Both central and local factors play an important role in the regulation of skeletal muscle blood flow during exercise. The skeletal muscle pump is thought to be crucial in coordinating the local and systemic blood flow responses during exercise (Rowell, 1993). During the concentric phase of muscle contraction there is expulsion and central mobilization of peripheral venous blood, thus facilitating venous return and increasing stroke volume (SV) and cardiac output 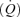 . Indeed, a single muscle contraction has been shown to be effective at emptying the venous vessels and enhancing the central translocation of more than 40% of the intramuscular blood volume (Stewart et al. 2004). By improving
. Indeed, a single muscle contraction has been shown to be effective at emptying the venous vessels and enhancing the central translocation of more than 40% of the intramuscular blood volume (Stewart et al. 2004). By improving 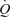 potential to match that of active muscle vasodilatation, muscle pump-induced displacement of blood centrally can indirectly promote higher exercise hyperaemia.
potential to match that of active muscle vasodilatation, muscle pump-induced displacement of blood centrally can indirectly promote higher exercise hyperaemia.
Additionally, the skeletal muscle pump is hypothesized to directly enhance local blood flow in the active muscle. The onset of exercise hyperaemia is rapid and can be significantly elevated within 1 s following a single contraction. The skeletal muscle pump may be involved in the rapid onset of hyperaemia by increasing the pressure gradient across the muscle vascular bed (via reduced venous pressure) following muscle relaxation (Sheriff et al. 1993). It is also thought to cause mechanical deformation of the vascular wall during muscle lengthening and shortening that can elicit dilatation of intramuscular arteries and arterioles. Both human and animal studies support an independent mechanically driven vasodilator pathway during the onset of exercise hyperaemia.
In a recent study in The Journal of Physiology, González-Alonso et al. (2008) evaluated the role of the skeletal muscle pump and vasodilatation on cardiovascular function during exercise in humans. The primary aim of the study was to partition the influence of leg vasodilatation and muscle mechanical contributions to skeletal muscle hyperaemia and cardiovascular function during exercise. In order to achieve this aim, González-Alonso et al. infused incremental doses of ATP into the femoral artery and measured central cardiovascular responses, specifically central venous pressure (CVP), SV and 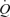 . In addition, leg and systemic haemodynamics were measured during incremental one-legged knee-extensor exercise. González-Alonso and colleagues demonstrated that femoral artery infusion of ATP matched the increase in
. In addition, leg and systemic haemodynamics were measured during incremental one-legged knee-extensor exercise. González-Alonso and colleagues demonstrated that femoral artery infusion of ATP matched the increase in 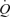 and leg blood flow (LBF) observed during incremental knee-extensor exercise without altering CVP or muscle metabolism. These findings suggest that skeletal muscle vasodilatation drives the increase in
and leg blood flow (LBF) observed during incremental knee-extensor exercise without altering CVP or muscle metabolism. These findings suggest that skeletal muscle vasodilatation drives the increase in 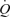 during exercise. Interestingly the increase in
during exercise. Interestingly the increase in 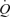 with graded ATP infusions was associated with progressive elevations in heart rate (HR) and SV, whereas, the same increase in
with graded ATP infusions was associated with progressive elevations in heart rate (HR) and SV, whereas, the same increase in 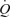 observed during exercise was completely driven by a curvilinear increase in HR above 30% of peak power.
observed during exercise was completely driven by a curvilinear increase in HR above 30% of peak power.
González-Alonso and colleagues next systematically addressed the contribution of the skeletal muscle pump and mechanical deformation on local and systemic exercise hyperaemia. Specifically, they compared the local and systemic responses during passive and active knee-extensor exercise, cyclic thigh compressions alone or in combination with passive and voluntary exercise and separate femoral vein and artery ATP infusion. Passive knee-extensor exercise resulted in small increases in leg muscle and systemic perfusion. Furthermore, superimposing thigh compressions upon exercise increased LBF when exercise was passive but not during voluntary exercise. Neither of the passive exercise conditions (with and without thigh compressions) altered 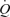 , mean arterial pressure (MAP), or leg oxygen consumption.
, mean arterial pressure (MAP), or leg oxygen consumption.
The main novel findings of González-Alonso et al. are that (a) arterial ATP infusion in the leg matches the increase in cardiac output observed during one-legged knee extensor exercise at the same LBF, without inducing changes in CVP, and (b) passive knee extensor exercise and mechanical compressions to the thigh caused leg blood flow to increase minimally. From these findings, González-Alonso and colleagues concluded that the muscle pump is not compulsory for sustaining venous return, CVP, SV and 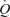 or maintaining muscle blood flow during one legged exercise in humans.
or maintaining muscle blood flow during one legged exercise in humans.
Until recently, the muscle pump was assumed to play a fundamental role in maintaining venous return, end-diastolic volume and SV during upright exercise in humans (Rowell, 1993). González-Alonso et al. are the first to indicate that during local vasodilatation in humans LBF, SV and 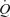 increased to that observed during exercise, suggesting that venous return can be augmented by vasodilatation alone. Therefore, the muscle pump may not be as obligatory during exercise as previously thought (Rowell, 1993).
increased to that observed during exercise, suggesting that venous return can be augmented by vasodilatation alone. Therefore, the muscle pump may not be as obligatory during exercise as previously thought (Rowell, 1993).
The increase in 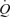 during exercise and ATP-induced vasodilatation demonstrated by González-Alonso and colleagues were, however, driven by different mechanisms. During exercise, the majority of the augmentation in
during exercise and ATP-induced vasodilatation demonstrated by González-Alonso and colleagues were, however, driven by different mechanisms. During exercise, the majority of the augmentation in 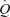 was driven by HR because SV plateaued at around 30% of peak power. Conversely, during ATP infusion the majority of the increase in
was driven by HR because SV plateaued at around 30% of peak power. Conversely, during ATP infusion the majority of the increase in 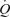 was driven by elevations in SV. González-Alonso et al. suggest that SV was blunted during exercise due to tachycardia; however, at a similar heart rate during ATP infusion SV was ∼20 ml higher compared to exercise. It is possible; therefore, that HR was not limiting stroke volume during exercise and may be associated with the increase in afterload that occurs during exercise. Additionally, CVP and MAP remained unchanged during ATP infusion, whereas MAP increased throughout exercise and CVP was elevated at the end of exercise. Therefore, González-Alonso and colleagues suggest that vasodilatation alone can increase SV and
was driven by elevations in SV. González-Alonso et al. suggest that SV was blunted during exercise due to tachycardia; however, at a similar heart rate during ATP infusion SV was ∼20 ml higher compared to exercise. It is possible; therefore, that HR was not limiting stroke volume during exercise and may be associated with the increase in afterload that occurs during exercise. Additionally, CVP and MAP remained unchanged during ATP infusion, whereas MAP increased throughout exercise and CVP was elevated at the end of exercise. Therefore, González-Alonso and colleagues suggest that vasodilatation alone can increase SV and 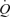 without augmenting CVP. Increased CVP promotes right atrial filling by increasing the pressure gradient between the right atrium and the central veins. Consequently, SV must have increased via several other mechanisms such as a reduction in afterload or sympathetically mediated increases in cardiac contractility. However, there was no evidence of alterations in afterload or cardiac sympathetic activity during ATP infusion. The increase in SV during ATP infusion therefore remains to be elucidated. It is possible that differences in SV between conditions may be explained by the validity of the model flow method to estimate SV in different haemodynamic conditions.
without augmenting CVP. Increased CVP promotes right atrial filling by increasing the pressure gradient between the right atrium and the central veins. Consequently, SV must have increased via several other mechanisms such as a reduction in afterload or sympathetically mediated increases in cardiac contractility. However, there was no evidence of alterations in afterload or cardiac sympathetic activity during ATP infusion. The increase in SV during ATP infusion therefore remains to be elucidated. It is possible that differences in SV between conditions may be explained by the validity of the model flow method to estimate SV in different haemodynamic conditions.
In addition, the effect of changes in intrathoracic pressure during exercise on cardiac function and venous return should be considered. In this context, changes in intrathoracic pressure during exercise may alter right ventricular filling, therefore the muscle pump may be fundamental in forcing blood from the peripheral veins into the thorax at greater intrathoracic pressures, which does not occur during ATP infusion alone. However, the fact remains that at rest local vasodilatation alone appears to increase 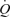 to that demonstrated during exercise.
to that demonstrated during exercise.
It is noteworthy that at LBF greater than 7 l min−1, leg vascular conductance was greater during local ATP infusion than during exercise. This suggests that a greater amount of vasodilatation was needed to produce the same blood flow during ATP infusion. During exercise there was an increase in MAP, which assisted in meeting the flow demand of the active muscle. Consequently, there was less need for vasodilatation during exercise to produce the same leg blood flow compared to the ATP infusion condition. A lack of vasoconstriction in the leg during ATP infusion may also account for the greater conductance compared to during exercise. It is possible that circulating ATP plays a pivotal role in the control of muscle blood flow by causing vasodilatation and abolishing α-adrenergic vasoconstriction. Indeed, infusion of ATP into the resting leg has been shown to fully blunt the effects of increased sympathetic vasoconstrictor activity via tyramine infusion (Rosenmeier et al. 2004). The mechanism by which ATP overrides the increases in vasoconstrictor tone remains uncertain at this time. Another potential explanation for the difference in leg vascular conductance between the exercise and ATP infusion is the amount of tissue that is being dilated with each condition. With exercise the dilatation is restricted to the active muscles, whereas during ATP infusion the dilatation can occur in the majority of the leg.
Despite an in-depth discussion and explanation of the findings from this study, the main question remains: what causes the increase in 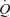 and specifically SV during ATP-induced vasodilatation? The large increase in SV alone suggests that venous return is increased during local vasodilatation and increased LBF. González-Alonso and colleagues point out that it cannot be mediated via a redistribution of blood to the central circulation as (a) the control leg blood flow and vascular conductance were unchanged with arterial ATP infusion, (b) blood flow in the non-exercising and non-infused bodily tissues is the same with graded ATP infusion and exercise, and (c) vascular conductance in the non-exercising and non-infused tissue remained unchanged with increasing rates of ATP infusion. Whilst the authors eloquently point out what mechanisms could not have increased preload and therefore SV, they do not provide insight to what mechanisms could have contributed to elevations in SV.
and specifically SV during ATP-induced vasodilatation? The large increase in SV alone suggests that venous return is increased during local vasodilatation and increased LBF. González-Alonso and colleagues point out that it cannot be mediated via a redistribution of blood to the central circulation as (a) the control leg blood flow and vascular conductance were unchanged with arterial ATP infusion, (b) blood flow in the non-exercising and non-infused bodily tissues is the same with graded ATP infusion and exercise, and (c) vascular conductance in the non-exercising and non-infused tissue remained unchanged with increasing rates of ATP infusion. Whilst the authors eloquently point out what mechanisms could not have increased preload and therefore SV, they do not provide insight to what mechanisms could have contributed to elevations in SV.
In conclusion, González and colleagues are the first to demonstrate that ATP-induced vasodilatation alone can increase 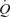 to that observed in one-legged knee extensor exercise. The authors therefore suggest that the muscle pump may not play a fundamental role in increasing venous return, preload and
to that observed in one-legged knee extensor exercise. The authors therefore suggest that the muscle pump may not play a fundamental role in increasing venous return, preload and 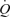 during exercise. Since the study utilized a one-legged exercise model, the conclusions drawn may underestimate the role of the muscle pump during upright whole-body exercise. Furthermore, the mechanisms by which local vasodilatation augmented
during exercise. Since the study utilized a one-legged exercise model, the conclusions drawn may underestimate the role of the muscle pump during upright whole-body exercise. Furthermore, the mechanisms by which local vasodilatation augmented 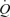 remain unknown. Future studies should aim to examine changes in cardiac filling and venous pressures during ATP-induced vasodilatation.
remain unknown. Future studies should aim to examine changes in cardiac filling and venous pressures during ATP-induced vasodilatation.
Acknowledgments
The authors thank Dr Michael J. Joyner for his critical evaluation of this manuscript and helpful suggestions. Additionally, the authors apologize for not citing all relevant articles due to reference limitations.
References
- González-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol. 2008;586:2405–2417. doi: 10.1113/jphysiol.2008.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. Central circulatory adjustments to dynamic exercise. [Google Scholar]
- Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump? Am J Physiol Heart Circ Physiol. 1993;265:H1227–H1234. doi: 10.1152/ajpheart.1993.265.4.H1227. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Medow MS, Montgomery LD, McLeod K. Decreased skeletal muscle pump activity in patients with postural tachycardia syndrome and low peripheral blood flow. Am J Physiol Heart Circ Physiol. 2004;286:H1216–H1222. doi: 10.1152/ajpheart.00738.2003. [DOI] [PubMed] [Google Scholar]


