke a stable MEP was marked on the scalp to ensure identical placement of the coil throughout the experiments. Stimulus intensity was given as a percentage of maximum stimulator output (%MSO). According to the IFCN guidelines (Rossini et al. 1994; Rothwell et al. 1999), resting motor threshold (RMT) was defined as the minimum stimulus intensity capable of inducing MEPs greater than 100 μV peak-to-peak amplitude in at least 5 out of 10 consecutive trials in the relaxed muscle, and the active motor threshold (AMT) was defined as the minimum stimulus intensity able to evoke a MEP bigger than 200 μV peak-to-peak amplitude in at least 5 out of 10 consecutive trials during isometric contraction of the tested muscle at 10% of MVC. Rate of TMS stimulation was less than once every 5 s.
Experimental procedures
Experiment 1. Effects of different intensities of the conditioning stimulus (S1) on SICI and ICF at rest and during voluntary contraction
All 16 subjects enrolled in the study participated in this experiment. Each subject participated in two separate sessions, carried out at approximately the same time on two consecutive days, with the resting and active muscle conditions assigned randomly between the days. In each session SICI and ICF were assessed with the classical subthreshold S1 suprathreshold S2 protocol described by Kujirai et al. (1993). Four conditioning pulse intensities were used (60, 70, 80, 90% of AMT). S2 intensity was adjusted to elicit a MEP of 1 mV peak-to-peak amplitude in the right FDI. Interstimulus intervals (ISIs) of 2 and 3 ms for SICI and of 10 and 15 ms for ICF were examined in a randomized order. Twelve unconditioned MEPs and 12 conditioned MEPs at each ISI were recorded in each experimental block. Thus, 60 trials were collected at each conditioning pulse intensity. The mean amplitudes of each conditioned MEP are expressed as a percentage of the mean test MEP.
Experiment 2. Time course of SICI at rest and during voluntary contraction
This experiment examined the time course of SICI in both resting and active muscular states in eight subjects. Nine ISIs, from 1 ms to 5 ms in 0.5 ms steps, were studied using four S1 intensities (60, 70, 80, 90% of AMT) and an S2 intensity able to evoke a 1 mV test MEP. Twelve responses for each ISI and 12 responses to the test stimulus given alone were collected and averaged for a total of 120 trials for each of the four S1 intensities tested. To avoid fatigue in the active condition, short breaks were provided during each experimental block. The size of the conditioned MEPs are expressed as a percentage of the mean test MEP.
Experiment 3. Effects of different levels of muscle contraction on SICI
In this experiment the effects of three different levels of background EMG activity (10, 25 and 50% of MVC) on SICI were studied in eight subjects. For 10% MVC, the active motor threshold (AMT10%) was defined, as noted above, as the lowest stimulus intensity (% MSO) required to produce MEPs greater than 200 μV in at least 5 out of 10 consecutive stimuli. For 25% and 50% MVC, the AMT (AMT25% and AMT50%, respectively) was defined as the minimum stimulus intensity that produced at least five MEPs from 10 consecutive trials, with a peak-to-peak amplitude greater than the 95% confidence interval of the prestimulus mean EMG activity (Mills & Nithi, 1997). Based on the results of Expts 1 and 2, an S1 intensity of 70% AMT was used with ISIs of 1, 2 and 3 ms. Each block of stimulation consisted of 48 pulses (12 pulses each for the three ISIs and 12 pulses for the test alone) delivered in a randomized order. Three experimental sessions were performed. In the first (Expt 3a), SICI was evaluated at each ISI and at each level of contraction with the intensity of S1 and S2 adjusted to the level of muscle contraction. In particular, S1 was set at 70% of AMT10%, at 70% of AMT25% and at 70% of AMT50%, respectively; S2 was set to obtain a mean MEP of 1 mV peak to peak during FDI activation at 10% MVC (S21mV(10%)), at 25% MVC (S21mV(25%)) and at 50% MVC (S21mV(50%)). In the second and third experimental sessions the importance of the S1 and S2 dimension was further studied at a contraction level of 25% MVC (Expt 3b) and of 50% MVC (Expt 3c) by combining different S1 and S2 intensities. In more detail: in Expt 3b the contraction level was kept constant at 25% MVC while two S1 intensities (70% of AMT25% and 70% AMT10%) were combined with two S2 intensities (S21mV(25%) and S21mV(10%)); in Expt 3c the contraction level was kept constant at 50% MVC while two S1 intensities (70% of AMT50% and 70% AMT10%) were combined with two S2 intensities (S21mV(50%) and S21mV(10%)). A total of four conditions were evaluated in this way in Expts 3b and 3c. To avoid fatigue a break was given when needed.
Experiment 4. Effects of different S1 amplitudes on SICI at 2.5 ms ISI
Four subjects took part in this experiment which was aimed at testing, in the active state and at a 2.5 ms ISI, the influence of the test MEP amplitude on SICI. Requested background EMG activity was 10% MVC. S1 intensities were set at 70% and 90% AMT and S2 intensity was adjusted to obtain a mean test MEP of 0.2, 1, and 2 mV, respectively. A total amount of 24 pulses (12 conditioned and 12 unconditioned MEPs) were delivered in a randomized order for each block. A total of six blocks were collected. Conditioned MEP amplitude was expressed as a percentage of the test MEP induced by the S2 given alone.
Experiment 5. SICF at rest and during muscular activation
The same eight subjects studied in Expt 2 participated in two experimental sessions. In each experimental session both resting and active muscle conditions were studied. In the active state the level of contraction was 10% MVC. In the first experimental session (Expt 5a), the paired-pulse protocol described by Ziemann et al. (1998b) was applied to test SICF. In particular, S1 intensity was set to produce a MEP with a mean peak-to-peak amplitude of 1 mV when given alone, while the S2 intensity was set at 90% AMT. In the second experimental session (Expt 5b) a SICI-like protocol using an S1 pulse intensity set at 100% AMT and an S2 whose intensity was able to elicit a 1 mV MEP was applied. In each session, nine ISIs (ranging from 1 ms to 5 ms in 0.5 ms steps) were evaluated and a total of 120 pulses (12 pulses for each of the nine ISIs and 12 pulses for the MEP test alone) were delivered in a randomized order. The amplitude of the conditioned MEP was expressed as a percentage of the mean size of the test MEP evoked by the suprathreshold pulse (S1 in Expt 5a and S2 in Expt 5b) in the same block.
Experiment 6. Effects of different levels of muscle activation and of different S2 intensities on SICF
This last experiment was conducted in four of the subjects studied in Expt 5 to measure how muscular activity modulates the SICF system. Three levels of voluntary contraction (10, 25 and 50% MVC) were tested. On the basis of the results obtained in Expt 5 only four ISIs were evaluated (1, 1.5, 2 and 3 ms). The intensity of S1 was set to elicit an MEP of 1 mV amplitude and two subthreshold S2s were used (90% and 70% of AMT10%, respectively). Six blocks of 60 pulses each were collected.
Data analysis
Statistical analysis (repeated measures ANOVA) was performed using the software SPSS release 13 (SPSS Inc., Chicago, IL, USA). Compound symmetry was evaluated testing the sphericity with Mauchly's test. The Greenhouse-Geisser correction was used to compensate for non-spherical data. In the case of significant F values, post hoc analysis (Student's paired t test with Bonferroni correction for multiple comparisons) was applied. A P value < 0.05 was considered significant. All the values are expressed as means ± standard error of the mean (s.e.m.).
In Expt 1, firstly the effects of voluntary contraction and of different S1 intensities on SICI and ICF were tested with three-way ANOVA using MUSCLE STATES (resting and active), S1 INTENSITIES (60, 70, 80, 90% of AMT) and ISIs (2, 3, 10, 15 ms) as within-subject factors. Secondly, a two-way ANOVA was applied separately for the resting and active muscle state to determine, for each S1 INTENSITY, the significance of both the MEP inhibition at 2 and 3 ms ISIs and MEP facilitation at 10 and 15 ms ISIs in comparison with the test MEP. Then a two-way ANOVA (S1 INTENSITY × ISIs) was applied separately for the resting and active conditions, to detect which S1 intensity was able to induce the largest inhibition at 2 and 3 ms ISIs. Finally a three-way ANOVA comparing MUSCLE STATE (resting and active), S1 INTENSITIES (60, 70, 80, 90% of AMT) and SICI ISIs (2 and 3 ms) was performed to compare for each S1 intensity the total amount of inhibition between muscle states. In Expt 2 the time course was studied with three-way ANOVA using MUSCLE STATES (rest and active), S1 INTENSITIES (60, 70, 80, 90% of AMT) and ISIs (from 0.5 to 5 ms) as within-subject factors. Experiment 3a was analysed with a two-way ANOVA comparing three different LEVELS OF CONTRACTION (10, 25, 50% MVC) with ISIs (1, 2 and 3 ms). The importance of the S1 and S2 intensity at 25% MVC (Expt 3b) and at 50% MVC (Expt 3c) was assessed with a three-way ANOVA comparing LEVELS OF CONTRACTION, CONDITIONS (S1 and S2 COMBINATIONS) and ISIs. In Expt 4 two-way ANOVA was performed using S2 INTENSITIES (0.2, 1 and 2 mV), S1 INTENSITIES (70 and 90% of AMT) as within-subject factors. In Expt 5a the effects induced by muscle activity on SICF were evaluated with a two-way ANOVA comparing MUSCLE STATES (resting and active), and ISIs (from 1 to 5 ms). In Expt 6, a three-way ANOVA comparing S2 INTENSITY (70 and 90% AMT), LEVELS OF CONTRACTION (10, 25, 50% MVC) and ISIs (1, 1.5, 2 and 3 ms) was performed. Prestimulus EMG activities at each ISI and among S1 intensities were compared using repeated measures ANOVA in all experiments where the FDI was activated.
Results
MEPs were recorded from the right FDI of all 16 subjects. The mean S2 intensity used to elicit a 1 mV test MEP was 52.6 ± 3.1% of MSO at rest and 44 ± 2.5% during muscle contraction at 10% MVC. RMT was 41 ± 1.8% while AMT was 34.4 ± 1.9%.
Experiment 1. Effects of different intensities of the conditioning stimulus (S1) on SICI and ICF at rest and during voluntary contraction
With the muscle at rest, there was significant inhibition (SICI) of the conditioned MEP at ISIs = 2 or 3 ms if the intensity of the conditioning stimulus (S1) was 70, 80 or 90% AMT. The strongest suppression occurred when S1 = 90% AMT; there was no suppression at S1 = 60% AMT. Facilitation (ICF) was present at ISI = 10 or 15 ms, particularly with S1 = 80 or 90% AMT (Fig. 1A). During contraction at 10% MVC, there was no ICF, and SICI was strongest with S1 = 70% AMT (Fig. 1B). There was no significant variation in either AMT or the intensity of stimulation able to evoke a 1 mV test MEP (expressed in percentage of MSO) between the two testing sessions.
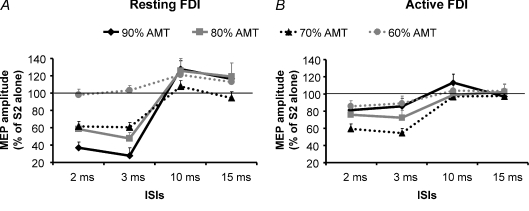
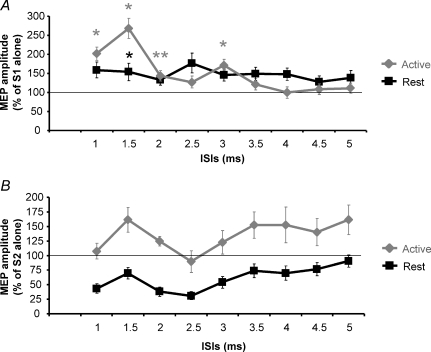
Figure 1. SICI and ICF assessed in the cortical representation of the first dorsal interosseus (FDI) muscle in resting and active conditions.
Mean data obtained from 16 subjects are reported. The effects of four different intensities of the conditioning stimulus (S1) (90% AMT (♦), 80% AMT (▪), 70% AMT (▴), 60% AMT (•)) on the size of the conditioned MEP were evaluated in the FDI muscle relaxed (A) and activated at 10% MVC (B). At rest the best inhibition was obtained delivering a S1 of 90% AMT while during contraction the most efficacious S1 was 70% AMT. At ICF ISIs the highest S1 intensity was able to induce a significant facilitation only in the relaxed muscle. The abscissa indicates interstimulus intervals (ISIs) while the ordinate indicates mean MEP amplitude expressed as a percentage of the S2 given alone. The thin horizontal lines indicate the 100% level. Error bars represent s.e.m.
These conclusions were confirmed in the statistical analysis. A three-way ANOVA comparing MUSCLE STATE (resting and active), S1 INTENSITIES (60, 70, 80, 90% of AMT) and ISIs (2, 3, 10 and 15 ms) showed a significant effect of S1 INTENSITY (F(3,45)= 5, P = 0.005) and of ISI (F(2.7,41.2)= 61.4, P < 0.0001) and a significant S1 INTENSITY × ISI × MUSCLE STATE interaction (F(5.4,80.4)= 6.6, P < 0.0001). We therefore analysed the resting and active data separately with two-way ANOVAs. At rest, there was a significant effect of S1 INTENSITY (F(3,45)= 7.6, P = 0.001) and ISI (F(4,60)= 63.4, P < 0.0001) and a significant S1 INTENSITY × ISI interaction (F(4,73.8)= 13.2, P < 0.0001). Post hoc analysis showed significant inhibition at ISI = 2 ms (P < 0.0001) and 3 ms (P < 0.0001) for S1 intensities from 90% to 70% AMT. There was more inhibition for S1 = 90% AMT than at 80% (P = 0.003), 70% (P < 0.0001) or 60% (P < 0.0001) AMT. A significant facilitation was found only with S1 = 90% AMT (10 ms: P = 0.006; 15 ms: P = 0.03). A two-factor ANOVA in the active muscle showed a significant effect of S1 INTENSITY (F(2,30.5)= 3.4, P = 0.04) and ISI (F(2.3,33.9)= 13, P < 0.0001) but no significant interaction (P = 0.08) between them. Post hoc analysis showed significant inhibition of the conditioned MEP at ISI = 2 ms (P < 0.0001) and 3 ms (P < 0.0001) only when the S1 intensity was 70% AMT. The amount of inhibition with S1 = 70% AMT was also significantly different from inhibition at S1 = 90% (P = 0.01), 80% (P = 0.04) and 60% (P = 0.0002).
In a final analysis we conducted a three-way ANOVA (MUSCLE STATES × S1 INTENSITIES × ISIs) on the same data using only ISI = 2 and 3 ms. This showed a significant effect of MUSCLE STATE (F(1,55)= 5.2, P = 0.04), S1 INTENSITY (F(1.4,20.9)= 21.5, P < 0.0001) and a significant MUSCLE STATE × ISI × S1 INTENSITY interaction (F(1.9,28.9)= 20.4, P < 0.0001). Post hoc analysis showed that SICI was significantly different between muscle states with S1 = 90% AMT (2 msrestversus 2 msactive: P < 0.0001; 3 msrestversus 3 msactive: P < 0.0001) or 80% AMT (2 msrestversus 2 msactive: P = 0.04; 3 msrestversus 3 msactive: P = 0.01) but there was no significant difference in SICI between resting and active states when S1 = 70% AMT. A repeated measures ANOVA during muscular activation revealed no differences in prestimulus EMG activity at each ISI or S1 intensity.
Experiment 2. Detailed time course of SICI at rest and during voluntary contraction
Experiment 1 was repeated focusing on the detailed time course of SICI from ISI = 1–5 ms in steps of 0.5 ms. In confirmation of the previous results, at rest (Fig. 2A) the largest inhibition was produced by the highest intensity S1 (90% AMT), being maximal at ISI = 1 and 2.5 ms. SICI was less marked for S1 = 70 or 80% and absent (apart from ISI = 1 ms) at 60% AMT. During voluntary contraction (Fig. 2B), the largest SICI occurred with S1 = 70% AMT gradually decreasing for S1 = 80 or 90% AMT.
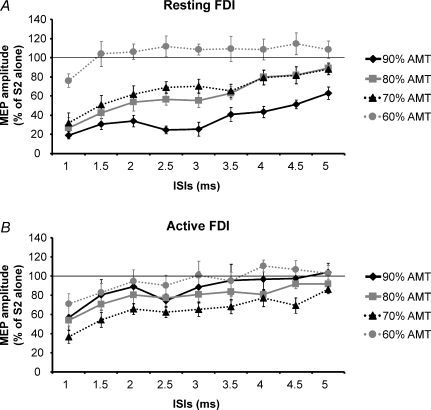
Figure 2. Time course of SICI studied in the resting FDI (A) and during slight (10% MVC) muscular contraction (B).
Data were obtained from 8 subjects and four intensities of the conditioning stimulus (S1) (90% AMT (♦), 80% AMT (▪), 70% AMT (▴), 60% AMT (•)) were used. S2 was set to induce a test MEP of 1 mV peak to peak. At rest the inhibition was bigger with a S1 of 90% AMT at all ISIs tested, the deepest inhibition being observed at 1 and 2.5 ms. During contraction, the 70% AMT was confirmed to be the optimal S1 intensity to obtain the maximal SICI. Ordinate indicates mean conditioned MEP amplitude expressed as a percentage of the test MEP amplitude, taken as 100% (thin horizontal line) and abscissa reports ISIs. Error bars represent s.e.m.
A three-way ANOVA comparing MUSCLE STATES (resting and active), S1 INTENSITIES (60, 70, 80, 90% of AMT) and ISIs (from 0.5 to 5 ms) showed a significant effect of MUSCLE STATE (F(1,7)= 11.7, P < 0.01), S1 INTENSITY (F(3,21)= 20.5, P < 0.0001) and ISI (F(9,63)= 31.2, P < 0.0001) and a significant interaction between all three variables (F(27,189)= 1.8, P = 0.01). To highlight how MUSCLE STATE changes the effect of INTENSITY on SICI, we have replotted the data at S1 = 70 and 90% AMT in Fig. 3. With S1 = 90% AMT there is much greater SICI at rest than when active. In contrast, there is no difference between states with S1 = 70% AMT.
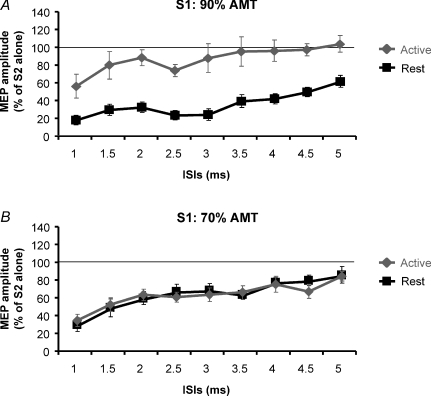
Figure 3. Comparison of SICI induced by a conditioning stimulus of 90% AMT (A) and of 70% AMT (B) in the resting and active conditions.
Note that while with a S1 of 90% AMT there was a big discrepancy in SICI curves between these two muscular conditions, when 70% AMT was used, the two curves overlapped. Abscissa indicates interstimulus intervals (from 1 to 5 ms in steps of 0.5 ms). MEP size after paired stimulation is expressed as a percentage of the control MEP in the ordinate. The thin horizontal line indicates the 100% level. Error bars represent s.e.m.
Experiment 3. Effects of different levels of muscle contraction on SICI
In the first experimental session (Expt 3a) the absolute intensities of the test and conditioning stimuli were adapted to the level of muscle activation (10, 25, 50% MVC). Thus, the mean S2 intensity used to evoke a test MEP of 1 mV peak to peak was 41 ± 2.5% (S21mV(10%)), 37 ± 2.6% (S21mV(25%)) and 31 ± 2.3% (S21mV(50%)), respectively. The intensity of S1 = 70% AMT; AMT values were 32 ± 2.2% (AMT10%), 29 ± 2.1% (AMT25%) and 25 ± 1.8% (AMT50%). The mean prestimulus EMG activities were 63.2 ± 2.9 μV, 93.8 ± 4.3 μV and 154.3 ± 6.5 μV at 10, 25 and 50% MVC, respectively.
The data in Fig. 4 show that inhibition was present at 10% MVC but not at higher levels of contraction (apart from ISI = 1 ms at 25% MVC). A two-way ANOVA comparing the three different LEVELS OF CONTRACTION at the three ISIs showed a significant effect of CONTRACTION LEVEL (F(1.2,8)= 15.6, P = 0.0003) and ISI (F(3,21)= 9.7, P = 0.0003) and a significant interaction between these two factors (F(6,42)= 9.7, P < 0.0001). Post hoc analysis with Bonferroni correction revealed that inhibition of the conditioned MEP observed when the muscle was activated at 10% MVC was different from that observed at contraction levels of 25% MVC (P = 0.003) and of 50% MVC (P = 0.0004) and that SICI was significant at all ISIs tested (1 ms: P = 0.002; 2 ms: P = 0.02; 3 ms: P = 0.01). On the contrary, at 25% MVC the inhibition of the conditioned MEP was significant only at 1 ms ISI (P = 0.04), while no significant inhibition was observed at any ISI when muscle activation was 50% MVC.
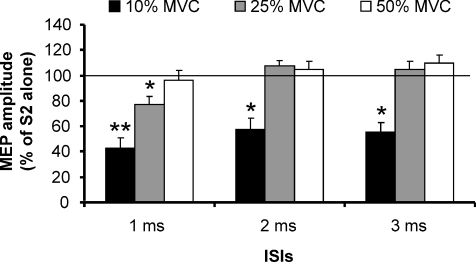
Figure 4. Effects of different levels of muscle contraction on SICI obtained delivering a S1 of 70% AMT.
Three levels of contraction (10% MVC, black columns; 25% MVC, grey columns; 50% MVC, white columns) were evaluated in 8 subjects. A significant inhibition of the conditioned MEP was observed during the lowest level of contraction in all the ISIs tested (1, 2 and 3 ms). During 25% MVC a significant inhibition was observed only at 1 ms ISI. During strong contraction (50% MVC) no inhibition of the conditioned MEP was observed. On the ordinate the conditioned MEP amplitude is expressed as a percentage of the control MEP. The thin horizontal line indicates the 100% level. Error bars represent s.e.m. Asterisks indicate significant differences (*P < 0.05; **P < 0.01).
In order to test whether the lack of effect at higher contraction strengths was the result of changing the stimulus intensities compared with testing at 10% MVC we conducted two control experiments. In Expt 3b (results not shown) the contraction level was 25% MVC. Two different S1 intensities (70% of AMT25%= 20 ± 1.5% and 70% AMT10%= 23 ± 1.5%) were combined with two S2 intensities (S21mV(25%)= 37 ± 2.6%, which evoked an MEP of 1.22 ± 0.04 mV; and S21mV(10%)= 41 ± 2.5%, which evoked an MEP of 2.66 ± 0.17 mV). In Expt 3c (results not shown) the contraction level was 50% MVC and the intensities of S1 were 70% of AMT50%= 18 ± 1.2% and 70% AMT10%= 23 ± 1.5%. S2 intensities were: S21mV(50%)= 31 ± 2.3%, which evoked an MEP of 1.28 ± 0.08 mV; and S21mV(10%)= 41 ± 2.5%, which evoked an MEP of 4.9 ± 0.04 mV. A three-way ANOVA comparing two CONTRACTION LEVELS, four CONDITIONS (S1 and S2 intensities) and three ISIs showed a significant effect of CONTRACTION LEVELS (F(1,7)= 16.3, P = 0.005) and of ISIs (F(3,21)= 15.9, P = 0.0001). No effects of the different S1 and S2 intensities (CONDITIONS) were observed.
Experiment 4. Effects of different test MEP amplitudes on SICI at 2.5 ms ISI in active muscle
With the smallest test MEP used (0.2 mV) no inhibition was seen either with S1 = 70% AMT or with S1 = 90% AMT. With 1 mV and 2 mV test MEP, both S1 intensities were able to inhibit MEPs particularly with S1 = 70% AMT. Two-way ANOVA (S2 INTENSITY × S1 INTENSITIES × ISI showed a significant effect of S2 INTENSITY (F(1,3)= 20.7, P = 0.02).
Experiment 5. SICF at rest and during muscular activation
In Expt 5a SICF was evoked using an S1 intensity that when given alone evoked a 1 mV MEP whilst S2 was set at 90% AMT. Figure 5A shows that with the muscle at rest, there was little facilitation at any interval, whereas there were two peaks when SICF was tested during muscle contraction. This was confirmed in the two-way ANOVA with main factors of MUSCLE STATE (resting and active), and ISIs (from 1 to 5 ms). There was a significant main effect of ISIs (F(9,63)= 12.6, P < 0.0001) and a significant MUSCLE STATE × ISIs interaction (F(9,63)= 10, P < 0.0001). A post hoc analysis comparing test MEP amplitude and conditioned MEP amplitude at all ISIs was then performed separately for resting and active conditions. This analysis showed significant facilitatory peaks during muscular contraction (1 ms: P = 0.03; 1.5 ms: P = 0.02; 2 ms: P = 0.01; 3 ms: P = 0.04) but not when the muscle was kept at rest. A second post hoc analysis performed to compare the amplitudes of the conditioned MEPs obtained in the resting and active conditions showed a significant difference only at 1.5 ms ISI (resting versus active: P = 0.02).
Figure 5. SICF assessed in the cortical representation of the first dorsal interosseus (FDI) muscle in resting and active conditions.
A, SICF mean data obtained from 8 subjects keeping the FDI relaxed (black line) or during slight muscular contraction at 10% MVC (grey line). In both conditions, S1 intensity was set to produce a MEP with a mean peak-to-peak amplitude of 1 mV when given alone, while S2 intensity was set at 90% AMT. Only during muscle contraction were significant facilitatory peaks observed (1, 1.5, 2 and 3 ms, respectively). On the contrary no significant peaks were seen at rest. Grey asterisks indicate that in the active state the conditioned MEP was significantly (*P < 0.05; **P < 0.01) bigger than the test MEP (S1). The black asterisk indicates that at 1.5 ms ISI the conditioned MEPs obtained in the resting and active conditions were significantly different (P < 0.05). B, in the same subjects a paired-pulse stimulation in which the S1 stimulus intensity was set at 100% AMT and the S2 at an intensity able to evoke 1 mV MEP amplitude was delivered at rest and during contraction of the FDI at 10% MVC. At rest (black line) the conditioned MEP was inhibited at all ISIs, while during contraction a facilitation was observed at all ISIs, apart from 1 and 2.5 ms. ISIs are reported in abscissa; ordinate indicates mean MEP amplitude expressed as percentage value of the suprathreshold pulse alone (S1 in experiment in A and S2 in experiment in B); thin horizontal lines indicate the 100% level and error bars represent s.e.m.
For comparison, Expt 5b used a typical SICI protocol at the same ISIs with an S1 pulse of 100% AMT preceding a S2 stimulus that, when given alone, evoked a test MEP of 1 mV peak to peak. Inhibition was seen only when the muscle was at rest, while a clear facilitation was observed during muscle contraction at all the ISIs tested except for 1 and 2.5 ms ISIs (Fig. 5B).
Experiment 6. Effects of different levels of muscle activation on SICF
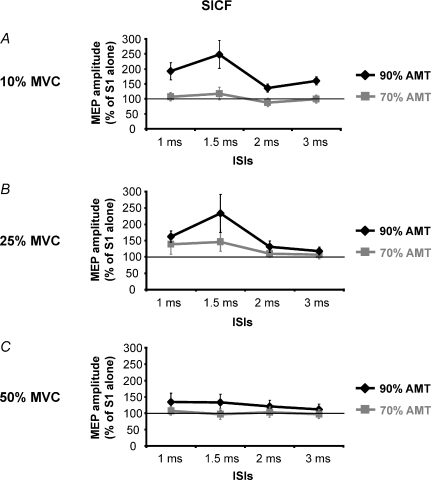
In this experiment S1 intensity was set at a value able to induce a 1 mV MEP, while two intensities of S2 (70% and 90% of AMT) were used at those ISIs (1, 1.5, 2 and 3 ms) where significant facilitation was seen in Expt 5a. Figure 6 shows how the level of muscular activity from 10 to 50% MVC modulated SICF. There was no facilitation at any level (10%, 25%, 50% MVC) of muscle contraction when S2 = 70% AMT. However, when S2 = 90% AMT, MEPs were facilitated at ISI = 1 and 1.5 ms for contractions at 10 and 25% MVC, but not at 50% MVC. Three-way ANOVA (S2 INTENSITY × CONTRACTION LEVELS × ISIs) showed a significant effect of ISIs (F(4,12)= 5.5, P = 0.009) and a significant interaction between S2 INTENSITY × ISIs (F(4,12)= 9.1, P = 0.001) and CONTRACTION LEVELS × ISIs (F(8,24)= 2.3, P = 0.48).
Figure 6. Effects of different levels of contraction on SICF.
Those ISIs where significant facilitatory peaks were previously found (Expt 5a, Fig. 6) were tested in four subjects. Three contraction levels (A: 10% MVC; B: 25% MVC; C: 50% MVC) were evaluated. For each of these levels of contraction, two S2 intensities, 90% AMT (black line) and 70% AMT (grey line), were evaluated. S1 was kept constant at 1 mV. Only using a S2 of 90% AMT was a MEP facilitation seen at 10% and 25% MVC. This facilitation was gradually reduced in amplitude with increasing contraction level and disappeared at 50% MVC. With a S2 of 70% AMT no significant facilitation was observed at any contraction level. Abscissa reports ISIs; the ordinate reports conditioned MEP amplitude which is expressed as a percentage value of the control MEP (S1). The thin horizontal line indicates the 100% level. Error bars represent s.e.m.
Discussion
It is well known that SICI and ICF are reduced during voluntary muscle contraction compared to rest (Ridding et al. 1995; Fisher et al. 2002; Roshan et al. 2003). The present work confirms this, but only for S1 ≥ 80% AMT. SICI evoked by S1 = 70% AMT was the same at rest as during activity. We also found that the recruitment of SICF was enhanced during weak voluntary contraction: there was no SICF when S2 = 90% AMT at rest, whereas it was clear during a 10% MVC. We argue that when an S1 intensity ≥ 80% AMT is used and the target muscle is active, SICF is recruited at the same time as SICI and this may contribute to the reduced inhibition that is usually observed.
Importance of a low intensity conditioning pulse (S1) in the assessment of SICI during muscle activation
SICI is usually evaluated with S1 = 80–90% AMT or 80% RMT (Kujirai et al. 1993; Ziemann et al. 1996c) and S2 at an intensity that when given alone elicits a test MEP with a mean amplitude of 1 mV peak to peak (Kujirai et al. 1993; Sanger et al. 2001; Roshan et al. 2003). In addition, SICI is studied with the target muscle relaxed because during active contraction, SICI is markedly reduced or abolished (Ridding et al. 1995; Fisher et al. 2002; Roshan et al. 2003; Jaberzadeh et al. 2007). Many mechanisms may contribute to the reduction of SICI during muscle activation, including superimposition of additional excitatory effects or reduced importance of later I waves in evoking MEP activation (Ridding et al. 1995; Hanajima et al. 1998; Reynolds & Ashby, 1999; Ridding & Rothwell, 1999; Zoghi et al. 2003). However, if the amount of inhibition is indeed reduced during contraction then it has been speculated that it could be involved in the ‘fractionation’ of muscular activity, by reducing inhibitory influences on the contracting muscle whilst maintaining inhibition on non-contracting muscles (Ridding et al. 1995; Reynolds & Ashby, 1999; Zoghi et al. 2003; Zoghi & Nordstrom, 2007).
In subjects at rest, our data confirm much existing literature. The largest SICI was induced by the highest intensity conditioning pulse that we used (S1 = 90% AMT) and the same held true for ICF (Figs 1A and 2A). SICI was more prominent at 1 ms and 2.5 ms, confirming the presence of two phases of SICI, as previously described (Fisher et al. 2002; Roshan et al. 2003; Hanajima et al. 2003). In active muscle we found that SICI could be evoked when S1 = 70% AMT and that the amount of inhibition was the same as in resting muscle. Interestingly, a similar result occurred in experiments of Zoghi et al. (2003; Fig. 7) comparing SICI at ISI = 3 ms at rest and during muscular activity. They found that when the intensity of the test stimulus was adapted to match the size of the control MEP in the resting and active (force level = 3 N) states, a S1 of 70% AMT induced the same amount of inhibition in the two different conditions. In contrast, Ridding et al. (1995) had reported the best S1 intensity to get SICI in active muscle was 90 or 95% AMT and that S1 = 70% AMT had no effect. A likely reason for this is the small number (n= 2) of people in whom different intensities were tested by those authors. In fact, three of our subjects exhibited the largest SICI with S1 = 80% or 90% AMT both in the resting and active states; similar inter- and intra-individual variability of the SICI system was reported in relaxed muscle by Orth et al. (2003). A result more comparable to ours was reported by Ilic et al. (2002) who examined different S1 and S2 intensities at an ISI of 1.5 ms in active muscle. They found that when the S2 was set at an intensity of 130% AMT (which is comparable to ours), there was inhibition when S1 = 70% AMT although in their data this was equal to that induced by S1 = 90% AMT.
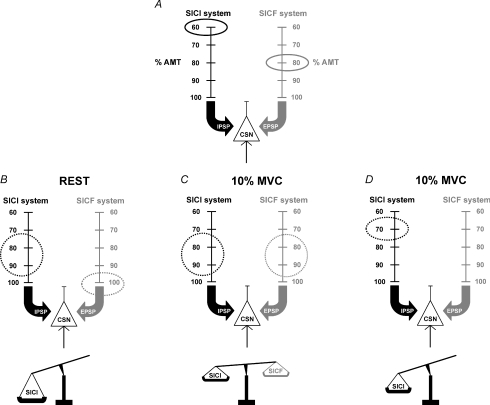
Figure 7. The model summarizes the effects of volitional contraction and of the intensity of the conditioning stimulus on SICI and SICF assessed in the FDI motor cortex.
A, SICI inhibitory system (black) and SICF facilitatory system (grey) exert their influence on corticospinal neurons (CSN) through the production of IPSPs and EPSPs, respectively. Threshold of both systems, expressed in percentage of AMT was 60% for SICI and 80% for SICF. B, at rest the S1 optimal intensity to obtain maximal SICI was 80% or 90% AMT while for SICF it was around 100% AMT. When the classical SICI paired-pulse protocol was applied using a S1 of 80–90%, only the SICI system was activated, because this intensity was too weak to activate the SICF system. Then a strong pure SICI effect was obtained in this condition. C, during slight contraction (10% MVC) the SICF system was facilitated; then it was susceptible to being affected at lower intensity. A S1 stimulus of 80–90% AMT was then able to activate both SICI and SICF systems. Inhibitory effects on the CSN are antagonized by the facilitatory ones thus resulting in little change in the conditioned MEP amplitude, with a slight preponderance of inhibition. D, during the same level of contraction (10% MVC) the delivery of a S1 of 70% AMT induced only the activation of the SICI system because it was not strong enough to simultaneously activate the SICF system, even if the latter was facilitated by muscular activity. A pure SICI effect was then obtained. The absence of activation of the antagonistic facilitatory system during contraction allowed the same entity of inhibition in the resting and active conditions to be obtained (see text for more details).
Fisher et al. (2002) showed that at an ISI of 1 ms the largest inhibition of the conditioned MEP in active muscle was obtained using a low intensity S1 (22% MSO) whereas a higher intensity S1 was needed (30% MSO) in the resting state. These intensity values are comparable to ours: 70% AMT = 24% MSO and 90% AMT = 31% MSO in the present experiments. These authors did not find any inhibition at ISI = 2.5 ms during voluntary contraction. However, this is likely to be due to the low intensity (0.2 mV) of the test stimulus they used. Indeed, our Expt 4 showed that when the amplitude of the test MEP was set at 0.2 mV there was no SICI with S1 = 90% or 70% AMT, whereas inhibition was clear with larger test MEPs (1 and 2 mV). It seems likely that at an MEP of 0.2 mV in active muscle is produced without recruitment of later I waves by the test stimulus. Since these are the ones targeted by SICI, no inhibition can be observed. The preferential effect of SICI on late I waves probably accounts for the results of Expt 3 in which SICI was present only at 10% MVC whereas it was abolished at 25% and 50% MVC. Zoghi & Nordstrom (2007) also noted that there was no reduction of SICI at low contraction levels, even though the effect was clear during strong contractions. Di Lazzaro et al. (1998c) showed that at high levels of muscle contraction, I waves were facilitated. We presume that at 25% and 50% MVC this facilitation allowed a 1 mV MEP to be evoked by early I waves only, whereas at 10% MVC some late I waves were still necessary so that SICI could still be observed. Interestingly, SICI at ISI = 1 ms was present at both 10% and 25% MVC, suggesting that its mechanism differs from SICI at longer intervals (Fisher et al. 2002).
Finally, we note that the present FDI data are in agreement with those recently obtained in active masseter muscles (Ortu et al. 2008) where among different S1 intensities, the only intensity able to inhibit the conditioned MEP was 70% AMT.
Interaction of SICI and SICF
At short ISIs (1–5 ms) two types of interaction can be observed between pairs of TMS pulses, SICI and SICF. SICF occurs at discrete interstimulus intervals of about 1.1–1.5 ms, 2.3–2.9 ms and 4.1–4.4 ms. Since the interpeak latencies between these facilitatory intervals is about 1.5 ms, approximately the same as that observed between I waves recorded in the corticospinal tract, it is thought that SICF originates in the same neural elements responsible for the generation of the I waves (Tokimura et al. 1996; Ziemann et al. 1998b). SICI and SICF appear to be independent systems. Single motor unit (SMU) studies and epidural cervical recordings have shown that SICI reflects the inhibition of I3 waves (Di Lazzaro et al. 1998a,b; Hanajima et al. 1998; Ilic et al. 2002) while SICF reflects the facilitation of I1 waves (Hanajima et al. 2002) or I2 waves (Ilic et al. 2002). SICI is thought to be a GABAergic effect whereas SICF is thought to be glutamatergic.
A number of observations indicate that SICI and SICF interact. Neuropharmacological data show that some glutamate antagonists enhance SICI (Ziemann et al. 1998a; Schwenkreis et al. 1999, 2000) and that GABAA agonists reduce SICF (Ziemann et al. 1996a, 1998c; Ilic et al. 2002). A systematic variation of S1 and S2 intensities can produce a transition from inhibition to facilitation and vice versa (Awiszus et al. 1999; Fisher et al. 2002; Ilic et al. 2002). In subjects at rest, SICI can be evoked with S1 = 60% AMT whereas SICF is only activated with S1 = 80% AMT (Ilic et al. 2002). Thus, in the resting condition the usual S1 = 80–90% AMT activates only or mostly the SICI system and the net result on the corticospinal neuron output is a relatively pure inhibition.
Our present data show that the recruitment of SICF is facilitated during slight voluntary contraction (10% MVC), but less so or not at all at 25% and 50% MVC. Thus S2 = 90% AMT produced SICF during 10% MVC but not at rest or at 50% MVC. As noted above, voluntary contraction increases the excitability of cortical I wave circuits, particularly at high levels of contraction. The initial facilitation at 10% MVC, is likely to be due to subthreshold depolarization of the interneurones mediating SICF. The reason for the gradual disappearance at 25% and 50% MVC is more speculative. One possibility is that at high contraction levels there is so much activity in the SICF circuit that a ‘busy-line’ phenomenon occurs, making SICF impossible to observe.
Conclusions
The effects of voluntary contraction on SICI are complex. At low contraction levels, SICI with S1 ≥ 80% AMT is reduced compared to rest by a superimposed recruitment of SICF. However, at higher contraction strengths the reduced SICI must involve another mechanism. As suggested by Hanajima et al. (1998), a likely contributing factor is reduced involvement of late I waves in generating 1 mV MEPs at ≥ 25% MVC. Indeed increasing the test stimulus intensity to produce larger MEPs tends to restore a small amount of SICI. Finally, because of the threshold difference between activation of SICI and SICF circuits, ‘pure’ SICI can be studied with S1 = 70% AMT. At these intensities, SICI is unaffected by weak voluntary contraction. Recently Zoghi & Nordstrom (2007) observed no difference in the amount of SICI at rest and during small contractions using an S1 intensity (80% of AMT) slightly higher than ours but with a lower level of muscle contraction (2–5% MVC). Again, this supports the idea that a combination of contraction level and S1/S2 intensities may critically affect the balance between SICI and SICF circuits.
The model illustrated in Fig. 7 summarizes this relationship in more detail. The final output of the corticospinal neuron is influenced by IPSPs and EPSPs generated, respectively, by inhibitory and excitatory cortical interneurons in the SICI and SICF circuits (Fig. 7A). The vertical scales show S1 intensity values expressed as percentage of the AMT and the circled values are the lowest intensities which have been demonstrated (Ilic et al. 2002) to be able to activate SICI (60% AMT) and SICF (80% AMT) systems. In the resting condition (Fig. 7B) the optimal S1 intensity inducing the largest SICI is 80–90% AMT (Kujirai et al. 1993; Ziemann et al. 1996c; Ilic et al. 2002) while that inducing the largest SICF is around 100% AMT (Tokimura et al. 1996) or higher (Ziemann et al. 1998b). In this state, the commonly used S1 intensity of 80–90% AMT is effective in activating the SICI system but subthreshold for the SICF system and the net effect is inhibition. During slight contraction (Fig. 7C) the recruitment of the SICF system is more efficient. In this condition S1 = 80–90% AMT is able to activate both SICI and SICF. The result is that SICI is reduced by concomitant SICF. If the intensity of S1 is reduced to 70% (Fig. 7D) SICI is activated in the absence of SICF.
In conclusion our study demonstrates that (1) the reduction of SICI during contraction in comparison with rest may not be entirely due to reduction in cortical GABAergic inhibition but also to superimposition of a concurrent facilitation (SICF) that is recruited during muscle contraction; (2) at low contraction levels, low intensity (70% AMT) conditioning stimuli can test SICI independently of effects on SICF. The application of this novel finding in studies comparing SICI during a small voluntary effort and rest may provide a less contaminated measure than those recorded at higher stimulus intensities. For instance, it may be especially helpful in the study of those movement disorders in which it is not possible to maintain a complete muscular relaxation.
Acknowledgments
The authors are very grateful to Mr Richard Symonds and Mr Peter Owbridge for their valuable technical assistance. E. Ortu was supported by grants from the Regione Autonoma della Sardegna (Master and Back Program). The work was funded by the Medical Research Council.
References
- Awiszus F, Feistner H, Urbach D, Bostock H. Characterisation of paired-pulse transcranial magnetic stimulation conditions yielding inhibition or I-wave facilitation using a threshold-hunting paradigm. Exp Brain Res. 1999;129:317–324. doi: 10.1007/s002210050901. [DOI] [PubMed] [Google Scholar]
- Chen R, Garg R. Facilitatory I wave interaction in proximal arm and lower limb muscle representations of the human motor cortex. J Neurophysiol. 2000;83:1426–1434. doi: 10.1152/jn.2000.83.3.1426. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol. 1998a;109:397–401. doi: 10.1016/s0924-980x(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998b;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol. 1998c;508:625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Furubayashi T, Iwata NK, Shiio Y, Okabe S, Kanazawa I, Ugawa Y. Further evidence to support different mechanisms underlying intracortical inhibition of the motor cortex. Exp Brain Res. 2003;151:427–434. doi: 10.1007/s00221-003-1455-z. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK, Kanazawa I. Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans. J Physiol. 2002;538:253–261. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaberzadeh S, Pearce SL, Miles TS, Turker KS, Nordstrom MA. Intracortical inhibition in the human trigeminal motor system. Clin Neurophysiol. 2007;118:1785–1793. doi: 10.1016/j.clinph.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KR, Nithi KA. Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve. 1997;20:570–576. doi: 10.1002/(sici)1097-4598(199705)20:5<570::aid-mus5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 2003;114:2362–2369. doi: 10.1016/s1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- Ortu E, Deriu F, Suppa A, Giaconi E, Tolu E, Rothwell JC. Intracortical modulation of cortical-bulbar responses for the masseter muscle. J Physiol. 2008;586:3385–3404. doi: 10.1113/jphysiol.2008.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology. 1999;53:730–735. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Afferent input and cortical organisation: a study with magnetic stimulation. Exp Brain Res. 1999;126:536–544. doi: 10.1007/s002210050762. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. Report of an IFCN committee. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:97–103. The International Federation of Clinical Neurophysiology. [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenkreis P, Liepert J, Witscher K, Fischer W, Weiller C, Malin J-P, Tegenthoff M. Riluzole suppresses motor cortex facilitation in correlation to its plasma level. Exp Brain Res. 2000;135:293–299. doi: 10.1007/s002210000532. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Witscher K, Janssen F, Addo A, Dertwinkel R, Zenz M, Malin JP, Tegenthoff M. Influence of the N-methyl-d-aspartate antagonist memantine on human motor cortex excitability. Neurosci Lett. 1999;270:137–140. doi: 10.1016/s0304-3940(99)00492-9. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998a;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996a;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996b;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between cortical inhibition and facilitation in human motor cortex. J Physiol. 1996c;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998b;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wischer S, Hildebrandt J, Paulus W. Pharmacological control of facilitatory I-wave interaction in the human motor cortex. Electroencephalogr Clin Neurophysiol. 1998c;109:321–330. doi: 10.1016/s0924-980x(98)00023-x. A paired transcranial magnetic stimulation study. [DOI] [PubMed] [Google Scholar]
- Zoghi M, Nordstrom MA. Progressive suppression of intracortical inhibition during graded isometric contraction of a hand muscle is not influenced by hand preference. Exp Brain Res. 2007;177:266–274. doi: 10.1007/s00221-006-0669-2. [DOI] [PubMed] [Google Scholar]
- Zoghi M, Pearce SL, Nordstrom MA. Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle. J Physiol. 2003;550:933–946. doi: 10.1113/jphysiol.2003.042606. [DOI] [PMC free article] [PubMed] [Google Scholar]