Abstract
Studies in humans and animals have demonstrated that maternal stress during fetal development can lead to altered hypothalamic-pituitary-adrenal (HPA) axis function and behaviour postnatally. We have previously shown adult male guinea pigs that were born to mothers exposed to a stressor during the phase of rapid fetal brain growth (gestational days (GD) 50, 51 and 52; prenatal stress (PS)50) exhibit significantly increased basal plasma cortisol levels. In contrast, male guinea pig offspring whose mothers were exposed to stress later in gestation (GD60, 61 and 62; PS60) exhibited a significantly higher plasma cortisol response to activation of the HPA axis. In the present study, we hypothesized that the endocrine changes in HPA axis function observed in male guinea pig offspring would be reflected by altered molecular regulation of the HPA axis. Corticosteroid receptors in the hippocampus, hypothalamus and pituitary were measured, as well as corticotropin-releasing hormone (CRH), pro-opiomelanocortin (POMC) and adrenal enzymes in the paraventricular nucleus, pituitary and adrenal cortex, respectively, by in situ hybridization and Western blot. PS50 male offspring exhibited a significant reduction in glucocorticoid receptor (GR) mRNA (P <0.01) in the CA3 region of the hippocampus and significantly increased POMC mRNA (P <0.05) in the pituitary, consistent with the increase in basal HPA axis activity observed. In line with elevated activity of the HPA axis, both PS50 and PS60 male offspring exhibited significantly higher steroidogenic factor (SF)-1 (P <0.001) and melanocortin 2 receptor (MC2-R) mRNA (P <0.001) in the adrenal cortex. This study demonstrates that short periods of prenatal stress during critical windows of neuroendocrine development affect the expression of key regulators of HPA axis activity leading to the changes in endocrine function observed in prenatally stressed male offspring. Further, these changes are dependent on the timing of the maternal stressor, a pattern that is emerging in human studies.
Altered development in early life is associated with an increased risk for the development of pathophysiology after birth. One of the mechanisms underlying this relationship is thought to involve exposure to excess glucocorticoids during critical windows of neuroendocrine development. Indeed, it has recently been demonstrated in humans that increased maternal cortisol levels late in gestation were associated with increased infant negative temperament (Davis et al. 2007). Additionally, maternal anxiety during pregnancy has been linked with sex-specific effects on hypothalamic-pituitary-adrenal (HPA) axis activity and symptoms of depression in adolescents (Van den Bergh et al. 2007). Therefore, prospective human studies have demonstrated that maternal stress leads to altered HPA axis function postnatally.
Consistent with human data, animal studies have also demonstrated that prenatal stress leads to altered HPA axis activity and behaviour in a sex-specific manner. Studies in rats have demonstrated profound effects of repeated prenatal stress on HPA axis function. Unpredictable noise and light stress throughout gestation resulted in offspring that exhibited increased basal plasma corticosterone levels (Weinstock et al. 1998). The circadian rhythm of corticosterone secretion was altered in offspring of mothers exposed to varying stressors throughout gestation (Koehl et al. 1999). Behaviours such as anxiety and spatial learning and memory are also affected by prenatal stress in the rat. Offspring born to mothers exposed to stress throughout pregnancy exhibited altered anxiety behaviour (Bosch et al. 2006) while stress during the last week of gestation resulted in offspring that exhibited deficits in spatial learning and memory (Wu et al. 2007). Compared to humans, the rat's brain is relatively immature at term as a large component of neuroendocrine development occurs postnatally in this species (Dobbing & Sands, 1979). As the timing of the stressors were varied in the above studies, it is difficult to discern the specific developmental window targeted with the stressor. This is important as human studies are beginning to show that the timing of the stress is critical to determining outcome (Huizink et al. 2003). Therefore, it is critical to establish the effects of stress during specific critical windows of brain development in order to attempt to determine the mechanism by which the endocrine and behavioural phenotypes are altered in offspring.
Studies in non-human primates have demonstrated altered HPA axis activity and stress-related behaviour in offspring of mothers stressed during pregnancy (Clarke et al. 1994; Lyons et al. 2000; Coe et al. 2003). The stress paradigms employed by non-human primate studies span a range of neurodevelopmental landmarks. Therefore, as in rats, specific times of maximal fetal vulnerability and the long-term effects of prenatal stress, during specific periods of brain development, on HPA axis activity are currently unknown. The guinea pig, like humans, gives birth to neuroanatomically mature offspring and landmarks of brain and neuroendocrine growth are well characterized (Dobbing & Sands, 1970). In the guinea pig, the period of rapid fetal brain growth occurs around gestational day (GD) 50 (75% of gestation) and in the human this phase is initiated at 28–36 weeks (80%) (Dobbing & Sands, 1970, 1979). In contrast, the phase of maximal brain growth in the rat does not occur until postnatal days (PND) 5–8 (Dobbing & Sands, 1979). Guinea pigs also have a long gestation (∼70 days), which allows very specific aspects of fetal development to be targeted. Indeed, we have previously shown that male guinea pig offspring whose mothers were exposed to a moderate stressor exhibit effects on HPA axis activity and behaviour; however, these changes were dependent on the specific timing of the stressor (Kapoor & Matthews, 2005). In the guinea pig it has also been shown that prenatal exposure to the synthetic glucocorticoid dexamethasone resulted in altered hippocampal mineralocorticoid receptor (MR) mRNA, pituitary POMC and GR mRNA in adult male offspring (Banjanin et al. 2004). However, no studies have investigated the effect of acute periods of prenatal stress at very specific windows of neuroendocrine development on molecular regulation of the HPA axis in guinea pig offspring. Therefore, we hypothesized that the endocrine changes in HPA axis function observed in adult male guinea pig offspring exposed to acute periods of prenatal stress (Kapoor & Matthews, 2005) result from altered molecular regulation of the HPA axis at the level of the brain, pituitary and adrenal, and that these effects differ with the timing of maternal insult.
Methods
Animals and treatments
Female guinea pigs (400–500 g) (Hartley strain, Charles River Canada, St Constant, PQ, Canada) were mated in our animal facility as previously described (Dean & Matthews, 1999). Food (Guinea Pig Chow 5025, Ralston Purina International, Leis Pet Distributing Inc., Wellesley, ON, Canada) and water were available ad libitum. The animals were kept in a 12 h : 12 h light–dark cycle, with lights off at 19.00 h. All studies were performed according to protocols approved by the Animal Care Committee at the University of Toronto, in accordance with the Canadian Council for Animal Care.
Pregnant guinea pigs were exposed to a strobe light (Mini Strobe, Progear Warehouse, OH, USA) in a dark environment for 2 h, from 09.00 h to 11.00 h, on GD50, 51 and 52 (PS50; n= 12) or GD60, 61 and 62 (PS60; n= 9). A control group (n= 12) of pregnant guinea pigs were left undisturbed throughout gestation except for routine maintenance. All animals were allowed to deliver normally. Normal litter size is 2–3.
There was no effect of prenatal stress on maternal weight gain, birth weight or litter size (Kapoor & Matthews, 2005). After birth, offspring were routinely weighed and subjected to open field and endocrine testing as previously published (Kapoor & Matthews, 2005). In order to facilitate plasma sampling, the carotid artery of male offspring was catheterized under isoflurane (2–3%). Male guinea pig offspring born to mothers that had been exposed to a high frequency strobe light for 2 h per day on GD 50–52 (during the period of rapid fetal brain growth) exhibited significantly higher basal plasma cortisol levels over a 24 h period, increased anxiety behaviour and significantly lower plasma testosterone levels. In contrast, male guinea pig offspring born to mothers that had been exposed to the same stress in late gestation (GD 60–62) exhibited a significantly higher plasma cortisol response to activation of the HPA axis by an adrenocorticotrophin (ACTH) challenge and exposure to a stressor (Kapoor & Matthews, 2005).
Upon completion of endocrine tests, animals were left undisturbed for at least 72 h before being killed by decapitation. Brains, pituitaries and adrenal glands were rapidly dissected and frozen (−80°C). Prior to freezing, brains were sagittally hemisected at an angle and slightly off midline, such that the left block contained the left hippocampus and the entire hypothalamus. The right hippocampus was dissected from the remaining block. Tissue was rapidly frozen and stored at −80°C until further processing. All male offspring were used for the current study; however, animal numbers for the endocrine data reported previously (Kapoor & Matthews, 2005) are variable due to intermittent loss of catheter patency.
In situ hybridization (ISH)
The method of ISH has been described in detail previously (Matthews & Challis, 1995; Matthews, 1998). Coronal cryosections of brain, pituitary and adrenals (10 μm) were mounted onto poly l-lysine-coated slides, dried and fixed in paraformaldehyde (4%). Oligonucleotide probes were synthesized by Sigma Genosys (Oakville, Canada). The use of the antisense glucocorticoid receptor (GR), mineralocorticoid receptor (MR), pro-opiomelanocortin (POMC), corticotropin-releasing hormone (CRH), melanocortin 2 receptor (MC2-R), CYP17, steroidogenic factor 1 (SF-1) and steroidogenic acute regulatory protein (StAR) oligonucleotide probes have been previously described (Dean & Matthews, 1999; Go et al. 2001; McCabe et al. 2001; Owen & Matthews, 2003; Banjanin et al. 2004). The arginine vasopressin (AVP) oligonucleotide probe was complementary to bases 273–317 of the Homo sapiens AVP gene (GenBank Ref. NM_000490.3). The CYP11A1 oligonucleotide probe was complementary to bases 6382–6338 of the Cavia porcellus whole genome shotgun (GenBank Ref. AAKN01000000) which displays 93% homology to the mouse CYP11A1 gene (GenBank Ref. NM_019779). The probes were labelled with [35S]-deoxyadenosine-5′-α-thiophosphate (1300 Ci mmol−1, Perkin-Elmer, Woodbridge, Canada) using terminal deoxynucleotidyl transferase (TdT; 15 U μl−1; Invitrogen Canada Inc., Burlington, Canada) to a specific activity of 1.0 × 109 c.p.m. μg−1. Labelled probes in hybridization buffer were applied to slides which were incubated overnight (42°C). After washing in 1 × standard sodium citrate (SSC; 30 min, 22°C), and 1 × SSC (30 min, 55°C) the slides were dehydrated and exposed to autoradiographic film (Biomax, Kodak) for variable time periods (hippocampal GR, 21 days; hippocampal MR, 14 days; pituitary GR, 7 days; anterior lobe POMC, 3 days; intermediate lobe POMC, 17 h; PVN CRH, 6 weeks; PVN GR, 6 weeks; adrenal MC2-R, 5 days; CYP17, 1 day; SF-1, 4 days; StAR, 3 days). A sense oligonucleotide probe was also run in each ISH to ensure that non-specific binding was not occurring. All sections to be compared were processed in the same ISH and exposed simultaneously to allow direct comparisons to be made. 14C standards (American Radiochemical, St Louis, MO, USA) were used to ensure that the measurements being made fell into the linear range of the autoradiographic film. The relative optical density (ROD) of the signal on autoradiographic film was quantified, after subtraction of background values, with a computerized image analysis system (Imaging Research, St Catharines, Canada). GR mRNA levels were measured in the CA1/2, CA3 regions of the hippocampus, dentate gyrus (DG), hypothalamic paraventricular nucleus (PVN) and anterior pituitary gland. MR mRNA levels were measured in hippocampus (as for GR) and the DG. AVP and CRH mRNA was measured in hypothalamic PVN. POMC mRNA was measured in the intermediate lobe and anterior lobe (superior and inferior regions) of the pituitary gland. MC2-R, CYP17, SF-1, CYP11A1 and StAR mRNA expression was measured throughout the entire adrenal cortex. ROD was measured in at least six sections per region per animal, and averaged for statistical analysis. All analyses were undertaken by an operator blinded to treatment. For molecular analysis, there was some variability with animal numbers as samples were excluded if the tissue was not of optimal integrity.
Western blot
Western blotting was performed as previously described (Owen & Matthews, 2003). Hippocampi were homogenized in ice-cold RIPA lysis buffer and the homogenate centrifuged (4°C, 10 000 g, 10 min). Protein concentration of the supernatant was determined by the Bradford method (Bradford, 1976). Samples were then denatured and separated by SDS-PAGE (8%) and transferred to a nitrocellulose membrane (Bio-Rad, Mississauga, Canada). Nitrocellulose membranes were blocked overnight (4°C) in skimmed milk (5% w/v) phosphate-buffered saline with Tween 20 (PBS-T). Membranes were then washed and incubated with a primary antibody in 5% skimmed milk PBS-T. Primary antibodies used were as follows: rabbit anti-MR (1 : 200; MCR, H-300, sc-11412, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-GR (1 : 500; H-300, sc-8992, Santa Cruz Biotechnology) and rabbit anti-tubulin (1 : 10 000; T-3526, Sigma-Aldrich Corp.). All antibodies have been characterized previously for use in the guinea pig (Owen & Matthews, 2003). Each primary antibody was run on a separate day. Membranes were then washed and incubated in goat anti-rabbit-horseradish peroxidase secondary antibody (1 : 5000, 1 h, 23°C; Perkin-Elmer). Blots were exposed to Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer) and bands were visualized by exposure to Kodak Blue X-OMAT film for 30 s to 1 min (Perkin-Elmer). Membranes were stripped in Restore Western Blot Stripping buffer (Pierce, MJS Biolynx, Mississauga, Canada). The absolute optical density (AOD) of primary antibody was analysed with computerized imaging software. Primary signals for hippocampal MR and GR were standardized to the signal for tubulin (Sigma-Aldrich Corp.). Expression levels for hippocampal proteins are the ratio of primary antibody to tubulin signal, respectively. All Western blots were performed a minimum of two times for each animal and the data were pooled to derive a mean value for each animal.
Statistical analysis
All data were expressed as mean ± standard error of the mean (s.e.m.). For all tests, significance was set at P < 0.05. Hippocampal MR and GR mRNA was analysed by two-way (treatment × region) multivariate analysis of variance (ANOVA). All other data were statistically analysed using one-way (treatment) ANOVA. ANOVAs were followed by Dunnett's method of post hoc comparison.
Results
Hippocampus
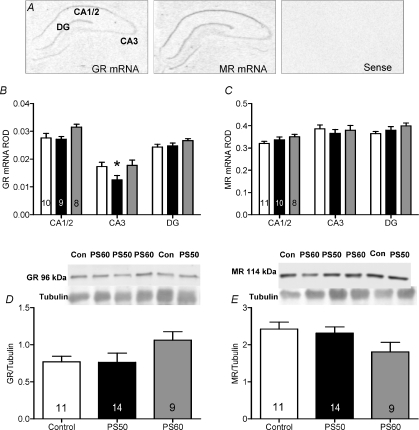
Analysis by two-way ANOVA revealed significant regional differences in GR mRNA expression in the hippocampus (P <0.0001; Fig. 1B). Expression of GR mRNA was highest in the CA1/2 region and lowest in the CA3 region. There was also a significant effect of prenatal stress in the CA3 region with GR mRNA expression significantly lower in PS50 male guinea pig offspring compared to controls (P <0.01; Fig. 1B). Western blot analysis revealed no difference in GR protein expression in whole hippocampal homogenates (Fig. 1D). For MR mRNA expression there was also a significant effect of hippocampal subfield (P <0.0001), but there was no effect of prenatal stress (Fig. 1C). There was also no effect of prenatal stress on hippocampal MR protein levels (Fig. 1E).
Figure 1. Hippocampal glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) mRNA and protein expression in male offspring whose mothers were exposed to a high frequency strobe light for 2 h on gestational days 50, 51 and 52 (PS50; black bars), gestational days 60, 61 and 62 (PS60; grey bars) or left undisturbed throughout pregnancy (control; open bars).
A, representative images of GR, MR and sense in situ hybridization. B, relative optical density (ROD) of GR mRNA. C, ROD of MR mRNA. D, GR protein levels with representative Western blot image above. E, MR protein levels with representative Western blot image above. Animal numbers are indicated within bars. *P < 0.05 compared to control.
Hypothalamus
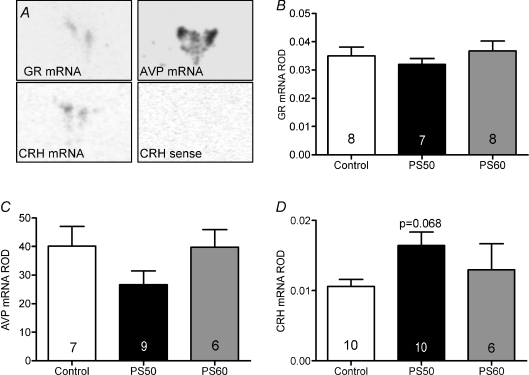
Analysis by one-way ANOVA revealed no significant effect of prenatal treatment on GR mRNA levels in the PVN (Fig. 2B). There was also no difference in AVP mRNA expression levels in the total PVN (data not shown) or in the parvocellular region of the PVN (Fig. 2C). There was a strong trend towards increased CRH mRNA in the PVN in PS50 male offspring compared to controls (P= 0.068; Fig. 2D).
Figure 2. Hypothalamic expression of glucocorticoid receptor (GR) in the paraventricular nucleus (PVN), arginine vasopressin (AVP) mRNA in the PVN, corticotropin-releasing hormone (CRH) mRNA in the PVN, and sense CRH mRNA in male offspring whose mothers were exposed to stress at PS50 and PS60 (as described in Fig. 1), or left undisturbed throughout pregnancy (control).
A, representative in situ hybridization images of AVP, CRH and GR mRNA in the PVN. Relative optical density (ROD) by in situ hybridization of GR mRNA (B), AVP mRNA (C) and CRH mRNA (D). Animal numbers are indicated within bars.
Pituitary
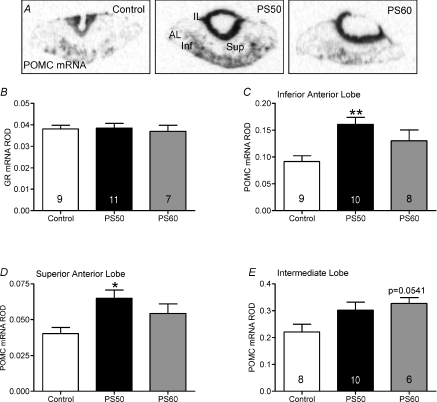
There was no significant effect by one-way ANOVA of prenatal treatment on GR mRNA levels in the anterior pituitary (Fig. 3B). POMC mRNA was significantly increased in the anterior lobe of the pituitary gland in PS50 male offspring (P <0.05; data not shown). Further, POMC mRNA was found significantly increased in both the inferior (P <0.01) and superior (P <0.05) regions of the anterior lobe in PS50 male offspring (Fig. 3C and D). There was also a strong trend (P= 0.0541) towards increased POMC mRNA in the intermediate lobe of PS60 male offspring compared to controls (Fig. 3E).
Figure 3. Pituitary pro-opiomelanocortin (POMC) mRNA expression in male offspring whose mothers were exposed to stress at PS50 and PS60 (as described in Fig. 1), or left undisturbed throughout pregnancy (control).
A, representative in situ hybridization images of POMC mRNA expression in control, PS50 and PS60 pituitary. Relative optical density (ROD) of glucocorticoid receptor (GR) mRNA in the anterior lobe (B), POMC mRNA in the inferior anterior lobe (C), POMC mRNA in the superior anterior lobe (D) and POMC mRNA in the intermediate lobe (E). Animal numbers are indicated within bars. *P < 0.05, **P < 0.01, compared to control. IL, intermediate lobe; AL, anterior lobe; Sup, superior anterior lobe; Inf, inferior anterior lobe.
Adrenal cortex
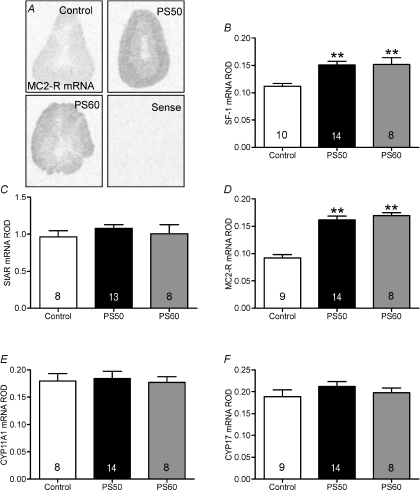
SF-1 and MC2-R mRNA were significantly increased in the adrenal cortex in both PS50 and PS60 male offspring (P <0.001; Fig. 4B and D). There was no significant effect of prenatal stress on adrenal StAR, CYP17 or CYP11A1 mRNA (Fig. 4C, E and F).
Figure 4. SF-1, StAR, MC2-R, CYP11A1 and CYP17 mRNA expression in adrenal gland of male offspring whose mothers were exposed to stress at PS50 and PS60 (as described in Fig. 1), or left undisturbed throughout pregnancy (control).
A, representative in situ hybridization images of melanocortin 2 receptor (MC2-R) mRNA expression in the adrenal. Relative optical density (ROD) of steroidogenic factor (SF)-1 mRNA (B), steroidogenic acute regulatory protein (StAR) mRNA (C), MC2-R mRNA (D), CYP11A1 mRNA (E) and CYP17 mRNA (F). Animal numbers are indicated within bars. **P < 0.01, compared to control.
Discussion
The current study has demonstrated that acute periods of moderate maternal stress during discrete periods of neuroendocrine development alters HPA axis activity by affecting regulation at the level of the brain, pituitary and adrenal in male guinea pig offspring. Further, these changes closely correlate with endocrine data that we have previously reported (Kapoor & Matthews, 2005). Male offspring whose mothers were exposed to stress during the period of rapid fetal brain growth (PS50) exhibited a significant reduction in GR mRNA in the CA3 region of the hippocampus, a trend towards increased CRH mRNA in the hypothalamic PVN and significantly increased POMC mRNA in the anterior lobe of the pituitary. These males also exhibited significantly higher SF-1 and MC2-R mRNA in the adrenal cortex. We have previously demonstrated that PS50 male offspring exhibit increased basal plasma cortisol levels over a 24 h period (Kapoor & Matthews, 2005). In line with this, the reduction in hippocampal GR mRNA demonstrated in the current study would facilitate a decrease in negative feedback of the HPA axis thereby leading to increased basal plasma cortisol levels. In contrast, male offspring whose mothers were exposed to stress during very late gestation (PS60) exhibited significantly higher SF-1 (P <0.001) and MC2-R mRNA (P <0.001) in the adrenal cortex. This was consistent with the significantly higher plasma cortisol response to an ACTH challenge and exposure to a stressor exhibited in PS60 male offspring (Kapoor & Matthews, 2005).
No previous studies have investigated the effect of prenatal stress on hippocampal GR or MR expression in guinea pigs. Studies in rats have been inconsistent, probably due to differences in the prenatal stress paradigm or the protocol used to assess MR and/or GR levels during postnatal life. Rat offspring born to mothers that were exposed to restraint stress during the last week of gestation exhibited increased basal corticosterone levels at PND 21 and a prolonged corticosterone response to stress at PND 90. This was associated with decreased hippocampal MR and GR densities at both ages (Henry et al. 1994). This study would suggest that endocrine changes in HPA axis activity are mediated by changes in negative feedback in the brain. Adult female rat offspring born to mothers exposed to restraint stress during the last week of gestation exhibited increased free corticosterone levels, but this was not associated with changes in hippocampal GR binding (McCormick et al. 1995). However, in the latter study, hippocampal MR was not measured, and potential changes in the MR may have been mediating the alterations in basal corticosterone secretion. Aged female rat offspring, born to mothers exposed to restraint stress during the last week of pregnancy exhibited increased basal and stress-induced corticosterone secretion and this was associated with a decrease in hippocampal GR binding (Szuran et al. 2000). Finally, in adult male rat offspring born to mothers that were exposed to restraint stress during the last week of pregnancy, a blunted HPA axis response to stress was associated with increased hippocampal GR immunoreactivity (Richardson et al. 2006). Therefore, studies in rats have produced mixed results in the specific endocrine and molecular phenotype, but have consistently demonstrated that changes in HPA axis activity are associated with changes in hippocampal negative feedback.
In the current study, GR mRNA was significantly lower in the CA3 region of the hippocampus in PS50 male offspring; however, there was no significant effect of prenatal stress on GR protein levels. This discrepancy is most likely due to the fact that entire hippocampal homogenates were used for the Western blot analysis, therefore any specific hippocampal subfield differences were probably masked. While the relative contribution of the CA3 hippocampal subfield on HPA axis negative feedback is unknown, it has been shown that the CA3 region is particularly vulnerable to the effects of prolonged stress. Indeed, chronic corticosterone exposure in rats led to atrophy of CA3 neurons (de Kloet et al. 2005). Furthermore, male rat offspring born to mothers exposed to restraint stress during the last week of gestation exhibited decreased CA3 cell dendritic tree size, but no change in the dendritic morphology of CA1 pyramidal cells (Hosseini-Sharifabad & Hadinedoushan, 2007). Differences in hippocampal morphology have also been observed in non-human primates. Juvenile rhesus monkeys born to mothers exposed to stress either during early or late gestation exhibited reduced hippocampal volume and decreased neurogenesis in the dentate gyrus of the hippocampus (Coe et al. 2003). These studies demonstrate that the hippocampus is vulnerable to the effects of prenatal stress. This is of particular importance as the hippocampus is involved in HPA axis negative feedback as well as behaviours such as spatial learning and memory (Janus, 2004).
It is well established that testosterone has an inhibitory effect on HPA axis activity (Viau, 2002). In our previous study, we observed dramatically reduced plasma testosterone levels in PS50 male offspring compared to control males. We hypothesized that the reduction in testosterone may mediate the increased basal plasma cortisol that we observed in these animals. Indeed, it has been shown that male rats that had been gonadectomized and given high levels of testosterone exhibited decreased levels of AVP. Although testosterone was decreased by 70% in PS50 male offspring compared to control male offspring in the animals analysed in current study (Kapoor & Matthews, 2005), we failed to identify an effect of prenatal stress on AVP mRNA in either the entire PVN or more specifically in the parvocellular region of the PVN. While AVP is found in magnocellular and parvocellular neurons of the PVN, parvocellular AVP neurons represent the primary source of AVP released at the median eminence (Engelmann et al. 2004). Unfortunately, analysis of AVP content in the median eminence was not possible in the present study, therefore whether the decreased plasma testosterone levels affected AVP protein levels in this region is not known.
The trend towards increased CRH mRNA in the PVN of PS50 male offspring would facilitate increased production of ACTH. Indeed, we have shown significantly higher levels of plasma ACTH at 05.00 h in PS50 male offspring (Kapoor & Matthews, 2005). The significant increase in POMC mRNA in the anterior pituitary gland of PS50 male offspring that we report in the present study would suggest that prenatal stress at 50 days of gestation results in increased pituitary corticotroph drive in adulthood. The increased POMC mRNA expression in the inferior region of the anterior lobe of the pituitary compared to the superior region has been previously described in a number of species (Matthews et al. 1994; Banjanin et al. 2004).
There was also a trend towards increased POMC mRNA expression in the intermediate lobe of the pituitary gland in PS60 male offspring. While basal plasma ACTH was significantly increased at one time point in these animals (Kapoor & Matthews, 2005), POMC produced by the intermediate lobe of the pituitary gland is post-translationally processed to α-melanocyte-stimulating hormone and β-endorphin rather than ACTH (Kjaer et al. 1995). Hence, the relative contribution of increased POMC mRNA in the intermediate lobe of the pituitary in PS60 male offspring towards ACTH secretion is probably minimal in the adult guinea pig.
Both PS50 and PS60 male offspring exhibited significantly elevated SF-1 and MC2-R mRNA in the adrenal cortex. SF-1 is an orphan nuclear receptor that has been shown to positively regulate transcriptional expression of MC2-R, as well as StAR and the CYP enzymes. SF-1 appears to be auto-regulated as there is a SF-1 binding site on the SF-1 gene promoter, and inhibition of SF-1 activity has been shown to reduce expression of endogenous SF-1 secretion (Li et al. 2004). In line with these findings, we demonstrated that elevated SF-1 mRNA was associated with significantly higher MC2-R mRNA expression. An increase in MC2-R production would increase sensitivity of the adrenal cortex to ACTH, representing a possible mechanism by which PS50 male offspring exhibit increased basal plasma cortisol levels and PS60 male offspring exhibit increased activated plasma cortisol levels. Although the administration of maternal stress during different windows of fetal brain development resulted in a different endocrine phenotype, the increase in adrenal glucocorticoid biosynthesis may represent a common pathway by which glucocorticoid secretion is increased. To our knowledge there are no other studies investigating prenatal stress and the expression of steroidogenic enzymes.
In conclusion, this study demonstrates that short periods of prenatal stress during discrete, critical windows of neuroendocrine development differentially affects expression of key factors involved in HPA axis function in adult male guinea pig offspring. Maternal stress applied during the period of rapid brain growth (GD50, 51 and 52) or during very late gestation (GD60, 61 and 62) have very different effects on HPA axis activity and we have demonstrated that these changes in activity appear modulated by altered molecular regulation of the HPA axis. Further, it appears that stress during the period of rapid brain growth is a particularly vulnerable period for programming as these males demonstrated functional changes in HPA axis activity in the hippocampus, hypothalamus, anterior pituitary and adrenal cortex. Understanding the mechanisms by which prenatal stress leads to the endocrine and behavioural phenotypes will allow us to begin to develop interventions that prevent and/or reverse the negative consequences of early life programming.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada (S.G.M.). A.K. was supported by scholarships from The Genesis Research Foundation–Obstetrics and Gynaecology Ontario Graduate Scholarships in Science and Technology and the Canadian Institutes of Health Research.
References
- Banjanin S, Kapoor A, Matthews SG. Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function and blood pressure in mature male guinea pigs. J Physiol. 2004;558:305–318. doi: 10.1113/jphysiol.2004.063669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Kromer SA, Neumann ID. Prenatal stress: Opposite effects on anxiety and hypothalamic expression of vasopressin and corticotropin-releasing hormone in rats selectively bred for high and low anxiety. Eur J Neurosci. 2006;23:541–551. doi: 10.1111/j.1460-9568.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Wittwer DJ, Abbott DH, Schneider ML. Long-term effects of prenatal stress on HPA axis activity in juvenile rhesus monkeys. Dev Psychobiol. 1994;27:257–269. doi: 10.1002/dev.420270502. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Dean F, Matthews SG. Maternal dexamethasone treatment in late gestation alters glucocorticoid and mineralocorticoid receptor mRNA in the fetal guinea pig brain. Brain Res. 1999;846:253–259. doi: 10.1016/s0006-8993(99)02064-8. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 1970;17:115–123. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: An old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Go KS, Lingas R, Wheeler MB, Irwin DM, Matthews SG. Decreased CRH mRNA expression in the fetal guinea pig hypothalamus following maternal nutrient restriction. Brain Res. 2001;896:179–182. doi: 10.1016/s0006-8993(01)02089-3. [DOI] [PubMed] [Google Scholar]
- Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Hosseini-Sharifabad M, Hadinedoushan H. Prenatal stress induces learning deficits and is associated with a decrease in granules and CA3 cell dendritic tree size in rat hippocampus. Anat Sci Int. 2007;82:211–217. doi: 10.1111/j.1447-073X.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. 2003;44:810–818. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- Janus C. Search strategies used by APP transgenic mice during navigation in the morris water maze. Learn Mem. 2004;11:337–346. doi: 10.1101/lm.70104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo-pituitary-adrenal axis activity in male guinea pig offspring. J Physiol. 2005;566:967–977. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer A, Knigge U, Bach FW, Warberg J. Stress-induced secretion of pro-opiomelanocortin-derived peptides in rats: Relative importance of the anterior and intermediate pituitary lobes. Neuroendocrinology. 1995;61:167–172. doi: 10.1159/000126837. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- Li LA, Chang YC, Wang CJ, Tsai FY, Jong SB, Chung BC. Steroidogenic factor 1 differentially regulates basal and inducible steroidogenic gene expression and steroid synthesis in human adrenocortical H295R cells. J Steroid Biochem Mol Biol. 2004;91:11–20. doi: 10.1016/j.jsbmb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Mobley BW, Nickerson JT, Schatzberg AF. Early environmental regulation of glucocorticoid feedback sensitivity in young adult monkeys. J Neuroendocrinol. 2000;12:723–728. doi: 10.1046/j.1365-2826.2000.00505.x. [DOI] [PubMed] [Google Scholar]
- McCabe L, Marash D, Li A, Matthews SG. Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol. 2001;13:425–431. doi: 10.1046/j.1365-2826.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Dynamic changes in glucocorticoid and mineralocorticoid receptor mRNA in the developing guinea pig brain. Brain Res Dev Brain Res. 1998;107:123–132. doi: 10.1016/s0165-3806(98)00008-x. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Challis JR. Levels of pro-opiomelanocortin and prolactin mRNA in the fetal sheep pituitary following hypoxaemia and glucocorticoid treatment in late gestation. J Endocrinol. 1995;147:139–146. doi: 10.1677/joe.0.1470139. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Han X, Lu F, Challis JR. Developmental changes in the distribution of pro-opiomelanocortin and prolactin mRNA in the pituitary of the ovine fetus and lamb. J Mol Endocrinol. 1994;13:175–185. doi: 10.1677/jme.0.0130175. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144:2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: Effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- Van Den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: A prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2007;33:536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Poltyrev T, Schorer-Apelbaum D, Men D, McCarty R. Effect of prenatal stress on plasma corticosterone and catecholamines in response to footshock in rats. Physiol Behav. 1998;64:439–444. doi: 10.1016/s0031-9384(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Wu J, Song TB, Li YJ, He KS, Ge L, Wang LR. Prenatal restraint stress impairs learning and memory and hippocampal PKCb1 expression and translocation in offspring rats. Brain Res. 2007;1141:205–213. doi: 10.1016/j.brainres.2007.01.024. [DOI] [PubMed] [Google Scholar]