Abstract
To better understand how the brainstem reticular formation controls and coordinates trunk and hindlimb muscle activity, we used optical recording to characterize the functional connections between medullary reticulospinal neurons and lumbar motoneurons of the L2 segment in the neonatal mouse. In an isolated brainstem–spinal cord preparation, synaptically induced calcium transients were visualized in individual MNs of the ipsilateral and contralateral medial and lateral motor columns (MMC, LMC) following focal electrical stimulation of the medullary reticular formation (MRF). Stimulation of the MRF elicited differential responses in MMC and LMC, according to a specific spatial organization. Stimulation of the medial MRF elicited responses predominantly in the LMC whereas stimulation of the lateral MRF elicited responses predominantly in the MMC. This reciprocal response pattern was observed on both the ipsilateral and contralateral sides of the spinal cord. To ascertain whether the regions stimulated contained reticulospinal neurons, we retrogradely labelled MRF neurons with axons coursing in different spinal funiculi, and compared the distributions of the labelled neurons to the stimulation sites. We found a large number of retrogradely labelled neurons within regions of the gigantocellularis reticular nucleus (including its pars ventralis and alpha) where most stimulation sites were located. The existence of a mediolateral organization within the MRF, whereby distinct populations of reticulospinal neurons predominantly influence medial or lateral motoneurons, provides an anatomical substrate for the differential control of trunk and hindlimb muscles. Such an organization introduces flexibility in the initiation and coordination of activity in the two sets of muscles that would satisfy many of the functional requirements that arise during postural and non-postural motor control in mammals.
The brainstem reticular formation, via the reticulospinal (RS) descending system, plays a pivotal role in the activation and modulation of the spinal circuits involved in the control of posture and locomotion (Deliagina et al. 2006; Drew et al. 2004 and Rossignol, 1996 for reviews). However, little is known about the underlying neural mechanisms that allow the RS system to control and coordinate the two functions.
Investigating the RS system in neonatal rodents may shed some light on the organization of the locomotor and postural networks. Reticulospinal neurons, which are generated relatively early during brain development (Altman & Bayer, 1980a, b; Bélanger et al. 1997), are among the first to develop axonal projections (Auclair et al. 1993, 1999; Easter et al. 1993; de Boer-van Huizen & ten Donkelaar, 1999) and to extend axons into the lower thoracic and lumbar segments (Kudo et al. 1993; Lakke, 1997). These events occur several days before birth. By the time of birth, RS projections in the rat are already functional and capable of producing mono- and polysynaptic responses in lumbar MNs (Floeter & Lev-Tov, 1993; Brocard et al. 1999a). Quadrupedal locomotion and postural control, on the other hand, are acquired postnatally (Westerga & Gramsbergen, 1990; Jamon & Clarac, 1998; Brocard et al. 1999b; Jiang et al. 1999a; Vinay et al. 2002) although with different developmental time courses (Gramsbergen, 1998; Jamon & Clarac, 1998; Vinay et al. 2002). Rats, for instance, have the ability to perform quadrupedal locomotion and use their hindlimbs to lift their belly between P5 and P6, before postural control with proper synchronization between trunk and limb muscles is attained (Jamon & Clarac, 1998; Jamon, 2006). It has been postulated that this relative independence in the acquisition of the two functions may stem, at least partly, from developmental differences in descending projections from supraspinal structures (Gramsbergen, 1998; Vinay et al. 2002). A developmental mechanism that establishes separate or partially overlapping descending systems for the regulation of trunk and hindlimb motoneurons (MNs) would also provide, if the separation of those systems were maintained, the differential control necessary for regulating and integrating posture and locomotion in the adult.
To study the organization of supraspinal descending systems, we have started to characterize connections between the medullary reticular formation (MRF) and trunk and hindlimb MNs in the upper lumbar spinal cord of the neonatal mouse using calcium imaging to rapidly monitor the synaptic activation of large numbers of individual MNs simultaneously. In this regard we have used the recording of calcium transients as a general indicator of postsynaptic activity. A fluorescent calcium probe was applied to the ventral roots rather than to the preparation as a whole so that we could selectively monitor evoked activity in MNs. We have focused on MNs located in the L2 segment because of the pivotal role of this segment in the generation of the locomotor rhythm (see Falgairolle et al. 2006 and references therein). We demonstrate that functional connections are established from the MRF to individual spinal neurons in the neonatal mouse. Our results support the existence of two reciprocally organized RS projections exerting control over trunk and hindlimb musculature with differential predominance. Parts of this work have been published previously in abstract form (Szokol et al. 2007).
Methods
Brainstem–spinal cord preparation
Experiments were performed on isolated brainstem–spinal cord preparations (n= 63) from neonatal (postnatal day (P) 0 to P5) mice. We used primarily Balb/c mice (Taconic, Denmark) but to test the generality of our findings we also performed some experiments (n= 6) on ICR (Harlan, France) and NZEG or Sim1-lacZ mice (the latter obtained originally from M. Goulding, Salk Institute). Animals were deeply anaesthetized with isoflurane, decerebrated (by transecting the brain just rostral to the superior colliculus) and eviscerated. To expose the brainstem and the spinal cord, both a craniotomy and a laminectomy were performed. The brainstem–spinal cord with attached ventral roots was then gently dissected out in ice-cold (1–5°C), oxygenated (95% O2 and 5% CO2) sucrose- or glycerol-based dissecting solutions containing (in mm): sucrose 188, NaCl 25, KCl 2, d-glucose 25, CaCl2 0.15, MgSO4 1, NaH2PO4 1.2, Hepes 10, NaHCO3 25 or glycerol 250, KCl 2, d-glucose 11, CaCl2 0.15, MgSO4 2, NaH2PO4 1.2, Hepes 5, NaHCO3 25. In a few control experiments, a Vaseline barrier at C2 was made to isolate the brainstem and the spinal cord in separate bath compartments. The tightness of this barrier was verified by adding phenol red to one of the compartments.
All efforts were made to minimize the number of animals used and their suffering in accordance with the European Communities Council directive 86/609/EEC and the National Institutes of Health guidelines for the care and use of animals. All procedures were approved by the National Animal Research Authority in Norway.
Loading of calcium dye into MNs, optical recordings and light scattering
Crystals of calcium green-1 (CG-1) conjugated dextran amine (CGDA; Mr 3000, Molecular Probes, Eugene, OR, USA) were applied to the transected motor axons of the L2 ventral root on one or both sides of the cord using tungsten or stainless steel pins (Glover, 1995). The cuts to the L2 ventral roots were made close to the root entry to reduce the time required to label the MN somata. The dissecting solution was then replaced by oxygenated artificial cerebrospinal fluid (ACSF) containing (in mm): NaCl 128, KCl 3, d-glucose 11 CaCl2 2.5, MgSO4 1, NaH2PO4 1.2, Hepes 5, NaHCO3 25. Retrograde labelling continued in the dark at room temperature (23–25°C) for 3 h. This period was sufficiently long to allow retrograde labelling of the MNs in the LMCs and the MMCs.
The brainstem–spinal cord preparation was transferred to a recording chamber (total volume of 7 ml) where it was pinned to the silicone rubber substrate (Sylgard; Dow Corning) with the ventral side up and superfused with oxygenated ACSF (see above). The perfusion rate (3–4 ml min−1) was adjusted so that total volume exchange occurred within 2 min. The CGDA-labelled MNs were visualized with a 40× water immersion objective (LUMPlanFl, 0.8 NA, Olympus, Norway) through the ventral white matter using an epi-fluorescence microscope (Axioskop, Carl Zeiss, Oberkochen, Germany) equipped with a 100 W mercury lamp driven by a DC power supply (PAN35-20A, Kikusui Electronics Corp., Yokohama, Japan) and excitation and emission filters (BP 450–490 nm and LP 515 nm, respectively). Changes in fluorescence intensity attributable to intracellular calcium transients were detected using a CCD camera (Cascade 650, Photometrics, Texas Instruments, USA) mounted on a video zoom adaptor. Video images were acquired using image-processing software (Metamorph 5.0, Universal Imaging Corporation/Molecular Devices, USA). Because epi-illumination intensity was lowered to minimize bleaching and phototoxicity during the recording sessions, we used a low frame rate (4 Hz), high gain (3) and a binning factor of 2.
To evaluate the extent to which light scattering from activated neurons could give false positive responses in adjacent recorded neurons, we analysed light scattering in the x–y plane (n= 9). We found that responses recorded from single MNs spatially isolated from the main population of labelled MNs fell off around 50% over a distance of about 30 μm, i.e. about one motoneuron soma diameter (mean of 24.6 ± 3.0 μm in P3 mice, Li et al. 2005) and by 75% over a distance of 55 μm.
Electrical stimulation
Electrical stimulation of the MRF was delivered with a monopolar tungsten microelectrode (Parylene-coated, shaft diameter 0.254 mm, tip diameter 1–2 μm, impedance 0.1 MΩ at 1 kHz; WPI, USA) using a Grass stimulator (S88, Grass Technologies/Astro-Med Inc., West Warwick, RI, USA) coupled to a constant current unit (CC-1, Astro-Med Inc., West Warwick, RI, USA). The electrode was mounted on a hydraulic microdrive (MO-103, Narishige, Tokyo, Japan), aligned parallel to the ventral midline in the horizontal plane, at an angle of 30 deg from the horizontal in the sagittal plane, with the tip pointing caudally (see Fig. 1), and positioned at various distances from the midline (50–850 μm) and from the convergence of the vertebral arteries to the basilar artery (1.2–2 mm). Positioning of the electrode was performed under the microscope using a 100 W halogen lamp (Carl Zeiss), and before each descent, the electrode entry at the ventral surface of the brainstem was photographed. The electrode was then slowly driven into the tissue until activation of MNs upon stimulation was observed (which could require up to five tracks).
Figure 1. Isolated brainstem–spinal cord preparation.
A, low magnification micrograph of a brainstem–spinal cord preparation isolated from a P4 mouse (side view, ventral side up) showing the position and orientation of the stimulating electrode. Left inset: MNs in the MMC and LMC of the L2 segment labelled retrogradely with CGDA and viewed from the ventral surface of the cord. Right inset: Parasagittal section of the brainstem (ventral side up) showing the trajectory of a DiI-coated electrode. B and C, effective stimulation parameters for evoking Ca2+ responses in iLMC and iMMC. The graphs on the left display the amplitude–frequency curves when stimulating at 2 times threshold (T) with a 5 s train (filled circles) and the response amplitudes as a function of the train duration when stimulating at 2T and 10 Hz (individual open circles). Each response in the amplitude–frequency curves is an average (6–30 MNs) expressed as a percentage of the response at 10 Hz. The graphs on the right display the amplitudes induced by 5 s, 10 Hz stimulation as a function of the current at threshold. All values are means ± standard error of the mean.
At each stimulation site (usually four sites per track), stimuli were delivered as two stimulation trains (5 s duration, 200 μs pulse at 10 Hz, 25–200 μA) 10 s and 70 s after the onset of the 120 s optical recording session. The intertrain interval of 60 s was chosen to minimize the possibility that synaptic depression might affect the response evoked by the second train (Floeter & Lev-Tov, 1993) and to minimize bleaching. Stimulation strength was expressed in multiples of threshold (T) for evoking a detectable increase in fluorescence (see Data Analyses). This implies that for each pair of stimulation site-motor column examined (i.e. medial MRF-iMMC, -iLMC, -coMMC, -coLMC and/or lateral MRF-iMMC, -iLMC, -coMMC, -coLMC) a minimum of two recordings were made (one at threshold and one between 1.5 and 2.5 T). At the end of the experiments, electrolytic lesions (60 μA for 3 s at P0 or 5 s at P4, cathodal followed by anodal DC) were made at the most superficial and the deepest stimulation sites along the last electrode track.
In a series of control experiments, we stimulated the MLF, the raphe nuclei, the trigeminal and the vestibular nerves. For electrical stimulation of the MLF and the raphe nuclei, we used the same electrode and parameters as for the MRF stimulation, and placed the electrode directly above the midline before insertion into the tissue. For electrical stimulation of cranial nerves, we used suction electrodes.
Localization of stimulation sites
Histological processing of the brainstems was performed with the following procedure: 12–20 h fixation in 4% paraformaldehyde, cryoprotection with 20% sucrose, embedding in tissue-Tek OCT, freezing, cryostat sectioning (serial sections of 50 μm either in the transverse or the sagittal plane, n= 34 and 19, respectively), nuclear staining with Harris haematoxylin (Histolab, Gothenburg, Sweden) and mounting under a coverslip in a gelatin–glycerol medium. In 34 experiments we used the locations of the electrolytic lesions to confirm the positions of the stimulation sites. In 20 of these experiments, the electrode was also coated with the fluorescent dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI, solution saturated in absolute alcohol, Molecular Probes) to leave a trace of the electrode trajectories (DiCarlo et al. 1996). The positions of the electrolytic lesions and the DiI traces were measured from photographs of the serial sections (ProgRes C14 camera from Jenoptik mounted on an Olympus AX70 microscope). In 19 preparations, we could not use the locations of the electrolytic lesions to confirm the position of the stimulation sites (lesions were either not produced or too large). Nevertheless, in these cases we could estimate the mediolateral positions by measuring the entry point of the electrode from surface photographs of the brainstem (see above). Effective stimulation sites were plotted on three standard transverse sections at different anteroposterior levels from a P2 mouse. Differences in the size of the brainstem between the different age groups were taken into account when plotting the sites onto the standardized sections.
Retrograde labelling of brainstem neurons
In 10 additional preparations, brainstem neurons with axons running in the ventral and/or ventrolateral and dorsolateral funiculi (VF and VLF and DLF) were labelled retrogradely using tetramethyl rhodamine dextran-amine (RDA, 3 kDa, Molecular Probes) according to Glover (1995). Because retrograde labelling over long distances in vitro is impracticable, we applied the tracer at the C1–C3 level. The application was performed immediately after cutting the descending axons. Labelling continued in oxygenated, superfused ACSF for 12–18 h at room temperature in the dark. The preparations were processed following the histological procedure described above and cut in the transverse plane. Both the labelled neurons in the brainstem and the dye application site were photographed. For the results presented in Fig. 7, counterstaining with methylene blue (300 mg in 100 ml distilled water for 10–20 s, Difco Laboratories, West Molesey, Surrey, UK) was performed subsequent to the photography to provide additional information about anatomical landmarks.
Figure 7. Effects of spinal cord lesions on responses evoked by medial MRF stimulation.
A, box-and-whisker plots (left) showing the responses that remain after lesion, expressed as a percentage of the control responses. The specific lesions are shown in the corresponding connectivity diagrams (right) where connections rendered ineffective by the lesions are shown as light-grey elements. The organization of the connectivity diagrams relies on 5 assumptions explained in the text. The upper diagram shows connections needed to explain the residual responses following an ipsilateral lesion, the middle diagram adds connections needed to explain the residual responses following a contralateral lesion, and the lower diagram adds connections needed to explain the residual responses following a contralateral lesion. B, diagram of putative crossed and uncrossed pathways between medullary RS neurons and L2 motor columns and commissural interneurons. The grey rectangles (omitted in A for the sake of clarity) indicate that RS neurons may act through mono- and/or polysynaptic pathways. For each of the motor columns, the principal connections deduced from these experiments are shown as thicker lines.
Data analyses
The timing markers for the brainstem stimulation and the gating pulse from the CCD camera were recorded at 500 Hz (Digidata 1320A, Molecular Devices, Union City, USA). To synchronize the electrical and the optical recordings, a digital pulse from the Digidata was sent to the CCD camera.
In each experiment, separate digital apertures (termed regions of interest, abbreviated ‘ROIs’) were manually placed over the somata of six MNs in each of the MMC and LMC on either the ipsilateral side, contralateral side or both (Fig. 2). The selection of the motoneuron somata, which was made according to labelling intensity and availability in the same focus plane, was always performed before the stimulation and therefore was blind to the effect of stimulation. The mean distance between ROIs was 45 ± 12 μm in the MMC and 36 ± 8.0 μm in the LMC and the smallest distance between two ROIs was 17 μm. To test whether the six-MN sample was representative for the entire population, we examined all labelled MNs in selected preparations (n= 11). The results indicated that virtually all labelled MNs exhibited calcium transients in response to MRF stimulation with the same response pattern. This was true whether the digital apertures were positioned over an individual soma or over a larger region that encompassed many MN somata. The fluorescence intensity averaged over all the pixels in each ROI was measured in each image frame. Differences in labelling intensity between the different MNs was accounted for by expressing the Ca2+ transients in each ROI as changes in fluorescence during stimulation divided by the baseline fluorescence before stimulation (ΔF/F= (F−Fbaseline)/Fbaseline). For pseudocolour-coded representation (Figs 2A and 3A), the pixel intensities were filtered in Metamorph (LP filter 3 × 3) and assigned to a rainbow type colour index.
Figure 2. Stimulation of the medial MRF evokes larger Ca2+ responses in iLMC.
A, pseudocolour representations of the first 28 s of two 120 s recording sessions (frame exposure of 250 ms) showing the Ca2+ fluorescence in the iMMC (top row) and iLMC (bottom row) before, during and after the first of two trains of stimuli (5 s duration, 200 μs pulse at 10 Hz, 60 μA = 2.4T) delivered to the medial MRF in a P1 mouse. The locations of the manually defined ROIs over six individual motoneurons in the iMMC and iLMC are shown in the first and last frames (open circles). B, waveforms of the changes in fluorescence in iMMC (green) and iLMC (red) for the entire recording session. Each waveform is an average of the waveforms from the six ROIs. The time windows during which stimulation occurred are indicated by the grey shading. The arrows indicate the presence of spontaneous bursts of activity. Such spontaneous activity was routinely observed in neonatal preparations and was often largest in the LMC. Evoked responses that were obviously contaminated by spontaneous activity were not analysed in this study (see Methods). As shown in supplementary video 3A, the same difference in response amplitude (larger in iLMC than in iMMC) was seen when MNs in the iLMC and iMMC were recorded simultaneously instead of sequentially as illustrated here. C, plot of the normalized responses in the iMMC (green) and iLMC (red). Each point represents the average responses from the two trains for each MN/ROI, normalized to the mean response in iLMC. The mean response in iMMC and iLMC are shown as horizontal bars.
Figure 3. Stimulation of the lateral MRF evokes larger Ca2+ responses in iMMC.
A, pseudocolour representations of the first 28 s of two recording sessions showing the Ca2+ fluorescence before, during and after the first of two trains of stimuli (40 μA = 2T) delivered to the lateral MRF in a P1 mouse. B, waveforms of the changes in fluorescence in iMMC (green) and iLMC (red) for the entire recording session. The same difference in response amplitude (larger in iMMC than in iLMC) was seen when MNs in the iLMC and iMMC were recorded simultaneously (supplementary video 3B) instead of sequentially as shown here. C, plot of the normalized responses in the iMMC (green) and iLMC (red). For other details see Fig. 2.
Data were converted to text files using a custom made program, imported into Clampfit 9.0 (Molecular Devices) and expressed as waveforms. The magnitudes of the responses were measured by calculating the area under the waveforms. Responses that did not exceed 90% of the prestimulus baseline or that were contaminated by spontaneous activity (of common occurrence in neonatal preparations) were not measured. For each recording, the responses from the two stimulation trains were averaged, except for a few instances where the response from the second train was significantly smaller (P < 0.05, Mann–Whitney, n= 6), in which case only the response to the first train was analysed. Unless stated otherwise, data are given as means ±s.d.
Results
The MRF was stimulated in 53 preparations in which optical recordings were obtained from MNs in the ipsi- and/or contralateral MMC and LMC. The region explored was located medial to the spinal trigeminal nucleus, ventral to the hypoglossal nucleus and dorsal to the inferior olive, and included the gigantocellularis reticular nucleus including its pars alpha and ventralis. The MRF is a major centre for the integration of descending commands for the control of postural muscle tone and locomotion (Takakusaki et al. 2004; Takakusaki, 2008 for revent reviews) and we focused on the ventral region of the MRF because of its involvement in the control of hindlimbs in particular (Drew et al. 1986; Perreault et al. 1993).
Determination of effective stimulation parameters
On the basis of preliminary experiments, we defined two regions in the ventral MRF, one more medial and the other more lateral. As detailed in the next section, these two regions (referred to hereafter as ‘medial MRF’ and ‘lateral MRF’) were easily distinguished by their differential effects in MMC and LMC. To determine effective stimulation parameters for the medial and lateral MRF we examined specifically the responses in the motor column in which each region produced the predominant effect (n = 5, respectively). We found that trains containing > 10 stimuli were required to evoke detectable changes in Ca2+ fluorescence. We also found that 5 s trains were more effective than trains of shorter duration (shown as individual open circles in the graphs on the left of Fig. 1B and C). Prolonging the train duration to 10 s only slightly increased the responses to medial MRF stimulation and increased the responses to lateral MRF stimulation by about 40%. With 5 s train stimulation, the amplitudes of the evoked responses increased with increasing stimulation frequency (curves in the graphs on the left of Fig. 1B and C). At 10 Hz, MRF stimulation produced clearly detectable and stable responses. Increasing the frequency to 20 Hz produced only small increments in the amplitude of the evoked responses. However, at this frequency, stimulation gave noticeably less reliable responses and failures started to appear. From this series of experiments, we concluded that within the range of parameters tested, 5 s trains at 10 Hz were effective and adequate stimulation parameters. We also tested responses induced by threshold (T) stimulation and found that less current was required to evoke a detectable response at ≥ 10 Hz than at lower frequencies (graphs on the right of Fig. 1B and C). Thus, all MRF stimulation experiments reported in this study were performed with 5 s trains at 10 Hz using usually current strength between 1.5 and 2.5T but never surpassing 200 μA.
Differential responses in MMC and LMC from medial and lateral MRF stimulation
The medial and the lateral MRF were stimulated in the same preparation in 17 experiments. The medial MRF was usually stimulated first but to ensure that any progressive changes in the level of excitability of the preparation did not bias the results, in 25% of the experiments we stimulated the lateral MRF first.
On the ipsilateral side of the lumbar spinal cord, stimulation of the medial MRF (46 preparations, total of 47 effective sites) elicited changes in Ca2+ fluorescence in both iMMC and iLMC. However, the changes in fluorescence evoked in the iMMC were always smaller than those evoked in the iLMC. Two examples are illustrated in Fig. 2 and in the corresponding supplementary videos 1A and B (sequential recording of the two columns) and supplementary video 3A (simultaneous recording of the two columns), respectively. The pseudocolour representation of fluorescence intensity in Fig. 2A shows that a train of stimuli to the medial MRF produced much larger changes in fluorescence in LMC (bottom row) than in MMC (top row). This differential effect can best be seen by comparing the waveforms in Fig. 2B which represent averages from manually defined ROIs (open circles in the first and last frames in Fig. 2A). The value of the averaged response in the MMC, expressed as a ratio of the value in the LMC (set to 100%), was 0.06 ± 0.02 (Fig. 2C).
In contrast, the responses evoked by stimulation of the lateral MRF (21 preparations, total of 23 effective sites) were always larger in the iMMC than in the iLMC (Fig. 3 with corresponding supplementary videos 2A and B and supplementary video 3B). In the example of Fig. 3, the changes in fluorescence from the baseline level prior to stimulation were much larger for the MMC than for the LMC (Fig. 3A and B). The normalized mean iMMC/iLMC ratio in this experiment was 3.26 ± 1.36 (Fig. 3C). To show that the reciprocal pattern was not dependent on the specific stimulation parameters used, we went back to the data obtained during the testing and selection of effective stimulation parameters and found that the same reciprocal response pattern was obtained in the frequency range of 1–10 Hz and the train duration range of 1–10 s (20 Hz could not be tested due to the presence of response failures).
Optical recordings from the contralateral motor columns during stimulation of the medial (25 preparations, total of 25 effective sites) and lateral MRF (8 preparations, total of 8 effective sites) were also obtained. Similar to what we observed ipsilaterally, stimulation of the medial MRF always produced larger responses in the coLMC whereas stimulation of the lateral MRF always produced larger responses in the coMMC.
As shown in Fig. 4, there was no overlap between the distributions of the response amplitudes recorded in MMC and LMC with stimulation in the medial and lateral MRF. Ipsilaterally, the mean ratio of MMC response/LMC response was 0.36 ± 0.25 and 2.67 ± 1.79 for medial and lateral MRF stimulation, respectively (Fig. 4A). Contralaterally, a similar response pattern was obtained, with a mean ratio of MMC response/LMC response of 0.48 ± 0.25 for medial MRF stimulation and 3.22 ± 1.58 for lateral MRF stimulation (Fig. 4B). A direct comparison in six preparations where both the ipsi- and contralateral MMC and LMC were labelled and recorded and both medial and lateral MRF were stimulated showed that the mean response amplitudes elicited by the medial MRF (but not the lateral MRF) in iMMC and iLMC were significantly larger than those evoked in coMMC and coLMC, respectively (Mann–Whitney, U = 36, P = 0.002).
Figure 4. Medial and lateral MRF stimulations elicit reciprocal responses in the MMC and LMC both ipsi- and contralaterally.
A, cumulative distribution of the normalized Ca2+ responses evoked ipsilaterally (iMMC/iLMC) by stimulation of the medial and lateral MRF. Each point shows the mean response in a single preparation (responses of 6 mN to two trains) with standard deviation indicated by the bar. The grand mean was 0.36 ± 0.25 for the medial MRF distribution and 2.67 ± 1.79 for the lateral MRF distribution. B, cumulative distribution of the normalized Ca2+ responses evoked contralaterally (coMMC/coLMC). The grand mean was 0.48 ± 0.25 for the medial MRF distribution and 3.22 ± 1.58 for the lateral MRF distribution. Crosses: experiments which were performed in other wild-type or transgenic mice (see Methods).
After histological confirmation, we found that all of the effective stimulation sites within the MRF were distributed within 1 mm from the midline (mean mediolateral position of the stimulation sites in medial and lateral MRF was 275 ± 16 μm (s.e.m.) and 635 ± 33 μm (s.e.m.), respectively). To each stimulation site we assigned a specific symbol according to the motor column in which it produced the predominant response. As shown for three different datasets in Fig. 5A–C, the stimulation sites fell into two distinct groups along the mediolateral axis. In the most medial group, predominant responses were evoked in the LMC (filled squares). In the lateral group, predominant responses were evoked in the MMC (open circles). Not surprisingly, stimulation at intermediates sites induced responses in MMC and LMC that were similar. Although we cannot exclude entirely the possibility of current spread from one MRF region to the other (for an estimation of actual current spread, see section below), it is unlikely to be the sole reason for the concomitant activation of both motor columns during medial and lateral MRF stimulation. Indeed, when we compared the location of the five sites in the medial MRF that produced the smallest MMC/LMC ratios to the location of the five sites of the lateral MRF that produced the largest MMC/LMC ratios, we found these sites were distributed along the entire mediolateral axis of the respective medial and lateral stimulated areas (as opposed to clustering in the most medial part of the medial MRF and the most lateral part of the lateral MRF, respectively).
Figure 5. MRF stimulation sites giving rise to responses in trunk versus limb MNs are segregated along the mediolateral axis.
A, stimulation sites recovered from transverse brainstem sections (32 sites) and plotted directly on standardized sections (from a P2 mouse) at three different anteroposterior levels (250 μm apart). In all of these experiments the locations of the stimulation sites were confirmed histologically from electrolytic lesions. Note that the overlap of stimulation sites with the inferior olive is a visual artifact due to the fact that the standardized section differs from the sections recovered from recorded preparations. In fact, only two stimulation sites were located in the inferior olive, the others were dorsal to the inferior olive. Filled squares and open circles indicate effective sites that evoked predominant Ca2+ responses in LMC and MMC, respectively. The open triangle on the most caudal section indicates an ineffective site. B, stimulation sites recovered from parasagittal sections of the brainstem (15 effective, 4 ineffective sites), plotted on a single standardized transverse section. As for the dataset shown in A, the locations of the stimulation sites were confirmed histologically from electrolytic lesions. Note that this is a projection such that the sites are not at their actual anteroposterior positions. C, plot of 26 additional effective stimulation sites for which electrolytic lesions were ambiguous but mediolateral positions could be obtained from photographs of the electrode entry point (see Methods). The scale is the same for A–C. All three datasets show the same segregation into two clusters along the mediolateral axis. D, amplitude of Ca2+ responses (5 s train, at 2T) plotted as function of the depth of the electrode along a trajectory in the medial MRF, expressed as a percentage of the maximal response in iLMC. Because the electrode track is at a 30 deg angle to the longitudinal axis, to relate these depths to the transverse sections in A or B the bottom scale must be used. In doing so, the 0 mm value must be positioned at the ventral surface of the brainstem. 7N: facial nucleus; 10N: dorsal motor nucleus of the vagus; 12N: hypoglossal nucleus; Amb: ambiguus nucleus; cc: central canal; Gi: gigantocellularis reticular nucleus (Giα: pars alpha); IO: inferior olive nucleus; Pyr: pyramidal tract.
Altogether, these results indicate a mediolateral organization within the MRF according to which wholly or partially segregated regions differentially regulate MNs controlling trunk and hindlimb muscles.
Effective stimulation sites and relationship to various populations of descending neurons
Stimulation of the ventral surface of the brainstem (n = 10) never elicited Ca2+ responses in MMC or LMC, and stimulation of the MRF produced similar Ca2+ responses before and after isolating the brainstem from the spinal cord by a Vaseline barrier (n = 2). This indicates that MRF stimulation did not directly activate lumbar MNs by current spread and that the responses evoked must be due to the activation of descending axons.
As expected from focal electrical stimulation, clear bell-shaped curves were obtained when we plotted the response amplitudes as a function of the depth of the electrode (Fig. 5D). The narrowness of the curves at 50% of response amplitude suggests that structures located within a radius < 500 μm from the electrode tip were activated to a larger degree than structures located outside this volume. In agreement with this, it can be estimated that the volume of the MRF directly excited by our stimulations (average current of 129 μA and 124 μA for the medial and lateral MRF, respectively) would be encompassed by a sphere of average radius of about 387 and 380 μm for medial and lateral MRF, respectively, assuming similar electrical properties of medullary tissue and morphological properties of reticulospinal neurons in the neonatal and adult rat MRF (see Hentall et al. 1984).
There are many neuron populations in and near the MRF that send axons to the lumbar spinal cord. Although it is most likely that MRF stimulation predominantly activated medullary RS neurons, other populations of neurons and axons passing through or near the MRF must be considered. These include the medially located raphespinal neurons, pontine RS neurons and axons, dorsolaterally located vestibulospinal axons and laterally located trigeminospinal axons. A contribution from the ventrally located corticospinal axons is however, unlikely since ventral surface stimulation never elicited changes in fluorescence in either MMC or LMC. Moreover, corticospinal axons reach the lumbar cord only after P4 (Hsu et al. 2006).
To investigate the possibility of a contribution from raphespinal, pontine RS, trigeminospinal and lateral vestibulospinal neurons, we performed two sets of control experiments. First, we stimulated ventrally at the midline to activate the raphe nuclei and dorsally at midline to activate the pontine RS axons in the medial longitudinal fascicle (n = 2). Second, we stimulated the vestibular (n = 2) and the trigeminal nerves (n = 2). In all of these experiments, we recorded from both the iMMC and iLMC, and we used ventral MRF stimulation as a control.
Stimulation of the midline at most ventral locations, which would presumably activate raphespinal neurons, evoked responses in both iMMC and iLMC with a slightly larger response in iLMC. Although the response pattern was similar to that evoked by the medial MRF in the same preparation, the response amplitudes were much smaller (response ratios ≤ 0.05 in iMMC and ≤ 0.16 in iLMC, not shown). Stimulation of the midline at the most dorsal locations, which would presumably activate pontine RS axons in the medial longitudinal fascicle, also produced responses in iMMC and iLMC. Responses were again much smaller than following subsequent medial MRF stimulation (response ratios ≤ 0.05 in iMMC and ≤ 0.06 iLMC, not shown), and moreover showed a different response pattern, with responses of similar amplitudes in iMMC and iLMC. Altogether these results suggest that neither of these sources of descending inputs contribute much, if at all, to the responses induced by stimulation of the medial MRF.
Stimulation of the vestibular nerve (2T) elicited responses in both the iMMC and iLMC. A direct comparison of the response amplitudes evoked by stimulation of the nerve and the MRF is not possible due to the use of different types of electrodes (see Methods). However, the response pattern from vestibular nerve stimulation with predominant responses in iLMC (normalized ratios iMMC/iLMC of 0.42 and 0.31, not shown) differed from that elicited by lateral MRF stimulation in which predominant responses are in iMMC. On the basis of these results, we suggest that stimulation of vestibulospinal inputs through direct activation of the lateral vestibular nucleus is probably not responsible for the responses evoked by stimulation of the lateral MRF. However, we cannot exclude the possibility of a contribution to the responses evoked by stimulation of the medial MRF by vestibulospinal axons that potentially pass through the medial MRF. Ruling out this possibility must await information about the trajectories of the vestibulospinal axons within the medulla of neonatal mice.
Stimulation of the trigeminal nerve (2T), on the other hand, elicited responses in both iMMC and iLMC with predominant responses in the iMMC, as for lateral MRF stimulation (normalized ratios iMMC/iLMC of 1.50 and 2.50). To explore further the possibility of a contribution from the trigeminospinal pathway, we tested whether a transverse section of the most lateral part of the medulla, which interrupts transmission along this pathway (Vinay et al. 1995), would affect the responses evoked by the lateral MRF. Interruption of the trigeminal pathway did not suppress the responses or alter the pattern normally elicited by stimulation of the lateral MRF.
Altogether, these control experiments indicate that the responses elicited by MRF stimulation are due primarily to the activation of medullary RS neurons as opposed to other nearby populations of neurons or descending axons.
Candidate populations of medullary RS neurons
Although RS neurons have been mapped by us and others in adult and embryonic rats (Reed et al. 2008 and references therein; Auclair et al. 1999) and mice (Auclair et al. 1999; VanderHorst & Ulfhake, 2006), such mapping has not been performed in neonatal rodents. Comparison to the adult and embryonic maps alone is insufficient to determine which RS neuron groups reside at or near the sites of stimulation in the present study. So, to assess which populations of medullary RS neurons might be responsible for the differential responses elicited by MRF stimulation, and to determine which RS neuron populations to focus on in future studies (see Discussion), we retrogradely labelled medullary neurons by applying RDA to the VF and/or VLF or to the ventral part of DLF (n = 12). These funiculi together encompass all known RS projections. Labelling was performed at cervical levels (C1–C3) rather than at L2 because retrograde labelling over long distances in the isolated in vitro preparation cannot be attained before the health of the preparation deteriorates. In this context, it is relevant to mention that we have attempted retrograde labelling in fixed preparations using DiI (n = 15) but the quality of the labelling obtained was insufficient to provide a clear picture. Anatomical and physiological evidence in adult rats and cats (Peterson et al. 1975; Reed et al. 2008; Lefler et al. 2008) indicate that at least one out of two RS neurons that project to cervical segments send axon collaterals to lumbar segments, and vice-versa. Since on this basis labelling biased towards RS neurons that project solely to cervical segments seems unlikely, we believe that our labelling from cervical levels would necessarily include populations of RS neurons that project to the lumbar cord.
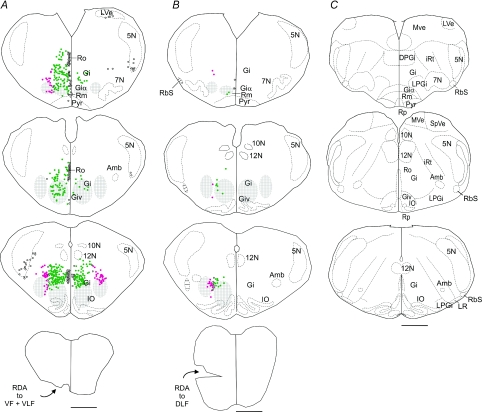
Figure 6 shows the plots of the resulting distributions of neurons labelled retrogradely from the VF and VLF in combination or from the ventral DLF. On the ipsilateral side, application to the VF and VLF in combination (n = 2, Fig. 6A) labelled neurons in the regions corresponding mainly to the gigantocellularis reticular nucleus with its pars alpha and ventralis, the spinal trigeminal nucleus, the raphe nucleus obscurus and magnus, and the lateral vestibular nucleus. On the contralateral side, a few labelled neurons were found in the gigantocellularis reticular nucleus and the spinal trigeminal nucleus. With the exception of the lateral vestibular nucleus, application restricted specifically to the VLF (n= 2) labelled neurons in the same regions as the VF (n= 4) but more neurons were labelled and these extended more caudally. Labelling of axons in the ventral DLF (n= 2, Fig. 6B) gave the least extensive retrograde labelling. In one experiment, neuron clusters labelled from the DLF were found only on the ipsilateral side in the region corresponding to the gigantocellularis reticular nucleus (along the entire anteroposterior extent of the inferior olive) and the spinal trigeminal nucleus. In the other experiment (not shown), some labelled neurons were also seen in the contralateral gigantocellularis reticular nucleus. As expected from encroachment on the DLF, in both experiments rubrospinal fibres were also labelled.
Figure 6. Retrograde labelling of brainstem neurons with descending axons.
A and B, line drawings of transverse sections made from low magnification photomicrographs of representative transverse sections through the medulla showing the distribution of neurons that were retrogradely labelled by application of RDA to VF + VLF or to DLF at the level of C1–C3 in P1 mice. Only the labelled neurons in the region of the medulla where we applied electrical stimulation are shown. In each experiment, the RDA application site (lesion) is visible in the caudal-most drawing. Note, however, that the application site was actually more extensive longitudinally. For the sake of clarity, each dot represents 1–5 retrogradely labelled neurons. After plotting of the labelled neurons, the sections were counterstained with methylene blue. The most discernible internal structures (Amb, 7N, 10N, 12N and IO) were traced directly from the stained sections. The borders of the structures that were less conspicuous (LV, 5 N and LR) are only rough estimates. Cross-hatched areas have been positioned on each side of the midline to indicate the regions containing the effective stimulation sites in the medial and lateral MRF. Labelled neurons are shown as green or red dots according to whether they are in register with the medial or lateral cross-hatched areas, respectively. The RS neurons located outside these areas including in the boundary region between the two areas were not assigned any colour. C, to help orientation, additional structures are delineated in standard P0 mouse sections, adapted and modified from Paxinos et al. (2007). 5N: spinal trigeminal nucleus; DPGi: dorsal paragigantocellularis nucleus; Giv: gigantocellularis pars ventralis; LPGi: lateral paragigantocellularis nucleus; LVe: lateral vestibular nucleus; LR: lateral reticular nucleus; iRt: intermediate reticuløar nucleus; MVe: medial vestibular nucleus; RbS: rubrospinal tract; Rm: raphe nucleus magnus; Ro: raphe nucleus obscurus; Rp: raphe nucleus pallidus; SpVe: spinal vestibular nucleus. For other abbreviations see Fig. 5. Scale bars are 500 μm.
To facilitate the comparison between Figs 5 and 6, we have placed crossed-hatched areas on each side of the midline in Fig. 6 to indicate the respective locations of the effective stimulation sites in the medial and lateral MRF. Without any regards to their dorsoventral position, the retrogradely labelled RS neurons which fell within the mediolateral extents of these two areas were coloured in green and red, respectively. As shown, the gigantocellularis reticular nucleus, which contained the largest contingent of RS neurons, emerges as the most likely candidate for mediating the responses elicited by medial and lateral MRF stimulations. Altogether the data provide new information about RS systems in the neonatal mouse and anatomical confirmation as to which RS neuron groups reside at the sites stimulated.
Effect of spinal lesions on responses elicited by medial MRF stimulation
As a first step towards defining the axon trajectories of the RS neurons that mediate differential responses in MMC and LMC, we made lesions in the spinal cord prior to stimulation of the MRF. We decided to focus on the spinal trajectories of the medial MRF-derived inputs for two reasons: (1) these provide the strongest contribution to the MNs innervating limb muscles, and (2) these exhibit a clear asymmetry in the responses elicited in both MMC and LMC, with the responses being stronger ipsilaterally than contralaterally. Thus, to determine the respective contributions of ipsi- and contralaterally descending RS axons to the responses in MMC and LMC (Fig. 7), we stimulated the medial MRF after ipsi- (n= 3) or contralateral hemisections at C4–C5 (n= 3) or longitudinal section of the midline at L2 (n= 3). The effects of these lesions on ipsi- and contralateral MNs are described in consecutive order.
The effects of each lesion are shown in Fig. 7A. The bar graphs (left) show the residual response as a percentage of the response before the lesion shown in the corresponding connectivity diagram (right). The scheme used in the connectivity diagrams is based on the following assumptions: (1) ipsi- and contralateral descending axons can provide inputs to MNs located on the same side as the axons descend (designated as ‘uncrossed inputs’ below), (2) ipsi- and contralateral descending axons can provide inputs to MNs located on the opposite side as the axons descend, either through commissural collaterals or commissural interneurons (designated as ‘crossed inputs’ below), (3) the schematic connections may represent both mono- and polysynaptic connections and include both excitatory and inhibitory components, (4) connections that are not necessary to account for the remaining responses are not shown, and (5) convergent inputs do not necessarily summate linearly. Note that the terms crossed and uncrossed in the presentation below refer to the behaviour of axons or their collaterals after the axons have descended into the spinal cord, not at their origins within the brainstem (contralateral descending axons are of course by definition crossed at the level of their parent somata).
Starting with the top row, we see that ipsilateral hemisection completely abolishes responses in the iLMC but has no effect on responses in the coMMC. Thus, crossed inputs from the descending axons are not required to account for the responses in these motor columns (hence these crossed inputs are not shown in the diagram). On the other hand, responses in iMMC and coLMC are diminished substantially, suggesting that responses in both of these motor columns arise from a combination of crossed and uncrossed inputs.
Moving to the second row, we see that contralateral hemisection diminishes responses in all four motor columns. The decrease in response in the iMMC is complementary to that seen after ipsilateral hemisection, and is therefore consistent with the connectivity scheme from the top row. The decrease in the response in coMMC indicates that contralateral uncrossed inputs, though sufficient to generate a maximal response (see top row), are supplemented by ipsilateral crossed inputs. Similarly, the decrease in response in iLMC indicates that contralateral crossed inputs, deemed unnecessary in the scheme in the top row, must be present, but must therefore be dependent on the presence of ipsilateral uncrossed inputs for their expression. The decrease in response in the coLMC is consistent with the conclusion that responses in this motor column arise from a combination of crossed and uncrossed inputs.
Moving to the last row, we see that longitudinal section of the midline at L2 diminishes responses in all motor columns except coMMC. This is consistent with the conclusion that contralateral uncrossed inputs are sufficient to generate a maximal response in coMMC. The decrements in the other three motor columns are consistent with the conclusion that responses in these columns arise from a combination of crossed and uncrossed inputs. However, the magnitude of the decrements indicates that these inputs do not summate linearly. Rather, it is likely that the various inputs to any given motor column influence each other, giving rise to nonlinear and to some extent permissive interactions.
A summary diagram of potential synaptic interactions between the medial MRF and L2 MN and commissural INs is presented in Fig. 7B. Based on the lesion data, we propose that the connections that provide predominant drive (thicker lines) are from uncrossed inputs to the ipsilateral motor columns, from crossed inputs to the coMMC and from crossed and uncrossed inputs to coLMC. Although the diagram is clearly an oversimplification of the potential synaptic interactions taking place (see for example Bannatyne et al. 2003 and Jankowska, 2008 concerning the organization of commissural interneurons), it nevertheless provides a platform for formulating relevant questions about the role of spinal interneurons in mediating the responses in MNs.
Discussion
Using optical imaging in an isolated neonatal brainstem–spinal cord preparation, we have obtained novel information about the functional connections between RS neurons and lumbar MNs. Our results reveal the existence of a functional connectivity pattern organized along the mediolateral axis of the ventral MRF wherein medial and lateral RS neurons activate both MMC and LMC MNs but with differential predominance. Medial RS neurons activate predominantly LMC MNs whereas lateral RS neurons activate predominantly MMC MNs.
This reciprocally organized RS connectivity pattern provides an anatomical substrate that permits a differential control over trunk and hindlimb muscles. Such an organization introduces flexibility in the coordination of activity in the two sets of muscles that would satisfy many of the functional requirements that arise during motor control (see below for a more detailed discussion of functional implications). Both postural and non-postural activities overlap in terms of muscles used and thus it is not surprising that medial and lateral RS neurons also display some degree of overlap in the control of trunk and hindlimb MNs.
Origin of the changes in Ca2+ signals
Evidence indicates that somatic Ca2+ transients in spinal motoneurons and cortical pyramidal neurons are good indicators of action potential activity (Fetcho & Malley, 1995; Lev-Tov & O'Donovan, 1995; Smetters et al. 1999), although in some other neurons Ca2+ transients may also indicate subthreshold events (Mochida & Glover, 2004; Lin et al. 2007). Spinal MNs in neonatal mice harbour a number of different voltage-sensitive calcium channels (Jiang et al. 1999b; Carlin et al. 2000), calcium-permeable ligand-gated channels (Fukaya et al. 2005) and metabotropic glutamatergic and/or cholinergic receptors (Miles et al. 2007), which all could contribute to an increase in intracellular Ca2+ either through calcium influx or through release of calcium from internal stores. A substantial contribution via activation of GABAA and glycine receptors is unlikely since GABA and glycine lose the ability to depolarize mouse lumbar motoneurons already by birth (Delpy et al. 2008).
One factor that may contribute to changes in Ca2+ signals is light scattering. In the present study, light scattering from motoneuron somata decreases by 75% over a distance of 55 μm. With an average distance of 174 ± 18 μm (n= 3) between the MMC and the LMC in animals where the motor columns are lying closest to each other (P0 mice), it is unlikely that light scattering from the somata of one motor column contaminated substantially the signals recorded from the somata of the other motor column. The extent to which dendrites that extend from one column to the other would contribute is, however, more difficult to evaluate because the dimensions of the dendrites are at the limit of the resolution of our optical recording system. Still, since only a fraction of the dendritic tree would be involved, it is most likely that such a contribution would be small. We believe that the response in the non-predominant motor column is mostly the result of synaptic activation, but the only way to resolve the issue is to measure directly its contribution at the single cell level using for example intracellular recording.
Another factor that might contribute to the observed spatial pattern of Ca2+ signals is the spread of Ca2+ and/or Ca2+-regulating second messengers through gap junctions (Roerig & Feller, 2000). Functional electrical coupling between MNs has been demonstrated in the embryonic and neonatal mouse spinal cord but its role in coordinating the activity of different groups of MNs remains uncertain (Hanson & Landmesser, 2003; Personius et al. 2007). In particular, the degree of coupling between the LMC and the MMC has not been characterized. A contribution from the spread of Ca2+ signals through gap junctions therefore remains to be determined.
Evidence for polysynaptic descending connections
In contrast with the situation in the adult mouse (Alstermark & Ogawa, 2004), descending volleys in the neonatal mouse spinal cord cannot be easily monitored. Despite numerous attempts, we were unable to record a descending volley from the activated RS axons. As a result, we could not accurately determine the latencies of the evoked Ca2+ transients. Floeter & Lev-Tov (1993) confronted the same difficulty in the neonatal rat and suggested that the problem arises because descending axons are not yet well fasciculated. Although it may be possible in the future to circumvent this difficulty with more sophisticated recording electrodes, the fact remains that Ca2+ transients are long lasting, and their recording, which requires integration over time and relatively low frame rates, does not provide enough temporal resolution to discriminate reliably between mono- and polysynaptic events.
Polysynaptic connections between RS neurons and spinal MNs have been found to predominate over monosynaptic connections both in the neonatal rat and in the adult mouse (Floeter & Lev-Tov, 1993; Brocard et al. 1999a; Alstermark & Ogawa, 2004). In agreement with these findings, and most probably reflecting the need for temporal summation and/or facilitation, we observed that trains containing > 10 stimuli were required to evoke detectable Ca2+ responses. Thus, we suggest that at least some of the responses we have recorded were mediated polysynaptically via spinal interneurons. To investigate the issue further we are currently examining functional connections between descending axons and various populations of lumbar interneurons, exploiting the same approach as used in the present study.
Mediolateral organization of the MRF and control of movement
The mediolateral organization revealed in this study would facilitate both differential regulation and integration of activity in the MMC and LMC. In the mouse, little information is available regarding the target muscles of the MMC MNs in specific spinal segments. It is likely, however, that the innervation pattern is similar to that of the rat and that MMC MNs of the L2 segment innervate dorsal axial muscles such as the lateral longissimus (Brink et al. 1979). The function of these muscles is to stabilize the lumbar vertebral column and help maintain proper posture during movement. LMC MNs in the L2 segment of the mouse innervate proximal hindlimb muscles, most of which are bifunctional: quadriceps femoris (hip flexion – knee extension), adductors and gracilis (hip adduction – knee flexion), pectineus (hip adduction and flexion), psoas (hip flexion) and perhaps semimembranosus (hip extension – knee flexion) (McHanwell & Biscoe, 1981).
Differential descending control over these trunk and hindlimb muscles is likely to be an essential feature in the normal progression of various types of automatic and voluntary movements, as well as in postural adjustments to unexpected perturbations. For instance, coordination between the activity of back and hindlimb muscles is important during locomotion and has been shown to be a critical factor in the development of a smooth walking pattern (Geisler et al. 1996). The existence of differentially regulated descending inputs to trunk and hindlimb muscles might also underlie the temporal disparity in the development of posture and locomotion in rodents (Gramsbergen, 1998; Jamon & Clarac, 1998; Vinay et al. 2002). This is probably physiologically relevant already in the neonate since training is required to coordinate trunk and limb musculature as the limbs become strong enough to bear weight (Bril & Ledebt, 1998; Gramsbergen, 1998).
As novel functional requirements arise throughout life, a mediolateral organization of the MRF could also provide a basis upon which to build more complex integrative processing between posture and movement. The anatomical pattern we demonstrate here could even become subordinate to physiological mechanisms such as differential discharge pattern and activity-dependent remodelling. In this regards, a recent study by Schepens & Drew (2004) in adult cats making reaching movements indicates the existence of functionally distinct populations of RS neurons: those whose discharge activity contributes to the initiation of postural adjustments preceding a movement and others whose activity contributes to the initiation of the movement and to the production of the postural responses that accompany that movement. Many of these were located in the gigantocellularis reticular nucleus but the possibility of a differential mediolateral distribution into spatially and functionally distinct cell groups was not investigated.
Activation of trunk and hindlimb muscles from the ventral MRF has been demonstrated in the adult rat (Femano et al. 1984; Cottingham et al. 1987, 1988; Mileykovskiy et al. 2002) and adult cat (Peterson et al. 1979; Drew & Rossignol, 1990). None of these studies reported a mediolateral division in the pattern of trunk versus hindlimb activation, but it has been noted that stimulation of lateral regions induces tonic hindlimb extension in both the adult rat and the cat (Kinjo et al. 1990; Drew & Rossignol, 1990, and see a discussion by Drew & Rossignol, 1990). Whether these lateral regions correspond to the lateral MRF region we have stimulated in the newborn is not clear. It therefore remains to be determined if the mediolateral organization within the MRF of newborn mice we report here remains as conspicuous in adulthood. Several developmental studies of medullary neuron populations have noted that neuron clusters can become more dispersed as the grey matter expands with growth of the neuropil (Glover & Petursdottir, 1991; Díaz et al. 2003), so a comparison of the newborn and the adult will probably require more precise stimulation, perhaps at the level of single RS neurons.
Identifying the candidate RS neurons: future directions
It is well established that during embryonic stages the brainstem neuroepithelium undergoes a systematic patterning of neuron populations along the anteroposterior and dorsoventral axes through the action of developmental patterning genes (Lumsden & Krumlauf, 1996; Glover, 2000; Pasqualetti et al. 2007). Through morphogenetic changes, the original dorsoventral axis of the embryonic brainstem deflects laterally to become more aligned with the mediolateral axis of the neonatal and adult brainstem. A mediolateral organization of functionally distinct RS populations as suggested by the stimulation sites observed here is therefore probably related to early patterning events that establish different neuron types along the dorsoventral axis. Although the relationship between neuron populations and dorsoventral patterning gene expression has not been as well characterized in the brainstem as in the spinal cord, there are clear indications that certain genes are related to RS neurons in the chicken embryo (Cepeda-Nieto et al. 2005). Since the embryonic organization of RS neurons has been well described in the mouse and rat (Auclair et al. 1999), it should be possible in future studies to link the candidate neuron populations in the neonate to genetically defined neuron populations in the embryo. This would open up possibilities for using molecular genetic techniques to target RS neurons for gene expression-based tracing, optical recording and stimulation, and functional manipulation.
Conclusion
To conclude, we have used the advantages of optical recording in the neonatal brainstem–spinal cord preparation to demonstrate what is likely to be a key feature of organization in the descending control of motor systems. As a basis upon which to build more complex integrative processing between posture and movement signals according to novel functional requirements arising throughout life, the topographical relationship shown here linking the MRF to the lumbar spinal cord goes a long way towards satisfying the requirement for a system that can exercise both independent and integrated control of trunk and limb muscles in different functional contexts.
Acknowledgments
We are grateful to Kobra Sultani for technical assistance. We thank S. Rossignol and S. Arber for their helpful comments on the manuscript. This work was supported by grants to M-C.P. from the Medical Faculty of University of Oslo and the Norwegian Research Council and a grant to J.C.G. from HFSP. K.Sz. was supported by a fellowship from The Christopher and Dana Reeve Foundation.
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2008.158105/DC1
References
- Alstermark B, Ogawa J. In vivo recordings of bulbospinal excitation in adult mouse forelimb motoneurons. J Neurophysiol. 2004;92:1958–1962. doi: 10.1152/jn.00092.2004. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat. J Comp Neurol. 1980a;194:1–35. doi: 10.1002/cne.901940102. I. Thymidine-radiographic study of the time of origin of neurons of the lower medulla. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat. J Comp Neurol. 1980b;194:37–56. doi: 10.1002/cne.901940103. II. Thymidine-radiographic study of the time of origin of neurons of the upper medulla, excluding the vestibular and auditory nuclei. [DOI] [PubMed] [Google Scholar]
- Auclair F, Bélanger MC, Marchand R. Ontogenetic study of early brain stem projections to the spinal cord in the rat. Brain Res Bull. 1993;30:281–389. doi: 10.1016/0361-9230(93)90256-b. [DOI] [PubMed] [Google Scholar]
- Auclair F, Marchand R, Glover JC. Regional patterning of reticulospinal and vestibulospinal neurons in the hindbrain of mouse and rat embryos. J Comp Neurol. 1999;411:288–300. doi: 10.1002/(sici)1096-9861(19990823)411:2<288::aid-cne9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger MC, Auclair F, Bertrand L, Marchand R. The early neuronal organization predicts the path followed by some major axonal bundles in the embryonic brainstem. Neuroscience. 1997;78:259–270. doi: 10.1016/s0306-4522(96)00484-8. [DOI] [PubMed] [Google Scholar]
- Bril B, Ledebt A. Head coordination as a means to assist sensory integration in learning to walk. Neurosci Biobehav Rev. 1998;22:555–563. doi: 10.1016/s0149-7634(97)00044-4. [DOI] [PubMed] [Google Scholar]
- Brink EE, Morrell JI, Pfaff DW. Localization of lumbar epaxial motoneurons in the rat. Brain Res. 1979;170:23–41. doi: 10.1016/0006-8993(79)90938-7. [DOI] [PubMed] [Google Scholar]
- Brocard F, Vinay L, Clarac F. Gradual development of the ventral funiculus input to lumbar motoneurons in the neonatal rat. Neuroscience. 1999a;90:1543–1554. doi: 10.1016/s0306-4522(98)00550-8. [DOI] [PubMed] [Google Scholar]
- Brocard F, Vinay L, Clarac F. Development of hindlimb postural control during the first postnatal week in the rat. Brain Res Dev Brain Res. 1999b;117:81–89. doi: 10.1016/s0165-3806(99)00101-7. Brain Res. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jiang Z, Brownstone RM. Characterization of calcium currents in functionally mature mouse spinal motoneurons. Eur J Neurosci. 2000;12:1624–1634. doi: 10.1046/j.1460-9568.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- Cepeda-Nieto AC, Pfaff SL, Varela-Echavarría A. Homeodomain transcription factors in the development of subsets of hindbrain reticulospinal neurons. Mol Cell Neurosci. 2005;28:30–41. doi: 10.1016/j.mcn.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Cottingham SL, Femano PA, Pfaff DW. Electrical stimulation of the midbrain central gray facilitates reticulospinal activation of axial muscle EMG. Exp Neurol. 1987;97:704–724. doi: 10.1016/0014-4886(87)90127-0. [DOI] [PubMed] [Google Scholar]
- Cottingham SL, Femano PA, Pfaff DW. Vestibulospinal and reticulospinal interactions in the activation of back muscle EMG in the rat. Exp Brain Res. 1988;73:198–208. doi: 10.1007/BF00279673. [DOI] [PubMed] [Google Scholar]
- de Boer-van Huizen RT, ten Donkelaar JH. Early development of descending supraspinal pathways: a tracing study in fixed and isolated rat embryos. Anat Embryol. 1999;199:539–547. doi: 10.1007/s004290050251. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Zelenin PV, Beloozerova IN. Neural bases of postural control. Physiology (Bethesda) 2006;21:216–225. doi: 10.1152/physiol.00001.2006. [DOI] [PubMed] [Google Scholar]
- Delpy A, Allain AE, Meyrand P, Branchereau P. NKCC1 cotransporter inactivation underlies embryonic development of chloride-mediated inhibition in mouse spinal motoneuron. J Physiol. 2008;586:1059–1075. doi: 10.1113/jphysiol.2007.146993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz C, Glover JC, Puelles L, Bjaalie J. The relationship between hodological and cytoarchitectonic organization in the vestibular complex of the 11-day chicken embryo. J Comp Neurol. 2003;457:87–105. doi: 10.1002/cne.10528. [DOI] [PubMed] [Google Scholar]
- DiCarlo JJ, Lane JW, Hsiao SS, Johnson KO. Marking microelectrode penetrations with fluorescent dyes. J Neurosci Methods. 1996;64:75–81. doi: 10.1016/0165-0270(95)00113-1. [DOI] [PubMed] [Google Scholar]
- Drew T, Dubuc R, Rossignol S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol. 1986;55:375–401. doi: 10.1152/jn.1986.55.2.375. [DOI] [PubMed] [Google Scholar]
- Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res. 2004;143:251–261. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. J Neurophysiol. 1990;64:767–781. doi: 10.1152/jn.1990.64.3.767. I. Movements evoked by microstimulation. [DOI] [PubMed] [Google Scholar]
- Easter SS, Jr, Ross LS, Frankfurter A. Initial tract formation in the mouse brain. J Neurosci. 1993;13:285–299. doi: 10.1523/JNEUROSCI.13-01-00285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falgairolle M, de Seze M, Juvin L, Morin D, Cazalets JR. Coordinated network functioning in the spinal cord: An evolutionary perspective. J Physiol Paris. 2006;100:304–316. doi: 10.1016/j.jphysparis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Femano PA, Schwartz-Giblin S, Pfaff DW. Brain stem reticular influences on lumbar axial muscle activity. Am J Physiol. 1984;246:389–395. doi: 10.1152/ajpregu.1984.246.3.R389. I. Effective sites. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, O'Malley DM. Visualization of active neural circuitry in the spinal cord of intact zebrafish. J Neurophysiol. 1995;73:399–406. doi: 10.1152/jn.1995.73.1.399. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Lev-Tov A. Excitation of lumbar motoneurons by the medial longitudinal fasciculus in the in vitro brain stem spinal cord preparation of the neonatal rat. J Neurophysiol. 1993;70:2241–2250. doi: 10.1152/jn.1993.70.6.2241. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Hayashi Y, Watanabe M. NR2 to NR3B subunit switchover of NMDA receptors in early postnatal motoneurons. Eur J Neurosci. 2005;21:1432–1436. doi: 10.1111/j.1460-9568.2005.03957.x. [DOI] [PubMed] [Google Scholar]
- Geisler HC, Westerga J, Gramsbergen A. The function of the long back muscles during postural development in the rat. Behav Brain Res. 1996;80:211–215. doi: 10.1016/0166-4328(96)00024-1. [DOI] [PubMed] [Google Scholar]
- Glover JC. Retrograde and anterograde axonal tracing with fluorescent dextrans in the embryonic nervous system. Neurosci Prot. 1995;30:1–13. [Google Scholar]
- Glover JC. Neuroepithelial ‘compartments’ and the specification of vestibular projections. Prog Brain Res. 2000;124:3–21. doi: 10.1016/S0079-6123(00)24004-1. [DOI] [PubMed] [Google Scholar]
- Glover JC, Petursdottir G. Regional specificity of developing reticulospinal, vestibulospinal, and vestibulo-ocular projections in the chicken embryo. J Neurobiol. 1991;22:353–376. doi: 10.1002/neu.480220405. [DOI] [PubMed] [Google Scholar]
- Gramsbergen A. Posture and locomotion in the rat: independent or interdependent development? Neurosci Biobehav Rev. 1998;22:547–553. [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J Neurosci. 2003;23:587–600. doi: 10.1523/JNEUROSCI.23-02-00587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentall ID, Zorman G, Kansky S, Fields HL. Relations among threshold, spike height, electrode distance, and conduction velocity in electrical stimulation of certain medullospinal neurons. J Neurophysiol. 1984;51:968–977. doi: 10.1152/jn.1984.51.5.968. [DOI] [PubMed] [Google Scholar]
- Hsu JY, Stein SA, Xu XM. Development of the corticospinal tract in the mouse spinal cord: a quantitative ultrastructural analysis. Brain Res. 2006;21:16–27. doi: 10.1016/j.brainres.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Jamon M. The early development of motor control in neonate rat. C R Palevol. 2006;5:657–666. [Google Scholar]
- Jamon M, Clarac F. Early walking in the neonatal rat: a kinematic study. Behav Neurosci. 1998;112:1218–1228. doi: 10.1037//0735-7044.112.5.1218. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Rev. 2008;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Carlin K, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res. 1999a;816:493–499. doi: 10.1016/s0006-8993(98)01199-8. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Rempel J, Li J, Sawchuk MA, Carlin KP, Brownstone RM. Development of L-type calcium channels and a nifedipine-sensitive motor activity in the postnatal mouse spinal cord. Eur J Neurosci. 1999b;11:3481–3487. doi: 10.1046/j.1460-9568.1999.00765.x. [DOI] [PubMed] [Google Scholar]
- Kinjo N, Atsuta Y, Webber M, Kyle R, Skinner RD, Garcia-Rill E. Medioventral medulla-induced locomotion. Brain Res Bull. 1990;24:509–516. doi: 10.1016/0361-9230(90)90104-8. [DOI] [PubMed] [Google Scholar]
- Kudo N, Furukawa F, Okado N. Development of descending fibers to the rat embryonic spinal cord. Neurosci Res. 1993;16:131–141. doi: 10.1016/0168-0102(93)90080-a. [DOI] [PubMed] [Google Scholar]
- Lakke EA. The projections to the spinal cord of the rat during development: a timetable of descent. Adv Anat Embryol Cell Biol. 1997;135:1–143. doi: 10.1007/978-3-642-60601-4. [DOI] [PubMed] [Google Scholar]
- Lefler Y, Arzi A, Reiner K, Sukhotinsky I, Devor M. Bulbospinal neurons of the rat rostromedial medulla are highly collateralized. J Comp Neurol. 2008;506:960–978. doi: 10.1002/cne.21586. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A, O'Donovan MJ. Calcium imaging of motoneuron activity in the en-bloc spinal cord preparation of the neonatal rat. J Neurophysiol. 1995;74:1324–1334. doi: 10.1152/jn.1995.74.3.1324. [DOI] [PubMed] [Google Scholar]
- Li Y, Brewer D, Burke RE, Ascoli GA. Developmental changes in spinal motoneuron dendrites in neonatal mice. J Comp Neurol. 2005;483:304–317. doi: 10.1002/cne.20438. [DOI] [PubMed] [Google Scholar]
- Lin BJ, Chen TW, Schild D. Cell type-specific relationships between spiking and [Ca2+]i in neurons of the Xenopus tadpole olfactory bulb. J Physiol. 2007;582:163–175. doi: 10.1113/jphysiol.2006.125963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse. Philos Trans R Soc Lond B Biol Sci. 1981;293:477–508. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–2453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Muscle tone facilitation and inhibition after orexin-A (hypocretin-1) microinjections into the medial medulla. J Neurophysiol. 2002;87:2480–2489. doi: 10.1152/jn.2002.87.5.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida H, Glover JC. 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2004. Development of functional synaptic connection between vestibulo-occular projections neurons and occulomotor motoneurons in the chicken embryo. Program No. 946.17. [Google Scholar]
- Pasqualetti M, Díaz C, Renaud JS, Rijli FM, Glover JC. Fate-mapping the mammalian hindbrain: segmental origins of vestibular projection neurons assessed using rhombomere-specific Hoxa2 enhancer elements in the mouse embryo. J Neurosci. 2007;27:9670–9681. doi: 10.1523/JNEUROSCI.2189-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Halliday G, Watson C, Koutcherov Y, Wang HQ. Atlas of the Developing Mouse Brain at E17.5, P0 and P6. San Diego: Elsevier; 2007. [Google Scholar]
- Perreault M-C, Drew T, Rossignol S. Activity of medullary reticulospinal neurons during fictive locomotion. J Neurophysiol. 1993;69:2232–2247. doi: 10.1152/jn.1993.69.6.2232. [DOI] [PubMed] [Google Scholar]
- Personius KE, Chang Q, Mentis GZ, O'Donovan MJ, Balice-Gordon RJ. Reduced gap junctional coupling leads to uncorrelated motor neuron firing and precocious neuromuscular synapse elimination. Proc Natl Acad Sci U S A. 2007;104:11808–11813. doi: 10.1073/pnas.0703357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BW, Maunz RA, Pitts NG, Mackel RG. Patterns of projection and branching of reticulospinal neurons. Exp Brain Res. 1975;23:333–351. doi: 10.1007/BF00238019. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp Brain Res. 1979;36:1–20. doi: 10.1007/BF00238464. [DOI] [PubMed] [Google Scholar]
- Reed WR, Shum-Siu A, Magnuson DS. Reticulospinal pathways in the ventrolateral funiculus with terminations in the cervical and lumbar enlargements of the adult rat spinal cord. Neuroscience. 2008;151:505–517. doi: 10.1016/j.neuroscience.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerig B, Feller MB. Neurotransmitters and gap junctions in developing neural circuits. Brain Res Rev. 2000;32:86–114. doi: 10.1016/s0165-0173(99)00069-7. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movements. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 173–216. [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol. 2004;92:2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- Smetters D, Majewska A, Yuste R. Detecting action potentials in neuronal populations with calcium imaging. Methods. 1999;18:215–221. doi: 10.1006/meth.1999.0774. [DOI] [PubMed] [Google Scholar]
- Szokol K, Glover JC, Perreault M-C. 2007 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2007. Differential activation of lumbar motoneurons by reticulospinal inputs in neonatal mouse. Program No. 924.10. [Google Scholar]
- Takakusaki K. Forebrain control of locomotor behaviors. Brain Res Rev. 2008;57:192–198. doi: 10.1016/j.brainresrev.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Oohinata-Sugimoto J, Saitoh K, Habaguchi T. Role of basal ganglia-brainstem systems in the control of postural muscle tone and locomotion. Prog Brain Res. 2004;143:231–237. doi: 10.1016/S0079-6123(03)43023-9. [DOI] [PubMed] [Google Scholar]
- VanderHorst VGJM, Ulfhake B. The organization of the brainstem and spinal cord of the mouse: relationship between monoaminergic, cholinergic and spinal projection systems. J Chem Neuroanat. 2006;31:2–36. doi: 10.1016/j.jchemneu.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Vinay L, Brocard F, Clarac F, Norreel JC, Pearlstein E, Pflieger JF. Development of posture and locomotion: an interplay of endogenously generated activities and neurotrophic actions by descending pathways. Brain Res Rev. 2002;40:118–129. doi: 10.1016/s0165-0173(02)00195-9. [DOI] [PubMed] [Google Scholar]
- Vinay L, Cazalets JR, Clarac F. Evidence for the existence of a functional polysynaptic pathway from trigeminal afferents to lumbar motoneurons in the neonatal rat. Eur J Neurosci. 1995;7:143–151. doi: 10.1111/j.1460-9568.1995.tb01028.x. [DOI] [PubMed] [Google Scholar]
- Westerga J, Gramsbergen A. The development of locomotion in the rat. Dev Brain Res. 1990;57:163–174. doi: 10.1016/0165-3806(90)90042-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.