Abstract
Previous human studies have shown divergent results concerning the effects of exercise training on myocardial blood flow (MBF) at rest or during adenosine-induced hyperaemia in humans. We studied whether these responses are related to alterations in adenosine A2A receptor (A2AR) density in the left-ventricular (LV) myocardium, size and work output of the athlete's heart, or to fitness level. MBF at baseline and during intravenous adenosine infusion, and A2AR density at baseline were measured using positron emission tomography, and by a novel A2AR tracer in 10 healthy male endurance athletes (ET) and 10 healthy untrained (UT) men. Structural LV parameters were measured with echocardiography. LV mass index was 71% higher in ET than UT (193 ± 18 g m−2versus 114 ± 13 g m−2, respectively). MBF per gram of tissue was significantly lower in the ET than UT at baseline, but this was only partly explained by reduced LV work load since MBF corrected for LV work was higher in ET than UT, as well as total MBF. The MBF during adenosine-induced hyperaemia was reduced in ET compared to UT, and the fitter the athlete was, the lower was adenosine-induced MBF. A2AR density was not different between the groups and was not coupled to resting or adenosine-mediated MBF. The novel findings of the present study show that the adaptations in the heart of highly trained endurance athletes lead to relative myocardial ‘overperfusion’ at rest. On the other hand hyperaemic perfusion is reduced, but is not explained by A2AR density.
Since oxygen extraction in the myocardium is high, increases in myocardial blood flow (MBF) are of importance for matching substrate delivery to demands of the heart (Feigl, 1983). It is known that in human skeletal muscle, blood flow capacity is increased through endurance training (Snell et al. 1987; Martin et al. 1987). Endurance training also enhances coronary blood flow capacity in laboratory animals (Laughlin et al. 1998), but no consensus has been reached on whether endurance-trained athletes (ET) have a better MBF reserve than untrained (UT) humans. While some studies suggest that ET have a supranormal coronary blood flow reserve (Toraa et al. 1999; Hildick-Smith et al. 2000; Kjaer et al. 2005), some do not support the view (Heiss et al. 1976; Radvan et al. 1997; Kalliokoski et al. 2002). A possible interfering factor in human studies is the size of the athlete's heart. Extreme phenotypes of the athlete's heart can structurally resemble hypertrophic cardiomyopathy, which is associated with reduced MBF reserve (Camici & Crea, 2007). However, it is not known to what extent the adaptations in the athlete's heart (Maron & Pelliccia, 2006) reflect maladaptive cardiac hypertrophy or how MBF and flow reserve are affected. Typically, endurance-trained athletes with varying left ventricle (LV) mass are reported to have similar or reduced basal MBF compared to untrained humans, despite similar or increased MBF reserve as determined by adenosine-induced vasodilatation (Heiss et al. 1976; Radvan et al. 1997; Toraa et al. 1999; Hildick-Smith et al. 2000; Kalliokoski et al. 2002; Kjaer et al. 2005; Laaksonen et al. 2007) However, pronounced LV hypertrophy may impact total MBF and flow reserve per unit ventricular mass.
In addition to the importance of global MBF and blood flow reserve in health and disease, the regulatory aspects of MBF are also of great interest. It is well recognized that control of MBF involves a dynamic interplay of neural, mechanical and metabolic mechanisms (Feigl, 1983; Duncker & Bache, 2008). Metabolic regulation is still considered to exert a major role for matching flow to the local energy demand in the myocardium by altering vascular resistance in the coronary circulation (Camici & Crea, 2007; Duncker & Bache, 2008). The role of adenosine as a metabolic regulator of MBF in humans is apparent (Edlund & Sollevi, 1995; Namdar et al. 2006), although some animal studies cast a doubt in this belief, as recently reviewed (Tune et al. 2004). The effects of adenosine are mediated via four different adenosine receptors; A1, A2A, A2B and A3 (Shryock & Belardinelli, 1997). Adenosine is a widely used agent to study coronary perfusion and evidence from animal studies suggests that adenosine-induced vasodilatation in the coronary circulation is mediated primarily through A2A receptors (A2ARs) (Belardinelli et al. 1998; Hein et al. 1999, 2001). However, no study has sought to determine whether this is also the case in humans in vivo. Interestingly, the density of A2ARs in the human heart has been found to be higher in endurance-trained triathletes (Mizuno et al. 2005). However, it is not known whether A2AR density and MBF are interrelated in humans, especially during adenosine-induced hyperaemia, and whether increased LV mass in athletes has an effect on A2AR density.
The purpose of the present study was to investigate the relation of MBF at rest and during adenosine-induced vasodilatation to A2AR density and LV mass in endurance-trained athletes and untrained men. It was hypothesized that ET have higher MBF reserve and adenosine A2AR density in the LV. Additionally, based on animal studies addressing adenosine receptor expression implicating an important role of A2AR in adenosine-induced coronary vasodilatation, it was expected that A2AR density and MBF would be proportional, thus subjects with higher adenosine A2AR density also exhibit a larger capacity to increase MBF during hyperaemia.
Methods
Subjects
Twenty healthy men volunteered for the study (Table 1). Ten of them were highly trained endurance athletes (ET) and 10 were untrained men (UT). The subjects were apparently healthy as determined by health questionnaire and medical screening by a doctor in addition to pre-ECG evaluation. The subjects were not under any medication and were normotensive non-smokers with no history of hypercholesterolaemia and no family history of coronary disease. The purpose, nature and potential risks were verbally explained to the subjects before they gave their written informed consent to participate. The study was performed according to the Declaration of Helsinki and was approved by the Ethical Committee of the Hospital District of South-Western Finland.
Table 1.
Characteristics of the subjects
| ET | UT | P value | |
|---|---|---|---|
| Age (years) | 24.6 ± 3.6 | 25.7 ± 4.2 | 0.54 |
| Height (cm) | 182.3 ± 7.5 | 179.3 ± 6.6 | 0.35 |
| Weight (kg) | 78.1 ± 6.6 | 78.1 ± 7.0 | 1 |
| BMI (kg m−2) | 23.4 ± 0.9 | 24.4 ± 2.9 | 0.33 |
| BSA (m2) | 1.99 ± 0.13 | 1.97 ± 0.09 | 0.60 |
| Body fat (%) | 9.7 ± 2.4 | 19.0 ± 3.9 | <0.001 |
| Powermax (W kg−1) | 4.7 ± 0.3 | 3.5 ± 0.4 | <0.001 |
| Powermax (W) | 367 ± 26 | 271 ± 35 | <0.001 |
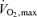 (ml kg−1 min−1) (ml kg−1 min−1) |
61.7 ± 5.0 | 46.2 ± 3.2 | <0.001 |
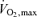 (l min−1) (l min−1) |
4.7 ± 0.3 | 3.5 ± 0.3 | <0.001 |
BMI, body mass index; BSA, body surface area; Powermax, highest workload in 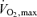 test.
test.
The ET participants in the study were cross-country skiers, who also trained and competed in other endurance sport events such as running, cycling and orienteering. The training volume of their last training season was on average 626 ± 61 h in a year. The ET had been training on a regular basis for 12.3 ± 3.9 years, 8.7 ± 1.4 times and 12.8 ± 1.5 h a week primarily in endurance exercise at various intensities. They had started sport training at 12.5 ± 2.4 years of age, competed in cross-country skiing for 15.6 ± 4.5 years, and won numerous medals especially in national junior championships, and also at the senior level. Thus, the athletes that participated in the present study were among the best Finnish athletes in cross-country skiing or orienteering. The UT men, on the other hand, had been performing physical activity only occasionally, less than three times per week, mainly walking, jogging, gym, ball games and swimming, for less than one hour at a time, in addition to other physical activities such as commuting by bike or walking.
Study design
Before the PET experiments, an echocardiography (ECHO) study was performed as described below. PET studies were performed at least 2 h after a light breakfast. The subjects were instructed to abstain from caffeinated drinks and to avoid exhausting exercise 48 h prior to the study. Before the PET experiment, an antecubital vein was cannulated for the administration of tracers. For blood sampling, a radial artery cannula was placed under local anaesthesia in the opposite hand. Thereafter, the subject was positioned into the PET scanner in a supine position with the thoracic region in the gantry. In the PET study (as a PET study), myocardial blood flow (MBF) was measured at rest and during adenosine administration with PET and 15O-labelled water [15O]H2O), which was produced as previously described (Sipiläet al. 2001). After the MBF measurements, A2AR density was measured with a recently developed tracer, [7-methyl-11C]-(E)-8-(3,4,5-trimethoxystyryl)-1,3,7-trimethylxanthine [11C]TMSX) and PET (Mizuno et al. 2005).
PET measurements
The PET imaging was performed using either ECAT EXACT HR + PET scanner (Siemens/CTI, Knoxville, TN, USA) or GE Advance PET scanner (General Electric Medical System, Milwaukee, WI, USA). The subjects as a whole and subgroups studied were evenly distributed between these scanners but the same scanner was used for a single subject. Before the emission scans, a transmission scan for the correction of photon attenuation was performed. MBF measurements were performed first at baseline and then during adenosine infusion. At baseline, [15O]H2O (1070 ± 130 MBq) was infused intravenously and a dynamic PET scanning was performed. After radioactivity decay, a similar perfusion measurement was repeated (1029 ± 120 MBq of [15O]H2O) during 7 min of intravenous infusion of adenosine (140 μg min−1 (kg body weight)−1). Distribution volume (DV) of A2ARs in the cardiac left ventriculum (LV), thus the density of A2ARs in LV, was determined according to the established principles of Mizuno et al. (Mizuno et al. 2005) by intravenous bolus injection (430 ± 130 MBq) of [11C]TMSX tracer and measured uptake of the tracer with dynamic PET imaging for 60 min in two-dimensional mode. Plasma radioactivity was measured from the arterial blood and unaltered [11C]TMSX in the plasma was analysed by HPLC (Mizuno et al. 2005). Adenosine A2AR DV values were obtained and are reported (and related correlations) from 16 subjects (9 athletes and 7 untrained men).
PET data analysis
Regions of interest (ROIs) encompassing the anterior, septal and lateral myocardial walls, and all three together – the whole LV – were drawn manually on six transaxial slices of the left ventricle (LV), and another ROI was drawn in the LV cavity. MBF was calculated using the previously introduced methods employing the single-compartment model (Iida et al. 1995). Measured MBF reserve was defined as a ratio of the perfusion during adenosine administration to the flow at rest. Total MBF in the whole LV was determined as the product of the measured MBF and the LV mass measured with ECHO. In addition, for normalizing the effect of different LV work loads on MBF observed between the groups, individual measured baseline MBF was corrected by multiplying it with LV work obtained from the whole subject population and divided by the individual LV work. Coronary resistance was calculated by dividing the mean arterial pressure (MAP) by the respective perfusion value, and coronary vascular resistance index was defined as vascular resistance during adenosine infusion divided by resistance at baseline. DV of A2AR was calculated with Logan's graphical analysis for irreversible tracers (Logan et al. 1990) using the time–activity curves (TACs) of myocardium and the metabolite, spillover and partial volume-corrected TAC of plasma.
Echocardiographic measurements and analyses
ECHO measurements and analyses for LV parameters were performed using a commercially available ultrasound scanner (Acuson 128 XP-10 ultrasound system, Siemens, USA) according to the recommendations of the American Society of Echocardiography by an experienced specialist in cardiology as previously described (Kalliokoski et al. 2002). Total LV work output was calculated as a product of MAP and cardiac output. Relative LV work output per unit mass was calculated by dividing total LV work output by LV mass.
Other measurements
A continuous incremental 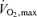 bicycle test with electrically braked cycle ergometer and direct respiratory measurements was performed for the evaluation of fitness level. Volumes of oxygen consumed
bicycle test with electrically braked cycle ergometer and direct respiratory measurements was performed for the evaluation of fitness level. Volumes of oxygen consumed 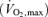 during exercise were obtained from minute ventilation, and maximal load at the end of the test was measured. For the UT, the fitness test was performed at Paavo Nurmi Centre, University of Turku, Turku, Finland with Ergoline 800 S ergometer (Ergoline, Bitz, Germany) and
during exercise were obtained from minute ventilation, and maximal load at the end of the test was measured. For the UT, the fitness test was performed at Paavo Nurmi Centre, University of Turku, Turku, Finland with Ergoline 800 S ergometer (Ergoline, Bitz, Germany) and 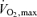 was measured with a Medikro 202 gas analyser (Medikro Oy, Kuopio, Finland). Since the ET were recruited from Central Finland, the fitness test for them was performed with a Monark ergomedic 839E ergometer (Monark Exercise AB, Vansbro, Sweden) and
was measured with a Medikro 202 gas analyser (Medikro Oy, Kuopio, Finland). Since the ET were recruited from Central Finland, the fitness test for them was performed with a Monark ergomedic 839E ergometer (Monark Exercise AB, Vansbro, Sweden) and 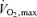 was obtained with Medgraphics Cardiorespiratory diagnostic system (Medical Graphics Corporation, St Paul, USA) at the Department of Biology of Physical Activity, University of Jyväskylä, Finland. Additionally, percentage body fat (skinfolds) was measured and a specific questionnaire concerning physical activity and health state was obtained from the subjects.
was obtained with Medgraphics Cardiorespiratory diagnostic system (Medical Graphics Corporation, St Paul, USA) at the Department of Biology of Physical Activity, University of Jyväskylä, Finland. Additionally, percentage body fat (skinfolds) was measured and a specific questionnaire concerning physical activity and health state was obtained from the subjects.
Statistical analysis
Statistical analysis was performed using SAS/STAT statistical software (version 8.2, SAS Institute Inc., Cary, NC, USA). Two-way ANOVA for repeated measurements was used for the analysis of statistical differences between the values obtained at baseline, during adenosine and [11C]TMSX administration, and between the groups. Student's two-tailed t test was used for comparisons of group differences in body composition, fitness test parameters and echocardiographic measurements. Correlation values were calculated using Pearson's correlation coefficient. P values < 0.05 were considered statistically significant. All data are shown as mean ±s.d.
Results
Characteristics of the subjects
The groups were closely matched in age, height, weight and body mass index (BMI) (Table 1), but differed in body fat percentage, fitness level and the LV structural parameters as determined by ECHO (Table 2). On average, both the total LV mass and the body surface area (BSA)-normalized LV mass were 71% higher in ET compared to UT subjects.
Table 2.
Echocardiography parameters of the study subjects
| ET | UT | P value | |
|---|---|---|---|
| EDD-l (mm) | 106.6 ± 7.1 | 96.9 ± 4.2 | <0.001 |
| EDD (mm) | 58.1 ± 2.5 | 53.2 ± 3.1 | <0.01 |
| SWd (mm) | 12.4 ± 0.8 | 9.0 ± 0.9 | <0.001 |
| PWd (mm) | 12.3 ± 0.7 | 9.1 ± 0.9 | <0.001 |
| AWd (mm) | 12.3 ± 0.7 | 9.0 ± 0.9 | <0.001 |
| LV mass (g) | 385 ± 43 | 225 ± 34 | <0.001 |
| LV mass index (g m−2) | 193 ± 18 | 114 ± 13 | <0.001 |
| SV (ml) | 110 ± 8 | 91 ± 10 | <0.001 |
| CO (l min−1) | 5.8 ± 1.1 | 5.6 ± 0.9 | 0.60 |
| EF (%) | 66.0 ± 4.5 | 66.3 ± 3.9 | 0.87 |
| FS (%) | 37.1 ± 2.9 | 37.3 ± 3.6 | 0.89 |
| E/A ratio | 1.8 ± 0.2 | 2.2 ± 0.6 | 0.07 |
EDD-l, longitudinal end-diastolic diameter; EDD, end-diastolic diameter; SWd, septal wall thickness in diastole; PWd, posterior wall thickness in diastole; Awd, anterior wall thickness in diastole; SV, stroke volume; CO, cardiac output; EF, ejection fraction; FS, fractional shortening; E/A ratio, ratio between early and late diastolic filling rate.
Haemodynamic parameters during imaging
Heart rate, blood pressure, and LV work values during the PET measurements are shown in Table 3. Adenosine infusion increased heart rate, but had no effect on blood pressure parameters. All haemodynamic parameters except systolic blood pressure were similar between the groups during the imaging procedures.
Table 3.
Haemodynamic variables during the PET measurements
| Rest | ADO | [11C]TMSX | ||||
|---|---|---|---|---|---|---|
| ET | UT | ET | UT | ET | UT | |
| HR (beats min−1) | 47 ± 7*** | 59 ± 8 | 80 ± 9*** | 103 ± 11† | 47 ± 6*** | 61 ± 8 |
| BPs (mmHg) | 123 ± 7* | 115 ± 11 | 127 ± 8* | 117 ± 10 | 126 ± 12* | 115 ± 11 |
| BPd (mmHg) | 64 ± 5 | 68 ± 7 | 66 ± 5 | 67 ± 8 | 70 ± 9 | 69 ± 8 |
| MAP (mmHg) | 84 ± 4 | 84 ± 8 | 86 ± 5 | 84 ± 9 | 89 ± 9 | 84 ± 8 |
| LV work (mmHg l min−1 g−1) | 1.1 ± 0.2*** | 2.0 ± 0.4 | 2.0 ± 0.2*** | 3.5 ± 0.6† | 1.2 ± 0.2*** | 2.1 ± 0.5 |
| Total LV work (mmHg l min−1) | 433 ± 79 | 448 ± 82 | 753 ± 89† | 765 ± 93† | 474 ± 103 | 456 ± 85 |
| MBF (ml min−1 g−1) | 0.56 ± 0.13*** | 0.69 ± 0.09 | 2.75 ± 0.75*** | 3.48 ± 0.94 | — | — |
| Normalized MBF (ml min−1 g−1) | 0.79 ± 0.16** | 0.56 ± 0.13 | — | — | — | — |
| Total MBF (ml min−1) | 217 ± 61*** | 155 ± 28 | 1056 ± 272*** | 777 ± 223 | — | — |
| Coronary resistance (mmHg ml min−1 g−1) | 156 ± 32* | 122 ± 15 | 34 ± 11 | 25 ± 7 | — | — |
Rest, ADO and [11C]TMSX represent values at baseline, during adenosine and [11C]TMSX infusions, respectively. HR, heart rate; BPs, systolic blood pressure; BPd, diastolic blood pressure; MAP, mean arterial blood pressure; LV work, left ventricular myocardial work; MBF, myocardial blood flow. Adenosine increased HR as well as relative and total LV work significantly from rest to ADO in both groups, †P < 0.001. Percentage increase in these parameters was similar between the groups (∼75%). However, adenosine induced no changes in BPs, BPd and MAP, but ET had higher BPs, and lower HR and LV work in every measurement,
P < 0.001,
P < 0.01,
P < 0.05 compared to UT.
Myocardial blood flow (Table 3) (Figs 1–3)
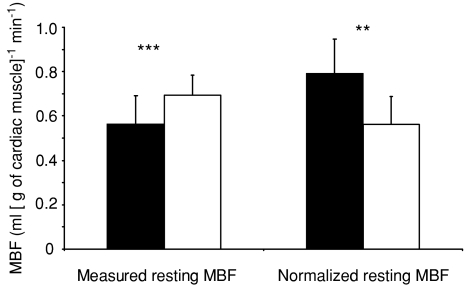
Figure 1. Measured and normalized myocardial blood flow (MBF) at rest.
Filled bars, male endurance athletes (ET); open bars, healthy untrained men (UT). **P < 0.001 and ***P < 0.001. After considering the significantly lower myocardial work load of ET, ET showed significantly higher MBF per gram of cardiac tissue at rest.
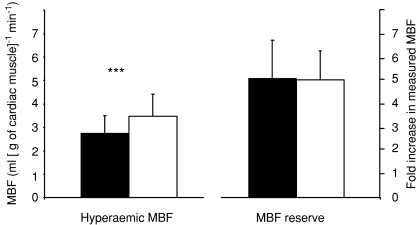
Figure 3. Adenosine-induced hyperaemic myocardial bood flow (MBF) and measured MBF reserve.
Filled bars, ET; open bars, UT. Adenosine increased MBF significantly compared to resting MBF (Fig. 1) in both groups, P < 0.001. Endurance athletes had significantly lower hyperaemic MBF, but their MBF reserve calculated from measured MBF values was similar compared to untrained men. ***P < 0.001.
Adenosine infusion increased MBF ∼5-fold both in the whole LV (Fig. 3) as well as in all three regions of it (Fig. 2) as compared to the resting values. ET had lower measured MBF per gram of tissue both at rest and during adenosine infusion (Figs 1 and 3), but the MBF reserve was similar between the groups (Fig. 3). When calculated for the whole LV, total LV MBF was substantially higher in ET than UT both at baseline (217 ± 61 versus 155 ± 28 ml min−1, respectively, P < 0.001) and during adenosine (1056 ± 272 versus 777 ± 223 ml min−1, P < 0.001). When MBF was normalized for myocardial work, baseline MBF was significantly higher in ET than in UT (P < 0.01) (Fig. 1). Coronary resistance was higher in ET than UT at baseline (156 ± 32 versus 122 ± 15 mmHg ml min−1 g−1, respectively, P < 0.04), but the difference was not statistically significant during adenosine-induced hyperaemia (34 ± 11 and 25 ± 7 mmHg ml min−1 g−1 in ET and UT, respectively, P= 0.17) and coronary vascular resistance index was similar in both groups (0.23 ± 0.08 in ET and 0.21 ± 0.06 in UT, P= 0.65).
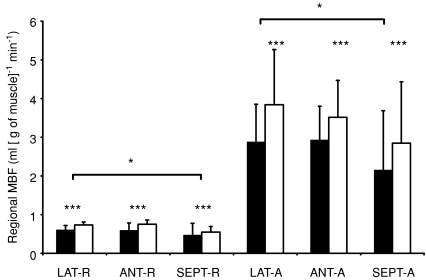
Figure 2. Regional MBF.
ET (filled bars) had lower MBF in all three regions of LV compared to UT men (open bars) both at rest and during adenosine-induced hyperaemia (ADO), ***P < 0.001. LAT-R, MBF in lateral part of LV at rest and during ADO (LAT-A); ANT-R, MBF in anterior part of LV at rest and during ADO (ANT-A); SEPT-R, MBF in septal part of LV at rest and during ADO (SEPT-A). Among all subjects, MBF in lateral region of LV was higher compared to septum, *P < 0.05 both at rest and during ADO, but not compared to anterior region (P= 0.94). There was no statistically significant difference in MBF between anterior and septal regions (P= 0.06).
Adenosine A2AR density
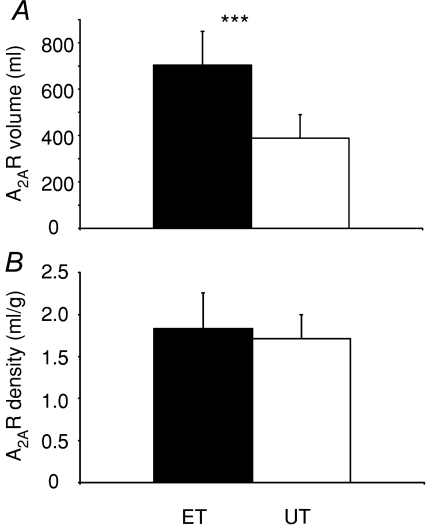
The total volume of cardiac A2AR in LV was significantly higher in ET compared to UT (Fig. 4A), but the DV of A2AR (receptor density) did not differ between the groups (Fig. 4B). Spillover and partial volume-corrected DV values did not differ from the reported uncorrected values (P= 0.7). Only a small fraction of [11C]TMSX was metabolized during the scan duration (< 20% at 60 min). There were no differences in metabolism (P= 0.7) nor in Area under curve (AUC) of TACs (P= 0.4) between the groups (data not shown).
Figure 4. Total volume (A) and density (B) of myocardial adenosine A2AR.
No difference in A2AR density was found, but ET had a higher total volume of A2ARs.
Relations of measured parameters
In the whole subject population, LV mass and LV mass index correlated negatively with MBF at rest (r=−0.46, P= 0.04 and r=−0.49, P= 0.03, respectively) and almost significantly during adenosine infusion (r=−0.43, P= 0.057 and r=−0.42, P= 0.067, respectively), but not with MBF reserve. In addition, a negative correlation between MBF at rest and 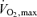 (r=−0.59, P < 0.01) was observed. However, significance levels of these correlations were not reached when parameters were correlated separately in the two groups.
(r=−0.59, P < 0.01) was observed. However, significance levels of these correlations were not reached when parameters were correlated separately in the two groups.
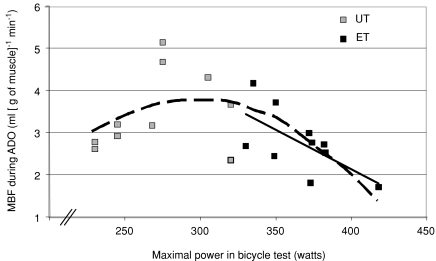
Measured MBF at rest or during adenosine administration in the LV did not correlate significantly with A2AR DV values in the whole study population or within the groups (P not significant in any group). However, in all subjects, DV in the whole LV tended to be negatively related to measured MBF reserve (r=−0.46, P= 0.07). In addition, there was a tendency for a negative relation of whole LV [11C]TMSX DV values and LV mass in ET (r=−0.63, P= 0.07) but not in UT (r=−0.38, P= 0.27). Finally, the fitter the athlete was, the lower the MBF during adenosine-induced hyperaemia (Fig. 5).
Figure 5. Relationship between maximal sustained power obtained during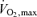 bicycle test (Wmax) and myocardial blood flow during adenosine infusion (MBF during ADO).
bicycle test (Wmax) and myocardial blood flow during adenosine infusion (MBF during ADO).
In UT, Wmax was not related to MBF during ADO (r= 0.28, P= 0.44, grey squares), but in ET these parameters were inversely related (black squares and line, r= 0.64, P= 0.04). Interestingly, if the subjects are considered showing a continuum of inborn and acquired fitness, a curvilinear relation between fitness level and MBF during adenosine-induced hyperaemia in the whole subject population is observed (dashed line, y= 0.0002x2+ 0.0981x− 10.824, r2= 0.42, P= 0.002).
Discussion
Human studies have shown divergent results concerning the effects of exercise training on MBF in healthy subjects and furthermore, the mechanisms of the detected changes have remained unresolved. We measured myocardial perfusion at rest and during adenosine-induced hyperaemia in absolute terms in untrained and trained subjects. Furthermore, we studied whether the training responses are related to structural or functional changes in the athlete's heart or alterations in cardiac A2AR density. The results show that elite endurance athletes had significantly lower MBF per gram of tissue both at rest and during adenosine-induced vasodilatation as compared with untrained but relatively fit subjects. Due to clearly increased cardiac mass in the athletes the whole heart total MBF is, however, significantly increased while the flow reserve is unchanged. Furthermore, when MBF is related to the myocardial work at rest, i.e. the energy demands of heart, MBF per gram of tissue was also significantly higher in athletes than untrained men, suggesting relative overperfusion of elite athletes’ myocardium. Finally, no differences in adenosine A2AR density was found between the groups and A2AR density was not coupled with MBF.
Structural and functional changes in the athlete's heart are considered a favourable physiological phenomenon with no known harmful consequences except for increased incidence of atrial fibrillation at older age (Karjalainen et al. 1998). However, extreme forms of myocardial hypertrophy due to heredity and vigorous physical conditioning can resemble a structural heart disease such as hypertrophic cardiomyopathy, which is associated with reduced MBF reserve (Camici & Crea, 2007) and substantially increased risk of cardiac event (Maron & Pelliccia, 2006; Thompson et al. 2007). LV cavity dimensions and wall thicknesses of athletes in the present study were in the near upper normal limits observed previously in world-class athletes (Pelliccia et al. 1991, 1999). LV mass normalized to BSA ranged in athletes from 159 g m−2 to 221 g m−2, even exceeding the upper normal limits found among athletes (Pelliccia et al. 1991, 1999; Rodriguez Reguero et al. 1995; Pluim et al. 2000).
Myocardial blood flow at rest
To the best of our knowledge, there are no reported studies of MBF in athletes with as high LV mass as the subjects had in the present study. Untrained subjects in the present study had good fitness, as judged by their relatively low heart rate and high 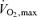 , highlighting our findings with respect to the two groups. Compared to the previous PET study where highly trained rowers were studied (Kjaer et al. 2005), our athletes had on average 51% higher LV mass index (128 versus 193 g m−2, respectively). In line with the study by Kjaer and colleagues, uncorrected MBF was significantly lower in ET than UT at rest in the present study. In other previous human studies with PET, uncorrected MBF has been similar between the trained and untrained groups at rest (Radvan et al. 1997; Toraa et al. 1999; Kalliokoski et al. 2002; Laaksonen et al. 2007) despite the larger LV mass in athletes. Given the known close relationship between LV work output, myocardial oxygen consumption, and myocardial blood flow, it is necessary to relate the measured blood flow values to LV work load. When this was done in the present study, MBF at rest was significantly higher in ET than UT. This leads to two alternative consequences: athletes had either (1) impaired myocardial efficiency of work with comparable myocardial oxygen extraction between the groups or (2) reduced oxygen extraction with unchanged efficiency of work.
, highlighting our findings with respect to the two groups. Compared to the previous PET study where highly trained rowers were studied (Kjaer et al. 2005), our athletes had on average 51% higher LV mass index (128 versus 193 g m−2, respectively). In line with the study by Kjaer and colleagues, uncorrected MBF was significantly lower in ET than UT at rest in the present study. In other previous human studies with PET, uncorrected MBF has been similar between the trained and untrained groups at rest (Radvan et al. 1997; Toraa et al. 1999; Kalliokoski et al. 2002; Laaksonen et al. 2007) despite the larger LV mass in athletes. Given the known close relationship between LV work output, myocardial oxygen consumption, and myocardial blood flow, it is necessary to relate the measured blood flow values to LV work load. When this was done in the present study, MBF at rest was significantly higher in ET than UT. This leads to two alternative consequences: athletes had either (1) impaired myocardial efficiency of work with comparable myocardial oxygen extraction between the groups or (2) reduced oxygen extraction with unchanged efficiency of work.
Myocardial oxygen extraction and efficiency have been sparsely studied in athletes mainly due to methodological difficulties. Takala and colleagues found no differences in myocardial efficiency and oxygen extraction between ET and UT subjects during insulin clamp at rest (Takala et al. 1999). A recent report of a study performed with monozygotic twins discordant for physical activity and fitness showed that myocardial oxygen extraction tended to be lower and efficiency higher at rest in the more active and fitter group, despite relatively small difference in fitness level between the groups (Hannukainen et al. 2007).
Additionally, in a study by Heiss and colleagues (Heiss et al. 1976), resting oxygen extraction also tended to be lower in endurance athletes than in untrained subjects (calculated from the individual data presented in the article). Thus, although solid evidence on the relation between flow and oxygen extraction in the present study is lacking the data from this study point to the conclusion that the athletes may have lower myocardial oxygen extraction at rest, which may then, in turn, allow a higher increase in oxygen extraction whenever myocardial demand is elevated, such as during strenuous exercise. This conclusion is also supported by the well-established fact that fit endurance athletes have substantially higher resting cardiac vagal tone, a well-documented direct coronary vasodilator, which may indeed allow them to take advantage of their larger stroke volume ‘overperfusing’ more relaxed and bigger coronary arteries, and ultimately, myocardium. This issue however, definitely warrants further research.
Myocardial blood flow during hyperaemia and blood flow reserve
The finding of a similar measured MBF reserve between the groups is concordant with many (Radvan et al. 1997; Kalliokoski et al. 2002; Hannukainen et al. 2007), but not all (Toraa et al. 1999; Kjaer et al. 2005) previous studies. Although there is some evidence from animal studies that endurance training could lead to improved MBF capacity (Laughlin et al. 1998) our findings suggest that endurance-trained athletes expressing remarkable LV hypertrophy have no supranormal MBF reserve. Coronary vasculature may simply increase in parallel with increases in exercise-induced LV mass (Hudlicka, 1982), which is also supported by the known close connection between the size of epicardial coronary arteries and LV mass (Rodriguez & Robbins, 1959; Lewis & Gotsman, 1973; O'Keefe et al. 1987; Leung et al. 1991; Dodge et al. 1992). Yet, it has also been found already decades ago that enlargement of even the healthy coronary arteries may not necessarily keep pace with that of the cardiac mass (Roberts & Wearn, 1941). In this respect, considering the reduced hyperaemic MBF per gram of tissue in our athletes, our findings suggest that pronounced myocardial hypertrophy in healthy athletes closely resembles hypertrophic cardiomyopathy in terms of blood flow (Knaapen et al. 2008). Regarding the reduced hyperaemic MBF per gram of myocardium in ET, it was also found that the fitter the athlete, the lower the MBF during adenosine-induced hyperaemia. The mechanism of this is unclear but may involve differing effects of nervous regulation during adenosine stimulation (Kaufmann et al. 2007). The possibility also exists that due to a few seconds half-life of adenosine (Moser et al. 1989) caused by its rapid cellular uptake through high-affinity nucleoside transporters (Loffler et al. 2007) especially in endothelium and erythrocytes abundant in athletes (Heinicke et al. 2001), the efficiency of a standard dose of adenosine inducing hyperaemia may not be similar in athletes and untrained men. However, it is unlikely that the degradation of adenosine would be that much different between the groups. Yet, our observation of the effect of fitness level of the subjects on adenosine-induced hyperaemia (Fig. 5) suggests that fitness may at least partly explain previously observed divergent inter-individual responses to intravenously infused adenosine (Chan et al. 1992). Another issue that could have modulated the response to adenosine is cardiac A2AR density. In particular, many animal studies addressing adenosine receptor expression have implicated an important role for A2AR in adenosine-induced coronary vasodilatation.
Adenosine A2AR density
In the present study in humans myocardial A2AR density and MBF were not tightly coupled and receptor density was not different between the groups. Athletes are, however, found to have higher total volume of A2ARs due to their significantly larger LV mass despite similar receptor density. Many animal studies suggest that coronary vasodilatation caused by adenosine is solely or at least primarily mediated via A2ARs (Belardinelli et al. 1998; Hein et al. 1999, 2001). Although direct human studies attempting to elucidate the coupling of adenosine-induced vasodilatation and A2ARs are lacking, it has been shown that specific A2AR agonists cause MBF increases comparable to adenosine with fewer side-effects (Udelson et al. 2004; Iskandrian et al. 2007), supporting the essential role of A2ARs also in the vasodilatation of adenosine in humans. In the present study, however, there was a trend of inverse relationship between A2AR density and MBF reserve among all the subjects, which may suggest that the amount/density of A2ARs may not be the most important factor in adenosine-induced myocardial hyperaemia in humans. In addition to a likely role of other adenosine receptors modulating (Shryock & Belardinelli, 1997) or directly evoking vasorelaxation, such as A2B (Talukder et al. 2003), the divergent inter-individual effects of intravenously infused adenosine (Chan et al. 1992) may obscure a direct comparison between our non-invasive in vivo correlation approach of examing the role of A2ARs as a predominant adenosine receptor responsible for adenosine-induced vasodilatation in humans. Moreover, it has to also be considered that A2Rs are not completely cell membrane-fixed receptors but rather, are up-regulated from the cytosol when needed (Milojevic et al. 2006). Thus, it may appear that there are, indeed, no differences between the groups at rest, but strenuous exercise for instance may induce receptor up-regulation that could be variable. On the other hand, continuous stimulus due to extensive infusion of adenosine may also induce A2AR down-regulation or inactivation (stimulus desensitization), and possibly more extensively in ET, potentially also explaining their reduced hyperaemic MBF. Finally, one possible reason for poor coupling of A2AR density and MBF is that the extent of adenosine-induced hyperaemia is not due solely to the amount/density of A2ARs, but also the sensitivity and activity of different signalling cascades downstream of A2AR activation is also important. These factors associated with adenosine-induced vasodilatation in humans may have precluded a direct correlation between MBF and A2AR density in the present study.
The current method measures total cardiac A2ARs, but cannot separate the receptors at specific tissue compartments. The density of A2ARs is high in myocardial resistance vessels and microcirculation, where the vasodilator action of adenosine also happens. This tracer probably binds mostly to the cell surface receptors of endothelial cells, but may also reach smooth and cardiac muscle cells. Since our findings were in contrast to a recent study in which a higher density of A2ARs in ET triathletes was observed (Mizuno et al. 2005), we tested whether possible differences in arterial input curves or tracer metabolism affected the findings, but no differences were found to exist between the groups in these calculations.
In conclusion, the novel findings of the present study indicate pronounced structural changes in the heart of highly trained endurance athletes leading to myocardial ‘overperfusion’ at rest. This is probably associated with a lower oxygen extraction in athletes, although inefficiency caused by maladaptive cardiac hypertrophy cannot be totally excluded. Furthermore, the density of adenosine A2ARs is similar in athletes as compared to sedentary people and this is in accord with a similar minimal coronary resistance during adenosine-induced hyperaemia. Finally, adenosine-induced hyperaemia and adenosine A2AR density are not directly coupled and linked with changes in myocardial perfusion.
Acknowledgments
The study was conducted within the Centre of Excellence in Molecular Imaging in Cardiovascular and Metabolic Research – supported by the Academy of Finland, University of Turku, Turku University Hospital and Abo Academy. The authors want to thank the personnel of the Turku PET Centre for their excellent assistance during the study. The present study was financially supported by the Academy of Finland (Grants 108539 and 214329), The Ministry of Education (Grant 74/627/2006 and 58/627/2007), The Finnish Cultural Foundation and its South-Western Foundation, The Finnish Foundation for Cardiovascular Research, The Finnish Sport Research Foundation, Turku University Foundation, Turku University Hospital (EVO), and Ella and Georg Ehrnrooth Foundation. The help of Marko Keskitalo during the  measurements performed in Jyväskylä is also deeply appreciated by the authors.
measurements performed in Jyväskylä is also deeply appreciated by the authors.
There are no conflicts of interest concerning this work.
References
- Belardinelli L, Shryock JC, Snowdy S, Zhang Y, Monopoli A, Lozza G, Ongini E, Olsson RA, Dennis DM. The A2A adenosine receptor mediates coronary vasodilation. J Pharmacol Exp Ther. 1998;284:1066–1073. [PubMed] [Google Scholar]
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- Chan SY, Brunken RC, Czernin J, Porenta G, Kuhle W, Krivokapich J, Phelps ME, Schelbert HR. Comparison of maximal myocardial blood flow during adenosine infusion with that of intravenous dipyridamole in normal men. J Am Coll Cardiol. 1992;20:979–985. doi: 10.1016/0735-1097(92)90201-w. [DOI] [PubMed] [Google Scholar]
- Dodge JT, Jr, Brown BG, Bolson EL, Dodge HT. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation. 1992;86:232–246. doi: 10.1161/01.cir.86.1.232. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- Edlund A, Sollevi A. Theophylline increases coronary vascular tone in humans: evidence for a role of endogenous adenosine in flow regulation. Acta Physiol Scand. 1995;155:303–311. doi: 10.1111/j.1748-1716.1995.tb09978.x. [DOI] [PubMed] [Google Scholar]
- Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- Hannukainen JC, Janatuinen T, Toikka JO, Jarvisalo MJ, Heinonen OJ, Kapanen J, Nagren K, Nuutila P, Kujala UM, Kaprio J, Knuuti J, Kalliokoski KK. Myocardial and peripheral vascular functional adaptation to exercise training. Scand J Med Sports. 2007;17:139–147. doi: 10.1111/j.1600-0838.2006.00548.x. [DOI] [PubMed] [Google Scholar]
- Hein TW, Belardinelli L, Kuo L. Adenosine A2A receptors mediate coronary microvascular dilation to adenosine: role of nitric oxide and ATP-sensitive potassium channels. J Pharmacol Exp Ther. 1999;291:655–664. [PubMed] [Google Scholar]
- Hein TW, Wang W, Zoghi B, Muthuchamy M, Kuo L. Functional and molecular characterization of receptor subtypes mediating coronary microvascular dilation to adenosine. J Mol Cell Cardiol. 2001;33:271–282. doi: 10.1006/jmcc.2000.1298. [DOI] [PubMed] [Google Scholar]
- Heinicke K, Wolfarth B, Winchenbach P, Biermann B, Schmid A, Huber G, Friedmann B, Schmidt W. Blood volume and hemoglobin mass in elite athletes of different disciplines. Int J Sports Med. 2001;22:504–512. doi: 10.1055/s-2001-17613. [DOI] [PubMed] [Google Scholar]
- Heiss HW, Barmeyer J, Wink K, Hell G, Cerny FJ, Keul J, Reindell H. Studies on the regulation of myocardial blood flow in man. I. Training effects on blood flow and metabolism of the healthy heart at rest and during standardized heavy exercise. Basic Res Cardiol. 1976;71:658–675. doi: 10.1007/BF01906411. [DOI] [PubMed] [Google Scholar]
- Hildick-Smith DJ, Johnson PJ, Wisbey CR, Winter EM, Shapiro LM. Coronary flow reserve is supranormal in endurance athletes: an adenosine transthoracic echocardiographic study. Heart. 2000;84:383–389. doi: 10.1136/heart.84.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudlicka O. Growth of capillaries in skeletal and cardiac muscle. Circ Res. 1982;50:451–461. doi: 10.1161/01.res.50.4.451. [DOI] [PubMed] [Google Scholar]
- Iida H, Takahashi A, Tamura Y, Ono Y, Lammertsma AA. Myocardial blood flow: comparison of oxygen-15-water bolus injection, slow infusion and oxygen-15-carbon dioxide slow inhalation. J Nucl Med. 1995;36:78–85. [PubMed] [Google Scholar]
- Iskandrian AE, Bateman TM, Belardinelli L, Blackburn B, Cerqueira MD, Hendel RC, Lieu H, Mahmarian JJ, Olmsted A, Underwood SR, Vitola J, Wang W. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14:645–658. doi: 10.1016/j.nuclcard.2007.06.114. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Nuutila P, Laine H, Luotolahti M, Janatuinen T, Raitakari OT, Takala TO, Knuuti J. Myocardial perfusion and perfusion reserve in endurance-trained men. Med Sports Exerc. 2002;34:948–953. doi: 10.1097/00005768-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Karjalainen J, Kujala UM, Kaprio J, Sarna S, Viitasalo M. Lone atrial fibrillation in vigorously exercising middle aged men: case-control study. BMJ. 1998;316:1784–1785. doi: 10.1136/bmj.316.7147.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann PA, Rimoldi OE, Gnecchi-Ruscone T, Luscher TF, Camici PG. Systemic nitric oxide synthase inhibition improves coronary flow reserve to adenosine in patients with significant stenoses. Am J Physiol Heart Circ Physiol. 2007;293:H2178–H2182. doi: 10.1152/ajpheart.01292.2006. [DOI] [PubMed] [Google Scholar]
- Kjaer A, Meyer C, Wachtell K, Olsen MH, Ibsen H, Opie L, Holm S, Hesse B. Positron emission tomographic evaluation of regulation of myocardial perfusion in physiological (elite athletes) and pathological (systemic hypertension) left ventricular hypertrophy. Am J Cardiol. 2005;96:1692–1698. doi: 10.1016/j.amjcard.2005.07.090. [DOI] [PubMed] [Google Scholar]
- Knaapen P, Germans T, Camici PG, Rimoldi OE, ten Cate FJ, ten Berg JM, Dijkmans PA, Boellaard R, van Dockum WG, Gotte MJ, Twisk JW, van Rossum AC, Lammertsma AA, Visser FC. Determinants of coronary microvascular dysfunction in symptomatic hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;294:H986–H993. doi: 10.1152/ajpheart.00233.2007. [DOI] [PubMed] [Google Scholar]
- Laaksonen MS, Kalliokoski KK, Luotolahti M, Kemppainen J, Teras M, Kyrolainen H, Nuutila P, Knuuti J. Myocardial perfusion during exercise in endurance-trained and untrained humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R837–R843. doi: 10.1152/ajpregu.00771.2006. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Oltman CL, Bowles DK. Exercise training-induced adaptations in the coronary circulation. Med Sports Exerc. 1998;30:352–360. doi: 10.1097/00005768-199803000-00004. [DOI] [PubMed] [Google Scholar]
- Leung WH, Stadius ML, Alderman EL. Determinants of normal coronary artery dimensions in humans. Circulation. 1991;84:2294–2306. doi: 10.1161/01.cir.84.6.2294. [DOI] [PubMed] [Google Scholar]
- Lewis BS, Gotsman MS. Relation between coronary artery size and left ventricular wall mass. Br Heart J. 1973;35:1150–1153. doi: 10.1136/hrt.35.11.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol. 2007;27:1004–1013. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(–)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation. 2006;114:1633–1644. doi: 10.1161/CIRCULATIONAHA.106.613562. [DOI] [PubMed] [Google Scholar]
- Martin WH, III, Montgomery J, Snell PG, Corbett JR, Sokolov JJ, Buckey JC, Maloney DA, Blomqvist CG. Cardiovascular adaptations to intense swim training in sedentary middle-aged men and women. Circulation. 1987;75:323–330. doi: 10.1161/01.cir.75.2.323. [DOI] [PubMed] [Google Scholar]
- Milojevic T, Reiterer V, Stefan E, Korkhov VM, Dorostkar MM, Ducza E, Ogris E, Boehm S, Freissmuth M, Nanoff C. The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol Pharmacol. 2006;69:1083–1094. doi: 10.1124/mol.105.015818. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Kimura Y, Tokizawa K, Ishii K, Oda K, Sasaki T, Nakamura Y, Muraoka I, Ishiwata K. Greater adenosine A2A receptor densities in cardiac and skeletal muscle in endurance-trained men: a [11C]TMSX PET study. Nucl Med Biol. 2005;32:831–836. doi: 10.1016/j.nucmedbio.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Moser GH, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. Am J Physiol Cell Physiol. 1989;256:C799–C806. doi: 10.1152/ajpcell.1989.256.4.C799. [DOI] [PubMed] [Google Scholar]
- Namdar M, Koepfli P, Grathwohl R, Siegrist PT, Klainguti M, Schepis T, Delaloye R, Wyss CA, Fleischmann SP, Gaemperli O, Kaufmann PA. Caffeine decreases exercise-induced myocardial flow reserve. J Am Coll Cardiol. 2006;47:405–410. doi: 10.1016/j.jacc.2005.08.064. [DOI] [PubMed] [Google Scholar]
- O'Keefe JH, Jr, Owen RM, Bove AA. Influence of left ventricular mass on coronary artery cross-sectional area. Am J Cardiol. 1987;59:1395–1397. doi: 10.1016/0002-9149(87)90927-1. [DOI] [PubMed] [Google Scholar]
- Pelliccia A, Culasso F, Di Paolo FM, Maron BJ. Physiologic left ventricular cavity dilatation in elite athletes. Ann Intern Med. 1999;130:23–31. doi: 10.7326/0003-4819-130-1-199901050-00005. [DOI] [PubMed] [Google Scholar]
- Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Medical. 1991;324:295–301. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- Pluim BM, Zwinderman AH, Van Der Laarse A, Van Der Wall EE. The athlete's heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101:336–344. doi: 10.1161/01.cir.101.3.336. [DOI] [PubMed] [Google Scholar]
- Radvan J, Choudhury L, Sheridan DJ, Camici PG. Comparison of coronary vasodilator reserve in elite rowing athletes versus hypertrophic cardiomyopathy. Am J Cardiol. 1997;80:1621–1623. doi: 10.1016/s0002-9149(97)00778-9. [DOI] [PubMed] [Google Scholar]
- Roberts JT, Wearn JT. Quantitative changes in the capillary-muscle relationship in human hearts during normal growth and hypertrophy. Am Heart J. 1941;21:617–633. [Google Scholar]
- Rodriguez Reguero JJ, Iglesias Cubero G, Lòpez de la Iglesia J, Terrados N, Gonzalez V, Cortina R, Cortina A. Prevalence and upper limit of cardiac hypertrophy in professional cyclists. Eur J Appl Physiol Occup Physiol. 1995;70:375–378. doi: 10.1007/BF00618486. [DOI] [PubMed] [Google Scholar]
- Rodriguez FL, Robbins SL. Capacity of human coronary arteries; a postmortem study. Circulation. 1959;19:570–578. doi: 10.1161/01.cir.19.4.570. [DOI] [PubMed] [Google Scholar]
- Shryock JC, Belardinelli L. Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am J Cardiol. 1997;79:2–10. doi: 10.1016/s0002-9149(97)00256-7. [DOI] [PubMed] [Google Scholar]
- Sipilä HT, Clark JC, Peltola O, Teräs M. An automatic [15O]H2O production system for heart and brain studies. J Labelled Comp Radiopharm. 2001;44:S1066–S1068. [Google Scholar]
- Snell PG, Martin WH, Buckey JC, Blomqvist CG. Maximal vascular leg conductance in trained and untrained men. J Appl Physiol. 1987;62:606–610. doi: 10.1152/jappl.1987.62.2.606. [DOI] [PubMed] [Google Scholar]
- Takala TO, Nuutila P, Katoh C, Luotolahti M, Bergman J, Maki M, Oikonen V, Ruotsalainen U, Gronroos T, Haaparanta M, Kapanen J, Knuuti J. Myocardial blood flow, oxygen consumption, and fatty acid uptake in endurance athletes during insulin stimulation. Am J Physiol Endocrinol Metab. 1999;277:E585–E590. doi: 10.1152/ajpendo.1999.277.4.E585. [DOI] [PubMed] [Google Scholar]
- Talukder MA, Morrison RR, Ledent C, Mustafa SJ. Endogenous adenosine increases coronary flow by activation of both A2A and A2B receptors in mice. J Cardiovasc Pharmacol. 2003;41:562–570. doi: 10.1097/00005344-200304000-00008. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA, III, Fulton JE, Gordon NF, Haskell WL, Link MS, Maron BJ, Mittleman MA, Pelliccia A, Wenger NK, Willich SN, Costa F. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115:2358–2368. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- Toraa M, Pouillard F, Merlet P, Friemel F. [Cardiac hypertrophy and coronary reserve in endurance athletes] Can J Appl Physiol. 1999;24:87–95. [PubMed] [Google Scholar]
- Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol. 2004;97:404–415. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- Udelson JE, Heller GV, Wackers FJ, Chai A, Hinchman D, Coleman PS, Dilsizian V, DiCarli M, Hachamovitch R, Johnson JR, Barrett RJ, Gibbons RJ. Randomized, controlled dose-ranging study of the selective adenosine A2A receptor agonist binodenoson for pharmacological stress as an adjunct to myocardial perfusion imaging. Circulation. 2004;109:457–464. doi: 10.1161/01.CIR.0000114523.03312.7D. [DOI] [PubMed] [Google Scholar]