Abstract
The tubular (t) system is essential for normal function of skeletal muscle fibre, acting as a conduit for molecules and ions within the cell. However, t system accessibility and interconnectivity have been mainly assessed in fixed cells where the t system no longer fully represents that of the living cell. Here, fluorescent dyes of different diameter were allowed to equilibrate within the t system of intact fibres from toad, mechanically skinned to trap the dyes, and then imaged using confocal microscopy to investigate t system accessibility and interconnectivity. Dual imaging of rhod-2 and a 500 kDa fluorescein dextran identified regions throughout the t system that differed in the accessibility to molecules of different molecular weight. Restrictions within the t system lumen occurred at the junctions of the longitudinal and transverse tubules and also where a transverse tubule split into two tubules to maintain their alignment with Z-lines of adjacent mis-registered sarcomeres. Thus, three types of tubule, transverse, longitudinal and Z, can be identified by their lumenal diameter in this network. The latter we define for the first time as a tubule with a narrow lumen that is responsible for the change in register. Stretch-induced t system vacuolation showed exclusive access of rhod-2 to these structures indicating their origin was the longitudinal tubules. Exposing the sealed t system to highly hypertonic solution reversed vacuolation of longitudinal tubules and also revealed that these tubules are not collapsible. Fluorescence recovery after photobleaching (FRAP) measurements of t system-trapped fluo-5 N showed interconnectivity through the t system along the axis of the fibre. However, diffusion occurred at a rate slower than expected given the known number of longitudinal tubules linking adjacent transverse tubules. This could be explained by the observed narrow opening to the longitudinal tubules from transverse tubules, reducing the effective cross-sectional area in which molecules could move within the t system.
In skeletal muscle fibres the tubular (t) system is the continuation of the surface membrane (sarcolemma), which invaginates into the cell and forms the main interface between the myoplasm and the extracellular environment. The t system primarily consists of transverse tubules; these elements of the t system form junctions with the internal Ca2+ store of the cell, the sarcoplasmic reticulum (SR). This arrangement is essential in the transduction of the signal from the propagating action potential through the transverse tubular voltage sensor to the SR Ca2+ release channel in the process known as excitation–contraction coupling (EC coupling; Melzer et al. 1995). Thus, the lumenal composition of the t system (i.e. the extracellular fluid within the cell) and t system architecture are essential in the normal function of EC coupling.
The accessibility of t system was described in some detail by imaging extracellularly applied horseradish peroxidase (HRP) with electron microscopy (e.g. Eisenberg & Eisenberg, 1968). This and similar work indeed showed that the majority of the t system consisted of transverse tubules and hence led to this membrane system being commonly referred to as the transverse tubular system. However, HRP is a relatively large molecule with a molecular weight of ∼44 kDa and may be excluded from tubules with a narrow diameter. Indeed, longitudinal tubules also form part of this membrane system. These structures were originally observed by the Italian microscopist E. Veratti at the beginning of the 20th century (later republished in English: Veratti, 1961) where he used a membrane stain to visualize transverse tubules and the interconnecting longitudinal tubules.
The longitudinal tubules of the t system represent less than 3% of the total membrane area of the t system in frog (Eisenberg, 1983). This translates to about 75–100 longitudinal connections across adjacent transverse tubules in a fibre of around 50 μm diameter, ensuring the t system is an internally connected network within the muscle fibre. Longitudinal tubules form junctions between adjacent transverse tubules (Veratti, 1961) and often run ‘in-series’ along the axis of the fibre (Launikonis & Stephenson, 2002a). Furthermore, Peachey & Eisenberg (1978) also described complex helicoidal structures in frog fibres, which provide longitudinal connections in a similar way in which a longitudinal change in register between the position of transverse tubules has been described in rat fibres (Launikonis & Stephenson, 2004). Similarly, the t system of cardiac muscle cells has been shown to be a network of interconnecting tubules in almost a rectangular array (Soeller & Cannell, 1999). In fact, these authors suggested that the membrane network be renamed ‘the sarcolemmal Z-rete’ to more aptly describe its architecture.
The skeletal muscle t system is important in distributing relevant ions and molecules in the maintenance of normal membrane potential (especially during periods of work; Nielsen et al. 2004; Shorten & Soboleva, 2007), Ca2+ homeostasis (Almers et al. 1981; Friedrich et al. 2001; Launikonis & Ríos, 2007) and during trafficking of molecules such as insulin and lactate into or out of the cell (Lännergren et al. 1999, 2000; Shorten et al. 2007). The transverse tubules provide the direct link between the extracellular space and the interior of the cell. However, this communication is limited in the axial direction for each transverse tubule. So the dissipation of any local ionic or molecular gradient created within the fibre will be limited by the route to the interstitial fluid provided by the transverse tubules. Distributing molecules and ions longitudinally within the t system of the fibre could be important. The ability of the longitudinal tubules to distribute molecules in the cell is not known.
It is also important to consider the role of t system vacuolation in skeletal muscle. This phenomenon is easily observed under certain conditions in amphibian muscle (Krolenko et al. 1995; Krolenko & Lucy, 2001) and to a lesser extent in mammalian muscle (Lännergren et al. 1999, 2000; Yeung et al. 2002). Therefore, it is highly relevant to determine where vacuoles form within the t system and how molecules and ions can be distributed throughout.
Mechanically skinned fibres, where the sarcolemma was removed with fine forceps causing the t system to seal over (Launikonis & Stephenson, 2004), have already been used to show that action potentials can propagate along the long axis of mammalian fibres throughout the t system (Posterino et al. 2000). We now wish to examine the accessibility and interconnectivity of the elements of the t system in skeletal muscle fibres to different sized molecules.
To perform this work we allowed two extracellularly applied fluorescent dyes of different molecular weights to equilibrate within the t system of the same intact fibre. Following this, the fibre was mechanically skinned to trap the dyes in the t system. Skinned fibres were bathed in an internal solution and imaged on a confocal microscope. Confocal imaging of skinned fibres confers a better image quality compared to similar techniques in intact fibres where dye must constantly surround the preparation, resulting in significant contamination from out-of-focus light (e.g. Launikonis & Stephenson, 2002b; Yeung et al. 2002). Clearer imaging of the t system in skinned fibres allowed easier discrimination between various tubules compared to imaging of intact cells.
Methods
Experiments were conducted at The University of Queensland, on Cane toads (Bufo marinus), which were stunned with a blow to the head and then double pithed in accordance with the procedures approved by the Animal Ethics Committee at the University of Queensland. Cane toads were obtained from Peter Krauss (Mareebra, Qld, Australia) and Alex Thorn (Brisbane, Qld, Australia). Twenty five toads were used in this study. Animal care regulations were adhered to as governed by the Animal Ethics Committee at the University of Queensland. The iliofibularis muscles were rapidly removed, blotted on filter paper and then pinned down in a Petri dish above a layer of Sylgard 184 (Dow chemicals, Midland, MI, USA) under a layer of paraffin oil.
The method of trapping dye in the t system of skinned fibres has been described in detail elsewhere (Lamb et al. 1995). Briefly, a small bundle of intact fibres was isolated and exposed to a Na+-based physiological solution containing fluorescent dye (see below). Single fibres were then isolated and mechanically skinned. Skinned fibres were then transferred to a custom-built experimental well which used a glass coverslip as a base.
Solutions
The dye-containing physiological solution was composed of (mm): NaCl, 112; KCl, 3.3; CaCl2, 2.5; MgCl2, 1, Hepes, 20 (pH adjusted to 7.4 with NaOH) and a combination of one or two fluorescent dyes. For a given experiment, different dyes were loaded into the t system. For FRAP experiments, fluo-5 N was added to the physiological solution at 1 mm and exposed to intact fibre bundles. In other experiments, rhod-2 and 500 kDa fluorescein dextran were loaded into the t system in the same physiological solution. The 500 kDa fluorescein dextran was subjected to a standard gel filtration method followed by concentration in a centrifugal filter and resuspended in the physiological solution (Larina et al. 2007). The concentration of fluorescein dextran added to the physiological solution was close to 7 mg ml−1. The [rhod-2] in the solution was 1 mm.
Rhod-2 is a high-affinity Ca2+ indicator with a Kd approaching 1 μm. The standard internal solution in the current experiments contained 100 nm Ca2+ and 1 mm Mg2+ and as such should not induce any significant leak of t system Ca2+ (Launikonis & Ríos, 2007). Nor should this solution induce release of [Ca2+]SR, thus avoiding activation of store-operated Ca2+ entry (SOCE). Furthermore, in SOCE experiments performed by Launikonis & Ríos (2007), the [Ca2+]t-sys never dropped below ∼0.1 mm. Therefore, we expect that [Ca2+]t-sys will consistently lie in the hundreds of micromolar range in the standard solution allowing rhod-2 to fluoresce at its maximum.
The standard internal solution contained (mm): K+, 117; Na+, 30; EGTA, 1; Ca2+, 0.0001; Mg2+, 1; Hepes, 60; ATP, 8; and creatine phosphate, 10. The pH of the standard solution was set to 7.1 with KOH. Osmolality was 260 ± 10 mosmol kg−1. In some experiments the standard internal solution was diluted 20% by addition of double-distilled water for a final osmolality of 208 mosmol kg−1 or sucrose was added to increase osmolality to 2600 mosmol kg−1.
Confocal imaging
Coverslips with dye-loaded preparations were placed on the stage above a water immersion objective (40×, NA 0.9 or 60×, NA 1.0) of a laser scanning confocal microscope (FV1000, Olympus, Tokyo, Japan). The xy pixel size was varied throughout experiments, and for the presented figures was: Fig. 1, 621 nm; Fig. 2, 310 nm; Fig. 3A, 414 nm; Fig. 3B, 103 nm, respectively; Fig. 4, 206 nm; Fig. 5, 207 nm; Fig. 6, 103 nm; Fig. 7, 621 nm. Two modes of imaging were performed: simultaneous imaging of two dyes trapped in the t system or imaging of one dye with a simultaneous bleaching of the dye in a smaller region of interest (ROI) with a second independently scanning laser (see below). All imaging was performed in xy mode. Simultaneous acquisition of two images was achieved by line-interleaving of two laser lines of 488 and 543 nm from a multi-line Ar and He–Ne lasers to excite fluorescein dextran and rhod-2, respectively. Emitted light was collected in the ranges 490–540 nm and 562–666 nm, respectively. Scanning of fluorescein dextran and rhod-2 was performed sequentially, such that the 488 and 543 nm lasers were turned on and off to reduce any bleed-through effects on the images taken.
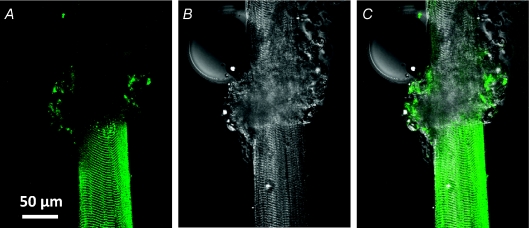
Figure 1. Extracellular dye is trapped in mechanically skinned fibres.
Confocal image of fluo-5 N fluorescence (A) and transmitted light image (B) of a partially skinned fibre from toad bathed in the standard internal solution. An overlay of A and B is shown in C. The skinned and intact sections are the lower and upper sections of the fibre, respectively. The rolled back sarcolemma amongst myofibrillar bundles is at the interface of the two sections. The intact preparation had been exposed to extracellular fluo-5 N in a physiological solution. Note that fluo-5 N is present in the skinned section, showing the banded pattern of the t system. ID: 062807a.
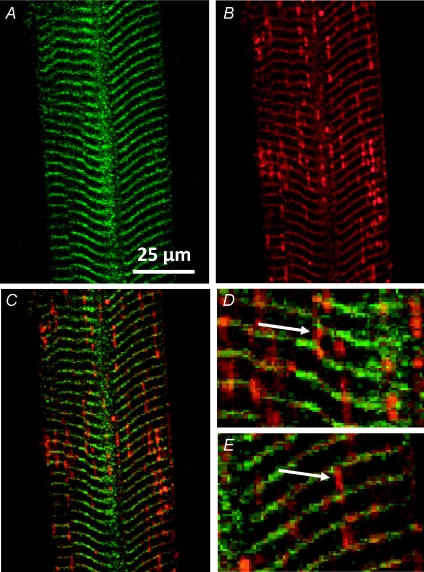
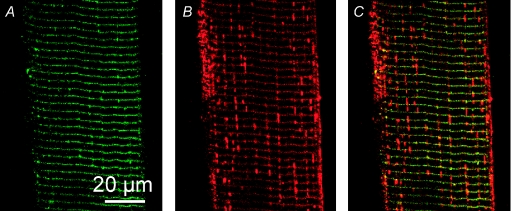
Figure 2. Large molecules cannot enter the longitudinal tubules of toad t system.
Images of 500 kDa fluorescein dextran (A) and rhod-2 (B) fluorescence signal from the t system of a skinned fibre. Overlays of A and B are shown in C–E. Arrows in D and E indicate different types of longitudinal tubules of the t system network. Note that these tubules only emit a rhod-2 fluorescence signal. Sarcomere length (SL) ≈ 4 μm. ID: 090607d.
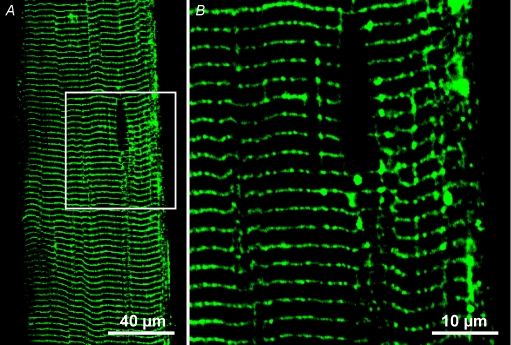
Figure 3. Low molecular weight fluorescein dextran is visible in longitudinal tubules.
3 kDa fluorescein dextran was trapped in the t system of a skinned fibre. The box in A indicates the area imaged at a smaller pixel size (B). Note that 3 kDa fluorescein dextran has entered longitudinal tubules. Also, note in A and B that tubules extend longitudinally from the darkened elliptical region that is most probably occupied by a nucleus. SL ≈ 2.5 μm. ID: 052508l and 052508m.
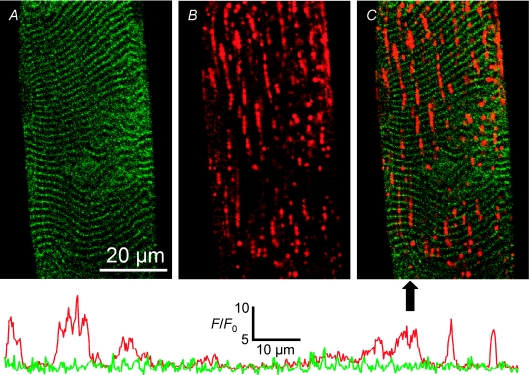
Figure 4. Stretch-induced vacuolation occurs in the longitudinal tubules.
Images of 500 kDa fluorescein dextran (A) and rhod-2 (B) fluorescence signal from the t system of a skinned fibre following stretch. An overlay of A and B is shown in C. The arrow below panel C indicates the position of the line profiles displayed below the images. Each line profile has been normalized to its own minimum (F0) and the absolute scale applies to both lines. The left of the line profile corresponds to the top of the image in C. The line colours correspond to the psuedocolours used in A and B. ID: 090607g.
Figure 5. Highly hypertonic solution does not collapse longitudinal tubules.
Fluorescence from t system trapped 500 kDa fluorescein dextran (A) and rhod-2 (B) in a toad fibre bathed in an internal solution of 2600 mosmol kg−1. An overlay of A and B is shown in C. Note that longitudinal tubules are still present under these conditions. SL ≈ 3.3 μm. ID: 032508i.
Figure 6. Hypotonic solution causes a uniform t system volume increase.
Images of 500 kDa fluorescein dextran (A) and rhod-2 (B) fluorescence signal from the t system of a skinned fibre bathed in an internal solution of 208 mosmol kg−1. An overlay of A and B is shown in C. Note that the fluorescein dextran is still largely excluded from the longitudinal connections. SL ≈ 3.0 μm. ID: 070708j.
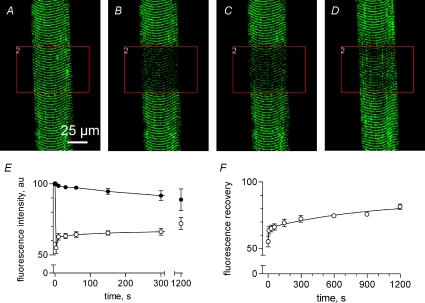
Figure 7. Fluo-5 N is mobile through the t system network.
The fibre was scanned with two independent lasers. The first laser excited the preparation at 488 nm to image fluo-5 N in the sealed t system. The area indicated by the box was illuminated with a second laser to bleach fluo-5 N: A, pre-bleach; B, bleach; C, 10 s post-bleach and D, 20 min post-bleach. Summary (mean ±s.e.m., n= 3) of t system fluorescence (E) in the region chosen for bleaching (^) and the rest of the fibre was used as reference (•). Note that the reference fluorescence decreased during the experiment and this is corrected for in F. SL ≈ 3.0 μm. ID: 082307l.
FRAP experiments were performed while continuously imaging the t system before, during and after photobleaching a ROI within the fibre. This was achieved by synchronizing laser stimulation of the preparation for bleaching and imaging with two independently scanning beams. Images of the t system were acquired by exciting with a multi-line Ar laser at 488 nm with a pixel size of 612 nm at 1.024 ms line−1 while collecting emitted light in the range 490–540 nm. During continuous imaging, a pre-selected ROI within the imaging area of the muscle preparation was stimulated with a blue diode laser at 405 nm. Laser power and duration was adjusted to bleach around 50% of the initial fluorescence in the t system. Typically, laser power was 80% and duration of stimulation was 2 s. This level of bleaching could be changed by varying laser power or duration of illumination. This chosen level of bleaching was used to avoid photodamage to the cell that could occur at more intense laser powers. The area to be bleached was typically 2500 μm2. The preparation was imaged once before initiation of bleaching to provide a reference, during the bleaching (typically 2 frames), and at 10 s immediately following the end of photobleaching. Thereafter, the preparation was scanned intermittently at pre-determined times for the following 30 min.
Stretch
To induce vacuoles, skinned fibres were stretched 5 times by approximately 40% above their slack length. This protocol allowed us to image vacuoles in 4 of 8 fibres prepared in this fashion.
Results
In the following section we show t system-trapped dyes of different molecular weights are not equally accessible to all parts of the t system of live cells. In each case, the fluorescent dyes were allowed to diffuse into the t system from the surrounding extracellular solution of intact fibres. Following an equilibration period of more than 10 min to a small bundle of fibres, a single fibre was isolated and mechanically skinned as previously described to trap the dyes in the t system (Lamb et al. 1995; Launikonis & Stephenson, 2004). The resealing nature of the t system following this procedure is starkly shown in Fig. 1.
Figure 1 shows a fibre from toad that has been partially skinned. The intact and skinned sections as well as the junction between them are visible. Prior to mechanical skinning, the intact fibre had been exposed to a physiological solution containing 1 mm fluo-5 N. Following skinning, the preparation was transferred to the standard internal solution (see Methods). Figure 1A is a confocal image of fluo-5 N fluorescence emitted from the preparation and Fig. 1B is the transmitted light image recorded simultaneously. Figure 1C is an overlay of A and B. The skinned section of the fibre corresponds to the lower half and the intact section the upper half of the preparation in the images. In the middle of the preparation are the rolled back sarcolemma and myofibrillar bundles. A droplet of oil is also present on the left side of the intact section of fibre (Fig. 1B).
The confocal image (Fig. 1A) clearly shows the fluo-5 N fluorescence emitting the regular banded pattern of the t system. This fluorescence pattern extends all the way to the point where the fibre has been skinned, indicating the t system has sealed everywhere where the sarcolemma has been removed. No fluorescence banded pattern is evident in the intact section of fibre because the dye has diffused out of the open t system into the bathing solution. The confocal image of the sealed t system (Fig. 1A) and transmitted light image of the skinned section of fibre (Fig. 1B) have the same defined edge (Fig. 1C) indicating that the t system seals precisely at the site where the outer myofibrillar bundles are stripped away. Some fluorescence is also evident within the rolled up sarcolemma between the intact and skinned section where Ca2+ and dye were trapped.
Accessibility of the t system
Different sized fluorescent dyes were trapped in the t system of muscle fibres in order to determine the accessibility of the various tubules of the t system to these molecules. Figure 2 shows images of the t system of a toad skinned fibre loaded with 500 kDa fluorescein dextran and rhod-2. Fluorescein dextran and rhod-2 fluorescence images are shown in Figure 2A and B, respectively. An overlay is shown in C. Both images A and B clearly show the transverse tubules at the position of the Z-line. However, only the rhod-2 signal is emitted from longitudinal structures. This shows that the large dye cannot enter the longitudinal tubules of the toad t system. This suggests there must at least be a restriction at both junctions of the longitudinal tubule with the transverse tubules it connects. Some swellings of the longitudinal tubules, which contain only rhod-2, appear close to the junction with the transverse tubules. This phenomenon has been reported before following mechanical skinning (Launikonis & Stephenson, 2002a) but are not always present (see Supplemental Fig. 1 for another example where fluorescence of rhod-2 in the longitudinal tubules remain relatively uniform throughout the preparation). This physical feature of the t system network was observed in 15 of 18 fibres examined. (i.e. three fibres allowed the fluorescein dextran to enter longitudinal elements of the t system network). This suggests that the diameter of the fluorescein dextran was close to that of the longitudinal tubule.
Figure 2D and E show enlarged regions from Fig. 2C. Two types of longitudinal connections linking transverse tubules are represented in these images. Figure 2D shows a transverse tubule at a point where it ‘forks’ into two separate transverse tubules. This ‘forking’ is necessary to compensate for the mis-alignment of adjacent sarcomeres, so that the t system can remain continuous while being positioned at the respective Z-line of adjacent sarcomeres (Peachey & Eisenberg, 1978). Image D shows exclusion of the 500 kDa fluorescein dextran at this junction. Therefore, there must be a restriction within the lumen of the transverse tubule either side of where the dextran signal ceases. In Fig. 2E, a longitudinal tubule that joins two adjacent transverse tubules also shows exclusion of the fluorescein dextran. It is also important to note here that rhod-2 salt loaded into the t system will not load into mitochondria. Furthermore, the longitudinal structures shown in Fig. 2 do not resemble the mitochondria of amphibian skeletal muscle fibres which do indeed run longitudinally, but are positioned near the edge of the fibre and are much thicker (Lännergren et al. 1999; Launikonis et al. 2005).
It is possible that a lower signal-to-noise ratio in the fluorescein dextran images compared to the rhod-2 images could result in an apparent exclusion of fluorescein dextran signal from the longitudinal tubules. To determine whether fluorescein dextran signal could be imaged in all t system compartments that rhod-2 was, a 3 kDa fluorescein dextran trapped in the sealed t system was imaged (Fig. 3). Indeed Fig. 3 (also see online Supplemental Fig. 3), shows that low molecular weight fluorescein dextran (3 kDa) can enter longitudinal tubules. This figure also shows tubules extending from a pole of the darkened elliptical region observed in Fig. 3A. The area within the white box in A encompassing this area is displayed at higher magnification in B. The darkened elliptical region most probably represents the position of a nucleus. The tubules extending longitudinally from the pole of the nucleus into the myofibril-free cytoplasmic space are occupied by Golgi apparatus and mitochondria (Voigt & Dauber, 2004). The presence of fluorescein dextran (Fig. 3) and lanthanum in electron microscopy studies (Voigt & Dauber, 2004) in these tubules indicates they are accessible from the transverse tubules.
Vacuolation of the t system
A well-known phenomenon in amphibian skeletal muscle is the reversible vacuolation of the t system in response to rapid removal of glycerol (Krolenko et al. 1995; Krolenko & Lucy, 2001). Such vacuoles can also form following fatigue or stretch (Lännergren et al. 1999, 2000; Yeung et al. 2002). To gain insight into whether defined regions of the t system are responsible for these local swellings, we examined toad fibres with t system-trapped rhod-2 and fluorescein dextran after inducing vacuolation. Vacuolation does not occur in response to osmotic stress induced by rapid removal of small molecules (such as glycerol or lactate) in mechanically skinned fibres. This is because the mechanism inducing water movement into the t system requires the differential permeability of the sarcolemma (which is absent) and t system to the small molecules (Launikonis & Stephenson, 2004). However, vacuoles often form in amphibian sealed t system (Launikonis & Stephenson, 2002a) and can be induced by stretch. Figure 2 shows how rhod-2 and fluorescein dextran trapped in the sealed t system allow the discrimination of longitudinal and transverse tubules in mechanically skinned fibres. Vacuolation was examined in these preparations to elucidate their tubular origin.
Figure 4 shows changes in the t system network in mechanically skinned fibres that were subjected to stretch (see Methods). The fluorescein dextran fluorescence in Fig. 4A shows that the transverse tubules remain intact following stretch, although some level of distortion is evident (cf. with Fig. 2). Importantly, dye remains trapped in the t system indicating that membrane integrity is maintained following stretch. Figure 4B represents the rhod-2 fluorescence signal from the sealed t system. No transverse tubules are easily observed in this image. The vast majority of the rhod-2 fluorescence signal was emitted from the vacuoles. An overlay of A and B is shown in C. The overlay shows that the vacuoles clearly run along the long axis of the fibre and fall between the transverse tubules.
As the dyes were trapped in the sealed t system, the local fluorescence signal should be roughly proportional to the volume the dye is occupying. The line profiles in Fig. 4 show the amplitudes of both rhod-2 and fluorescein dextran signals through a representative section of the fibre indicated by the arrow below Fig. 4C. The profile of the fluorescein dextran signal (green line) shows a roughly uniform profile of peaks and troughs that represent the transverse tubules in this cell. In contrast the rhod-2 profile shows amplitudes of up to 10 times above baseline fluorescence in some places.
Another example of stretch-induced vacuolation is shown in the online Supplemental Fig. 2 where no damage to the transverse tubules was evident. We observed vacuoles in 4 of 8 fibres following stretch.
Tubule volume changes under osmotic stress
The sealed t system of toad fibres acts as an almost perfect osmometer within the range of ∼190 to ∼640 mosmol kg−1 (Launikonis & Stephenson, 2004). As such, water fluxes across the t system membrane can be induced to manipulate t system volume. Furthermore, by applying osmotic pressures beyond this range, the ability of the tubules to withstand large forces can be observed (Launikonis & Stephenson, 2004). Figure 5 shows a mechanically skinned fibre exposed to a highly hypertonic solution (2600 mosmol kg−1) for more than 5 min. In Fig. 5, both the transverse and longitudinal tubules are visible indicating that both tubules can withstand this high pressure. Note that the fluorescein dextran remains excluded from the longitudinal tubules even under this high hydrostatic pressure. This behaviour was observed in all six fibres exposed to this high hydrostatic pressure.
As volume change occurred locally following stretch (Fig. 4), it was also pertinent to induce an increase in t system volume to observe whether volume change induced by a simple osmotic gradient occur uniformly or locally. Figure 6A and B shows a skinned fibre loaded with rhod-2 and 500 kDa fluorescein dextran in the same manner as in Figs 2–4 and bathed in an internal solution made 20% hypo-osmotic (208 mosmol kg−1). An overlay is shown in C. The t system structure remained the same (no further increases in the apparent diameter) as it was prior to exposure to hypo-osmotic solution. Some fluorescein dextran is observed in vesicles that do not join between adjacent transverse tubules. However the exclusion of fluorescein dextran from the longitudinal tubules is evident from the large amount of rhod-2 that independently occupied these structures. This suggests that the 20% increase in t system volume due to water uptake (Launikonis & Stephenson, 2004) occurs uniformly throughout the t system network. Thus, water entry into the t system due to an imposed simple osmotic gradient is a distinct volume-increasing mechanism than that imposed due to stretch (Fig. 4).
Interconnectivity of the t system lumen
The mobility of small molecules through the longitudinal connections of the skeletal muscle t system was assessed using FRAP in conjunction with dye trapped in the t system of mechanically skinned fibres. Figure 7 shows an example of a skinned fibre preparation with dye trapped in the t system that was continuously imaged before, during and after bleaching. Continuous imaging while bleaching a smaller region of the imaged preparation was achieved using a confocal microscope with two independently scanning lasers (see Methods). The area to be bleached is indicated by the box in Fig. 7. The area chosen for bleaching spans the short axis of the fibre.
Figure 7A shows the fluo-5 N fluorescence signal from the sealed t system immediately prior to bleaching. Figure 7B shows the same area of fibre after the dye was bleached –∼50% initial level in the region indicated by the box. Figure 7C and D shows the same area of fibre 10 s and 20 min post-bleach. Fluorescence recovery within the boxed area is noticeable at both times, indicating fluo-5 N moved continuously through the t system into the optical plane. Figure 7E plots the average change in fluo-5 N fluorescence from the sealed t system in three such experiments as represented in A–D. The filled symbols indicate the change in fluorescence in the areas of the t system outside the box that were not intensely bleached, which serve as reference. This fluorescence signal drops slowly due to lower-level bleaching caused by the scanning 488 nm laser line for imaging and slow extrusion of dye from the t system due to transport via anion exchangers (Launikonis & Stephenson, 2002a). The open symbols represent the average of the fluorescence change inside the box over the imaging period before and after the high-intensity bleaching occurred due to intense illumination by the 405 nm laser. Figure 7F represents the fluorescence recovery corrected from fluorescence loss indicated in the reference areas.
A fast and slow phase of recovery was observed. The fast phase of FRAP showed about 15% fluorescence recovery in the bleached area in approximately 7 s. This was very similar to the fluorescence recovery seen following bleaching of dye in the transverse tubules at the edge of the fibre only (that is, not completely across the short axis as in Fig. 7; not shown). A slow recovery phase was then followed, which lasted in the order of minutes. A sum of two exponential functions could be fitted to the points in F, with rate constants 0.244 ± 0.228 and 0.001 ± 0.001 s−1, respectively. From Fig. 7F it is also clear that there is a component of the t system fluorescence that does not recover. By bleaching the t system dye at lower laser intensities it was observed that the non-recoverable component also decreased. Thus, the non-recoverable component is probably due to a degree of photodamage to the cell.
The simplest explanation for the fast recovery phase of the fluo-5 N fluorescence signal following bleaching is that the dye is not bleached uniformly through the Z-axis of the fibre. Therefore, unbleached dye from above the optical section rapidly moves through the transverse tubules into the imaging plane, accounting for the fast phase. This fast FRAP phase could be eliminated by using wide-field illumination to bleach t system-trapped fura-2, as this causes dye to be bleached at all levels of the Z-axis (data not shown; details of this microscope set-up and bleaching protocol are in Larina et al. 2007). The slow recovery phase of the fluorescence in the bleached region of the t system (Fig. 7) must be due to the diffusion of dye from the t system outside the axial plane of the bleached region. This requires dye to move through the pathway provided by the longitudinal tubules through the t system network.
One can estimate the diffusion coefficient of the dye in the t system from the time it takes to reach half-way recovery considering that the dye diffuses predominantly along the transverse tubules from the region where the dye was not bleached to the region where the dye was bleached and where recovery was measured. Dye bleaching in the t system was assumed to occur uniformly within an optical slice of about 20 μm from the bottom of the preparation and the concentration of the dye in the t system located in the upper part of the preparation, about 30 μm above the optical section, was assumed not to be affected by bleaching, acting as an extending source of limited extent. Calculating dye concentration–distance diffusion curves for an extending source of limited extent and imposing the condition that there is no flow of the dye out of the sealed t system (see equation 2.17 in Crank, 1976), one could estimate that half-way recovery (trec) in the bleached region of the preparation (20 μm) would occur when:
where D is the dye diffusion coefficient and h is the thickness of the region where the dye was not bleached (30 μm). Since measured trec= 3–4 s, it follows that D= 22–30 μm2 s−1.
Discussion
This study has used a fluorescence size-exclusion assay to assess the accessibility within the lumen of the t system network of skeletal muscle fibres from toad. Importantly, dyes were applied to and equilibrated in intact, living muscle fibres ensuring examination of accessibility under conditions as close to that occurring in vivo as possible. It was found there are restrictions in the lumen in at least two distinct junctions that occur within the t system network. These are between the transverse and longitudinal tubules where they join at right angles; and also at the junctional points where one transverse tubule ‘forks’ into two transverse tubules to keep the transverse tubule aligned with the Z-line in adjacent, mis-registered sarcomeres. It was shown that the longitudinal tubules are the origin of stretch-induced vacuoles in toad skeletal muscle. Furthermore, while the t system network is clearly linked within the fibre, its tortuous path and narrow opening to longitudinal tubules allows only for the distribution of molecules within its lumen over no more than a couple of sarcomeres in minutes.
Tubules of the tubular system
The elements that make up the t system network in amphibian skeletal muscle fibres have been imaged before (e.g. Veratti, 1961; Peachey & Eisenberg, 1978; Launikonis & Stephenson, 2004). However, in this study we show for the first time that there are differences in the lumenal dimensions of the tubules that make up the t system network by trapping fluorescent markers with different molecular weights in the t system of live skeletal muscle fibres (Figs 2–5). This information now allows discrimination between tubules by their lumenal diameter.
The Stokes–Einstein radius for 500 kDa fluorescein dextrans can be calculated to 29 nm from the average molecular mass by using the formula: radius = 0.33(molecular mass)0.463 (Venturoli & Rippe, 2005). However, high molecular weight dextrans are known to be highly asymmetrical in shape and thus the Einstein–Stokes radius will be a significant overestimate. The minimum diameter of 500 kDa fluorescein dextran may be closer to 10 nm (Venturoli & Rippe, 2005). This value should only be used as a tentative estimation of the diameter of the lumen of the longitudinal tubules in amphibian skeletal muscle.
The longitudinal tubules did not allow entry of the 500 kDa fluorescein dextran but allowed the entry of the small rhod-2 molecules. These tubules were not collapsible under high hydrostatic pressure (Fig. 5), a feature shared with transverse tubules (Launikonis & Stephenson, 2004). It was not clear from the size-exclusion imaging of fluorescent dyes under normal resting conditions whether the tubules emitting only rhod-2 were restricted at their point of contact with the transverse tubules or whether they were simply narrow along the entire length of the tubule. However, the maintained exclusion of the fluorescein dextran during vacuolation of the longitudinal tubules clearly shows that the restriction at the longitudinal–transverse tubules junction remains regardless of the increasing diameter of the rest of the longitudinal tubules (Fig. 4). Consistent with this, increasing the sealed t system volume uniformly in a 20% hypo-osmotic bathing solution (Fig. 6; Launikonis & Stephenson, 2004) continued to show exclusion of the 500 kDa fluorescein dextran from tubules accessible to rhod-2. This suggests that although all tubules presumably increased their volume by 20% or more, a restriction excluding 500 kDa fluorescein dextran from rhod-2 accessible tubules remained. Thus, we suggest that a thickened membrane ‘collar’ exists at the point of contact between the longitudinal and transverse tubules. Note, it appears that there is slight staining of fluorescein dextran in some longitudinal tubules and this staining appears to fade away at further distances from the transverse tubules (Fig. 5). This would suggest that the ‘collar’ is actually funnel-like, narrowing as it enters the longitudinal tubule.
The membrane collars or different lumenal diameters that we have identified with our size-exclusion fluorescence imaging (Figs 2–6) can be used to define the length of distinct tubules within the t system network. These tubules clearly fall in to three categories: (1) transverse tubules, which are the predominate tubule of the t system and open to the sarcolemma and also form the junctions with SR; (2) longitudinal tubules, which run the length of a sarcomere and link two adjacent transverse tubules; and (3) the tubules that link one transverse tubule with two others to adjust for the change in register of sarcomeres. We introduce the term ‘Z tubule’ to describe this tubule that occurs across mis-registered sarcomeres and is clearly distinct from the transverse tubules but may be considered as another class of longitudinal tubule. The previously described helicoidal structures in frog skeletal muscle t system would therefore be formed from many individual Z tubules, creating the ‘stairwell’ effect (Peachey & Eisenberg, 1978).
Tubules were also observed in the myofibril-free space, which presumably were at the poles of a nucleus within a high density of Golgi and mitochondria (Fig. 3; Voigt & Dauber, 2004). The architecture of the tubules in this region suggests a physiological role for the t system. This would most probably be the transfer of ions and molecules between the intracellular and extracellular environments as EC coupling would not be required in a region without myofibrils.
Vacuolation of the t system
The phenomenon of reversible vacuolation is well-known in skeletal muscle fibres, especially in the amphibian (Krolenko et al. 1995; Lännergren et al. 1999, 2000). Here we show that vacuoles formed following stretch in toad skeletal muscle fibres are indeed swollen longitudinal tubules. The positioning of the stretch-induced vacuoles between the transverse tubules and the exclusive access of rhod-2 is consistent with this (Fig. 4). Exposing vacuolated longitudinal tubules to a highly hypertonic solution also removed the vacuoles by drawing water from them, ‘returning’ these structures to longitudinal tubules.
In mammalian skeletal muscle vacuoles that form following an eccentric contraction protocol have been suggested to be the result of t system disruptions or tears. A tear would allow external Na+ to enter the cell, which would lead to an increased Na+ pump activity and create an osmotic gradient (Yeung et al. 2002). The results in Fig. 4 suggest dye remains well trapped in the t system following stretch suggesting that if any membrane disruption occurred during stretch, it must be brief. As shown in skinned fibres, the transverse tubules seal over across a ∼55 nm gap effectively instantly (Lamb et al. 1995; Launikonis & Stephenson, 2004). Therefore, it would be expected any disruption in the t system membrane caused by stretch would seal over similarly. An alternative hypothesis accounting for possible Na+ entry and vacuole formation may require stretch-sensitive channel activation.
The thickened membrane at the junction of the transverse and longitudinal tubules, or membrane ‘funnel-like collar’, may have physiological implications during stretch. During such activities as eccentric contraction, stretching of the muscle fibre would exert force along the axis of the fibre that the t system must absorb (Yeung et al. 2002). It is clear that the transverse tubules maintain their position relative to the Z-line following stretch compared to the resting length (DiFranco et al. 2005). Therefore, stress must be placed largely upon the longitudinal and Z tubules and their connection with the transverse tubules as they increase in length under these conditions. A thick membrane collar between the transverse and longitudinal or Z tubules may strengthen this connection to help prevent membrane damage.
A further issue that is raised by the extensive t system vacuolation is that of the apparent increase in membrane surface area required for this to occur. The extent of this is emphasized by measurements showing the volume of the t system in a resting toad fibre increases from ∼1.4% of total cell volume (Endo, 1966; Launikonis & Stephenson, 2002b) up to ∼10% of total cell volume upon vacuolation (Krolenko & Lucy, 2001). The problem of where the membrane required to support the increase in surface area comes from and thus t system volume has been discussed in some detail by others (e.g. Lännergren et al. 1999). Here we show that the t system must be capable of a significant increase in membrane area during vacuolation even in the absence of the sarcolemma. One possible explanation for the increase in t system surface area in the absence of the sarcolemma may be the unfolding of caveolae that exist throughout the t system of skeletal muscle fibres (Murphy & Lamb, 2007).
Interconnectivity of the t system
The diffusion of molecules through the longitudinal tubules is restricted to small molecules (Fig. 2). Indeed, diffusion through the transverse tubules was found to occur in the order of seconds (Fig. 7), as expected (Almers et al. 1981; Friedrich et al. 2001; Yeung et al. 2002). Now by using mechanically skinned fibres we have been able to track the movement of a fluorescent dye through the t system along the axis of the fibre. Movement of dye through the transverse tubules was observed as the fast component of the FRAP experiment (Fig. 7). This translated into a diffusion coefficient of 22–30 μm2 s−1, which is 3–4 times slower than in free solution (∼90 μm2 s−1; see Kushmeric & Podolsky, 1969).
There was a 1000-fold decrease of the rate of diffusion of fluo-5 N through the t system network along the long axis of the fibre compared to that simply through the transverse tubules (Fig. 7). Assuming diffusion of fluo-5 N is similar through transverse and longitudinal tubules, this decrease in rate that dye can diffuse through the t system network depends on: (1) the frequency of longitudinal connections between transverse tubules and (2) the effective cross-sectional area of the t system in the longitudinal direction. About 2–3% of the total t system membrane area is made of longitudinal tubules (Eisenberg, 1983), giving 75–100 longitudinal tubules between transverse tubules in a ∼50 μm diameter fibre (equivalent to the size of preparations used here). Thus, at most only 2–3% of a transverse tubule surface area will allow diffusion of the dye along the long axis of the fibre (through the longitudinal tubules) towards the adjacent transverse tubule. This must decrease the rate of diffusion by ∼30–50 times. This does not fully account for the slow diffusion observed. The remaining reduction in dye diffusion rate must be due to the narrow openings to the longitudinal tubule lumen (which may be around 10 nm). This further decreases the effective t system cross-sectional area that the dye has to move through. This must increase diffusion time by up to an order of magnitude.
The observation that longitudinal tubules often run ‘in series’ across several sarcomeres (Fig. 2; Launikonis & Stephenson, 2002a) could allow for a more rapid diffusion of molecules across such a distance. These structures undoubtedly aid in the conductance of action potentials (Posterino et al. 2000). Furthermore, the slow diffusion of molecules through the longitudinal tubules may also allow these tubules to act as ‘storage pockets’ in the t system. For example, lactate may accumulate in these areas and be used later as a metabolic fuel for the cell. This may certainly be relevant during fatigue-induced t system vacuolation (Lännergren et al. 1999).
In this study we show that there are differences in the lumenal dimensions of the tubules that make up the t system network of living skeletal muscle fibres by assessing the accessibility of fluorescent markers with different molecular weights (Figs 2–5). It followed that diffusion of small molecules within the t system along the long axis the fibre was slower than predicted by the number of longitudinal tubules connecting transverse tubules, as the narrow opening to the longitudinal tubules reduced the effective cross-sectional area in which molecules could move within the t system. Furthermore, longitudinal tubules were found to be able to absorb high hydrostatic pressure as transverse tubules can (Launikonis & Stephenson, 2004) and were also shown to be the origin of vacuoles induced following stretch. Our technique of imaging two fluorescent molecules of different size also allowed us to define tubules of the t system network by their minimum lumenal diameter. We identified three tubules in the network: transverse, longitudinal and Z. The latter we define for the first time as a tubule with a narrow lumen that joins transverse tubules across mis-registered sarcomeres to maintain alignment of transverse tubules with the Z-line. In series, the Z tubules form the helicoidal arrangement originally described by Peachey & Eisenberg (1978).
Acknowledgments
We thank D. George Stephenson (La Trobe University, Melbourne) for helpful comments on the manuscript and Purnima Bhat (University of Queensland, Brisbane) for providing us with purified fluorescein dextran. B.S.L. was a C. J. Martin Fellow of the National Health and Medical Research Council (Australia). This work was supported by an Australian Research Council Discovery Grant and National Health and Medical Research Council (Australia) Project Grant to B.S.L.
Supplementa1 material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2008.155127/DC1
References
- Almers W, Fink RHA, Palade P. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol. 1981;312:177–207. doi: 10.1113/jphysiol.1981.sp013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crank J. The Mathematics of Diffusion. 2nd edn. Oxford: Clarendon Press; 1976. [Google Scholar]
- DiFranco M, Capote J, Vergara JL. Optical imaging and functional characterization of the transverse tubular system of mammalian muscle fibers using the potentiometric indicator di-8-ANEPPS. J Membr Biol. 2005;208:141–153. doi: 10.1007/s00232-005-0825-9. [DOI] [PubMed] [Google Scholar]
- Eisenberg BR. Quantitative ultrastructure of mammalian skeletal muscle. In: Peachey LD, editor. Handbook of Physiology, section 10, Skeletal Muscle. Bethesda, MD, USA: American Physiological Society; 1983. pp. 73–112. [Google Scholar]
- Eisenberg BR, Eisenberg RS. Selective disruption of the sarcotubular system in frog sartorius muscle. J Cell Biol. 1968;39:451–476. doi: 10.1083/jcb.39.2.451. A quantitative study with exogenous peroxidase as a marker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Entry of fluorescent dyes into the sarcotubular system of the frog muscle. J Physiol. 1966;185:224–238. doi: 10.1113/jphysiol.1966.sp007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich O, Ehmer T, Uttenweiler D, Vogel M, Barry PH, Fink RH. Numerical analysis of Ca2+ depletion in the transverse tubular system of mammalian muscle. Biophys J. 2001;80:2046–2055. doi: 10.1016/S0006-3495(01)76178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolenko SA, Amos WB, Lucy JA. Reversible vacuolation of the transverse tubules of frog skeletal muscle: a confocal fluorescence microscopy study. J Muscle Res Cell Mot. 1995;16:401–411. doi: 10.1007/BF00114505. [DOI] [PubMed] [Google Scholar]
- Krolenko SA, Lucy JA. Reversible vacuolation of T-tubules in skeletal muscle: mechanisms and implications for cell biology. Int Rev Cytol. 2001;202:243–298. doi: 10.1016/s0074-7696(01)02006-x. [DOI] [PubMed] [Google Scholar]
- Kushmeric MJ, Podolsky RJ. Ionic mobility in muscle cells. Science. 1969;166:1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Junankar PR, Stephenson DG. Raised intracellular [Ca2+] abolishes excitation–contraction coupling in skeletal muscle fibres of rat and toad. J Physiol. 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Bruton JD, Westerblad H. Vacuole formation in fatigued single muscle fibres from frog and mouse. J Muscle Res Cell Mot. 1999;20:19–32. doi: 10.1023/a:1005412216794. [DOI] [PubMed] [Google Scholar]
- Lännergren J, Bruton JD, Westerblad H. Vacuole formation in fatigued skeletal muscle fibres from frog and mouse: effects of extracellular lactate. J Physiol. 2000;526:597–611. doi: 10.1111/j.1469-7793.2000.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larina O, Bhat P, Pickett JA, Launikonis BS, Shah A, Kruger WA, Edwardson JM, Thorn P. Dynamic regulation of the large exocytotic fusion pore in pancreatic acinar cells. Mol Biol Cell. 2007;18:3502–3511. doi: 10.1091/mbc.E07-01-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Ríos E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J Physiol. 2007;583:81–97. doi: 10.1113/jphysiol.2007.135046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Properties of the vertebrate skeletal muscle tubular system as a sealed compartment. Cell Biol Int. 2002a;26:921–929. doi: 10.1006/cbir.2002.0942. [DOI] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Tubular system volume changes in twitch fibres from toad and rat skeletal muscle assessed with confocal microscopy. J Physiol. 2002b;538:607–618. doi: 10.1113/jphysiol.2001.012920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Osmotic properties of the sealed tubular system of toad and rat skeletal muscle. J Gen Physiol. 2004;123:231–247. doi: 10.1085/jgp.200308946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Zhou J, Royer L, Shannon TR, Brum G, Ríos E. Confocal imaging of [Ca2+] in cellular organelles by SEER, shifted excitation and emission ratioing of fluorescence. J Physiol. 2005;567:523–543. doi: 10.1113/jphysiol.2005.087973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Lamb GD. Higher expression of caveolin-3 in mechanically-skinned single fibres from slow-twitch muscle compared with fast-twitch muscle. Proc Aust Physiol Soc. 2007;38:53P. [Google Scholar]
- Nielsen OB, Ørtenblad N, Lamb GD, Stephenson DG. Excitability of the T-tubular system in rat skeletal muscle: roles of K+ and Na+ gradients and Na+–K+ pump activity. J Physiol. 2004;557:133–146. doi: 10.1113/jphysiol.2003.059014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey LD, Eisenberg BR. Helicoids in the T system and striations of frog skeletal muscle fibres seen by high voltage electron microscopy. Biophys J. 1978;22:145–154. doi: 10.1016/S0006-3495(78)85480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino GS, Lamb GD, Stephenson DG. Twitch and tetanic force responses and longitudinal propagation of action potentials in skinned skeletal muscle fibres of the rat. J Physiol. 2000;527:131–137. doi: 10.1111/j.1469-7793.2000.t01-2-00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorten PR, McMahon CD, Soboleva TK. Insulin transport within skeletal muscle transverse tubule networks. Biophys J. 2007;93:3001–3007. doi: 10.1529/biophysj.107.107888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorten PR, Soboleva TK. Anomalous ion diffusion within skeletal muscle transverse tubule networks. Theor Biol Med Model. 2007;4:18. doi: 10.1186/1742-4682-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller C, Cannell MB. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ Res. 1999;84:266–275. doi: 10.1161/01.res.84.3.266. [DOI] [PubMed] [Google Scholar]
- Venturoli D, Rippe B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. Am J Physiol Renal Physiol. 2005;288:F605–F613. doi: 10.1152/ajprenal.00171.2004. [DOI] [PubMed] [Google Scholar]
- Veratti E. Investigations on the fine structure of striated muscle fiber. J Biophys Biochem Cytol. 1961;10:1–59. doi: 10.1083/jcb.10.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt T, Dauber W. About the T-system in the myofibril-free sarcoplasm of the frog muscle fibre. Tissue Cell. 2004;36:245–248. doi: 10.1016/j.tice.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Yeung EW, Balnave CD, Ballard HJ, Bourreau JP, Allen DG. Development of T-tubular vacuoles in eccentrically damaged mouse fibres. J Physiol. 2002;540:581–592. doi: 10.1113/jphysiol.2001.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.