Abstract
Local control of ATP/ADP ratio is essential for efficient functioning of cellular ATPases. Since creatine kinase (CK) activity and mitochondrial content are reduced in heart failure (HF), and cardiomyocyte ultrastructure is altered, we hypothesized that these changes may affect the local energetic control of two major cardiac ATPases, the sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA) and the myosin ATPase. Heart failure was induced by aortic stenosis in rats. Electron microscopy confirmed that failing cardiomyocytes had intracellular disorganization, with fewer contacts between mitochondria and myofibrils. Despite normal SERCA protein content, spontaneous Ca2+ release measurements using Fluo-4 on saponin-permeabilized cardiomyocytes showed a lower SR loading in HF even when endogenous CK and mitochondria were fully activated. Similarly, in permeabilized fibres, SR Ca2+ loading supported by SR-bound CK and mitochondria was significantly reduced in HF (by 49% and 40%, respectively, 43% when both systems were activated, P < 0.05). Alkaline phosphatase treatment had no effect, but glycolytic substrates normalized calcium loading in HF to the sham level. The control by CK and mitochondria of the local ATP/ADP ratio close to the myosin ATPase (estimated by rigor tension) was also significantly impaired in HF fibres (by 32% and 46%, respectively). However, while the contributions of mitochondria and CK to local ATP regeneration were equally depressed in HF for the control of SERCA, mitochondrial contribution was more severely impaired than CK (P < 0.05) with respect to myofilament regulation. These data show that local energetic regulation of essential ATPases is severely impaired in heart failure, and undergoes a complex remodelling as a result of a decreased activity of the ATP-generating systems and cytoarchitecture disorganization.
Calcium cycling defects greatly contribute to cardiomyocyte dysfunction in heart failure (HF). A reduced calcium transient is typically observed in heart failure, and results from a reduced amount of calcium stored in the sarcoplasmic reticulum (SR). This is classically thought to be due to a reduced SR-ATPase (SERCA) function and/or enhanced calcium leak through ryanodine receptors (RyRs) (Marx et al. 2000; Bers, 2006; Belevych et al. 2007).
However, a number of additional factors may be involved in cardiac dysfunction, such as dysfunction of the contractile machinery, cytoarchitectural disorganization and energetic starvation. Data from several laboratories, including our own (Garnier et al. 2003; Ingwall & Weiss, 2004; Ventura-Clapier et al. 2004), indicate that ‘energy deficiency’ occurs in, and contributes to, many pathophysiological processes. Heart muscle is among the highest ATP consumer organs. It has the highest content of mitochondria and an efficient system of energy transfer kinases which ensure that energy consumption by ATPases is exactly matched by energy production. Failing hearts have respective reductions in the activity and content of creatine kinase and mitochondria, which correlate with reduced working capacity (De Sousa et al. 1999; Dzeja et al. 2000; Ventura-Clapier et al. 2004; Ingwall & Weiss, 2004). Among other pathological processes, the engagement of the SR Ca2+-ATPase pump in a futile cycle to maintain SR Ca2+ load against a large ryanodine receptor-mediated leak in HF is costly in terms of ATP consumption (Bers, 2006). Energy deficiency may therefore play a significant role in the pathogenesis of heart failure. Indeed, changes in energy charge (PCr/ATP ratio), which occur in the failing myocardium, represent a more accurate mortality prognosis than New York Heart Association class or ejection fraction (Neubauer et al. 1997).
The critical match between energy supply and consumption does not take place in a bulk phase. The adult cardiomyocyte is a highly organized cell with a crowded cytoplasm mainly composed of metabolically active and structured organelles that create microdomains at their boundaries with restricted diffusion and direct channelling of substrates to enzymes (Ventura-Clapier et al. 1998; Saks et al. 2006; Weiss et al. 2006). The efficient functioning of myofibrils and sarcoplasmic reticulum (SR), necessary for a proper contraction and relaxation, strongly depends on ATP supply and ADP withdrawal (high localized ATP/ADP ratio) in the vicinity of myofibrillar and SR ATPases (Dzeja & Terzic, 2003). Indeed, local ADP accumulation considerably inhibits ATPase activity. We and others have shown that in cardiomyocytes, this is achieved by phosphotransfer kinases and mitochondria much more efficiently than by simple diffusion of nucleotides through the bulk cytosol. Creatine kinase (CK) is bound to myofibrils and SR, and efficiently transfers the high-energy phosphate group of phosphocreatine (PCr) to ADP in the vicinity of the ATPases (Dzeja & Terzic, 2003). Mitochondria can themselves directly support myofibrillar/SR-Ca2+ ATPase function as efficiently as the bound CK system, and much better than cytosolic ATP alone (Kaasik et al. 2001; Saks et al. 2001). This indicates that a localized crosstalk between energy producing and energy consuming sites in the cardiac cell is required to ensure proper cardiac contractility. Local regulation and microcompartmentation are not unique to energy metabolism but are rather general properties for optimal regulation of the cell, because the same holds true for many processes, including calcium regulation between mitochondria and SR, subsarcolemmal macromolecular complexes, or second messenger signalling. Cytoskeleton structurally participates in these local regulations, because in models of cytoarchitectural disorganization, such as the muscle LIM protein (MLP) or the desmin knockout mice, mitochondria–SR direct energy transfer is impaired (Wilding et al. 2006). Cytoskeletal alterations participate in cellular remodelling and in the alteration of mechanical function in the failing heart (Hein et al. 2000), and probably can also affect these local processes.
Because both CK activity and mitochondrial content are reduced in HF (De Sousa et al. 1999; Ventura-Clapier et al. 2004; Ingwall & Weiss, 2004) and because the failing myocardium is known to exhibit cytoarchitectural disorganization that may compromise mitochondria, SR and myofilament interactions, we hypothesized that the localized control of ATPase activity by the local ATP/ADP ratio would be impaired and that this might contribute to contractile failure. Therefore, we examined the local regulation of ATPases by CK and mitochondria in situ in an animal model of heart failure. Since the energetic compartments controlling cellular ATPases are highly dependent on the intracellular architecture, our study was performed in saponin-permeabilized cells and fibres in which the cytoarchitecture is preserved. Our data reveal a striking relationship between function, energetics and cytoarchitecture within myocardium that participates in the contractile dysfunction of the cardiomyocyte.
Methods
All experiments performed conform to the European Community guiding principles in the care and use of animals (86/609/CEE, CE Off J no. L358, 18 December 1986), the local ethics committee (CREEA Ile-de-France Sud) guidelines, and the French decree no. 87–848 of October 19, 1987 (J Off République Française, 20 October 1987, pp. 12245–12248). Authorizations to perform animal experiments according to this decree were obtained from the French Ministère de l'Agriculture, de la Pêche et de l'Alimentation (no. 92–283, June 27, 2007).
Briefly, aortic stenosis was mimicked in weaned male rats (60–70 g) by placing a stainless steel haemoclip of 0.6 mm ID on the ascending aorta via a thoracic incision, as previously described (De Sousa et al. 1999). Control rats underwent the same procedure but without placement of the clip (sham operation). Animals were randomly assigned to one of two groups: sham-operated (sham, n= 9) or heart failure (HF, n= 9). Five months after surgery, rats were anaesthetized by intraperitoneal injection of sodium thiopental (150 mg kg−1). Hearts were excised and rinsed in ice-cold calcium-free Krebs solution equilibrated with 95% O2–5% CO2 for 10 min. Hearts were dissected, with part of the tissue used for mechanical experiment, and part used for electron microscopy or biochemical experiments.
Electron microscopy
Hearts from five sham and five failing rats were excised and rinsed in ice-cold calcium-free Krebs solution for 10 min and fixed with 2% glutaraldehyde in cacodylate buffer for 2 h as previously described (Wilding et al. 2006). The papillary muscles were dissected and fixed in 2% glutaraldehyde in cacodylate buffer for 45 min and postfixed by 1% osmium tetroxide in cacodylate buffer for 30 min at room temperature. Tissue was stained with 1% aqueous solution of uranyl acetate, dehydrated in graded ethanol series, and embedded into Durcupan (ACM Fluka). Three tissue blocks of papillary muscle from each animal were obtained. Longitudinal sections were cut from each tissue block at three randomly selected levels separated by more than 50 μm. Ultrathin longitudinal sections (70–75 nm thick) were cut on a RMC PowerTome Ultramicrotome (Boeckeler Instruments, Inc., Tucson, AZ, USA), placed on copper grids covered with formvar, stained with lead citrate and studied using JEM 1200 (JEOL Ltd, Tokyo, Japan) electron microscope. Images were recorded using a Gatan Dual Vision 300 W CCD camera (Gatan, Inc., Pleasanton, CA, USA).
Myofibrillar intrinsic properties and estimation of SR calcium uptake
Fibres (∼200 μm width) were dissected from papillary muscles and permeabilized for 30 min in saponin (50 μg ml−1), which preserves mitochondrial and SR function as previously described (De Sousa et al. 1999; Kaasik et al. 2001; Wilding et al. 2006) Fibres were mounted on a stainless-steel hook with a force transducer (AE 801, Microelectronics, Horton, Norway) as previously described (Kaasik et al. 2001). Fibres were immersed in 2.5 ml chambers arranged around a disc and placed into a temperature-controlled bath and positioned on a magnetic stirrer at 22°C. Sarcomere length was measured by laser diffraction and adjusted to 2.1–2.2 μm, which gives maximum twitch. SR calcium loading capacity was measured in sham and HF rat cardiac fibres as previously described (Minajeva et al. 1996; Kaasik et al. 2001). Briefly, SR calcium loading was initiated at pCa 6.5 in the presence of exogenous ATP, with or without endogenous mitochondrial ATP production and/or CK-system activation. Azide (2 mm) was used to block mitochondrial ATP production, and solutions lacking phosphocreatine (PCr) were used to block creatine kinase function. SR calcium release was elicited with 5 mm caffeine, and was detected by measuring the resulting contractile force transient. At the end of the experiment pCa–tension relationship was assessed by stepwise increase in calcium concentration in the presence of caffeine. The calcium concentration that gave half-maximal tension and the Hill coefficient were obtained by nonlinear fit to the Hill equation:
where T is the relative rigor tension, K a constant, and nH the Hill coefficient. They were used to calculate the [Ca2+]–time integral as an index of SR calcium load (De Sousa et al. 1999). The ATP-channelling solution (ATP + MITO) contained (in mm): ethylene glycol-bis(β-aminoethyl ether)N,N,N′,N′-tetra-acetic acid 10 (EGTA), 0.2 during calcium release; N,N-bis[2-hydroxyethyl]-2-aminoethanesulphonic acid (BES) 40 (pH 7.1); free Mg2+ 1; taurine 10; glutamic acid 5; malic acid 2; K2HPO4 3; dithiothreitol 0.5; P1,P5-diadenosine pentaphosphate 0.04 (to inhibit adenylate kinase activity); ruthenium red 0.005 (to block mitochondrial calcium uptake); and MgATP 3.16; ionic strength was adjusted to 160 with potassium methanesulphonate, and 6% dextran was added to maintain normal cell volume after skinning.
Finally, we tested the ability of glycolysis to increase further the SR function (Boehm et al. 2000), with further addition of 4 mm glyceraldehyde-3-phosphate (GAP), 4 mm phosphoenolpyruvate (PEP) and 4 mm nicotinamide adenosine dinucleotide (NAD).
pMgATP–rigor tension relationships in saponin-permeabilized cardiac fibres
pMgATP–rigor tension relationships were established by measuring the change in force during stepwise decreases in ATP concentration, until maximum rigor tension was obtained, with ATP alone, and with or without PCr and azide. Data were fitted using the Hill equation:
where T is the relative rigor tension, K a constant, and nH the Hill coefficient. This fitting procedure was used to calculate pMgATP50, at which half-maximum rigor tension is obtained. Efficacy of ATP/ADP ratio control by mitochondrial ATP channelling and CK was calculated as the difference between pMgATP50 with and without mitochondrial or CK activation.
Confocal imaging of spontaneous Ca2+ waves in saponin-skinned cardiac myocytes
SR calcium uptake for the different energy loading conditions was also estimated by analysing spontaneous Ca2+ release from the SR with Fluo-4 fluorescence (excitation wavelength 488 nm; emission 500–595 nm) (Yang & Steele, 2002). Ventricular myocytes were isolated from sham and HF adult rats as described before (Verde et al. 1999). For HF rats, 1 mg ml−1 hyaluronidase was added to facilitate tissue digestion. Cardiomyocytes were permeabilized with saponin (50 mg ml−1) at room temperature (22°C) for 3 min using basic internal solution (pCa 9), and then plated onto coverslips coated with Matrigel (Sigma) for attachment. Coverslips were attached on the stage of a Carl Zeiss LSM-510 confocal microscope using a recording chamber (Warner instruments, Hamden, CT, USA). Loading solutions were identical to those used for the permeabilized fibre experiments, except that [EGTA] was decreased to 100 μm (calcium concentration was estimated at 360 nm with calcium dye), and 4 μm Fluo-4 was added (Invitrogen). Images were acquired in line scan mode (at intervals of 3.5 or 7 ms) along the longitudinal axis of the cell. All the experiments were performed at 22°C.
Biochemical analysis and immunoblotting
Activities of total CK, citrate synthase (CS) and mitochondrial complex IV (COX) were measured using standard assays as previously described (De Sousa et al. 1999). For immunoblotting, protein extracts (50 μg) were separated on 8% SDS-polyacrylamide gels and subsequently transferred to Hybond nitrocellulose membranes (Amersham). Membranes were incubated for 2 h with antibodies against mitochondrial CK (mi-CK, gift from Dr Z. Khuchua, Nashville, TN, USA), SERCA2 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and calsequestrin (Affinity Bioreagents, Inc., Golden, CO, USA). Chemiluminescence was detected using an ECL+ kit (Amersham) and quantified using an image-analysis system (Bio-Rad). Calsequestrin was used to normalize the protein expression.
Statistics
Data are expressed as means ±s.e.m. Statistical differences between two or more groups were determined by analysis of variance (ANOVA), or by one-way ANOVA with repeated measures (for different SR loading conditions), followed by the Newman–Keuls post hoc test. Significance was defined by a P-value less than 0.05 (95% confidence).
Results
Anatomical data
After nearly five months of aortic banding, mortality was ∼60% in the HF group. The remaining HF animals showed decreased body weight, increased absolute heart weight and increased weights of heart, right and left ventricles and lungs normalized to tibia length, indicating severe heart failure (Table 1). Other anatomical signs of cardiac decompensation (ascites, pleural effusion and oedema) confirmed the presence of severe HF in this model.
Table 1.
Anatomical parameters in HF rats compared with sham
| Sham (n= 9) | HF (n= 9) | P* | |
|---|---|---|---|
| Aortic banding duration (months) | 4.9 ± 0.1 | 4.8 ± 0.1 | NS |
| Body weight (g) | 533 ± 23 | 429 ± 16 | <0.001 |
| Heart weight (g) | 1.5 ± 0.1 | 2.8 ± 0.1 | <0.001 |
| Tibia length (cm) | 4.55 ± 0.13 | 4.37 ± 0.06 | NS |
| Heart weight/tibia length (g cm−1) | 0.33 ± 0.03 | 0.65 ± 0.02 | <0.001 |
| Right ventricle weight/tibia length (g cm−1) | 0.058 ± 0.005 | 0.117 ± 0.005 | <0.001 |
| Left ventricle weight/tibia length (g cm−1) | 0.22 ± 0.02 | 0.36 ± 0.01 | <0.001 |
| Lung weight/tibia length (g cm−1) | 0.40 ± 0.02 | 1.02 ± 0.08 | <0.001 |
| Liver weight/tibia length (g cm−1) | 3.7 ± 0.2 | 3.2 ± 0.2 | 0.05 |
Values are means ±s.e.m.; n= number of animals. *Statistical differences between HF and sham values; NS: non significant.
Myofibrillar function of HF rats in saponin-permeabilized fibres
Myofibrillar function was assessed in saponin skinned cardiac fibres from sham and HF rats (Table 2). Active tension at pCa 4.5 (defined as the difference between maximal and resting tension) was 22% lower in HF rat heart than sham (P < 0.001), indicating contractile dysfunction. Passive tension was three-fold higher in saponin-skinned HF fibres than sham. Calcium sensitivity was unchanged.
Table 2.
Myofibrillar functions in saponin permeabilized cardiac fibres
| Sham | HF | P* | |
|---|---|---|---|
| Active tension (mN mm−2) | 37 ± 2 | 29 ± 2 | <0.001 |
| (n= 22) | (n= 23) | ||
| Resting tension (mN mm−2) | 4.9 ± 0.8 | 16.8 ± 2.1 | <0.001 |
| (n= 22) | (n= 23) | ||
| pCa50 | 5.82 ± 0.01 | 5.83 ± 0.01 | NS |
| (n= 12) | (n= 13) | ||
| nH | 4.8 ± 0.2 | 4.3 ± 0.1 | NS |
| (n= 12) | (n= 13) |
Values are mean ±s.e.m.; pCa50: pCa for half-maximal tension development. nH: Hill coefficient; NS: non significant.
Statistical differences between HF and sham values. n= number of fibres.
Metabolic enzymes involved in energy production and consumption
Enzyme activities of CK, CS and COX were decreased in banded rat heart by 38% (P < 0.01), 24% (P < 0.05) and 22% (P < 0.05), respectively (Fig. 1A and B). Because decreased mitochondrial CK (mi-CK) activity is a good indicator of the severity of the pathology (Nascimben et al. 1996), we also estimated mi-CK expression by Western blot (Fig. 1B). Compared to sham, mi-CK expression was decreased by 59% (P < 0.005). These results confirmed that mitochondrial energy production and CK-mediated energy transfer were reduced in our heart failure model, consistent with previous data showing impairment of maximal oxygen consumption rate (De Sousa et al. 1999).
Figure 1. Enzymes involved in energy production and consumption in sham and HF rats.
A, mitochondrial enzymes (CS, citrate synthase; COX, cytochrome oxidase; IU, μmol min−1). B, CK content (total CK activity IU, μmol min−1; mi-CK, Western blot analysis of mitochondrial CK; au: arbitrary units). C, representative Western blot of SERCA2 and mean values of SERCA2 expression levels. Calsequestrin was used as loading control (n= 8 in each group). *P < 0.05, **P < 0.005 versus sham.
Western Blot analysis showed that SERCA2 levels were unchanged in HF left ventricle (Fig. 1C).
Failing cardiomyocyte ultrastructure
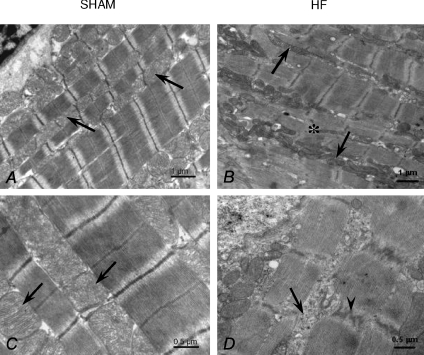
Compared with sham, the cytoarchitecture of failing rat heart myocytes was greatly disorganized, which made quantification of ultrastructural images difficult (Fig. 2). Myofibrils and mitochondria were affected; there were regions where the longitudinal orientation of myofibrils was lost, and regions that had widened, zig-zagging Z lines with fewer contacts between mitochondria and myofibrils. In these regions, it was very difficult to identify the membrane of the longitudinal sarcoplasmic reticulum. The size and distribution of mitochondria in failing myocytes were very heterogeneous. Mitochondrial clusters were present in the intermyofibrillar space, and in these clusters mitochondrial diameter varied greatly. Some mitochondria were small and round, whilst others were long and tubular, spanning more than two sarcomeres. In some large mitochondria, a circular arrangement of cristae was evident, together with signs of mitochondrial degradation.
Figure 2. Electron micrographs of papillary muscles from sham (A and C) and HF (B and D) rats.
A, longitudinal section of cardiomyocyte from sham rat with regular organization of myofibrils and rows of mitochondria (arrows). B, longitudinal section of cardiomyocyte from HF rat with irregular organization of myofibrils with small areas of the disorganized myofibrils (atsterisk). Mitochondria are extended along the long fibre axis (arrows) and form clusters in the intermyofibrillar space. C, detail showing mitochondria of regular shape (arrows) found close to the myofibrils in cardiomyocytes from sham rat. D, portion of cardiomyocyte from HF rat. Intermyofibrillar space is rich in cytosol (arrow). In the myofibrils irregular course of the Z-line is seen (arrowhead).
Energy regulation of sarcoplasmic reticulum ATPase (SERCA) function
Calcium wave experiments on saponin-permeabilized cardiac myocytes
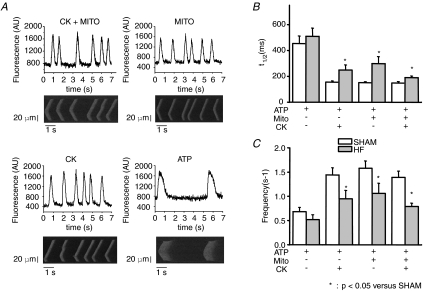
In saponin-skinned cardiac myocytes spontaneous calcium waves can be detected at low EGTA concentration, due to cyclic uptake and release of Ca2+ from the SR (Yang & Steele, 2002) (Fig. 3A). Wave frequency and decay time course of the calcium waves were highly dependent on the ability of mitochondria and CK to regulate SERCA. In sham myocytes, ATP alone as energy source was a poor substrate for SR calcium loading, because the half-time of the decay phase (t1/2) was almost 3-fold higher and wave frequency 3-fold lower in the absence of CK or mitochondrial substrates, indicating lower SR calcium content and less efficient calcium reuptake by SERCA. In HF myocytes, support by ATP diffusion alone remained poor (no significant change compared to sham), indicating no major change of intrinsic properties of SR in our conditions (Fig. 3B and C). However, calcium wave frequency was lower with CK and/or mitochondria substrates, and the descending phase of calcium waves was prolonged indicating lower calcium loading capacity of SERCA in HF versus sham rats in the presence of CK and mitochondrial substrates.
Figure 3. Confocal imaging of spontaneous Ca2+ waves.
A, Fluo-4 original (lower lane) and averaged (upper lane) signals from representative experiments showing spontaneous Ca2+ waves in saponin-skinned sham cardiomyocytes in different energy solutions: ATP alone (ATP) or with mitochondrial and CK system activation (ATP + CK + MITO), mitochondria (ATP + MITO) or CK (ATP + CK). [EGTA]= 100 μm, pCa 6.5. B and C, graphs showing the mean Ca2+ wave frequency (B) and the half-time of the descending phase of the wave (t1/2, C) in sham and HF rat myocytes (n= 10–15 cells for each condition from 3 different hearts).
Mechanical experiments on saponin-permeabilized cardiac fibres
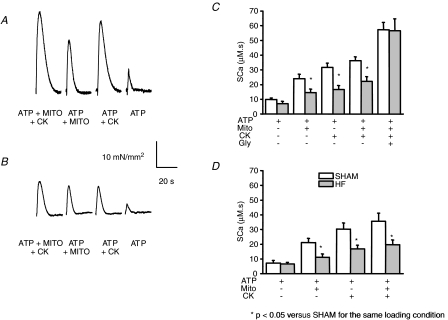
SR calcium content was also assessed in saponin-permeabilized sham and HF rat cardiac fibres, using the force transient induced by caffeine stimulation (Kaasik et al. 2001; Wilding et al. 2006). SR calcium loading over 5 min was performed in the presence of ATP and with CK and/or mitochondrial substrates. Caffeine-induced tension transients had decreased amplitude in HF cardiac fibres under all energetic conditions (Fig. 4A), consistent with reduced maximal active tension and myofibrillar disarray (Table 2) and with the cytoarchitecture disorganization. Calcium concentration–time transient (SCa), calculated using the force–calcium relationship for each fibre (see Methods), was used as an index of SR calcium content. Compared to sham, SCa was decreased in HF fibres by 43% (P < 0.005) after loading with ATP + Mito + CK solution (Fig. 4B), by 40% (P < 0.05) with mitochondrial direct ATP channelling (ATP + Mito), and by 49% (P < 0.005) with bound creatine kinase (ATP + CK) (Fig. 4B). Loading supported by exogenous ATP was again not significantly decreased.
Figure 4. SR calcium loading under different energetic conditions in sham and HF permeabilized cardiac fibres.
A and B, representative tension transients during SR calcium release in sham (A) and HF (B) cardiac fibres. The SR was loaded with calcium for 5 min under different energetic conditions (ATP + Mito + CK, ATP + Mito, ATP + CK, ATP, see Methods). C and D, calcium load was estimated by relating tension transients, elicited by caffeine stimulation to the calcium–tension curve for each fibre (SCa). C, energy supplied by ATP, by ATP + Mito, ATP + CK, ATP + mitos + CK, or by ATP + mitos + CK + glycolysis (Gly). *P < 0.05 versus Sham. D, same experiments as in C in the presence of alkaline phosphatase.
In order to test whether altered phosphorylation of proteins such as SERCA, phospholamban or the ryanodine receptor played a role in impaired SR calcium loading in our HF model, we preincubated some fibres for 1 h with 200 U ml−1 alkaline phosphatase, which causes protein dephosphorylation. Dephosphorylation had no significant effect on calcium loading in sham fibres, and did not restore SR loading to sham levels in HF fibres (Fig. 4C).
Finally, we tested whether SR function could be improved by the presence of an additional energy source (Fig. 4B). In sham fibres, activation of supplementary glycolytic pathways increased SR loading from 36 ± 3 to 57 ± 3 μm s (+55%). In HF fibres, the same level of SR loading was reached (57 ± 8 μm s), but the relative increase was larger (+155%), thus compensating for mitochondrial and CK impairment.
Function of actomyosin-ATPase
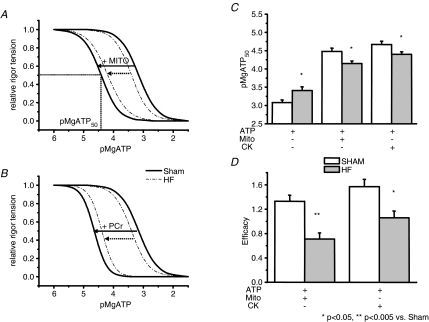
Rigor experiments were performed to assess the ability of mitochondrial direct ATP channelling and CK to control the ATP/ADP ratio in the vicinity of actomyosin-ATPase (myosin-ATPase). In the absence of calcium and PCr, and in the presence of azide (i.e. with non-working CK and mitochondria), a progressive decrease in MgATP concentration led to the development of rigor tension (Fig. 5A). The pMgATP that produced half-maximal rigor tension (pMgATP50) was shifted towards slightly higher values in HF, indicating greater accessibility of exogenous ATP to myosin-ATPase than in sham (Fig. 5B). When mitochondria or CK were activated (by removing azide or by adding PCr, respectively), pMgATP50 was increased due to local rephosphorylation of ADP by mitochondria or bound CK. This is the first demonstration that mitochondrial direct ATP channelling is effective at inhibiting rigor tension development in cardiac fibres. In HF fibres, the shift of MgATP50 was smaller than in shams, indicating a reduced efficacy of local ATP regeneration by mitochondria and CK. Mitochondrial efficacy declined from 1.30 ± 0.09 in sham to 0.75 ± 0.08 in HF (n= 9, P < 0.001), whereas CK efficacy declined to a lesser extent, from 1.48 ± 0.11 in shams to 1.08 ± 0.09 in HF (n= 9, P= 0.02; Fig. 5C). There was no statistical difference in the value of the Hill coefficient between groups, which ranged from 1.3 to 2.3.
Figure 5. Function of actomyosin-ATPase in sham and HF rat.
A and B, graphs showing relative pMgATP–rigor tension relationship without or with substrates for mitochondria (A) and creatine kinase (B). pMgATP–rigor tension relationship is established by a stepwise decrease in ATP concentration (in the absence of calcium) until maximum rigor tension was obtained. The curves represent the Hill equation fitting of data (see Methods) obtained with the mean of seven experiments in ventricular skinned fibres from sham (continuous line) and HF rat fibres (dashed lines). The shift observed by addition of mitochondrial substrates (+ MITO) or PCr (+ CK) represent the efficacy of both systems for the regulation of actomyosin ATPase function. C, means of pMgATP50 (ATP concentration needed to obtain the half-maximum rigor tension) estimated by rigor experiments. D, mean values of efficacy of mitochondria and CK for myosin ATPase regulation. *P < 0.05, **P < 0.005 versus sham.
Relative contribution of CK and mitochondria for ATPases regulation
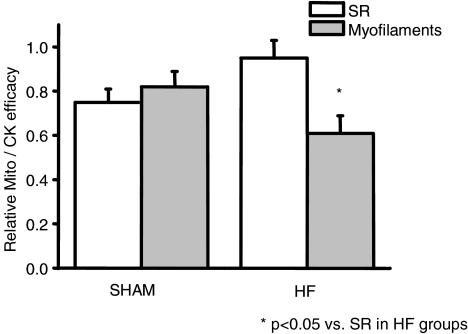
We compared the relative efficacy of mitochondria and CK in regulating SERCA and myosin-ATPase in sham and HF rats (Fig. 6). For this purpose, we calculated the ratio between mitochondria and CK efficacy relative to ATP, using the data from Figs 4 and 5. Both mitochondria and CK appeared to contribute equally to local ATP regeneration near the myofibrils and SR in sham rats. Interestingly, in HF rats, the relative contribution of both energetic systems to SR regulation remained the same, but a relative decrease in the mitochondrial contribution was observed (P < 0.05) with respect to myofilament regulation.
Figure 6. Relative efficacy of mitochondria and CK for regulating SR and myofibrils function in sham and HF.
For SR, the relative efficacy of energy sources was calculated as the ratio between SCa after loading in the presence of mitochondrial substrates (ATP + MITO) and SCa in the presence of PCr (ATP + PCr) (data from Fig. 4). For myofilaments, the relative efficacy is calculated as the ratio between efficacy of mitochondria and CK for myosin ATPase regulation (from Fig. 5). In sham hearts, the efficacy of mitochondria for regulating local ATP/ADP is similar in SR and in myofilaments (compared to CK), but appears to be more affected in myofilaments than in SR in HF.
Discussion
The aim of this work was to determine whether the ability of CK and mitochondria to control the ATP/ADP ratio in the vicinity of two main cardiac cellular ATPases, SERCA and myosin-ATPase, is modified in HF and whether this might contribute to cardiomyocyte dysfunction. Using two different approaches in saponin-skinned fibres and cells, we observed a global decrease in the efficacy of both energetic systems: in the presence of functional CK and/or mitochondria, SR calcium loading was lower in HF versus sham, and could be restored to the normal level only by activation of bound glycolytic enzymes. For myofibrils, CK and mitochondria were also less efficient in HF than in sham myocardium, causing contractile dysfunction. Energy deficiency and impairment of local control of ATP/ADP may thus contribute to the global contractile dysfunction in heart failure.
Methodological approaches to the study of local energy regulation
To investigate local energy regulation near SERCA and myofilament ATPases in a physiological setting, we used a unique method developed 10 years ago in our laboratory (Minajeva et al. 1996). It is based on the use of sarcolemmal membrane permeabilization with saponin, which preserves the integrity of the cellular architecture and organelles and allows a precise control of the composition of the intracellular medium. The intracellular medium is kept physiological and can be modified to control bulk energy supply, calcium concentration, pH, reactive oxygen species, and ion concentrations. Moreover, the ability of each energy source to control local ATP/ADP ratio can be investigated separately or in combination.
To assess SERCA function, we combined two complementary experimental approaches: in the first one, we used myofilaments from skinned fibres as an intracellular functional probe for calcium released by the SR; in the second one, we used skinned cardiomyocytes loaded with a fluorescent calcium indicator. In the fibres, SR calcium uptake capacity was assessed by recording the time integral of the tension transient induced by caffeine in the presence of optimal and constant energy supply (ATP + Mito + CK). A major advantage of this is that the myofilaments respond to ‘the functional calcium’, i.e. the calcium stored in the SR that can be mobilized and can reach myofilaments upon full activation of the ryanodine receptors. To circumvent possible confounding effects due to intrinsic myofibrillar changes in heart failure, the calcium sensitivity of the myofilaments was assessed for each fibre providing a tension–pCa calibration curve from which the amount of released calcium was calculated (Minajeva et al. 1996). In the skinned isolated myocytes, spontaneous SR calcium release and reuptake were elicited in the presence of a subthreshold calcium concentration. The resulting intracellular calcium waves develop with a frequency and t1/2 which reflect the efficacy of calcium uptake into the SR (Yang & Steele, 2002). Because the composition of the bulk cytosol can be precisely controlled, this approach allowed evaluation of the consequences for SR calcium release of a change in local concentrations of nucleotides or energy sources.
To assess energy compartmentation in myofilaments, we used a well-established approach of the dependency of rigor tension development on local control of ATP/ADP by CK in Triton X-100 (Ventura-Clapier et al. 1987). This approach was modified using saponin instead of Triton X-100, which allowed us for the first time to evaluate the role of mitochondrial energy supply.
Local energetic regulation of ATPases is impaired in the failing rat heart
Even when energy stores are kept constant, a decreased capacity for local energy regeneration may lead to dysfunction of cellular ATPases. In sham fibres or cardiomyocytes, ATP alone is not sufficient to sustain SR calcium load, and local withdrawal of ADP and regeneration of ATP by either CK or mitochondria or both (Kaasik et al. 2001) is necessary for efficient thermodynamic and kinetic control of ATPases. This is evidence that SR calcium loading capacity, and thus SR calcium content, is also dependent on local energy supply. In heart failure, SR loading was not different when ATP alone was provided. However, when the CK and/or mitochondrial systems were activated, SR loading was lower, indicating a further limitation of the energy supply due to local regeneration systems. The same holds true for cardiac myofilaments in HF, where either mitochondria- or CK-supported myofibrillar function was impaired. This impairment occurs despite a sharp decrease in the proportion of the fast myosin isoform in the failing heart, which should increase the economy of contraction (Herron & McDonald, 2002) and reduce energy demand. Altogether, these results indicate that local energy limitation occurs in HF which affects both calcium homeostasis and myofibrillar function.
An impaired local ATP/ADP ratio may not be the only reason for the observed decreased SR calcium release in HF. Although not always observed (Frank et al. 2002), there is evidence for a SERCA2 down-regulation in HF. In our model of heart failure, cardiac SERCA2 expression was normal. In the presence of ATP alone, no difference was observed in calcium loading between sham and HF. Interestingly, maximal activation of glycolysis was sufficient to restore a normal SR loading (i.e. HF matched sham levels), indicating (1) that energy supply is a limiting factor for SR function in HF, and (2) that glycolysis plays a more important role in HF in supporting SR function, to compensate for the impairment of CK (like it occurs in skeletal muscle, Janssen et al. 2003) and mitochondria. Glycolysis may also act by decreasing SR calcium leak (Zima et al. 2006), thus preserving the balance between load and leak, in the presence of all energy sources.
In recent years it has been emphasized that calcium leak due to hyperphosphorylation of ryanodine receptors or reduced phosphorylation level of phospholamban may participate in the decreased SR calcium content and calcium transient in heart failure (Frank et al. 2002; Lehnart et al. 2005). However, alkaline phosphatase had no significant effect on calcium loading in our experiments, suggesting that our results were not due to a change in SERCA expression level and/or protein phosphorylation. Yet, the contribution of phosphorylation may be underscored in our experiments because the permeabilization procedure may in itself lead to protein dephosphorylation, which could explain why alkaline phosphatase had no significant effect. This does not rule out the role of phosphorylation on altered calcium fluxes but provides evidence that local energy regulation could additionally take part in the defective SR function in HF. This is in line with the similar results obtained with myofibrillar ATPase where HF induced alterations in local control of energy supply by mitochondria and CK directly impacts myofibrillar function.
Cytoarchitecture and heart failure
In cardiac myocytes, specialized cellular functions are highly organized within structural and functional compartments (Saks et al. 1994; Ventura-Clapier et al. 1998; Weiss & Korge, 2001). Each subcellular compartment is a functional and structural entity (Saks et al. 2001). The role of the cytoskeleton in stabilizing cellular structure and functional integrity is well established (Clark et al. 2002). In heart failure, profound alterations of microtubules or intermediate filament protein expression may contribute to functional impairment (Hein et al. 2000; Belmadani et al. 2002; Wilding et al. 2005; Cooper, 2006) It was proposed that an increased distance between the calcium channel and RyR may affect the efficiency of excitation–contraction coupling in HF (Gomez et al. 1997). Cytoskeletal organization may also play a role in the regulation of energy exchanges (Milner et al. 2000; Appaix et al. 2003), since it is required for positioning mitochondria and anchoring myofibrils (Capetanaki, 2002). A recent study by our laboratory focused on two models of cytoarchitectural disorganization, the muscle LIM protein (MLP) and the desmin knock-out mouse: in both models, a specific alteration of mitochondria–SR interaction was observed (Wilding et al. 2006), suggesting that efficient energy transfer depends on the arrangement of organelles and myofibrils, independently of mitochondrial function and SR content.
In the present study, as in other models of heart failure (Sabbah et al. 1992), we found misalignment of mitochondria and myofibrils, heterogeneity of mitochondrial shape and size and mitochondrial degradation. These changes could be the basis for the decreased energy transfer between mitochondria and myosin-ATPase in addition to the decrease in energy production. Indeed, while in sham rats both CK and mitochondria contribute equally to SR and myofibrillar functions (Fig. 6), in HF heart, mitochondria were less efficient than CK in maintaining an adequate energy supply for contraction. Our data suggest that energetic remodelling occurs in HF, which alters the ratio of efficacy between the two main energy sources. Due to the large structure of myofibrils, the core of myofilaments may be more dependent on mitochondrial form, mass and architectural arrangement than SR, which is in closer physical interaction with mitochondria. On the other hand, because CK is closely bound to myofilaments and SR, CK efficacy will depend more on the amount of bound CK than on architectural design. Thus, in HF mitochondria could be more limiting than CK for the regulation of ATP/ADP ratio in the vicinity of myofibrils as compared to SR. Maintaining the close interaction between mitochondria and myofibrils appears to be crucial for optimal energetic regulation.
Implications for cardiac function
In vivo, the reduction in myocardial CK fluxes, the decrease in fatty acid utilization and the decrease in oxidative capacity are sufficient to compromise ATP buffering capacity and limit ATP availability to the myofibrils and SR calcium pump, and in turn affect both systolic and diastolic function (De Sousa et al. 1999; Ten Hove & Neubauer, 2007; Smith et al. 2006). Consequently, and in contrast to our experimental conditions, in vivo the concentrations of substrates and intermediates are no longer in excess, further enhancing energetic limitations. The importance of glycolytically produced substrates for mitochondrial oxidative phosphorylation is increased, but remains limited particularly under conditions of high workload (Ventura-Clapier et al. 2004). Impairment of CK- or mitochondria-mediated control of ATPases via the local ATP/ADP ratio demonstrated here will participate in impairment of SR calcium handling and contraction and thus excitation–contraction coupling. Importantly, in the healthy heart the two main energy-supplying systems (CK and direct mitochondrial energy channelling) show clear redundancy, creating a certain reserve in situations of increased energy demands of high workload. However, this is not any more the case in the failing heart as demonstrated here. Thus, the failing heart seems to be unable to meet high haemodynamic requirements of the body in part because of a lack of chemical energy available, defective energy distribution, and energy starvation (Ingwall & Weiss, 2004; Neubauer, 2007).
In conclusion, the present data suggest that there are intimate links between cytoarchitecture, energy metabolism and contractile activity ensuring efficient cardiac pump function. Depression of contractile force and myocardial function in heart failure may result from intricate alterations of calcium handling, cytoarchitectural disorganization and energetic starvation. Further experiments are needed in order to understand better the link between energy, cytoarchitecture and contraction and the need to maintain these components in harmony to ensure optimal cardiac cell function.
Acknowledgments
We thank Dr R. Fischmeister for continuous support and critical reading of the manuscript, Florence Lefebvre for preparation of isolated cells, the animal core facility of IFR141 for efficient handling and preparation of the animals, and Valérie Nicolas for help with the confocal microscopy. This work was supported by the Institut National de la Santé et de la Recherche Médicale, La Fondation de France, The Leverhulme Trust (J.R.W.), the Centre National de la Recherche Scientifique (F.J., R.V.-C.), APVT-51-31104 and VEGA 2/3189/25 (M.N.), Ministère des Affaires Etrangères STEFANIK (08093WL), the European Union Contract no. LSHM-CT-2005-018833/EUGeneHeart.
References
- Appaix F, Kuznetsov AV, Usson Y, Kay L, Andrienko T, Olivares J, Kaambre T, Sikk P, Margreiter R, Saks V. Possible role of cytoskeleton in intracellular arrangement and regulation of mitochondria. Exp Physiol. 2003;88:175–190. doi: 10.1113/eph8802511. [DOI] [PubMed] [Google Scholar]
- Belevych A, Kubalova Z, Terentyev D, Hamlin RL, Carnes CA, Gyorke S. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys J. 2007;93:4083–4092. doi: 10.1529/biophysj.107.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani S, Pous C, Ventura-Clapier R, Fischmeister R, Mery PF. Post-translational modifications of cardiac tubulin during chronic heart failure in the rat. Mol Cell Biochem. 2002;237:39–46. doi: 10.1023/a:1016554104209. [DOI] [PubMed] [Google Scholar]
- Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- Boehm E, Ventura-Clapier R, Mateo P, Lechene P, Veksler V. Glycolysis supports calcium uptake by the sarcoplasmic reticulum in skinned ventricular fibres of mice deficient in mitochondrial and cytosolic creatine kinase. J Mol Cell Cardiol. 2000;32:891–902. doi: 10.1006/jmcc.2000.1130. [DOI] [PubMed] [Google Scholar]
- Capetanaki Y. Desmin cytoskeleton: a potential regulator of muscle mitochondrial behavior and function. Trends Cardiovasc Med. 2002;12:339–348. doi: 10.1016/s1050-1738(02)00184-6. [DOI] [PubMed] [Google Scholar]
- Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- Cooper G., 4th Cytoskeletal networks and the regulation of cardiac contractility: microtubules, hypertrophy, and cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2006;291:H1003–H1014. doi: 10.1152/ajpheart.00132.2006. [DOI] [PubMed] [Google Scholar]
- De Sousa E, Veksler V, Minajeva A, Kaasik A, Mateo P, Mayoux E, Hoerter J, Bigard X, Serrurier B, Ventura-Clapier R. Subcellular creatine kinase alterations. Implications in heart failure. Circ Res. 1999;85:68–76. doi: 10.1161/01.res.85.1.68. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Redfield MM, Burnett JC, Terzic A. Failing energetics in failing hearts. Curr Cardiol Rep. 2000;2:212–217. doi: 10.1007/s11886-000-0071-9. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J Exp Biol. 2003;206:2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- Frank KF, Bolck B, Brixius K, Kranias EG, Schwinger RH. Modulation of SERCA: implications for the failing human heart. Basic Res Cardiol. 2002;97(Suppl.)(1):I72–I78. doi: 10.1007/s003950200033. [DOI] [PubMed] [Google Scholar]
- Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- Hein S, Kostin S, Heling A, Maeno Y, Schaper J. The role of the cytoskeleton in heart failure. Cardiovasc Res. 2000;45:273–278. doi: 10.1016/s0008-6363(99)00268-0. [DOI] [PubMed] [Google Scholar]
- Herron TJ, McDonald KS. Small amounts of a-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res. 2002;90:1150–1152. doi: 10.1161/01.res.0000022879.57270.11. [DOI] [PubMed] [Google Scholar]
- Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- Janssen E, Terzic A, Wieringa B, Dzeja PP. Impaired intracellular energetic communication in muscles from creatine kinase and adenylate kinase (M-CK/AK1) double knock-out mice. J Biol Chem. 2003;278:30441–30449. doi: 10.1074/jbc.M303150200. [DOI] [PubMed] [Google Scholar]
- Kaasik A, Veksler V, Boehm E, Novotova M, Minajeva A, Ventura-Clapier R. Energetic crosstalk between organelles: architectural integration of energy production and utilization. Circ Res. 2001;89:153–159. doi: 10.1161/hh1401.093440. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XH, Marks AR. Defective ryanodine receptor interdomain interactions may contribute to intracellular Ca2+ leak: a novel therapeutic target in heart failure. Circulation. 2005;111:3342–3346. doi: 10.1161/CIRCULATIONAHA.105.551861. [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Milner DJ, Mavroidis M, Weisleder N, Capetanaki Y. Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J Cell Biol. 2000;150:1283–1298. doi: 10.1083/jcb.150.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minajeva A, Ventura-Clapier R, Veksler V. Ca2+ uptake by cardiac sarcoplasmic reticulum ATPase in situ strongly depends on bound creatine kinase. Pflugers Arch. 1996;432:904–912. doi: 10.1007/s004240050214. [DOI] [PubMed] [Google Scholar]
- Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD. Creatine kinase system in failing and nonfailing human myocardium. Circulation. 1996;94:1894–1901. doi: 10.1161/01.cir.94.8.1894. [DOI] [PubMed] [Google Scholar]
- Neubauer S. The failing heart – An engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1992;24:1333–1347. doi: 10.1016/0022-2828(92)93098-5. [DOI] [PubMed] [Google Scholar]
- Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T. Cardiac system bioenergetics: metabolic basis of the Frank–Starling law. J Physiol. 2006;571:253–273. doi: 10.1113/jphysiol.2005.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks VA, Kaambre T, Sikk P, Eimre M, Orlova E, Paju K, Piirsoo A, Appaix F, Kay L, Regitz-Zagrosek V, Fleck E, Seppet E. Intracellular energetic units in red muscle cells. Biochem J. 2001;356:643–657. doi: 10.1042/0264-6021:3560643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks VA, Khuchua ZA, Vasilyeva EV, Belikova OY, Kuznetsov AV. Metabolic compartmentation and substrate channelling in muscle cells. Role of coupled creatine kinases in in vivo regulation of cellular respiration – a synthesis. Mol Cell Biochem. 1994;133–134:155–192. doi: 10.1007/BF01267954. [DOI] [PubMed] [Google Scholar]
- Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation. 2006;114:1151–1158. doi: 10.1161/CIRCULATIONAHA.106.613646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Hove M, Neubauer S. MR spectroscopy in heart failure – clinical and experimental findings. Heart Fail Rev. 2007;12:48–57. doi: 10.1007/s10741-007-9003-8. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol. 2004;555:1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R, Kuznetsov A, Veksler V, Boehm E, Anflous K. Functional coupling of creatine kinases in muscles: species and tissue specificity. Mol Cell Biochem. 1998;184:231–247. [PubMed] [Google Scholar]
- Ventura-Clapier R, Mekhfi H, Vassort G. Role of creatine kinase in force development in chemically skinned rat cardiac muscle. J Gen Physiol. 1987;89:815–837. doi: 10.1085/jgp.89.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde I, Vandecasteele G, Lezoualc'h F, Fischmeister R. Characterization of the cyclic nucleotide phosphodiesterase subtypes involved in the regulation of the L-type Ca2+ current in rat ventricular myocytes. Br J Pharmacol. 1999;127:65–74. doi: 10.1038/sj.bjp.0702506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Korge P. The cytoplasm: no longer a well-mixed bag. Circ Res. 2001;89:108–110. [PubMed] [Google Scholar]
- Weiss JN, Yang L, Qu Z. Systems biology approaches to metabolic and cardiovascular disorders: network perspectives of cardiovascular metabolism. J Lipid Res. 2006;47:2355–2366. doi: 10.1194/jlr.R600023-JLR200. [DOI] [PubMed] [Google Scholar]
- Wilding JR, Joubert F, de Araujo C, Fortin D, Novotova M, Veksler V, Ventura-Clapier R. Altered energy transfer from mitochondria to sarcoplasmic reticulum after cytoarchitectural perturbations in mice hearts. J Physiol. 2006;575:191–200. doi: 10.1113/jphysiol.2006.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding JR, Schneider JE, Sang AE, Davies KE, Neubauer S, Clarke K. Dystrophin- and MLP-deficient mouse hearts: marked differences in morphology and function, but similar accumulation of cytoskeletal proteins. FASEB J. 2005;19:79–81. doi: 10.1096/fj.04-1731fje. [DOI] [PubMed] [Google Scholar]
- Yang Z, Steele DS. Effects of phosphocreatine on SR Ca2+ regulation in isolated saponin-permeabilized rat cardiac myocytes. J Physiol. 2002;539:767–777. doi: 10.1113/jphysiol.2001.012987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima AV, Kockskamper J, Blatter LA. Cytosolic energy reserves determine the effect of glycolytic sugar phosphates on sarcoplasmic reticulum Ca2+ release in cat ventricular myocytes. J Physiol. 2006;517:281–293. doi: 10.1113/jphysiol.2006.117242. [DOI] [PMC free article] [PubMed] [Google Scholar]